Abstract
The serine-threonine kinase mammalian target of rapamycin (mTOR) plays a major role in the regulation of protein translation, cell growth, and metabolism. Alterations of the mTOR signaling pathway are common in cancer, and thus mTOR is being actively pursued as a therapeutic target. Rapamycin and its analogs (rapalogs) have proven effective as anticancer agents in a broad range of preclinical models. Clinical trials using rapalogs have demonstrated important clinical benefits in several cancer types; however, objective response rates achieved with single-agent therapy have been modest. Rapalogs may be more effective in combination with other anticancer agents, including chemotherapy and targeted therapies. It is increasingly apparent that the mTOR signaling network is quite complex, and rapamycin treatment leads to different signaling responses in different cell types. A better understanding of mTOR signaling, the mechanism of action of rapamycin, and the identification of biomarkers of response will lead to more optimal targeting of this pathway for cancer therapy.
INTRODUCTION
mTOR Signaling and Cancer
mTOR signaling plays a key role in cell growth, protein translation, autophagy, and metabolism. Activation of mTOR contributes to the pathogenesis of many tumor types. Upstream, phosphatidylinositol 3′-kinase (PI3K)/Akt signaling is deregulated through a variety of mechanisms, including overexpression or activation of growth factor receptors such as human epidermal growth factor receptor 2 (HER-2) and insulin-like growth factor receptor (IGFR), mutations in PI3K and mutations/amplifications of Akt.1–4 Tumor suppressor phosphatase and tensin homolog deleted from chromosome 10 (PTEN) is a negative regulator of PI3K signaling. PTEN expression is decreased in many cancers, including breast, endometrial, thyroid, and prostate cancers; melanoma; and glioblastoma. PTEN may be downregulated through several mechanisms, including mutations, loss of heterozygosity, methylation, aberrant expression of regulatory microRNA, and protein instability. Activated mTOR signaling is also associated with tumor-predisposition syndromes: Cowden's syndrome (PTEN mutations), Peutz-Jeghers syndrome (LKB1 mutations), tuberous sclerosis (TSC1/2 mutations), and neurofibromatosis (NF1 mutations).5–8 Thus mTOR signaling is activated in conditions of proliferative dysregulation and in many cancer types.
Activation of mTOR results in phosphorylation of its effectors, the best studied of which are eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1). 4E-BP1 hyperphosphorylation leads to inhibition of 4E-BP binding to eukaryotic initiation factor 4E (eIF4E), activating translation. eIF4E is rate-limiting for cap-dependent translation. The translational efficiency of mRNA with highly complex 5′ untranslated regions is especially dependent on eIF4E.9 eIF4E enhances cell proliferation, survival, and angiogenesis by leading to selective translation of mRNA such as cyclin D1, Bcl-2, Bcl-xL and vascular endothelial growth factor (VEGF)9,10 as well as the nucleocytoplasmic transport of selected mRNA such as cyclin D1.11 S6K1 is a key regulator of cell growth. It phosphorylates ribosomal protein S6 and, in some models, enhances the translation of mRNAs possessing a 5′ terminal oligopyrimidine tract. S6K1 also phosphorylates other important targets, including insulin receptor substrate 1 (IRS-1), eukaryotic initiation factor 4B, programmed cell death 4, eukaryotic elongation factor-2 kinase, mTOR, glycogen synthase kinase 3, and S6K1 Aly/REF-like target.12 Both eIF4E and S6K1 are implicated in cellular transformation, and their overexpression has been linked to poor cancer prognosis.9,13,14 Rapamycin and its analogs bind FK506 binding protein, and this complex binds to mTOR, inhibiting downstream signaling. Rapamycin causes cell cycle arrest in a broad spectrum of tumor types. In addition to direct antitumor effects, rapamycin also inhibits endothelial cell proliferation, hypoxia inducible factor 1 and VEGF expression, angiogenesis, and vascular permeability.15,16 Taken together, these data demonstrate the importance of mTOR signaling in cancer and support a role for mTOR as an antitumor target.
mTOR Signaling Network
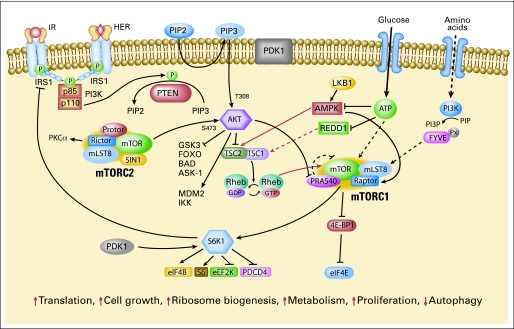
The intricate mTOR signaling network (Fig 1) needs to be better understood to effectively target the pathway. mTOR exists in two multiprotein complexes: mTOR complexes 1 and 2 (mTORC1 and mTORC2). mTORC1 consists of mTOR, mammalian LST8 (mLST8), proline-rich Akt substrate 40 (PRAS40), and raptor.17 PRAS40 has been proposed to be a negative regulator when bound to mTORC1.18 PRAS40 itself may be a substrate of mTOR that is phosphorylated on activation by upstream regulators and released from mTORC1.18 mTORC1 activation results in phosphorylation of 4E-BP1 and S6K1. mTORC2 consists of mTOR, mLST8 (GßL), mSIN1, PRR5 (protor), and rictor.19–23 mTORC2 phosphorylates Akt at Ser473 and has been proposed to regulate the ability of integrin-linked kinase to promote Akt phosphorylation.24–26 Akt Ser473 phosphorylation leads to full Akt activation and may affect its substrate specificity, with activation of Akt toward the Forkhead transcription factor FOXO and the apoptosis regulator BAD.19 mTORC2 has been proposed to regulate phosphorylation of PKCα, control actin cytoskeleton and is linked to cell migration.19,24,27
Fig 1.
The mammalian target of rapamycin (mTOR) signaling network. Arrows represent activation, bars represent inhibition. mTOR signaling regulates multiple critical cellular processes by integrating energy and nutrient stutus and PI3K/Akt signaling induced by growth factors and insulin.
mTOR signaling is regulated by growth factor signaling as well as nutrient (amino acid) and energy status. PI3K/Akt signaling regulates mTOR through phosphorylation/inactivation of mTOR's negative regulator TSC2.28–30 TSC2 contains a GTPase activating domain that inactivates Rheb GTPase, which associates with and directly activates mTORC1. Ras/MAPK signaling also inhibits TSC2.31 Furthermore, TSC2 is regulated by cellular energy sensor AMP kinase.7 When cellular energy stores are reduced or AMP levels increase, AMPK is activated, phosphorylating and activating TSC2 to inhibit mTOR signaling, reducing protein synthesis. Although the exact mechanism of nutrient signaling remains unclear, amino acids are thought to mediate mTORC1 signaling through class III PI3K hVps34.32
mTORC1 is rapamycin-sensitive; rapamycin results in dephosphorylation of 4E-BP1 and S6K1. In contrast, mTORC2 was originally thought to be rapamycin-insensitive.19,24 However, rapamycin regulates rictor phosphorylation, suggesting that components of mTORC2 may be regulated by rapamycin.33 Further, prolonged rapamycin treatment reduces mTORC2 levels and inhibits Akt activation in some cell lines.24,34
Rapamycin induces Akt activation in some models.35,36 Insulin-like growth factor I (IGF-I) and insulin-dependent induction of the PI3K/Akt pathway leads to feedback inhibition of signaling due to mTOR/S6K-mediated phosphorylation and degradation of IRS-1. Rapamycin-induced Akt activation has been attributed to loss of this negative-feedback loop.35,36 The effect of rapamycin on Akt may vary with drug dose, with lower doses leading to an increase in Akt activation and higher doses diminishing Akt activity.16,37 The effect on Akt also varies with cell type, with rapamycin leading to an increase in Akt phosphorylation in some cell lines, and no change or a decrease in others.38 The response of Akt may depend on the activity of upstream-signaling pathways and whether the mTORC2 complex is maintained.
INCORPORATION OF mTOR-TARGETED THERAPY INTO CLINICAL PRACTICE
Single-Agent Rapamycin Analogs in Clinical Trials
Cinical trials are ongoing with rapamycin and its analogs temsirolimus (Torisel, CCI-779, Wyeth Pharmaceuticals, Madison, NJ), everolimus (RAD001, Novartis, Basel, Switzerland). and AP23573 (Ariad Pharmaceuticals, Cambridge, MA) in various tumor types. Although mTOR plays a central role in many biologic processes, rapalogs have been generally well tolerated. Toxicities have included asthenia, mucositis, nausea, cutaneous toxicity, diarrhea, hypertriglyceridemia, thrombocyopenia, hypercholesterolemia, elevated transaminases, hyperglycemia, and pneumonitis.39–41 Toxicity was more common with higher doses in some studies.42
mTOR is now a validated therapeutic target for renal cell carcinoma (RCC). In a multicenter phase III trial, patients with previously untreated, poor-prognosis metastatic RCC were randomized to receive 25 mg of temsirolimus intravenously weekly, interferon alfa, or combination therapy.43 Patients who received temsirolimus alone had a significantly longer overall survival (OS) and progression-free survival (PFS) than patients who received interferon alone (Table 1). The OS in the combination group did not differ significantly from that of the interferon group. The median OS with temsirolimus, interferon, or the combination was 10.9, 7.3 and 8.4 months, respectively. The US Food and Drug Administration approved temsirolimus for the treatment of poor prognosis metastatic RCC in 2007. Recently, a randomized, double-blind, placebo-controlled phase III trial of everolimus was performed in patients with RCC whose disease progressed on VEGFR-targeted therapy.44 At the second interim analysis, the trial showed a significant difference in efficacy and was halted early. The hazard ratio was 0.3 (95% CI, 0.22 to0.4; P < .0001) and the median PFS was 4 months for the everolimus arm versus 1.8 months for the control arm. The probability of being progression-free at 6 months was 26% for everolimus and 2% for placebo.
Table 1.
Efficacy of Rapamycin Analogs in Selected Clinical Trials
| Study Treatment | Phase | Disease | No. of Patients | Objective Response (PR or CR, %) |
|---|---|---|---|---|
| Hudes, 200743 | III | RCC | ||
| Temsirolimus (25 mg IV qwk) | 209* | 8.6 | ||
| Interferon | 207* | 4.8 | ||
| Temsirolimus + interferon | 210* | 8.1 | ||
| Motzer, 200844 | III | RCC | ||
| Everolimus (10 mg po qd) | 272* | 1 | ||
| Placebo | 138* | 0 | ||
| Hess, 200848 | III | Mantle-cell lymphoma | ||
| Temsirolimus (175 mg × 3 doses, followed by 75 mg qwk) | 54* | 22 | ||
| Temsirolimus (mg × 3 doses, followed by 25 mg qwk) | 54* | 6 | ||
| Investigator's choice | 54* | 2 | ||
| Galanis, 2005105 | II | GBM | ||
| Temsirolimus (250 mg IV qwk) | 64† | 0‡ | ||
| Atkins, 200441 | II | RCC | ||
| Temsirolimus (250 mg IV qwk) | 37* | 8.1 | ||
| Temsirolimus (75 mg IV qwk) | 38* | 7.9 | ||
| Temsirolimus (25 mg IV qwk) | 36* | 5.6 | ||
| Witzig, 200546 | II | Mantle-cell lymphoma | ||
| Temsirolimus (250 mg IV qwk) | 34† | 38 | ||
| Chan, 200542 | II | Breast cancer | ||
| Temsirolimus (250 mg IV qwk) | 54* | 7.4 | ||
| Temsirolimus (75 mg IV qwk) | 55* | 10.9 | ||
| Margolin, 2005106 | II | Melanoma | ||
| Temsirolimus (250 mg IV qwk) | 33† | 3 | ||
| Duran, 200667 | II | Neuroendocrine | ||
| Temsirolimus (25 mg IV qwk) | 36* | 5.6 | ||
| Chawla, 200650 | II | Sarcoma | ||
| Deforolimus (12.5 mg IV qd × 5, every 2 wks) | 193† | 3¶ | ||
| Colombo, 200751 | II | Endometrial cancer | ||
| Deforolimus (12.5 mg IV qd × 5, every 2 wks) | 27† | 7∥ | ||
| Pandya, 200786 | II | SCLC | ||
| Temsirolimus (250 mg IV qwk) | 41† | 0 | ||
| Temsirolimus (25 mg IV qwk) | 44† | 2.3 | ||
| Smith, 200847 | II | Lymphoma (non–mantle-cell, non-Hodgkins') | ||
| Temsirolimus (25 mg IV qwk) | 74* | 35 | ||
| Rizzieri, 2008107 | II | Hematologic malignancies | ||
| Deforolimus (12.5 mg IV qd × 5, every 2 wks) | 52† | 10 | ||
| Slomovitz, 200862 | II | Endometrial | ||
| Everolimus (10 mg po qd) | 25† | 0¶ | ||
| Ansell, 200845 | II | Mantle-cell lymphoma | ||
| Temsirolimus (25 mg IV qwk) | 27† | 41 |
Abbreviations: PR, partial response; CR, complete response; RCC, renal cell carcinoma; IV, intravenously; po, by mouth; qd, every day; qwk, every week; GBM, glioblastoma multiforme; SCLC, small-cell lung cancer.
No. of intent-to-treat patients.
No. of assessable patients.
Thirty-six percent of patients had evidence of improvement on neuroimaging.
Stable disease in 25% of patients.
Stable disease in 26% of patients.
Stable disease in 44% of patients.
Rapalogs have been evaluated in several other cancer types (Table 1). They have shown clear evidence of single-agent activity in lymphoma. Phase II studies have shown objective response rates (ORR) of 38% to 41% in mantle-cell lymphoma45,46 and 35% in non–mantle-cell non-Hodgkin's lymphoma.47 A phase III trial in refractory mantle-cell lymphoma demonstrated a 22% ORR with temsirolimus given at 175 mg weekly for 3 weeks followed by 75 mg weekly, compared with 2% for the investigator's choice of therapy (P = .0019).48 PFS rates were 4.8 months with the 75-mg weekly temsirolimus and 1.9 months with investigators' choice treatment (P = .009).48 Rapamycin has led to regression of Kaposi's sarcoma in renal transplant recipients.49 In preliminary analysis of phase II trials, rapalogs have also shown promise in patients with sarcoma and endometrial cancer.50,51
Rapamycin has also been evaluated in syndromes of proliferative dysregulation. Clinical benefit has been reported with facial angiofibroma, renal angiolypomas, and lymphangiomyomatosis.52–54 Clinical trials are ongoing for patients with neurofibromatosis, Cowden's Syndrome, and tuberous sclerosis, as well as for sporadic lymphangiomyomatosis—a condition associated with somatic mutations in the tuberous sclerosis genes.
Overall rapalogs have achieved modest ORRs. For example, in metastatic poor-prognosis RCC, temsirolimus treatment was associated with an improvement in PFS and OS, but it was only associated with a 8.6% ORR.43 Though everolimus improved the PFS for RCC that progressed on VEGFR-targeted therapy, the ORR was 1%.44 Thus for rapalogs, high ORRs may not be needed to achieve clinical benefit. As demonstrated by preclinical studies,55 rapalogs used alone are cytostatic in most tumor types and clinically may primarily stabilize disease.
Patient Selection for Treatment With Rapamycin Analogs
Although mTOR signaling is commonly deregulated in cancer, rapalogs have failed to show any appreciable single agent activity in many tumor types. The clinical benefit seen in different tumor histologies have been attributed to rapamycin's effects on different oncogenic drivers: angiogenesis in renal cell carcinoma and Kaposi's sarcoma, t(11;14)(q13;q32) translocation with cyclin D1 overexpression in mantle-cell lymphoma, PTEN loss for endometrial cancer, and activation of IGF-1R signaling in sarcomas. However, these attributions have remained controversial. Further, the low ORRs seen with unselected patient cohorts demonstrate that histology-based patient selection is insufficient. Based on preclinical data, a variety of predictors of response have been proposed, but most have not yet been clinically validated. Correlative studies in many ongoing and completed clinical trials have been limited due to availability of evaluable samples and the small numbers of patients achieving objective responses. Thus there remains an urgent need to better understand rapamycin's mechanism of action and to identify predictive markers of response that can be used to prospectively select patients who will derive the greatest benefit from rapalogs.
Patients with decreased PTEN may especially benefit from rapalogs. mTOR inhibition reduces neoplastic proliferation and tumor size in PTEN± mice, demonstrating that mTOR is the major effector of oncogenic PI3K signaling.56 Studies with isogenic PTEN+/+ and PTEN−/− mouse cells and with human cell lines with defined PTEN status have shown that PTEN-deficient tumors are preferentially inhibited by mTOR inhibition.57–60 However, PTEN loss was not able to predict sensitivity to everolimus in glioblastoma orthotopic xenografts. The predictive role of PTEN in clinical trials remains controversial.61,62
Activation of PI3K signaling, regardless of mechanism (PTEN loss or activated receptor-tyrosine-kinase signaling), may sensitize tumors to mTOR inhibition.63 Tumor growth conferred by Akt activation is also reversed by mTOR inhibitors.57 Rapalogs also block tumor growth induced by oncogenic PIK3CA mutations,64 suggesting that activating PI3K mutations may also have predictive value.
Predictive markers have been assessed in few clinical trials to date (Table 2). In a phase II trial of temsirolimus in RCC, paraffin-embedded tissue was available from 20 patients, five with a response (one partial and four minor).65 A positive association of p-S6 (Ser235) expression and a trend toward positive expression of p-Akt (Ser473) was found. Patients without high p-Akt or p-S6 expression did not achieve a response. No correlation was seen between response and carbonic anhydrase IX, PTEN or Von-Hippel Lindau mutation status. Iwenofu et al assessed the predictive value of p-S6 (Ser235/236) in patients with sarcoma who received deforolimus (with or without chemotherapy).66 Among p-S6 high expressors there were eight patients (73%) with stable disease and three patients (27%) with progression; among low expressors there were three patients (33%) with stable disease and six (67%) experienced progression (P = .05). Biomarkers were assessed in a phase II trial of temsirolimus in neuroendocrine tumors using archival samples and pretreatment biopsies. Although high p-mTOR (S2448) and p-S6 (Ser235/236) in archival samples were not predictive of response, high p-mTOR on freshly procured pretreatment biopsies was predictive (P = .01), with a trend towards response with high p-S6 on the pretreatment samples.67
Table 2.
Potential Predictors and Pharmacodynamic Markers of Response in Clinical Trials
| Marker | Disease | Treatment | End Point |
|---|---|---|---|
| Cho, 200765 | Renal cell carcinoma | Temsirolimus | |
| High p-S6 (Ser235) | Response (PR or MR) | ||
| High p-Akt (Ser473; trend)* | |||
| Duran, 200667 | Neuroendocrine | Temsirolimus | |
| High p-mTOR (Ser2448) | Response (not defined) | ||
| High p-S6 (Ser235/236; trend) | Response (not defined) | ||
| Increase in p-Akt (Ser473) | Increased TTP | ||
| Decrease in p-mTOR (Ser 2448) | Increased TTP | ||
| Slomovitz, 200862 | Endometrial | Everolimus | |
| Low PTEN (trend) | SD (v PD) | ||
| Iwenofu, 200866 | Sarcoma | Deforolimus with or without adriamycin | |
| High p-S6 (Ser235/236) | SD (v PD) | ||
| Cloughesy, 200880 | Glioblastoma | Rapamycin | |
| Increase in p-PRAS40 (Thr246) | Decreased TTP |
Abbreviations: PR, partial response; MR, minor response; mTOR, serine-threonine kinase mammalian target of rapamycin; TTP, time to progression; PTEN, phosphatase and tensin homologue deleted from chromosome 10; SD, stable disease; PD, progressive disease.
Trend, or difference in marker expression between responders and nonresponders, did not reach statistical significance.
The assessment of markers of response remains an obstacle to predictive marker development. Immunohistochemistry (IHC) with PTEN and with phospho-specific antibodies such as p-Akt, p-S6K, and p-S6 is challenging. Their staining and quantification have not been standardized. Concerns exist about the stability of phosphoproteins.68 The results of phospho-marker testing may vary based on specimen acquisition and processing, and may be influenced by tumor heterogeneity. Further, clinicians often assess markers in the primary tumor to make therapeutic decisions for metastatic disease; however, concordance of p-Akt and p-4E-BP1 levels by IHC in primary breast tumors and matched distant metastases was found to be poor.69 This may reflect true biologic heterogeneity or may simply be a reflection of the poor reproducibility and process sensitivity of IHC with phospho-specific antibodies. An alternate approach may be more quantitative assays such as enzyme-linked immunosorbent array with fresh samples. Streamlining higher throughput strategies (eg, reverse-phase proteomic arrays) to quantitate the activity of several pathways simultaneously may be considered. Evaluating multiple markers may demonstrate a more robust evaluation of the oncogenic signaling drivers of each tumor. As transcriptional profiling becomes more commonplace, there is also a need to identify transcriptional profiles that correlate with mTOR activation and profiles predictive of response. Identification of genomic alterations that confer rapamycin sensitivity is also highly desirable, since genomic aberrations may be more reliably tested in paraffin.
Pharmacodynamic Markers of Target Inhibition
For mTOR, the two best studied targets are S6K1 and 4E-BP1; thus, most studies have concentrated on these proteins. Preclinically rapamycin and its analogs inhibit phosphorylation of 4E-BP1 and S6K1 in tumor, skin and peripheral blood mononuclear cells (PBMCs).70,71 4E-BP1 has been reported to be hypophosphorylated in PBMCs71 while S6K1 activity has little intrasubject variation (14%)70; thus, PBMC S6K1 activity has been pursued in most pharmacodynamic (PD) studies. Time and dose-dependent inhibition of S6K1 was demonstrated in PBMCs. In preclinical models, a correlation with antitumor effect and prolonged (≥ 7 days) PBMC-derived S6K1 activity has been observed.71 For everolimus, preclinical simulations suggest that the administration regimen has a greater influence on S6K1 activity in the tumor than PBMCs, with daily dosing exerting greater activity than weekly doses,72 sustained S6K inhibition occurring with ≥ 20-mg everolimus weekly and ≥ 5 mg daily.73 These findings highlight that although PBMC S6K1 activity is often measured as a PD marker, it is not a perfect readout of target inhibition in the tumor.
In an elegant phase I study of everolimus in solid tumors, pretreatment and on-treatment (day 22) tumor and skin biopsies were evaluated.74 mTOR signaling was inhibited at all dose and schedules tested (5 and 10 mg daily, and 20, 50, and 70 mg weekly). Dose- and schedule-dependent inhibition of mTOR was observed with near-complete inhibition of p-S6 and p-eIF4G at 10 mg/d and ≥ 50 mg/wk. The relative inhibition of these markers differed with different dose levels. With daily dosing, p-S6 was inhibited with both dose levels, while p-eIF4G inhibition was partial with 5 mg but complete with 10 mg. With weekly dosing, p-S6 inhibition was almost complete at all dose levels. Inhibition was sustained in biopsies obtained 24 hours before the next weekly dose. In contrast, p-eIF4G inhibition was complete at 24 hours for all dose levels, but was sustained only for ≥ 50 mg/wk. p-4E-BP1 inhibition was not observed in all patients. Although there was good concordance of pathway inhibition in tumor and skin, p-4E-BP1 reduction was more profound in skin than tumors. This study clearly demonstrates that inhibition of mTOR signaling may be dependent on dose and schedule, and downstream targets may not always be inhibited concordantly.
Pharmacodynamic Markers of Response
To identify the potential determinants of response to rapamycin, one needs to better understand the downstream effects of mTOR inhibition in rapamycin-sensitive versus -resistant tumors and better elucidate rapamycin's mechanism of action. These molecular changes can then be followed to determine whether a patient is responding early in the treatment course, either through serial biopsies of the tumor or through molecular imaging. These markers may not only assist in better prospective patient selection, but would also allow therapy to be modified early if there is no molecular response.
Differential intrinsic sensitivity to rapamycin and analogs is not explained by differences in blockade of mTOR signaling pathway, at least not by inhibition of S6K1 or S6 phosphorylation.57,58,63,75 A correlation was found between rapamycin-mediated decline in p-4E-BP1 T70 with growth inhibition in some xenograft models,76 but in other preclinical studies, inhibition of p-4E-BP1 did not correlate with sensitivity.57 Taken together, decrease of downstream signaling appears to be useful for determining whether a biologically relevant drug dose is achieved; however, this finding does not necessarily correlate with growth inhibition and thus is not a good PD marker of response.
Pathway inhibition may not be a useful marker of response because different components of downstream signaling have differing thresholds for inhibition and the critical mediators of rapamycin's growth inhibitory effect may not be measured. Thus, if one focused on p-S6 alone, mTOR signaling may appear to be inhibited, while different mTOR effectors have different sensitivity to mTOR inhibitors.74 The mTOR/4E-BP1 axis which regulates eIF4E availability and cap-dependent translation may be the major driver of rapamycin-mediated growth inhibition, especially since eIF4E is a growth-regulatory target itself10,77 and in some models confers rapamycin resistance.78 Alternately, the pathway may be active but may not be the oncogenic driver; thus, inhibition of the pathway may be insufficient to achieve a growth-inhibitory effect.
Additionally, mTOR inhibition may in turn activate compensatory pathways such as Akt and MAPK signaling,79 which may theoretically limit antitumor activity. However, Akt activation has been observed even in rapamycin-sensitive preclinical models; thus, the value in assessing Akt activation as a marker of rapamycin resistance remains unclear.
In a phase I trial for recurrent PTEN-deficient glioblastoma, Cloughesy et al80 evaluated p-PRAS40 (Thr246) as a biomarker of Akt activity in surgical specimens obtained after 1 week of rapamycin treatment. This study differed from the usual pretreatment and on-treatment biopsy design as untreated primary tumor surgical specimens (S1) were compared with recurrent tumors treated with rapamycin for 1 week before surgery (S2). S1 and S2 samples from nine patients who did not receive rapamycin, were used as control and did not show a change in p-Akt. Of 14 patients in the rapamycin study, seven had an increase in p-PRAS40 in their S2 sample (P = .0047). Patients were maintained on rapamycin postoperatively. An increase in S2 p-PRAS40 was associated with a shorter time to progression (P < .05). Although it can not be determined whether p-PRAS40 was prognostic or whether induction of p-PRAS40 was predictive of poor response, these findings highlight the importance of assessing the p-Akt and its phosphorylation targets as potential PD markers.
Identification of the major mediators of drug response will be critical to identify ideal PD markers of response. Preclinical studies have identified a variety of alterations that occur on rapamycin treatment that may reflect direct or indirect drug effects (Table 3). These changes, alone or in combination, may be pursued as PD markers of response. Potential PD markers of response may be prioritized by concentrating on alterations critical to rapamycin's growth inhibitory effect. For example, rapamycin decreases cyclin D1 levels in several models.63,81–83 Further, a decrease in cyclin D1 plays an important role in rapamycin-mediated growth inhibition.82,83 However, although rapamycin decreases cyclin D1 in rapamycin-sensitive but not rapamycin-resistant cells in some studies, others report no change in cyclin D1 expression in either sensitive or resistant cells.58,63 It is unlikely that any single marker will sufficiently separate responders from nonresponders. Evaluating a panel of rapamycin effectors may be preferable for PD monitoring. Molecular imaging with tracers that assess metabolic and proliferative function ([18F]fluorodeoxyglucose and [18F]fluorothymidine uptake) has also shown promise in preclinical models.15,84 Molecular imaging with novel tracers of pathway activity is also being pursued.
Table 3.
Selected Downstream Effects of Rapamycin
| Target | Rapamycin Effect | Reference No. |
|---|---|---|
| S6K1 (T389, T421/S424, T229) | Decrease | 12 |
| S6 (Ser235/236, Ser240/244) | Decrease | 74 |
| 4E-BP1 (Thr 37*, Thr 46*; T70. Ser 65) | Decrease | 74,108,109 |
| eIF-4G (Ser1108; Ser1148; Ser1192) | Decrease | 74,110,111 |
| eIF-4B (Ser 422) | Decrease | 112 |
| FOXO1 (Ser256) | Decrease | 19 |
| PRAS40 (Ser221, Ser183) | Decrease | 18 |
| SGK1 (Ser422, Thr2560) | Decrease | 113 |
| Cyclin D1 | Decrease | 34,63,81,83,115 |
| Cyclin D3 | Decrease | 75 |
| c-Myc | Decrease | 75,81 |
| GLUT-1 | Decrease | 34 |
| VEGF | Decrease | 90 |
| HIF-1α | Decrease | 90 |
| Ki-67 | Decrease | 80,91 |
| Dusp6 | Decrease | 116 |
| eEF2 (Thr56) | Increase† | 85 |
| eIF2α (Ser51) | Increase† | 85 |
| c-Jun (S63) | Increase‡ | 114 |
| p27 | Increase | 75 |
Abbreviations: S6K1, S6 kinase 1; 4E-BP1, 4E-binding protein 1; eIF, eukaryotic initiation factor; PRAS40, proline-rich Akt substrate 40; SGK1, Serum/glucocorticoid-regulated kinase; GLUT-1, glucose transporter protein; VEGF, vascular endothelial growth factor; HIF-1α, hypoxia inducible factor 1α; eEF2, eukaryotic elongation factor 2 kinase.
Although Thr 37/46 is phosphorylated in vitro by serine-threonine kinase mammalian target of rapamycin (mTOR), these residues are proposed to relatively resistant to rapamycin in the presence of serum, but they are sensitive to rapamycin under serum starvation.
At high micromolar concentrations.
In cells lacking functional p53.
Effect of Dose and Schedule Selection on Efficacy
The clinical development of rapalogs has focused on the effect of dose and schedule on target inhibition. However, the ideal dose and schedule for rapamycin and analogs to achieve antitumor effect remains controversial. Rapamycin and everolimus have both shown dose-dependent antitumor efficacy in xenograft models.71 Further, lower doses of rapamycin leads to Akt activation, whereas higher doses diminish p-Akt in some models.16,37 In addition, although lower concentrations of temsirolimus and rapamycin have a selective growth inhibitory effect, at higher micromolar concentrations they have a profound antiproliferative effect in all tested cell lines with a decline in global protein synthesis and an increase in phosphorylation of eukaryotic elongation factor-2 kinase and eIF2α.85 This highlights another means through which dose and schedule selection may affect clinical outcome.
Dosing regimens have been compared in a few randomized trials. In the phase II temsirolimus trial in RCC, 25-, 75-, and 250-mg intravenous doses were compared (Table 1): the ORR were 5.6%, 7.9%, and 8.1%, respectively.41 The time to progression was 6.3, 6.7, and 5.2 months, respectively, and median survival was 13.8, 11.0, and 17.5 months, respectively. These were not statistically different and the authors concluded that efficacy was not significantly influenced by dose level. Thus, the 25-mg dose was pursued for the RCC trials that led to US Food and Drug Administration approval. However, some clinical data suggest that dose may be relevant to efficacy.86 In the phase III trial of temsirolimus in mantle-cell lymphoma,48 the 75-mg weekly regimen had a significantly higher ORR compared with investigator's choice treatment, while the 25-mg weekly regimen did not (Table 1). Furthermore, the 75-mg regimen significantly prolonged PFS (4.8 v 1.9 months; P = .0009), while the improvement in PFS with the 25-mg regimen (3.4 months) did not reach statistical significance (P = .0618). Thus higher doses may be more effective in some tumor types. The ideal dose and schedule needs to be further studied.
COMBINATION OF mTOR-TARGETED THERAPIES AND OTHER ANTICANCER AGENTS
In clinical trials, rapalogs have predominantly led to disease stabilization rather than tumor regression. Thus, for most tumor types, mTOR-targeted therapies will likely be used in combination therapy, with the expectation that this may induce a cytotoxic rather than cytostatic response and subsequent tumor regression.
Combination With Chemotherapy
mTOR inhibitors have been found to be additive or synergistic with paclitaxel, carboplatin, cisplatin, vinorelbine, doxorubicin, and campthotecin.55,59,87,88 Compared with single agent therapy, the combination of rapamycin with chemotherapy enhances apoptosis in vitro and enhances antitumor efficacy in vivo.55,87–89 Ongoing clinical trials are currently evaluating the efficacy of rapamycin and its analogs in combination with a broad spectrum of chemotherapeutic agents.
Combination With IGF-IR Inhibitors
The rapamycin-induced Akt activation observed in some cancer cell lines and in clinical trials increased interest in overcoming this feedback loop activation by using mTOR inhibitors in combination with antagonists of upstream signaling such as IGF-IR inhibitors.35,36,90 IGF-IR inhibition prevents rapamycin-induced Akt activation and sensitizes tumor cells to mTOR inhibition in preclinical models.35,90 The combination of rapalogs and IGF-IR inhibitors are now being studied in clinical trials.
Combination With Octreotide
In neuroendocrine tumors, although a phase II trial with temsirolimus obtained a relatively low ORR, a phase II trial of everolimus in combination with octreotide demonstrated clinical efficacy with an ORR of 20% by intent-to-treat analysis.91 This may reflect differences between patient cohorts, differences in mTOR inhibition with different drug and dosing regimens, or may be attributable to the combination of mTOR inhibitors with octreotide in the latter trial. Somatostatin analogs such as octreotide decrease PI3K/Akt signaling in some models92 and thus theoretically may enhance rapamycin's antitumor activity. However, preclinical work in carcinoid cells demonstrated that although rapamycin causes significant growth inhibition in vitro and in vivo, it did not enhance rapamycin's antiproliferative effects and did not inhibit rapamycin-mediated Akt activation.93 Yet, preclinical models have clear limitations. Randomized prospective trials are being conducted to determine whether octreotide enhances the antitumor effects of mTOR inhibitors.
Combination With Trastuzumab
In HER-2–positive breast cancer cell lines, trastuzumab has been shown to inhibit feedback-loop activation of Akt.94 This is especially notable as PTEN loss is a known mediator of trastuzumab resistance,95,96 providing another rationale to use mTOR inhibitors to restore or enhance trastuzumab sensitivity. In vitro, low doses of everolimus significantly increased growth inhibition by trastuzumab, and in vivo everolimus enhanced the antitumor efficacy of trastuzumab by a modest amount.94 The combination of everolimus and trastuzumab is currently in clinical trials. A recent multicenter phase I trial of everolimus in combination with paclitaxel and trastuzumab in patients with HER-2–overexpressing metastatic breast cancer with prior resistance to trastuzumab demonstrated that the combination was well tolerated, with the preliminary evidence of efficacy.97
Combination With Antiestrogen Therapy
Akt/mTOR signaling has been associated with resistance to endocrine therapy in breast cancer,98 providing rationale for combining endocrine therapy with mTOR inhibitors. In preclinical models, rapalogs enhance the efficacy of selective estrogen receptor modulators tamoxifen, raloxifene, and ERA-923; estrogen receptor downregulator fulvestrant; and aromatase inhibitor letrozole.71,99–101 However, the interim analysis of a phase III randomized placebo controlled trial of letrozole with or without temsirolimus reported no improvement in PFS102; final analysis has not been published. The combination of everolimus with letrozole has been pursued with more promising results. A phase I study of everolimus with letrozole demonstrated some clinical responses.103 The combination of daily oral everolimus plus letrozole versus placebo plus letrozole was recently tested in a randomized phase II neoadjuvant trial in 270 postmenopausal women with estrogen receptor–positive breast cancer.61 The clinical response rate with everolimus and letrozole was significantly more than letrozole alone at the preplanned alpha of 0.1 (68% v 59%; P = .062). These results were confirmed by ultrasound (objective response 58% v 47%; P = .035). Cell cycle response was also higher in the combination arm (57% v 30% for Ki-67 ≤ 2 at day 15; P < .01). Thus, mTOR inhibition may increase the efficacy of endocrine therapy. However, everolimus was associated with an increase in grade 3/4 adverse events (22.6% in the combination arm v 3.8% in the letrozole arm). Although, the addition of everolimus to letrozole, a drug that has excellent baseline tolerability, increases adverse effects,61,103 this strategy may be warranted in patients with higher-risk hormone receptor–positive tumors, especially if predictors of response can be utilized to select patients most likely to benefit from this combination.
NEW mTOR-TARGETED THERAPIES
A new generation of mTOR inhibitors is being developed. In contrast to rapalogs, catalytic site inhibitors of mTOR inhibit both mTORC1 and mTORC2, and inhibition of mTORC2 will affect the activation of Akt. Agents such as BEZ235 (Novartis, East Hanover, NJ) and EX147 (Exelixis, San Francisco, CA) are dual PI3K/mTOR inhibitors and thus may bypass feedback loops, potentially increasing their efficacy compared with rapalogs. The tolerability and efficacy of these agents are currently being tested in clinical trials. In addition, other strategies to downregulate mTOR signaling, such as the use of antidiabetic drug metformin—an activator of AMPK104—are being pursued in clinical trials.
SUMMARY AND CONCLUSION
mTOR is now a validated target in the treatment of some tumor types. Careful patient selection and rational selection of combination therapies will enhance the success of mTOR therapies. Used effectively, mTOR inhibitors will play an important role in delivering more effective, personalized cancer therapy.
Acknowledgment
We thank Kristi M. Speights for editorial input and Dave Aten for the medical illustration. We also thank the investigators who have made valuable contributions to our knowledge about mTOR signaling whose work we have been unable to cite due to space limitations.
Footnotes
The authors' laboratory research is funded by National Institutes of Health (NIH) Grant No. NIH1 R01 CA112199 (F.M.-B.), American Institute of Cancer Research (F.M.-B.), Grant No. NIH K23 CA121994 (A.M.G.-A.), ASCO Career Development Award (A.M.G.-A.), and the Cancer Center Core Grant No. CA16672 from The University of Texas M. D. Anderson Cancer Center.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Stock Ownership: None Honoraria: Funda Meric-Bernstam, Novartis Research Funding: Funda Meric-Bernstam, Novartis, Abraxis, Merck; Ana Maria Gonzalez-Angulo, Novartis, Abraxis, Merck Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Funda Meric-Bernstam
Financial support: Funda Meric-Bernstam, Ana Maria Gonzalez-Angulo
Administrative support: Funda Meric-Bernstam
Collection and assembly of data: Funda Meric-Bernstam
Data analysis and interpretation: Funda Meric-Bernstam, Ana Maria Gonzalez-Angulo
Manuscript writing: Funda Meric-Bernstam, Ana Maria Gonzalez-Angulo
Final approval of manuscript: Funda Meric-Bernstam, Ana Maria Gonzalez-Angulo
REFERENCES
- 1.Zhou BP, Hu MC, Miller SA, et al. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem. 2000;275:8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 2.Chung J, Bachelder RE, Lipscomb EA, et al. Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: A survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165–174. doi: 10.1083/jcb.200112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui X, Zhang P, Deng W, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: Progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17:575–588. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 4.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 6.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 8.Johannessen CM, Reczek EE, James MF, et al. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A. 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Benedetti A, Graff JR. EIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 10.Soni A, Akcakanat A, Singh G, et al. EIF4E knockdown decreases breast cancer cell growth without activating Akt signaling. Mol Cancer Ther. 2008;7:1782–1788. doi: 10.1158/1535-7163.MCT-07-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Culjkovic B, Topisirovic I, Skrabanek L, et al. EIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol. 2005;169:245–256. doi: 10.1083/jcb.200501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jastrzebski K, Hannan KM, Tchoubrieva EB, et al. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura JL, Garcia E, Pieper RO. S6K1 plays a key role in glial transformation. Cancer Res. 2008;68:6516–6523. doi: 10.1158/0008-5472.CAN-07-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bärlund M, Forozan F, Kononen J, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J Natl Cancer Inst. 2000;92:1252–1259. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]
- 15.Thomas GV, Tran C, Mellinghoff IK, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 16.Phung TL, Ziv K, Dabydeen D, et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 2006;10:159–170. doi: 10.1016/j.ccr.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Harris TE, Lawrence JC., Jr Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)-mediated phosphorylation. J Biol Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacinto E, Facchinetti V, Liu D, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Yang Q, Inoki K, Ikenoue T, et al. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearce LR, Huang X, Boudeau J, et al. Identification of protor as a novel rictor-binding component of mTOR complex-2. Biochem J. 2007;405:513–522. doi: 10.1042/BJ20070540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frias MA, Thoreen CC, Jaffe JD, et al. MSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Martin J, Masri J, Bernath A, et al. Hsp70 associates with rictor and is required for mTORC2 formation and activity. Biochem Biophys Res Commun. 2008;372:578–583. doi: 10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 25.Hresko RC, Mueckler M. MTOR. RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 26.McDonald PC, Oloumi A, Mills J, et al. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–1624. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 27.Hernández-Negrete I, Carretero-Ortega J, Rosenfeldt H, et al. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J Biol Chem. 2007;282:23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- 28.Inoki K, Li Y, Zhu T, et al. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 29.Manning BD, Tee AR, Logsdon MN, et al. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- 30.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4:658–665. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 31.Roux PP, Ballif BA, Anjum R, et al. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nobukuni T, Joaquin M, Roccio M, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akcakanat A, Singh G, Hung MC, et al. Rapamycin regulates the phosphorylation of rictor. Biochem Biophys Res Commun. 2007;362:330–333. doi: 10.1016/j.bbrc.2007.07.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109:3509–3512. doi: 10.1182/blood-2006-06-030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly KE, Rojo F, She QB, et al. MTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi Y, Yan H, Frost P, et al. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4:1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- 37.Stoeltzing O, Meric-Bernstam F, Ellis LM. Intracellular signaling in tumor and endothelial cells: The expected and, yet again, the unexpected. Cancer Cell. 2006;10:89–91. doi: 10.1016/j.ccr.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Hidalgo M, Buckner JC, Erlichman C, et al. A phase I and pharmacokinetic study of temsirolimus (CCI-779) administered intravenously daily for 5 days every 2 weeks to patients with advanced cancer. Clin Cancer Res. 2006;12:5755–5763. doi: 10.1158/1078-0432.CCR-06-0118. [DOI] [PubMed] [Google Scholar]
- 40.Yee KW, Zeng Z, Konopleva M, et al. Phase I/II study of the mammalian target of rapamycin inhibitor everolimus (RAD001) in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2006;12:5165–5173. doi: 10.1158/1078-0432.CCR-06-0764. [DOI] [PubMed] [Google Scholar]
- 41.Atkins MB, Hidalgo M, Stadler WM, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 42.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 43.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 44.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 45.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: A phase II trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle-cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM, Pro B, Cisneros A, et al. Activity of single agent temsirolimus (CCI-779) in non–mantle-cell non-Hodgkin lymphoma subtypes. J Clin Oncol. 2008;26(suppl):457s. abstr 8514. [Google Scholar]
- 48.Hess G, Romaguera JE, Verhoef G, et al. Phase III study of patients with relapsed, refractory mantle-cell lymphoma treated with temsirolimus compared with investigator's choice therapy. J Clin Oncol. 2008;26(suppl):457s. doi: 10.1200/JCO.2008.20.7977. abstr 8513. [DOI] [PubMed] [Google Scholar]
- 49.Stallone G, Schena A, Infante B, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 50.Chawla SP, Tolcher AW, Staddon AP, et al. Updated results of a phase II trial of AP23573, a novel mTOR inhibitor, in patients (pts) with advanced soft tissue or bone sarcomas. J Clin Oncol. 2006;24(suppl):521s. abstr 9505. [Google Scholar]
- 51.Colombo N, McMeekin S, Schwartz P, et al. A phase II trial of the mTOR inhibitor AP23573 as a single agent in advanced endometrial cancer. J Clin Oncol. 2007;25(suppl):278s. abstr 5516. [Google Scholar]
- 52.Herry I, Neukirch C, Debray MP, et al. Dramatic effect of sirolimus on renal angiomyolipomas in a patient with tuberous sclerosis complex. Eur J Intern Med. 2007;18:76–77. doi: 10.1016/j.ejim.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Bissler JJ, McCormack FX, Young LR, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wienecke R, Fackler I, Linsenmaier U, et al. Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex. Am J Kidney Dis. 2006;48:e27–e29. doi: 10.1053/j.ajkd.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 55.Mondesire WH, Jian W, Zhang H, et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res. 2004;10:7031–7042. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 56.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten+/− mice. Proc Natl Acad Sci U S A. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- 59.Steelman LS, Navolanic PM, Sokolosky ML, et al. Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors. Oncogene. 2008;27:4086–4095. doi: 10.1038/onc.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeGraffenried LA, Fulcher L, Friedrichs WE, et al. Reduced PTEN expression in breast cancer cells confers susceptibility to inhibitors of the PI3 kinase/Akt pathway. Ann Oncol. 2004;15:1510–1516. doi: 10.1093/annonc/mdh388. [DOI] [PubMed] [Google Scholar]
- 61.Baselga J, van Dam PA, Greil R, et al. Improved clinical and cell cycle response with an mTOR inhibitor, daily oral RAD001 (everolimus) plus letrozole versus placebo plus letrozole in a randomized phase II neoadjuvant trial in ER+ breast cancer. J Clin Oncol. 2008;26(suppl):13s. abstr 530. [Google Scholar]
- 62.Slomovitz BM, Lu KH, Johnston T, et al. A phase II study of oral mammalian target of rapamycin (mTOR) inhibitor, RAD001 (everolimus) in patients with recurrent endometrial carcinoma (EC) J Clin Oncol. 2008;26(suppl):293s. abstr 5502. [Google Scholar]
- 63.Noh WC, Mondesire WH, Peng J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–1023. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 64.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103:1475–1479. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho D, Signoretti S, Dabora S, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2007;5:379–385. doi: 10.3816/CGC.2007.n.020. [DOI] [PubMed] [Google Scholar]
- 66.Iwenofu OH, Lackman RD, Staddon AP, et al. Phospho-S6 ribosomal protein: A potential new predictive sarcoma marker for targeted mTOR therapy. Mod Pathol. 2008;21:231–237. doi: 10.1038/modpathol.3800995. [DOI] [PubMed] [Google Scholar]
- 67.Duran I, Kortmansky J, Singh D, et al. A phase II clinical and pharmacodynamic study of temsirolimus in advanced neuroendocrine carcinomas. Br J Cancer. 2006;95:1148–1154. doi: 10.1038/sj.bjc.6603419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baker AF, Dragovich T, Ihle NT, et al. Stability of phosphoprotein as a biological marker of tumor signaling. Clin Cancer Res. 2005;11:4338–4340. doi: 10.1158/1078-0432.CCR-05-0422. [DOI] [PubMed] [Google Scholar]
- 69.Akcakanat A, Sahin A, Shaye AN, et al. Comparison of Akt/mTOR signaling in primary breast tumors and matched distant metastases. Cancer. 2008;112:2352–2358. doi: 10.1002/cncr.23456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peralba JM, DeGraffenried L, Friedrichs W, et al. Pharmacodynamic evaluation of CCI-779, an inhibitor of mTOR, in cancer patients. Clin Cancer Res. 2003;9:2887–2892. [PubMed] [Google Scholar]
- 71.Boulay A, Zumstein-Mecker S, Stephan C, et al. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka C, O'Reilly T, Kovarik JM, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 73.O'Donnell A, Faivre S, Burris HA, III, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 74.Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: A phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 75.Yu K, Toral-Barza L, Discafani C, et al. mTOR, a novel target in breast cancer: The effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–258. doi: 10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 76.Dudkin L, Dilling MB, Cheshire PJ, et al. Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res. 2001;7:1758–1764. [PubMed] [Google Scholar]
- 77.Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wendel HG, De Stanchina E, Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 79.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gera JF, Mellinghoff IK, Shi Y, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 82.Law M, Forrester E, Chytil A, et al. Rapamycin disrupts cyclin/cyclin-dependent kinase/p21/proliferating cell nuclear antigen complexes and cyclin D1 reverses rapamycin action by stabilizing these complexes. Cancer Res. 2006;66:1070–1080. doi: 10.1158/0008-5472.CAN-05-1672. [DOI] [PubMed] [Google Scholar]
- 83.Dong J, Peng J, Zhang H, et al. Role of glycogen synthase kinase 3beta in rapamycin-mediated cell cycle regulation and chemosensitivity. Cancer Res. 2005;65:1961–1972. doi: 10.1158/0008-5472.CAN-04-2501. [DOI] [PubMed] [Google Scholar]
- 84.Wei LH, Su H, Hildebrandt IJ, et al. Changes in tumor metabolism as readout for mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin Cancer Res. 2008;14:3416–3426. doi: 10.1158/1078-0432.CCR-07-1824. [DOI] [PubMed] [Google Scholar]
- 85.Shor B, Zhang WG, Toral-Barza L, et al. A new pharmacologic action of CCI-779 involves FKBP12-independent inhibition of mTOR kinase activity and profound repression of global protein synthesis. Cancer Res. 2008;68:2934–2943. doi: 10.1158/0008-5472.CAN-07-6487. [DOI] [PubMed] [Google Scholar]
- 86.Pandya KJ, Dahlberg S, Hidalgo M, et al. A randomized, phase II trial of two dose levels of temsirolimus (CCI-779) in patients with extensive-stage small-cell lung cancer who have responding or stable disease after induction chemotherapy: A trial of the Eastern Cooperative Oncology Group (E1500) J Thorac Oncol. 2007;2:1036–1041. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 87.Grünwald V, DeGraffenried L, Russel D, et al. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–6145. [PubMed] [Google Scholar]
- 88.Geoerger B, Kerr K, Tang CB, et al. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–1532. [PubMed] [Google Scholar]
- 89.Beuvink I, Boulay A, Fumagalli S, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 90.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 91.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: Results of a phase II study. J Clin Oncol. 2008;26:4311–4318. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Charland S, Boucher MJ, Houde M, et al. Somatostatin inhibits Akt phosphorylation and cell cycle entry, but not p42/p44 mitogen-activated protein (MAP) kinase activation in normal and tumoral pancreatic acinar cells. Endocrinology. 2001;142:121–128. doi: 10.1210/endo.142.1.7908. [DOI] [PubMed] [Google Scholar]
- 93.Moreno A, Akcakanat A, Munsell MF, et al. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer. 2008;15:257–266. doi: 10.1677/ERC-07-0202. [DOI] [PubMed] [Google Scholar]
- 94.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 95.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 96.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 97.André F, Campone M, Hurvitz SA, et al. Multicenter phase I clinical trial of daily and weekly RAD001 in combination with weekly paclitaxel and trastuzumab in patients with HER-2–overexpressing metastatic breast cancer with prior resistance to trastuzumab. J Clin Oncol. 2008;26(suppl):41s. abstr 1003. [Google Scholar]
- 98.Pérez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86:540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadler TM, Gavriil M, Annable T, et al. Combination therapy for treating breast cancer using antiestrogen, ERA-923, and the mammalian target of rapamycin inhibitor, temsirolimus. Endocr Relat Cancer. 2006;13:863–873. doi: 10.1677/erc.1.01170. [DOI] [PubMed] [Google Scholar]
- 100.Beeram M, Tan QT, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- 101.deGraffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 102.Chow LWC, Sun Y, Jassem J, et al. Phase III study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer. Presented at the 29th Annual San Antonio Breast Cancer Symposium; December 14-17, 2006; San Antonio, TX. [Google Scholar]
- 103.Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: Results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91. doi: 10.1016/j.ejca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 105.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: A North Central Cancer Treatment Group Study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 106.Margolin K, Longmate J, Baratta T, et al. CCI-779 in metastatic melanoma: A phase II trial of the California Cancer Consortium. Cancer. 2005;104:1045–1048. doi: 10.1002/cncr.21265. [DOI] [PubMed] [Google Scholar]
- 107.Rizzieri DA, Feldman E, Dipersio JF, et al. A phase II clinical trial of deforolimus (AP23573, MK-8669), a novel mammalian target of rapamycin inhibitor, in patients with relapsed or refractory hematologic malignancies. Clin Cancer Res. 2008;14:2756–2762. doi: 10.1158/1078-0432.CCR-07-1372. [DOI] [PubMed] [Google Scholar]
- 108.Gingras AC, Gygi SP, Raught B, et al. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 110.Raught B, Gingras AC, Gygi SP, et al. Serum-stimulated, rapamycin-sensitive phosphorylation sites in the eukaryotic translation initiation factor 4GI. Embo J. 2000;19:434–444. doi: 10.1093/emboj/19.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramírez-Valle F, Braunstein S, Zavadil J, et al. EIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol. 2008;181:293–307. doi: 10.1083/jcb.200710215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raught B, Peiretti F, Gingras AC, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. Embo J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hong F, Larrea MD, Doughty C, et al. MTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 114.Huang S, Shu L, Dilling MB, et al. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1) Mol Cell. 2003;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 115.Hashemolhosseini S, Nagamine Y, Morley SJ, et al. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998;273:14424–14429. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- 116.Bermudez O, Marchetti S, Pages G, et al. Post-translational regulation of the ERK phosphatase DUSP6/MKP3 by the mTOR pathway. Oncogene. 2008;27:3685–3691. doi: 10.1038/sj.onc.1211040. [DOI] [PubMed] [Google Scholar]