Abstract
Purpose
Iodine-131—metaiodobenzylguanidine (131I-MIBG) provides targeted radiotherapy with more than 30% response rate in refractory neuroblastoma, but activity infused is limited by radiation safety and hematologic toxicity. The goal was to determine the maximum-tolerated dose of 131I-MIBG in two consecutive infusions at a 2-week interval, supported by autologous stem-cell rescue (ASCR) 2 weeks after the second dose.
Patients and Methods
The 131I-MIBG dose was escalated using a 3 + 3 phase I trial design, with levels calculated by cumulative red marrow radiation index (RMI) from both infusions. Using dosimetry, the second infusion was adjusted to achieve the target RMI, except at level 4, where the second infusion was capped at 21 mCi/kg.
Results
Twenty-one patients were enrolled onto the study at levels 1 to 4, with 18 patients assessable for toxicity and 20 patients assessable for response. Cumulative 131I-MIBG given to achieve the target RMI ranged from 22 to 50 mCi/kg, with cumulative RMI of 3.2 to 8.92 Gy. No patient had a dose-limiting toxicity. Reversible grade 3 nonhematologic toxicity occurred in six patients at level 4, establishing the recommended cumulative dose as 36 mCi/kg. The median time to absolute neutrophil count more than 500/μL after ASCR was 13 days (4 to 27 days) and to platelet independence was 17 days (6 to 47 days). Responses included two partial responses, eight mixed responses, three stable disease, and seven progressive disease. Responses by semiquantitative MIBG score occurred in eight patients, soft tissue responses occurred in five of 11 patients, but bone marrow responses occurred in only two of 13 patients.
Conclusion
The lack of toxicity with this approach allowed dramatic dose intensification of 131I-MIBG, with minimal toxicity and promising activity.
INTRODUCTION
Neuroblastoma, an embryonal tumor in children, is derived from the peripheral sympathetic nervous system and is metastatic at diagnosis in half of patients. Patients with neuroblastoma have long-term survival of less than 40%, even with intensive multimodality therapy, including myeloablative treatments. Approximately 15% of patients have disease that is refractory to induction chemotherapy, and 50% of children will experience relapse even after attaining remission and undergoing myeloablative consolidation.1 New approaches that do not increase nonspecific toxicity are needed for such resistant tumors.
Iodine-131—metaiodobenzylguanidine (131I-MIBG) is a guanethidine derivative structurally resembling norepinephrine that is internalized in adrenergic tissues via the norepinephrine transporter and stored in neurosecretory granules, and therefore holds promise for cell-specific radiation treatment of neuroblastoma. Large numbers of children with refractory neuroblastoma have been treated with 131I-MIBG, with response rates of 20% to 37%.2–9 Toxicity has mainly been limited to myelosuppression, particularly thrombocytopenia. At activity levels greater than 12 mCi/kg, approximately one third of patients require support with autologous hematopoietic stem cells to avoid prolonged myelosuppression, especially thrombocytopenia.7,9–11 The total activity of 131I-MIBG administered per infusion has been limited by radiation safety concerns. A dose-response relationship is still controversial with 131I-MIBG. Our original phase I escalating-dose trial gave 3 to 18 mCi/kg, with a trend toward a higher response rate in those receiving more than 10 mCi/kg,7 whereas other small trials do not show this trend.12 There are some reports showing responses with small doses of 131I-MIBG repeated every 4 to 8 weeks,13 whereas our large phase II study in 164 patients showed responses in 37% of patients receiving 18 mCi/kg, but 25% for a smaller group without back-up stem cells treated with 12 mCi/kg.9 Most protocols using repetitive infusions of MIBG have spaced treatments at 2 to 4 months to allow hematologic recovery.14 Only one other small pilot study reported using two closely spaced infusions, but without dose escalation.15
We report here a phase I study based on the hypothesis that there is a relationship between delivered activity of 131I-MIBG and tumor response in patients with refractory neuroblastoma. We postulated that 131I-MIBG, given as two closely spaced infusions, may result in greater tumor radiation than is possible with a single 18 mCi/kg dose or from two widely spaced 18 mCi/kg infusions because residual isotope will remain in the MIBG-avid tumor from the first infusion, possibly supplementing the level from the second. We also hypothesized that the hematologic toxicity will be abrogated by autologous peripheral-blood stem-cell rescue (ASCR) after the second MIBG infusion.
PATIENTS AND METHODS
Patient Population
131l-MIBG was synthesized at University of California, San Francisco (San Francisco, CA; IND 32, 147) or the University of Michigan radiopharmacy (Ann Arbor, MI; IND 17, 239). Patients with high-risk neuroblastoma and age 1 to 30 years at diagnosis were eligible if they had refractory or relapsed disease, defined as stable disease at the end of at least 12 weeks of any induction therapy, bone marrow containing more than 100 tumor cells per 105 mononuclear cells by immunocytology after 12 weeks of induction therapy,16 or progressive disease at any time. All patients were required to have demonstrated MIBG uptake in skeletal or soft tissue tumor. Patients had adequate peripheral-blood stem cells (PBSCs; > 1.5 × 106 CD34+ cells/kg) without detectable tumor by immunocytology, or no tumor in bone marrow by routine morphology before PBSC harvest. Patients had normal organ function, absolute neutrophils count (ANC) more than 500/μL, platelets greater than 50,000/μL, and glomerular filtration rate or creatinine clearance of ≥ 60 mL/min/1.73 m2. Any amount of prior chemotherapy was allowed, providing at least 2 weeks had elapsed (3 months for prior myeloablative therapy) and full organ recovery occurred before enrollment. Prior MIBG therapy and prior total-body radiation were excluded, and a 6-month interval was required for large-fields radiation (whole lung, whole abdominal, or craniospinal). The protocol was sponsored by the New Approaches to Neuroblastoma Therapy (NANT) consortium (www.nant.org), and was approved by the US Food and Drug Administration (FDA). Patients received MIBG infusion at University of California (San Francisco, CA) or Children's Hospital of Philadelphia (Philadelphia, PA), and then returned to their respective NANT institutions for ASCR. Twenty-one patients were enrolled onto the study from 2001 to 2005. The study was approved by NANT institutional review boards, and informed consent was obtained for all patients. Participating NANT investigators and institutions are listed in Appendix Table A1 (online only).
Study Design
Patients received intravenous 131I-MIBG on day 0 and again on day 14, given over 2 hours with hydration, with thyroid protection with potassium iodide and potassium perchlorate, and a Foley catheter for bladder protection. Patients remained in a radiation-protected isolation room for 4 to 7 days, until radiation emissions met institutional regulations.17,18 On day 28, 2 weeks after the second infusion, autologous stem cells were infused, with granulocyte colony-stimulating factor 5 μg/kg daily until ANC was more than 1,500/μL for 3 days. The dose of radiation to the whole body and red marrow from the 131I-MIBG was calculated as described using multiple measurements from a ceiling-mounted Geiger counter and measured urinary excretion.17 The activity for the second dose of 131I-MIBG was adjusted such that the desired total red marrow radiation was delivered for the two infusions.
Total red marrow doses were escalated using a standard phase I design in cohorts of three to six patients from 4.0, 6.0, and 8.0 Gy (Table 1). After study initiation, the FDA mandated limiting the activity of the second infusion because of theoretical concerns about excessive liver dose, so not all patients reached the target red marrow radiation index (RMI). Toxicity was graded according to National Cancer Institute Common Toxicity Criteria version 2.0 (http://ctep.cancer.gov/reporting/ctc.html). Dose-limiting toxicity (DLT) included any grade 4 nonhematologic toxicity (excluding asymptomatic metabolic abnormalities, infection unless associated with other organ grade 3 toxicity, hearing, vomiting, fever, anorexia, and mood); and grade 3 renal, pancreatitis, CNS hemorrhage, ischemia, or aphasia. Hematologic DLT included grade 4 hemolysis, grade 4 failure to engraft, life-threatening hemorrhage, and platelet refractoriness. DLT was based on the two 131I-MIBG treatments. Any patient experiencing DLT after the first infusion and before the second would be considered to have DLT, and would not be eligible to receive the second infusion. Otherwise, to be assessable for toxicity, a patient must have received both 131I-MIBG infusions, received ASCR, been observed for at least 4 weeks after ASCR, and reached an ANC ≥ 500/μL and platelets ≥ 20,000/μL without transfusion, or have experienced DLT. Patients not assessable for toxicity were replaced.
Table 1.
Dose Escalation Schema
| Toxicity Level | Red Marrow Index (Gy)* | MIBG Dose (mCi/kg) |
||
|---|---|---|---|---|
| 1 | 2 | Cumulative | ||
| 1 | 4.0 | 12 | † | ∼24 |
| 2 | 6.0 | 15 | † | ∼30 |
| 3 | 8.0 | 18 | † | ∼36 |
| 4 | 8.0 | 21 | ≤ 21 | ∼42 |
Abbreviation: MIBG, metaiodobenzylguanidine.
Red marrow index was calculated from the whole body dose.
All second doses were adjusted such that the desired cumulative whole body dose was delivered, but an amendment for level 4 mandated by the US Food and Drug Administration restricted dose 2 so that it would not exceed dose 1.
Outcome Evaluation
Responses were assessed at day 56 by central review of MIBG scans (n = 20) and, for those with measurable tumor, computed tomography (CT) scans (n = 11) by a radiologist and nuclear medicine physician, blinded to patient identity and outcome. Response in measurable soft tissue lesions was evaluated according to Response Evaluation Criteria in Solid Tumors Group criteria.19 To quantify the response by MIBG scan, a score was assigned to all pretherapy and day 56 post-therapy MIBG scans, and response was defined as a relative score of ≤ 0.5.20,21 Overall response for all patients was assigned using NANT response criteria that incorporate a modification of the International Neuroblastoma Response Criteria,22 using Response Evaluation Criteria in Solid Tumors Group criteria for solid lesions, semiquantitative MIBG score for bone metastases, and only considering a bone marrow response if all tumor cleared because quantization was difficult due to the uneven involvement seen in neuroblastoma. Outcome was defined as overall survival (OS), calculated from treatment start to death as a result of any cause, and progression-free survival (PFS), calculated from treatment start until progression or death, regardless of subsequent nonprotocol therapy. OS and PFS were summarized using Kaplan-Meier plots.23 SEs of the OS/PFS estimates were based on Greenwood's formula.24
RESULTS
Toxicity and Dose Escalation
Twenty-one patients were enrolled onto the study; of these, 20 patients were assessable for response and 15 patients were assessable for dose escalation. The one patient not assessable for response received only one dose of MIBG due to technical issues. Patients (Appendix Table A2, online only) had highly refractory disease, with a median of three prior treatment regimens, including 12 who had relapsed after myeloablative therapy. Nineteen had bone lesions, 13 had bone marrow metastases, 11 had measurable soft tissue lesions, and six had elevated urine catecholamines at entry. No patient experienced DLT. The only nonhematologic toxicities greater than grade 2 were seen at level 4, including 10 grade 3 toxicities in six patients (Table 2). Nearly all patients experienced myelosuppression and required transfusions of blood and platelets, an expected toxicity of 131I-MIBG. The dose escalation was halted at level 4 due to institutional radiation safety limitations on the allowable activity per infusion (800 to 1,000 mCi) and the increased number of grade 3 toxicities seen. All assessable patients engrafted. The three patients at level 1 completed treatment without grade 3 or 4 nonhematologic toxicity.
Table 2.
Nonhematologic Toxicity Summary
| Toxicity Category | Dose Level and Maximum Grade Experienced |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 3) |
2 (n = 7) |
3 (n = 3) |
4 (n = 8) |
|||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Hepatic | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 3 | 1 | 3 | 0 |
| Constitutional symptoms | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Cardiovascular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Pulmonary | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Renal/genitourinary | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Infection/febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 0 |
| Pain | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Hemorrhage | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 |
| GI | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 0 |
| Coagulation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Endocrine | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Metabolic/laboratory | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 1 | 1 | 0 |
| Musculoskeletal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Neurology | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Any nonhematologic toxicity (No. of patients by highest grade)* | 1 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 6 | 0 |
NOTE. Including the toxicity “unlikely” related to treatment but excluding “unrelated” toxicities.
Each patient is accounted for once in each row, so a patient who has experienced grade 1 neurologic, grade 2 pain and hearing, grade 3 vomiting and diarrhea, and grade 4 hepatic toxicities is listed as having had a grade 4 nonhematologic toxicity as the greatest toxicity.
Seven patients were treated at level 2. Four were not assessable for dose escalation for the following reasons: one was not assessable for RMI and was replaced; three were treated on dose level 2 pending discussions with the FDA regarding adjustment of the second dose and addition of new dose levels. No patient experienced DLT, although one patient had grade 3 increase in partial thromboplastin time, unrelated to the MIBG treatment. Three patients were treated at dose level 3 without grade 3 or 4 nonhematologic toxicity.
Eight patients were treated at dose level 4. The first patient had grade 3 elevation of AST; the second patient had asymptomatic grade 3 hyperglycemia and grade 3 elevations of AST and ALT. The third patient had disease progression before platelet engraftment, thus becoming unassessable for engraftment or dose escalation. This latter patient also had grade 3 epistaxis, petechiae, and purpura. The patient was replaced in the study and the dose level expanded. The fourth patient had no grade 3 or 4 toxicity. The fifth patient experienced grade 3 pulmonary and GI toxicity, hematemesis, and febrile neutropenia, but no DLT. Due to a technical problem, this patient did not receive the second MIBG dose, had ASCR a week late, and was replaced. The sixth patient had grade 3 gamma-glutamyltransferase; the seventh patient had grade 3 nausea; the eighth patient (the sixth patient assessable for toxicity) had no grade 3 or 4 nonhematologic toxicity.
MIBG Dose and RMI
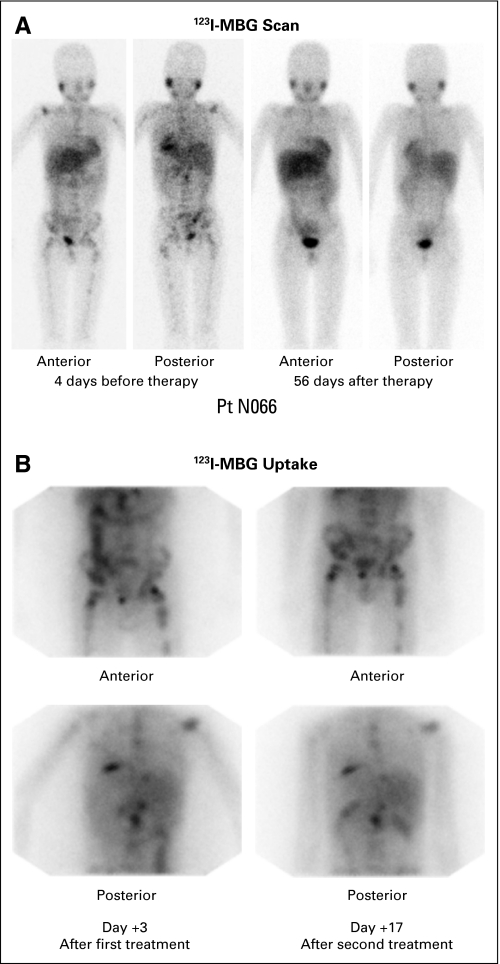
The targeted RMI for level 2 (6.0 Gy) and 3 (8.0 Gy) was not generally achievable with radiation safety compliant doses (Table 3). After discussions with the FDA, the protocol was amended to add two subsequent dose levels to be given at 21 mCi/kg for two treatments, and then 24 mCi/kg for two treatments. At these new dose levels, the dose of the second infusion was not permitted to exceed the dose of the first infusion. The dose escalation was halted at level 4 due to safety constraints, as previously discussed. The mean RMI per millicurie of 131I-MIBG for the first infusion was 0.0081 Gy/mCi (n = 21), and for the second infusion it was 0.0076 Gy/mCi. In 13 patients, the RMI decreased by 0.0019 Gy/mCi; in seven patients, the RMI increased by 0.0015 Gy/mCi. Figure 1 demonstrates the MIBG uptake in a patient responding to therapy (Fig 1A) with excellent isotope accumulation after each infusion (Fig 1B).
Table 3.
Median Cumulative MIBG Activity and Red Marrow Index in 20 Assessable Patients*
| Dose Level | No. of Patients |
Red Marrow Index (Gy) |
MIBG (mCi/kg) |
||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| 1 | 3 | 452 | 320 to 505 | 23 | 22 to 24 |
| 2 | 7 | 534 | 377 to 592 | 31 | 24 to 35 |
| 3 | 3 | 577 | 572 to 657 | 49 | 41 to 51 |
| 4† | 7 | 642 | 487 to 892 | 41 | 32 to 44 |
Abbreviation: MIBG, metaiodobenzylguanidine.
One of 21 patients at level 4 only received one infusion due to a technical problem and was excluded from this analysis.
A second dose at level 4 was capped at 21 mCi/kg.
Fig 1.
Patient N066 with refractory neuroblastoma treated at dose level 4 on day 0 (709 mCi; cumulative red marrow radiation index [RMI], 4.74 Gy) and day +14 (497 mCi; RMI, 4.18 Gy). (A) Iodine-123—metaiodobenzylguanidine (MIBG) scan pretherapy and on day +56 post therapy. Pretherapy scan showed multiple sites of skeletal uptake throughout the spine, pelvis, femurs, and humeri, with physiologic uptake in salivary glands, nasopharynx, liver, and bladder. The post- therapy day +56 scan showed only very faint uptake in the left proximal humerus and left pelvis and the usual physiologic uptake. (B) Iodine-131—MIBG uptake immediately after therapy 1 (day +3) and 2 (day +17).
Engraftment
Nineteen of the 21 patients were fully assessable for hematologic toxicity and engraftment. Of the 21 assessable patients for neutrophil engraftment, seven never had ANC less than 500/μL. The median time to ANC more than 500/μL was 13 days (range, 4 to 27 days) in the 14 assessable patients. Two patients never had platelet count less than 20,000/μL and one patient was not assessable for platelet engraftment due to disease progression and additional chemotherapy added at day 40. The median time to platelet transfusion independence was 17 days (range, 6 to 47 days) in 18 assessable patients.
Response, Event-Free Survival, and OS
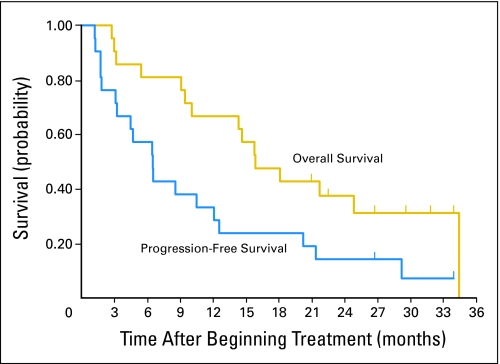
Twenty of 21 patients were fully assessable for response because one patient received only one of the two MIBG therapies for technical reasons (Table 4). Thirteen patients showed a decrease in extent of disease by MIBG scan; of these, nine patients (eight fully assessable) had a semiquantitative relative extension score post-therapy of ≤ 0.5, qualifying for partial response (PR) by MIBG response criteria (Fig 1). Eight of nine patients with relative extension scores less than 0.5 also had a relative intensity score less than 0.5, showing good correlation between the two evaluations. Two of 13 assessable patients had a CR in bone marrow (one cleared, one matured to ganglioneuroma), and five of 11 patients had a PR in CT-measurable lesions. Two patients had an overall PR, as evaluated by response in bone lesions by MIBG, without assessable bone marrow or soft tissue disease. Eight patients had MR, due to PR in one of the measures, but stable disease in the other parameters; most often a response by MIBG but persistent bone marrow disease. Three of 20 patients had disease stabilization and seven patients had progressive disease. Urine catecholamines were a less sensitive measure of disease than the radiologic evaluations, with only six of the patients having elevated vanillylmandelic acid or homovanillic acid values at study entry. The PFS at 6 and 12 months (mean ± SE) was 57% ± 11% and 33% ± 11% respectively, and the OS at 12 months and 18 months was 67% ± 10% and 48% ± 11%, respectively (Fig 2). When patients starting other therapy before progression were counted as an event, the median event-free survival was only 81 days, compared with PFS of 199 days (data not shown).
Table 4.
Response in All Patients (20 of 21 patients fully assessable) on Day 56
| Toxicity Level | Patient | MIBG |
BM Response | CT Response | Overall Response | |
|---|---|---|---|---|---|---|
| Response | Relative Score | |||||
| 1 | N0021 | PD | 1.2 | SD | SD | PD |
| N0025 | SD | 1.0 | PD | PR | PD | |
| N0026 | PD | 1.5 | — | PD | PD | |
| 2 | N0033 | PR | 0.4 | — | PR | PR |
| N0035 | PR | 0.4 | — | — | PR | |
| N0038 | CR | 0.0 | SD | — | MR | |
| N0042 | SD | 1.0 | CR | — | MR | |
| N0048 | PD | 1.5 | SD | PD | PD | |
| N0057 | SD | 0.8 | SD | — | SD | |
| N0058 | SD | 0.8 | — | — | SD | |
| N0047 | PR | 0.3 | SD | SD | MR | |
| 3 | N0054 | PD | 3.0 | — | — | PD |
| N0056 | PR | 0.4 | SD | SD | MR | |
| N0044 | SD | 0.7 | CR* | PR | MR | |
| N0066 | PR | 0.3 | SD | — | MR | |
| 4 | N0067 | PD | 2.0 | SD | PR | PD |
| N0069 | PR | † | PD | SD | PD | |
| N0070‡ | PR | 0.2 | SD | — | SD‡ | |
| N0076 | PR | 0.3 | SD | — | MR | |
| N0077 | PR | 0.4 | SD | PR | MR | |
| N0094 | SD | 1.0 | — | — | SD | |
NOTE: A dash indicates no assessable disease on study and at follow-up.
Abbreviations: 131I, iodine-131; MIBG, metaiodobenzylguanidine; BM, bone marrow; CT, computed tomography; CR, complete response; SD, stable disease; PD, progressive disease; PR, partial response; MR, mixed response.
BM went from neuroblastoma to all ganglioneuroma.
Scans unavailable for central review; PR by local assessment.
N0070 was the patient who received only one infusion of 131I-MIBG and was not counted in the summation of response for the 20 assessable patients.
Fig 2.
Overall survival and progression-free survival (PFS) of all 21 patients with median follow-up for all patients at 16 months, and surviving patients at 28 months. PFS was calculated regardless of subsequent therapy.
DISCUSSION
The results reported here showed that closely spaced infusions of 131I-MIBG can be administered safely using ASCR without dose-limiting nonhematologic toxicity and with rapid and reliable reconstitution of hematopoiesis. Using this approach, the activity of 131I-MIBG used in a prior phase II study was safely doubled, and red marrow radiation doses up to an average of 6.00 Gy were tolerated. To our knowledge, the only prior published study attempting this approach was the pilot study reported in eight children with relapsed neuroblastoma treated with 12 mCi/kg of 131I-MIBG repeated at a 2-week interval with a target whole-body dose of 4.00 Gy, also including intravenous topotecan days 1 through 5 and days 15 to 19, followed by ASCR on day 27.15 That study primarily reported the whole-body dosimetry, without toxicity and response data. Previous reports of multiple infusions of 131I-MIBG were without mandatory ASCR, and therefore the infusions were widely spaced to allow hematopoietic recovery. In a prior phase II study, 62 MIBG therapies were administered to 28 patients, with 24 patients receiving two infusions, two patients receiving three infusions, and two patients receiving four infusions. The median time between infusions was 3 months, and the hematologic toxicity was dose limiting.14 Kang et al12 studied eight patients, including four who received two infusions, and four who received three infusions at a median dose of 10 mCi/kg, with grade 3 hematologic toxicity in half of the patients. Other investigators have used multiple infusions at varying but often shorter intervals at much lower activity levels of 131I-MIBG, with set doses not adjusted by patient weight, either in refractory patients or as neoadjuvant therapy at diagnosis, with frequent hematologic toxicity.2,6,25,26
The striking finding of our study is the near universal tolerability of these high-activity infusions, as only one patient had a nonhematologic toxicity greater than grade 2 at the first three dose levels. Even at cumulative activity administered up to 50 mCi/kg, the only grade 3 nonhematologic toxicities occurred at level 4, and were reversible. The hematologic toxicity was abrogated in all patients by ASCR, with rapid engraftment of neutrophils and platelets, although most patients required temporary transfusion support. There was only one patient with grade 3 infection, and two instances of grade 3 hemorrhage.
This regimen clearly demonstrated activity against highly resistant neuroblastoma. Five of the patients had primary refractory disease, without response to conventional therapy, and the other 16 patients had metastatic relapsed disease after multiple regimens, including myeloablative therapy in 12 patients. Not surprisingly, the PFS in our double-infusion trial was short, despite the fact that eight patients began other therapy before progression. However, with stringent blinded central review, two patients had overall PR of their disease. Eight fully assessable patients had a PR in bone lesions by semiquantitative relative MIBG score ≤ 0.5, and 12 patients showed improvement by MIBG scan. Five of 11 patients with assessable soft tissue disease had a PR by CT scan review. Of the eight patients who had a mixed response to therapy, five patients had failed to clear their bone marrow disease despite response either by MIBG score or by CT scan. Of all 13 patients with disease in bone marrow on study, only two patients responded to the double infusion by clearing disease and two other patients developed new bone marrow disease. This is similar to the results of a phase II study of single-infusion MIBG, where the bone marrow tumor only cleared in 17 of 69 assessable patients, possibly because the physical properties of 131I are suboptimal for delivery of radiation to bone marrow single cells and micrometastases.9,27 Future trials with MIBG should include a combination of a chemotherapeutic agent with the radionuclide to improve effectiveness against bone marrow disease. An ongoing NANT study (N04-06) is testing the radiosensitizing cytotoxic camptothecin, irinotecan, with 131I-MIBG.
Decrease in expected red marrow dose was seen for the second infusion in 13 of 20 patients receiving the two rapid-sequence infusions, despite careful dosimetry estimates for the second dose. This suggests that either tumor-cell kill resulted in less isotope retention on the second therapy, or there was some persisting saturation of the norepinephrine transporter or the catecholamine storage granules at the time of the second therapy. However, accurate tumor dosimetry would be required to estimate the difference in tumor uptake, and despite multiple attempts, current methods and technology are too approximate to yield accurate measurements17,28–30 Furthermore, many of the patients on this study did not have measurable soft tissue lesions that would lend themselves to dosimetry with single-photon emission CT.
In conclusion, rapid-sequence double-infusion of 131I-MIBG with stem-cell support was feasible, with demonstrable activity against resistant neuroblastoma without significant toxicity at a cumulative dose of 36 mCi/kg, the level recommended for future studies. Efficacy may be further improved by combination with a cytotoxic or biologic agent to target bone marrow disease in addition to bone lesions. A randomized trial would be required to determine whether this approach was more beneficial than a single infusion or a more widely spaced treatment with 131I-MIBG.
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Table A1.
Participating NANT Consortium Investigators and Institutions
| Investigator | Institution |
|---|---|
| Judy G. Villablanca, MD | Children's Hospital Los Angeles |
| Katherine K. Matthay, MD | University of California San Francisco School of Medicine |
| Clare Twist, MD | Lucille Salter Packer Children's Hospital |
| John M. Maris, MD | Children's Hospital of Philadelphia |
| Susan Cohn, MD | Children's Memorial Hospital |
Abbreviation: NANT, New Approaches to Neuroblastoma Therapy.
Table A2.
Patient Characteristics (N = 21)
| Characteristic | Patients (N = 21) |
|
|---|---|---|
| No. of Patients Affected | Total No. of Patients Involved | |
| Time from diagnosis, months | ||
| Median | 26 | |
| Range | 6 to 80 | |
| Age at diagnosis, years* | ||
| Median | 3.9 | |
| Range | 1.7 to 11.2 | |
| Age at entry, years | ||
| Median | 6.5 | |
| Range | 2.8 to 13.5 | |
| Sex | ||
| Male | 9 | |
| Female | 12 | |
| MYCN amplification | 4 | 17 |
| Disease status | ||
| Primary refractory | 5 | |
| Relapsed or progressive | 16 | |
| Prior therapy | ||
| Median | 3 | |
| Range | 2 to 11 | |
| Prior ASCR | 12 | |
| Prior radiotherapy | 16 | |
| GFR > 100 mL/min/1.73 m2 | 17 | 21 |
| Disease sites | ||
| Bone | 19 | |
| Bone marrow | 13 | |
| Soft tissue | 12 | |
| Elevated urine VMA and/or HVA | 6 | 20 |
Abbreviations: ASCR, autologous stem-cell rescue; GFR, glomerular filtration rate; VMA, vanillylmandelic acid; HVA, homovanillic acid.
Only one patient at diagnosis and two patients at time on study were older than 10 years of age.
Footnotes
Supported in part by the National Institutes of Health Grants No. PO1 CA81403, R21 CA097758, CCSG CA82103, 2MO1 RR0127, M01-RR00240, as well by donations from the Campini Foundation, the Conner Research Fund, the Katie Dougherty Foundation, Kasle and Tkalcevik Neuroblastoma Research Fund, the Thrasher Research Fund, Alex's Lemonade Stand Foundation, the Evan T.J. Dunbar Neuroblastoma Foundation, Children's Neuroblastoma Cancer Foundation, Milken Family Foundation, and Pediatric Cancer Research Foundation.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Katherine K. Matthay, John Huberty, Randall A. Hawkins, Susan Groshen, Gregory Yanik, Janet Veatch, Judith G. Villablanca, John M. Maris
Administrative support: Alekist Quach, Janet Veatch, Patricia Brophy, Judith G. Villablanca
Provision of study materials or patients: Katherine K. Matthay, John Huberty, Suzanne Shusterman, Janet Veatch, Patricia Brophy, John M. Maris
Collection and assembly of data: Alekist Quach, John Huberty, Susan Groshen, Janet Veatch, Patricia Brophy, Judith G. Villablanca
Data analysis and interpretation: Katherine K. Matthay, Alekist Quach, Benjamin L. Franc, Randall A. Hawkins, Hollie Jackson, Susan Groshen, Gregory Yanik, Judith G. Villablanca, John M. Maris
Manuscript writing: Katherine K. Matthay, Judith G. Villablanca, John M. Maris
Final approval of manuscript: Katherine K. Matthay, Alekist Quach, John Huberty, Benjamin L. Franc, Randall A. Hawkins, Hollie Jackson, Susan Groshen, Suzanne Shusterman, Gregory Yanik, Janet Veatch, Patricia Brophy, Judith G. Villablanca, John M. Maris
REFERENCES
- 1.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 2.Garaventa A, Bellagamba O, Lo Piccolo MS, et al. 131I-metaiodobenzylguanidine (131I-MIBG) therapy for residual neuroblastoma: A mono-institutional experience with 43 patients. Br J Cancer. 1999;81:1378–1384. doi: 10.1038/sj.bjc.6694223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchinson RJ, Sisson JC, Miser JS, et al. Long-term results of [131I]metaiodobenzylguanidine treatment of refractory advanced neuroblastoma. J Nucl Biol Med. 1991;35:237–240. [PubMed] [Google Scholar]
- 4.Klingebiel T, Feine U, Treuner J, et al. Treatment of neuroblastoma with [131I]metaiodobenzylguanidine: Long-term results in 25 patients. J Nucl Biol Med. 1991;35:216–219. [PubMed] [Google Scholar]
- 5.Lashford LS, Lewis IJ, Fielding SL, et al. Phase I/II study of iodine 131 metaiodobenzylguanidine in chemoresistant neuroblastoma: A United Kingdom Children's Cancer Study Group investigation. J Clin Oncol. 1992;10:1889–1896. doi: 10.1200/JCO.1992.10.12.1889. [DOI] [PubMed] [Google Scholar]
- 6.Lumbroso J, Hartmann O, Schlumberger M. Therapeutic use of [131I]metaiodobenzylguanidine in neuroblastoma: A phase II study in 26 patients—“Societe Francaise d'Oncologie Pediatrique” and Nuclear Medicine Co-investigators. J Nucl Biol Med. 1991;35:220–223. [PubMed] [Google Scholar]
- 7.Matthay KK, DeSantes K, Hasegawa B, et al. Phase I dose escalation of 131I-metaiodobenzylguanidinewith autologous bone marrow support in refractory neuroblastoma. J Clin Oncol. 1998;16:229–236. doi: 10.1200/JCO.1998.16.1.229. [DOI] [PubMed] [Google Scholar]
- 8.Voûte PA, Hoefnagel CA, de Kraker J, et al. Results of treatment with 131 I-metaiodobenzylguanidine131 I-MIBG in patients with neuroblastoma. Future prospects of zetotherapy. Prog Clin Biol Res. 1991;366:439–445. [PubMed] [Google Scholar]
- 9.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 10.DuBois SG, Messina J, Maris JM, et al. Hematologic toxicity of high-dose iodine-131-metaiodobenzylguanidine therapy for advanced neuroblastoma. J Clin Oncol. 2004;22:2452–2460. doi: 10.1200/JCO.2004.08.058. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg SS, DeSantes K, Huberty JP, et al. Engraftment after myeloablative doses of 131I-metaiodobenzylguanidine followed by autologous bone marrow transplantation for treatment of refractory neuroblastoma. Med Pediatr Oncol. 1998;30:339–346. doi: 10.1002/(sici)1096-911x(199806)30:6<339::aid-mpo7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 12.Kang TI, Brophy P, Hickeson M, et al. Targeted radiotherapy with submyeloablative doses of 131I-MIBG is effective for disease palliation in highly refractory neuroblastoma. J Pediatr Hematol Oncol. 2003;25:769–773. doi: 10.1097/00043426-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hoefnagel CA, De Kraker J, Valdes Olmos RA, et al. [131I]MIBG as a first line treatment in advanced neuroblastoma. Q J Nucl Med. 1995;39:61–64. [PubMed] [Google Scholar]
- 14.Howard JP, Maris JM, Kersun LS, et al. Tumor response and toxicity with multiple infusions of high dose (131)I-MIBG for refractory neuroblastoma. Pediatr Blood Cancer. 2005;44:232–239. doi: 10.1002/pbc.20240. [DOI] [PubMed] [Google Scholar]
- 15.Gaze MN, Chang YC, Flux GD, et al. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother Radiopharm. 2005;20:195–199. doi: 10.1089/cbr.2005.20.195. [DOI] [PubMed] [Google Scholar]
- 16.Seeger RC, Reynolds CP, Gallego R, et al. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: A Children's Cancer Group Study. J Clin Oncol. 2000;18:4067–4076. doi: 10.1200/JCO.2000.18.24.4067. [DOI] [PubMed] [Google Scholar]
- 17.Matthay KK, Panina C, Huberty J, et al. Correlation of tumor and whole-body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with (131)I-MIBG. J Nucl Med. 2001;42:1713–1721. [PubMed] [Google Scholar]
- 18.Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: A new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol. 2006;24:500–506. doi: 10.1200/JCO.2005.03.6400. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 21.Messina JA, Cheng SC, Franc BL, et al. Evaluation of semi-quantitative scoring system for metaiodobenzylguanidine (mIBG) scans in patients with relapsed neuroblastoma. Pediatr Blood Cancer. 2006;47:865–874. doi: 10.1002/pbc.20777. [DOI] [PubMed] [Google Scholar]
- 22.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Miller RG., Jr . New York, NY: John Wiley & Sons; 1981. Survival Analysis; p. 51. [Google Scholar]
- 25.Klingebiel T, Berthold F, Treuner J, et al. Metaiodobenzylguanidine (mIBG) in treatment of 47 patients with neuroblastoma: Results of the German Neuroblastoma Trial. Med Pediatr Oncol. 1991;19:84–88. doi: 10.1002/mpo.2950190203. [DOI] [PubMed] [Google Scholar]
- 26.De Kraker J, Hoefnagel CA, Caron H, et al. First line targeted radiotherapy, a new concept in the treatment of advanced stage neuroblastoma. Eur J Cancer. 1995;31A:600–602. doi: 10.1016/0959-8049(95)00063-o. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro B, Sisson JC, Shulkin BL, et al. The current status of radioiodinated metaiodobenzylguanidine therapy of neuro-endocrine tumors. Q J Nucl Med. 1995;39:55–57. [PubMed] [Google Scholar]
- 28.Flower MA, Fielding SL. Radiation dosimetry for 131I-mIBG therapy of neuroblastoma. Phys Med Biol. 1996;41:1933–1940. doi: 10.1088/0031-9155/41/10/006. [DOI] [PubMed] [Google Scholar]
- 29.Smith SL, Vincent RM, Perkins AC, et al. Does simple estimation of 131I-metaiodobenzylguanidine uptake in patients with neural crest tumours correlate with clinical outcome? Nucl Med Commun. 2001;22:257–260. doi: 10.1097/00006231-200102000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Buckley SE, Saran FH, Gaze MN, et al. Dosimetry for fractionated (131)I-mIBG therapies in patients with primary resistant high-risk neuroblastoma: Preliminary results. Cancer Biother Radiopharm. 2007;22:105–112. doi: 10.1089/cbr.2007.301. [DOI] [PubMed] [Google Scholar]