Abstract
Purpose
For patients with stage II to IV laryngeal cancer, radiation therapy (RT) either alone or with concurrent chemotherapy provides the highest rate of organ preservation but can be associated with functional impairment. Thus, we studied the use of induction chemotherapy with or without conservation laryngeal surgery (CLS). Our objectives were to study the sensitivity of laryngeal cancer to platinum-based chemotherapy alone and to highlight the efficacy of CLS in this setting.
Patients and Methods
Thirty-one previously untreated patients with laryngeal cancer (T2-4, N0-1, M0), who were resectable with CLS, were enrolled. Patients received three to four cycles of paclitaxel, ifosfamide, and cisplatin (TIP) chemotherapy, and response was assessed histologically. Patients with partial response (PR) proceeded to CLS. Patients achieving pathologic complete response (pCR) received an additional three cycles of TIP and no other treatment.
Results
Thirty patients were assessable for response. With TIP chemotherapy alone, 11 patients (37%) achieved pCR, 10 of whom (33%) remain alive with durable disease remission and no evidence of recurrence over a median follow-up time of 5 years. Nineteen patients (63%) treated with TIP alone achieved PR. The overall laryngeal preservation (LP) rate was 83%, and only five patients (16%) required postoperative RT. No patient required a gastrostomy tube or tracheotomy.
Conclusion
Chemotherapy alone in selected patients with T2-4, N0-1 laryngeal cancer can provide durable disease remission at 5 years. For patients with PR, CLS provides a high rate of LP. This prospective study suggests that chemotherapy alone may cure selected patients with laryngeal cancer, warranting further prospective investigation.
INTRODUCTION
Historically, the role of surgery for patients with T2-3 laryngeal cancer was limited to a few select patients amenable to vertical partial laryngectomy or supraglottic laryngectomy. Thus, for decades, total laryngectomy (TL) was considered the only curative option for most intermediate- to advanced-stage laryngeal cancers. Radiation therapy (RT) alone provided an important alternative, but local control rates for T2 cancers of the glottic and supraglottic larynx ranged from 62% to 92%.1 Worse still, 70% of patients who experienced treatment failure with RT required TL.2,3
In 1991, the Veterans Affairs Laryngeal Cancer Study demonstrated equivalence in overall survival (OS) between patients treated with TL followed by postoperative RT (PORT) compared with patients treated with induction chemotherapy and RT.4 In 2003, the randomized prospective Radiation Therapy Oncology Group 91-11 study demonstrated that laryngectomy-free survival was superior for patients treated with either concurrent chemotherapy and RT (cRT) or induction chemotherapy followed by RT, compared with RT alone.5,6 However, RT with or without chemotherapy can be associated with significant long-term morbidity. An important minority of patients have an anatomically preserved organ7–9 but significant functional compromise, sometimes necessitating tracheotomy and/or gastrostomy. For patients treated with cRT who later require salvage surgery, postoperative complications are increased, and survival is significantly diminished.10
Recently, a variety of conservation laryngeal surgery (CLS) techniques have emerged.11 The functional compromise sometimes seen with primary RT and organ preservation approaches has led some centers to take a different approach, relying on induction chemotherapy followed by CLS. Laccourreye et al12 reported that platinum-based chemotherapy alone could achieve long-term locoregional control in selected patients with laryngeal squamous cell carcinoma (SCC). In a subset of patients who achieved a clinical complete response after induction chemotherapy, selected patients were treated with three more cycles of chemotherapy without surgery or RT. Follow-up publications confirmed a high rate of laryngeal preservation (LP) in larger cohorts.13,14 However, this experience has never been validated prospectively in another institution.
We evaluated this novel approach in patients with intermediate- to advanced-stage laryngeal SCC using a novel regimen of paclitaxel, ifosfamide, and cisplatin (TIP) or paclitaxel, ifosfamide, and carboplatin (TIC) developed at The University of Texas M. D. Anderson Cancer Center (M. D. Anderson).15–17 The primary objective was to determine whether chemotherapy either alone or followed by CLS would achieve long-term, durable remission for selected patients with laryngeal cancer, without compromising LP or OS.
PATIENTS AND METHODS
Eligibility Criteria
From 1997 to 2004, patients with histologically proven laryngeal SCC were screened for study eligibility by multidisciplinary evaluation and endoscopic staging via direct laryngoscopy. Patients selected for this study had stage II to IVa (T2-4, N0-1, M0) glottic or supraglottic SCC that was surgically resectable via CLS. Patients with T2 tumors were included when the tumor was considered unfavorable18 by the multidisciplinary tumor board (ie, at high risk for local failure with altered-fractionation RT alone).
Eligible patients had favorable performance level (Eastern Cooperative Oncology Group performance status of 0 or 1) and normal hematopoietic, hepatic, and renal function. Patients with nodal metastasis classified as N2 or greater and patients with distant metastatic disease were ineligible. The study protocol was approved by the M. D. Anderson Institutional Review Board. Eligible patients were required to sign an informed consent form approved by the Human Subjects Review Board.
Treatment and Chemotherapy Regimens
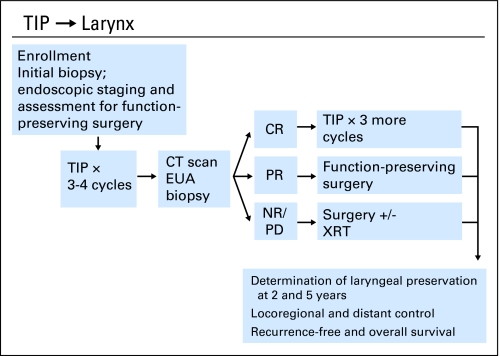
The treatment schema is shown in Figure 1. Patients received three to four cycles of TIP chemotherapy (paclitaxel 175 mg/m2 on day 1, ifosfamide 1,000 mg/m2 with mesna 420 mg/m2 on days 1 through 3, and cisplatin 60 mg/m2 on day 1). All patients received at least three cycles of chemotherapy before biopsy, and nine patients received four cycles. If, after three cycles, the patient had a good clinical partial response (PR), as defined by greater than 90% tumor reduction, a fourth cycle could be administered before rebiopsy at the discretion of the treating physician.
Fig 1.
Treatment schema for laryngeal preservation. TIP, paclitaxel, ifosfamide, and cisplatin; CT, computed tomography; EUA, examination under anesthesia; CR, complete response; PR, partial response; NR, no response; PD, progressive disease; XRT, radiotherapy.
Dose modifications were allowed (specified with the full regimen in Appendix Table A1, online only). If irreversible grade 2 nonhematologic toxicity, grade 4 hematologic toxicity for ≥ 7 days, or neutropenic fever with or without infection occurred, the next dose was reduced one level. The protocol was amended to allow for a change from cisplatin (60 mg/m2) to carboplatin (area under the curve 6) in subsequent cycles in case of the following adverse events: neuropathy ≥ grade 2; nausea or vomiting ≥ grade 3 to 4; or renal failure ≥ grade 2.
Clinical and Histologic Assessment of Pathologic Response
After high-resolution computed tomography (CT) scanning, patients returned to the operating room for operative direct laryngoscopy, tumor mapping, and biopsy of any residual abnormality. If no gross residual tumor was present, biopsies were taken at the site of the tumor epicenter (based on pretreatment videostroboscopy) and adjacent mucosa. For patients with an endoscopic and histologic complete response (CR), three additional cycles of chemotherapy were administered, followed by repeat endoscopy to confirm a final histologic CR. For patients with histologic residual disease, CLS was performed.
CLS
The surgical technique was determined by the location of the primary tumor. Patients with glottic cancers had transoral laser microsurgery, vertical partial laryngectomy, or supracricoid partial laryngectomy with cricohyoidoepiglottopexy. Patients with supraglottic cancers underwent transoral laser microsurgery, supraglottic horizontal partial laryngectomy, or supracricoid partial laryngectomy with cricohyoidopexy. Margin control was confirmed by frozen section to ensure complete tumor removal.
Follow-Up and Tumor Surveillance After Induction Chemotherapy
Patients with a pathologic CR (pCR) underwent monthly videostroboscopic examination for the first year after treatment and then fiberoptic laryngeal examination every 3 months in the second year and every 6 months during years 3 to 5. Patients with PR underwent laryngeal examination every 3 months after CLS for the first 2 years and every 6 months thereafter. CT scan of the head and neck was also performed every 3 months for the first 2 years after treatment and then yearly thereafter.
Statistical Analysis
An intent-to-treat analysis was performed. The primary end point of the study was LP, which was defined as native laryngeal speech and swallowing without TL 2 years after initiation of chemotherapy. Per protocol, if the treatment gave an LP rate of 60% or lower,1 it was considered a failure; otherwise, the regimen was considered a success. Exact binomial test was used to test the significance of the observed LP rate. The secondary end point was the response rate (clinical and pathologic) of TIP induction chemotherapy. Other end points were local, regional, and distant control; time from initiation of chemotherapy to TL; OS; and recurrence-free survival (RFS). OS and RFS were defined as the time from initiation of chemotherapy to death and disease recurrence, respectively (patients who had no disease recurrence but died of an unrelated cause were censored from the RFS function). The distribution of RFS and OS and the time to TL were estimated using the Kaplan-Meier method. Log-rank test was performed to test differences in survival between patients with different characteristics.19
RESULTS
Patients
Thirty-one patients were enrolled; the median age was 58 years (range, 20 to 79 years). Table 1 lists the clinicopathologic characteristics of the study population. The majority of patients were staged as T2 (n = 23) and N0 (n = 26). Nine patients were classified as T3 (n = 6) or T4 (n = 3), but all were amenable to LP via CLS before induction chemotherapy. Mean follow-up time was 65 months (median, 64 months; range, 26 to 108 months).
Table 1.
Demographic and Clinical Characteristics
| Characteristic | Patients |
|
|---|---|---|
| No. | % | |
| Sex | ||
| Female | 9 | 29 |
| Male | 22 | 71 |
| Race | ||
| Hispanic | 3 | 10 |
| White | 28 | 90 |
| Zubrod score | ||
| 0 | 11 | 35 |
| 1 | 20 | 65 |
| Primary site | ||
| Supraglottic larynx | 17 | 55 |
| Supraglottic larynx, NOS | 7 | 23 |
| Epiglottis | 9 | 29 |
| False cord | 1 | 3 |
| Glottic larynx | 14 | 45 |
| Tumor classification | ||
| T2 | 22 | 71 |
| T3 | 6 | 19 |
| T4 | 3 | 10 |
| Node classification | ||
| N0 | 25 | 81 |
| N1 | 6 | 19 |
| TNM stage | ||
| 0-I | 0 | 0 |
| II | 19 | 61 |
| III | 9 | 23 |
| IV | 3 | 16 |
Abbreviation: NOS, not otherwise specified.
Response to TIP Chemotherapy and Toxicity
The total number of TIP/TIC cycles administered was six in nine patients, five in one patient, four in nine patients, and three in 12 patients. No patient received more than six cycles of chemotherapy. Four patients changed their platinum regimen from TIP to TIC. Granulocyte colony-stimulating factor was not used prophylactically and was restricted to abrogating neutropenic fever with and without infection. Patients underwent a detailed endoscopic, radiographic, and pathologic assessment of response after three (n = 22) or four (n = 9) cycles of chemotherapy.
Of 31 eligible patients, 30 had assessable tumor response after study treatment; a single patient could not be evaluated secondary to a cerebrovascular event and could not undergo anesthesia. A small residual focus of tumor was suspected but not documented histologically.
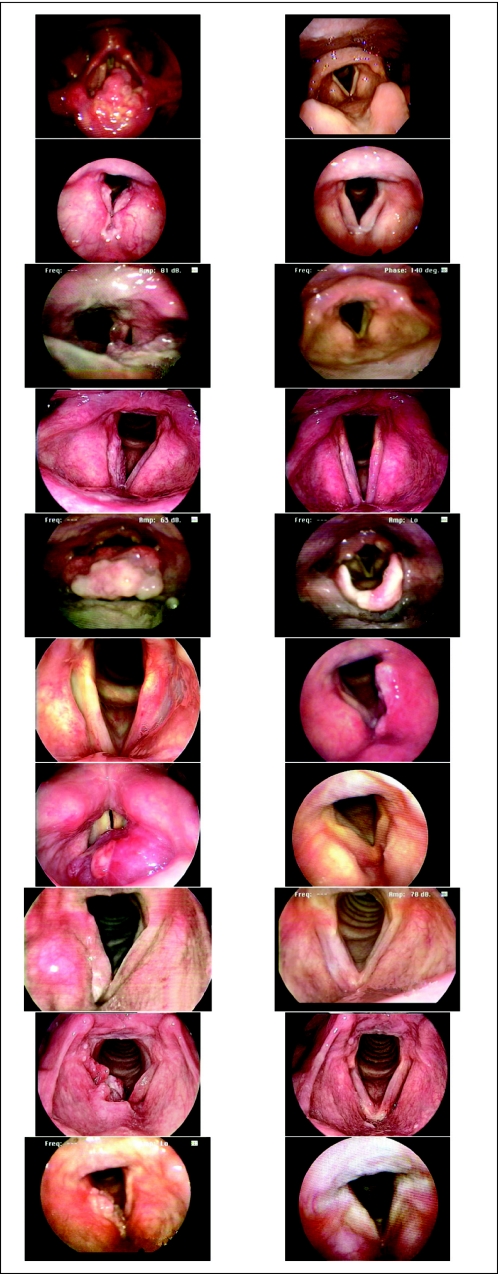
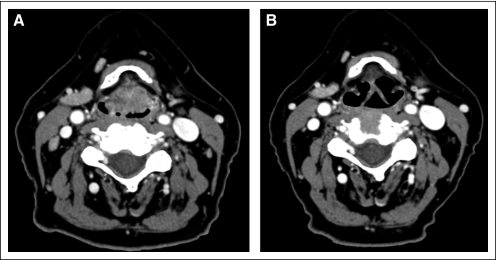
Of all assessable patients (n = 30), 11 patients (37%) had a pCR, and 19 patients (63%) had a PR. There were no patients with stable or progressive disease. There was no significant association between the clinically observed response to chemotherapy and demographic, clinical, or baseline pathologic factors. Figure 2 shows pre- and postchemotherapy endoscopic tumor staging imaging for the 10 patients with long-term CR. Figure 3 shows CT imaging of CR after six cycles of chemotherapy in a patient whose primary supraglottic tumor was radiographically staged as T3.
Fig 2.
Pre- and postchemotherapy endoscopic tumor staging imaging for the 10 long-term complete responders.
Fig 3.
Pre- and postchemotherapy computed tomographic imaging of a complete response in a patient with a T3 supraglottic tumor.
Table 2 lists the distributions of response to chemotherapy and T classification. Three patients with T4 tumors were accrued before 2002, staged according to American Joint Committee on Cancer 1997 criteria, and had thyroid cartilage invasion. Two of these patients had pCR and achieved long-term remission without surgery or RT. The third patient had a PR and underwent supracricoid laryngectomy with cricohyoidopexy. All three patients had LP.
Table 2.
Tumor Classification and Response to Chemotherapy
| Larynx Subsite and T Stage* | Response Status (No. of patients) |
Total No. of Patients | |
|---|---|---|---|
| CR† | PR | ||
| Glottic larynx | 4 | 10 | 14 |
| T2 | 3 | 9 | 12 |
| T3 | 0 | 1 | 1 |
| T4 | 1 | 0 | 1 |
| Supraglottic larynx | 7 | 9 | 16 |
| T2 | 5 | 4 | 9 |
| T3 | 1 | 4 | 5 |
| T4 | 1 | 1 | 2 |
Abbreviations: CR, complete response; PR, partial response.
T stage by American Joint Committee on Cancer.
All patients with CR had a mean follow-up time of greater than 5 years.
TIP/TIC chemotherapy was generally well tolerated. Table 3 lists the adverse events encountered. Dose reductions were made for five patients. Four patients required a one-level dose reduction, three patients had a two-level dose reduction, and one patient had a three-level reduction in the dose of paclitaxel for neuropathy. The major reasons for dose modification included neurotoxicity (n = 4), renal toxicity (n = 3), neutropenic fever (n = 1), intractable nausea and vomiting (n = 1), and generalized weakness and malaise (n = 1).
Table 3.
Adverse Event Reporting
| Adverse Event | All Grades |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Anemia | 23 | 74.2 | 11 | 35.5 | 11 | 35.5 | 1 | 3.2 | ||||
| Anorexia | 9 | 29.0 | 7 | 22.6 | 1 | 3.23 | 1 | 3.2 | ||||
| Dehydration | 3 | 9.7 | 2 | 6.5 | 1 | 3.2 | ||||||
| Diarrhea | 7 | 22.6 | 4 | 12.9 | 3 | 9.7 | ||||||
| Dysphagia | 18 | 58.0 | 14 | 45.2 | 4 | 12.9 | ||||||
| Fatigue | 24 | 77.4 | 12 | 38.7 | 7 | 22.6 | 5 | 16.1 | ||||
| Granulocytopenia | 21 | 67.7 | 3 | 9.7 | 2 | 6.5 | 7 | 22.6 | 9 | 29.0 | ||
| Fever, neutropenic | 1 | 3.2 | 1 | 3.2 | ||||||||
| Hearing | 14 | 45.2 | 9 | 29.0 | 1 | 3.23 | 3 | 9.7 | ||||
| Leukopenia | 20 | 64.5 | 6 | 19.4 | 4 | 12.9 | 9 | 29.0 | 1 | 3.2 | ||
| Nausea | 23 | 74.2 | 8 | 25.8 | 14 | 45.2 | 1 | 3.2 | ||||
| Peripheral neuropathy | 18 | 58.0 | 11 | 35.5 | 6 | 19.4 | 1 | 3.2 | ||||
| Thrombocytopenia | 9 | 29.0 | 4 | 12.9 | 2 | 6.5 | 2 | 6.5 | 1 | 3.2 | ||
| Vomiting | 13 | 41.9 | 8 | 25.8 | 3 | 9.7 | 2 | 6.5 | ||||
| Xerostomia | 5 | 16.1 | 3 | 9.7 | 2 | 6.5 | ||||||
LP
The LP rate 2 years after initiation of chemotherapy was 83% (95% CI, 65% to 94%), which is significantly higher than 60% (P = .01). None of the 11 patients with pCR required TL. Six patients underwent TL. Of these, four patients with PR required TL for local disease recurrence after chemotherapy and CLS. One patient requested cRT instead of CLS. Six weeks after the completion of cRT, persistent disease was noted, and the patient underwent TL. Finally, one patient had TL for subglottic spread of tumor. This patient's original diagnostic biopsy demonstrated subglottic spread of disease, which regressed clinically but was histologically present on postchemotherapy biopsy and was not considered suitable for partial laryngeal surgery after endoscopy after a third course of TIP.
Functional Outcomes
All 25 patients with LP had native speech and swallowing. None required a gastrostomy tube for nutrition or a tracheostomy for airway protection. However, 3 years after induction chemotherapy and supracricoid laryngectomy, a single patient required both gastrostomy and tracheostomy as a result of adverse effects from cRT for a metachronous thoracic esophageal neuroendocrine carcinoma.
Management of the Neck
No patients with CR had neck dissection, and none had delayed recurrence in the neck. Four of the 11 patients with CR had clinical N1 disease. Twelve of 19 patients who underwent CLS also underwent selective neck dissection; eight of these patients had primary tumors arising from the supraglottis, and four had tumors arising in the glottic larynx with transglottic spread. Three patients (cN0, n = 2; cN1, n = 1) had histologic evidence of regional metastasis. Two of these patients had only one pathologic node. The third patient was found to have a contralateral nodal metastasis without extracapsular spread and had PORT.
RT
Only five patients (16%) underwent RT. A single patient who refused surgery after three cycles of TIP had primary cRT. Three patients (with PR and CLS) had PORT after TL for surgical salvage. The patient with a cerebrovascular accident shortly after induction chemotherapy received RT for disease control.
Survival and Disease Control
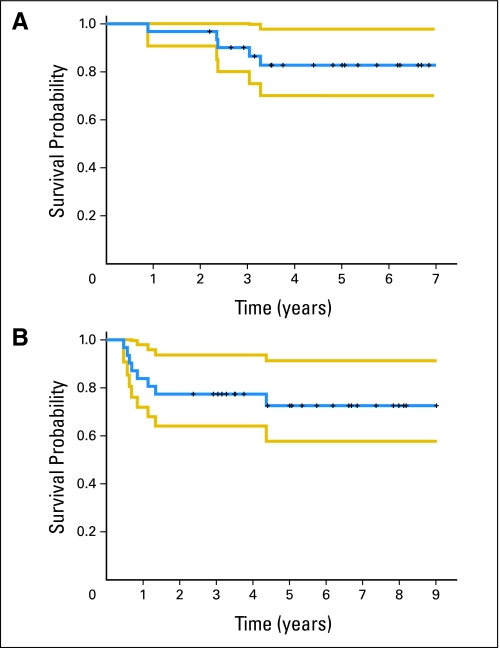
OS rates at 2 and 5 years were 97% (95% CI, 91% to 100%) and 83% (95% CI, 70% to 98%), respectively (Fig 4A). RFS rates at 2 and 5 years were 77% (95% CI, 64% to 94%) and 73% (95% CI, 58% to 91%), respectively (Fig 4B). All 31 patients were included in time-to-event analysis.
Fig 4.
(A) Overall survival. (B) Recurrence-free survival. The blue line represents the Kaplan-Meier estimate of survival (overall or recurrence free) and the yellow lines represent the 95% CI limits.
Eight patients experienced recurrences (local, n = 5; regional only, n = 1; local and regional, n = 2). Only one patient (9%) with pCR experienced a local recurrence and underwent salvage treatment with endoscopic CLS and PORT. Six patients with PRs experienced recurrences (local, n = 4; local and regional, n = 1; regional, n = 1). The patient who was not assessable for response developed regional recurrence. No patient developed distant metastasis. Metachronous primary tumors were found in two patients (papillary thyroid carcinoma and neuroendocrine carcinoma of the thoracic esophagus).
DISCUSSION
We report that selected patients with T2-4, N0-1 laryngeal cancer treated with induction chemotherapy, with or without CLS, achieve a high rate of LP (83%), meeting the primary end point of the study. More than one third of patients (11 of 30 patients; 37%) had a clinical, radiographic, and pathologic CR after three to four cycles of induction TIP chemotherapy alone. After receiving three additional cycles of TIP without surgery or RT, 10 of 11 patients had no local or regional recurrence over a median follow-up time of 5 years. No patient had more than six cycles of chemotherapy. Patients were observed by a multidisciplinary team and assessed monthly for evidence of tumor recurrence.
All patients with a PR who had CLS had excellent functional outcomes; none required a gastrostomy tube for nutrition or a tracheotomy for airway protection. RT was not needed for 84% of patients.
To achieve these results, patients in this prospective study were carefully selected. All patients underwent operative endoscopy to ensure that the laryngeal cancer could be resected via CLS. Eligibility was restricted to patients with stage N0-1 based on the experience of Laccourreye et al,12–14 which indicated that primary tumors may respond differently than nodal metastasis to chemotherapy. These rigorous selection criteria likely accounted for the slow rate of accrual over 7 years. Post-treatment follow-up and tumor surveillance were similarly fastidious. Monthly follow-up with laryngoscopy and CT scanning of the head and neck every 3 months for the first year were mandatory.
Despite an aggressive regimen, patient compliance with therapy was high, and toxicity was manageable with dose modification and close patient monitoring (Table 3). There were no grade 5 events. Grade 4 events were restricted to effects on the marrow, mainly neutropenia and neuropathy. Dose reduction was needed in only five patients. Four patients underwent a shift from cisplatin to carboplatin because of peripheral neuropathy and/or ototoxicity.
Most patients (71%) in this study had T2 laryngeal cancers. The recent American Society of Clinical Oncology Clinical Practice Guidelines for Larynx Preservation highlighted the difficulties in treating certain unfavorable T2 supraglottic and glottic laryngeal cancers.18 Hinerman et al20 reported a local control rate of 85% for T2 supraglottic carcinoma, but the vast majority of their patients had normal mobility of the vocal cords, suggesting, in general, a more favorable presentation. Reported control rates for T2 glottic tumors range from 70% to 80%, with control rates at the lower end of this range for patients with impaired vocal cord mobility.1,21
Although a widely used approach for patients with intermediate- to advanced-stage laryngeal cancer, the routine use of RT and the toxicity of platinum-based concurrent chemotherapy may result in a significant minority of patients with severe delayed toxicity,22 which may have a greater impact on long-term speech and swallowing function. Permanent tracheotomy and gastrostomy are not often necessary for patients treated with RT only or the smaller fields commonly used for T2 glottic cancer, but the incidence of debilitating late complications increases with larger fields used for supraglottic and advanced glottic SCC. In our study, all 25 CLS patients with LP had native speech and swallowing, without gastrostomy or tracheostomy.
In this study, 10 patients were treated exclusively with chemotherapy and remain alive with no evidence of recurrence after 5 years. This notable achievement probably relates in part to our selection criteria, which included only patients with laryngeal cancer amenable to CLS and with N0 or N1 regional disease and excellent performance status. Our data demonstrate proof of principle that selected squamous cancers of the head and neck may be cured with chemotherapy.
However promising these results are, we wish to emphasize that further multi-institutional phase III validation is required before such an approach is widely used. This single-institution phase II study was performed in a large quaternary referral center with expertise in CLS where close multidisciplinary follow-up is possible. Furthermore, it is important to stress that the use of this approach for the larynx or other head and neck tumor sites should be done with great care and only in the setting of a clinical trial. Laryngeal SCC may be more sensitive to cytotoxic drug therapy than some SCCs arising in the hypopharynx or oral cavity. Indeed, our experience suggests that this approach is an effective technique for achieving functional LP in centers with appropriate surgical expertise. Such an approach may obviate the need for concurrent chemoradiotherapy for unfavorable18 T2N0 and T3N0 laryngeal tumors.
The TIP regimen is highly active, but the development of more profoundly active, novel systemic approaches is currently under intense study. Randomized trials show increased activity for the traditional cisplatin and fluorouracil platform after the addition of a taxane.23–25 TIP was chosen as the regimen for this trial because of promising activity and tolerable toxicity in our previous phase I and II trials in patients with recurrent or metastatic head and neck cancer.15–17 In contrast to the potent triple-drug regimen of docetaxel, cisplatin, and fluorouracil, which was then being refined at the Dana-Farber Cancer Institute,26,27 our TIP regimen was efficient and effective, and most critically, repeated doses were tolerated by patients who were considered amenable to an organ preservation approach.
Future studies of chemotherapy in patients who are candidates for CLS should seek to identify biomarkers that predict which patients will benefit from chemotherapy alone as definitive treatment. The recent introduction of fiberoptic endoscopy will facilitate research biopsies.28 Microarray-based biomarker approaches can predict outcomes in other tobacco-related cancers such as lung cancer, even in the earlier stages of the disease.29 Identifying predictive biomarkers may enable us to move toward a model of personalized cancer medicine, in which the selection of therapy will be based on individual patient and tumor characteristics.30
Acknowledgment
We acknowledge Anthea Hammond, PhD; David L. Callender, MD, MBA; Helmuth Goepfert, MD, FACS; Kathleen A. Gillaspy, RN; Shirley Taylor, RN; Robert Byers, MD; Jeffrey N. Myers, MD, PhD, FACS; Fady Geara, MD, PhD; and William Morrison, MD.
Appendix
Table A1.
TIP Regimen and Definition of Dose Reduction Levels
| Agent | Day | Starting Dose (mg/m2) |
|||
|---|---|---|---|---|---|
| −3 | −2 | −1 | 0 | ||
| Paclitaxel | 1 | 85 | 110 | 135 | 175 |
| Cisplatin | 1 | 50 | 60 | 60 | 60 |
| Ifosfamide | 1-3 | 1,000 | 1,000 | 1,000 | 1,000 |
| Mesna | 1-3 | 720 | 720 | 720 | 720 |
NOTE. Patients received three to four cycles of TIP chemotherapy (paclitaxel 175 mg/m2 on day 1; ifosfamide 1,000 mg/m2 on days 1 to 3; and cisplatin 60 mg/m2 on day 1). Patients were premedicated with dexamethasone, cimetidine, and diphenhydramine before paclitaxel, which was administered intravenously over 3 hours by continuous infusion in a single dose. Ifosfamide was administered intravenously over 2 hours on days 1 to 3. Mesna was administered to reduce the incidence of hemorrhagic cystitis and hematuria. Cisplatin was administered intravenously over 2 hours on day 1 after completion of paclitaxel infusion. Treatment courses were repeated every 21 days or when blood counts recovered and nonhematologic toxicity resolved.
Abbreviation: TIP, paclitaxel, ifosfamide, and cisplatin.
Footnotes
Supported by peer-reviewed funding from Bristol-Myers Squibb Co's Investigator Initiated trials program, The University of Texas M. D. Anderson Cancer Center Support Grant No. CA 16672, discretionary funds obtained from patient donations and departmental funds; The University of Texas Faculty Incentive Award (F.C.H.), The University of Texas M. D. Anderson Recruitment Funds, Specialized Program of Research Excellence in Head and Neck Cancer Grant No. P50 CA97007 from the National Cancer Institute, National Institutes of Health (F.C.H.), and “Clinician Investigator Program in Translational Research” Grant No. K12 CA88084 (F.C.H.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: F. Christopher Holsinger, Bristol-Myers Squibb Co (C), Imclone Systems Inc (C); Fadlo R. Khuri, Sanofi-aventis (C) Stock Ownership: None Honoraria: F. Christopher Holsinger, Bristol-Myers Squibb Co, Imclone Systems Inc; Eduardo M. Diaz Jr, Bristol-Myers Squibb Co; Adam S. Garden, Sanofi-aventis; Dong M. Shin, Bristol-Myers Squibb Co, Sanofi-aventis, Imclone Systems Inc Research Funding: Dong M. Shin, Domantis, Ltd; Fadlo R. Khuri, Bristol-Myers Squibb Co, Sanofi-aventis, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eduardo M. Diaz Jr, Ann M. Gillenwater, Jan S. Lewin, Bonnie S. Glisson, J. Jack Lee, Dong M. Shin, Fadlo R. Khuri
Financial support: Merrill S. Kies, Waun Ki Hong, Fadlo R. Khuri
Administrative support: F. Christopher Holsinger, Waun Ki Hong, Fadlo R. Khuri
Provision of study materials or patients: F. Christopher Holsinger, Merrill S. Kies, Eduardo M. Diaz Jr, Bonnie S. Glisson, Adam S. Garden, Adel K. El-Naggar, Waun Ki Hong, Dong M. Shin, Fadlo R. Khuri
Collection and assembly of data: F. Christopher Holsinger, Eduardo M. Diaz Jr, Ann M. Gillenwater, Nebil Ark, J. Jack Lee, Waun Ki Hong, Fadlo R. Khuri
Data analysis and interpretation: F. Christopher Holsinger, Eduardo M. Diaz Jr, Ann M. Gillenwater, Jan S. Lewin, Lawrence E. Ginsberg, Nebil Ark, Heather Y. Lin, J. Jack Lee, Adel K. El-Naggar, Waun Ki Hong, Fadlo R. Khuri
Manuscript writing: F. Christopher Holsinger, Merrill S. Kies, Eduardo M. Diaz Jr, Ann M. Gillenwater, Adam S. Garden, Nebil Ark, Heather Y. Lin, J. Jack Lee, Waun Ki Hong, Dong M. Shin, Fadlo R. Khuri
Final approval of manuscript: F. Christopher Holsinger, Merrill S. Kies, Eduardo M. Diaz Jr, Ann M. Gillenwater, Jan S. Lewin, Lawrence E. Ginsberg, Bonnie S. Glisson, Adam S. Garden, Nebil Ark, Heather Y. Lin, J. Jack Lee, Adel K. El-Naggar, Waun Ki Hong, Dong M. Shin, and Fadlo R. Khuri
REFERENCES
- 1.Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786–1792. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 2.Viani L, Stell PM, Dalby JE. Recurrence after radiotherapy for glottic carcinoma. Cancer. 1991;67:577–584. doi: 10.1002/1097-0142(19910201)67:3<577::aid-cncr2820670309>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Holsinger FC, Funk E, Roberts DB, et al. Conservation laryngeal surgery versus total laryngectomy for radiation failure in laryngeal cancer. Head Neck. 2006;28:779–784. doi: 10.1002/hed.20415. [DOI] [PubMed] [Google Scholar]
- 4.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 5.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 6.Forastiere AA, Maor M, Weber RS, et al. Long-term results of Intergroup RTOG 91-11: A phase III trial to preserve the larynx—Induction cisplatin/5-FU and radiation therapy versus concurrent cisplatin and radiation therapy versus radiation therapy. J Clin Oncol. 2006;24(suppl):284s. abstr 5517. [Google Scholar]
- 7.Logemann JA, Rademaker AW, Pauloski BR, et al. Site of disease and treatment protocol as correlates of swallowing function in patients with head and neck cancer treated with chemoradiation. Head Neck. 2006;28:64–73. doi: 10.1002/hed.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Logemann JA, Pauloski BR, Rademaker AW, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008;30:148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutcheson KA, Barringer DA, Rosenthal DI, et al. Swallowing outcomes after radiotherapy for laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2008;134:178–183. doi: 10.1001/archoto.2007.33. [DOI] [PubMed] [Google Scholar]
- 10.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: The Radiation Therapy Oncology Group trial 91-11. Arch Otolaryngol Head Neck Surg. 2003;129:44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Holsinger FC, Weber RS. Swing of the surgical pendulum: A return to surgery for treatment of head and neck cancer in the 21st century? Int J Radiat Oncol Biol Phys. 2007;69:S129–S131. doi: 10.1016/j.ijrobp.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Laccourreye O, Brasnu D, Bassot V, et al. Cisplatin-fluorouracil exclusive chemotherapy for T1-T3N0 glottic squamous cell carcinoma complete clinical responders: Five-year results. J Clin Oncol. 1996;14:2331–2336. doi: 10.1200/JCO.1996.14.8.2331. [DOI] [PubMed] [Google Scholar]
- 13.Laccourreye O, Diaz EM, Jr, Bassot V, et al. A multimodal strategy for the treatment of patients with T2 invasive squamous cell carcinoma of the glottis. Cancer. 1999;85:40–46. [PubMed] [Google Scholar]
- 14.Laccourreye O, Veivers D, Hans S, et al. Chemotherapy alone with curative intent in patients with invasive squamous cell carcinoma of the pharyngolarynx classified as T1-T4N0M0 complete clinical responders. Cancer. 2001;92:1504–1511. doi: 10.1002/1097-0142(20010915)92:6<1504::aid-cncr1475>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Shin DM, Glisson BS, Khuri FR, et al. Phase II trial of paclitaxel, ifosfamide, and cisplatin in patients with recurrent head and neck squamous cell carcinoma. J Clin Oncol. 1998;16:1325–1330. doi: 10.1200/JCO.1998.16.4.1325. [DOI] [PubMed] [Google Scholar]
- 16.Shin DM, Khuri FR, Glisson BS, et al. Phase II study of paclitaxel, ifosfamide, and carboplatin in patients with recurrent or metastatic head and neck squamous cell carcinoma. Cancer. 2001;91:1316–1323. doi: 10.1002/1097-0142(20010401)91:7<1316::aid-cncr1134>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Shin DM, Glisson BS, Khuri FR, et al. Phase II study of induction chemotherapy with paclitaxel, ifosfamide, and carboplatin (TIC) for patients with locally advanced squamous cell carcinoma of the head and neck. Cancer. 2002;95:322–330. doi: 10.1002/cncr.10661. [DOI] [PubMed] [Google Scholar]
- 18.Pfister DG, Laurie SA, Weinstein GS, et al. American Society of Clinical Oncology clinical practice guideline for the use of larynx-preservation strategies in the treatment of laryngeal cancer. J Clin Oncol. 2006;24:3693–3704. doi: 10.1200/JCO.2006.07.4559. [DOI] [PubMed] [Google Scholar]
- 19.Woolson RF, Clarke WR. Statistical Methods for the Analysis of Biomedical Data. ed 2. New York, NY: Wiley; 2002. [Google Scholar]
- 20.Hinerman RW, Mendenhall WM, Amdur RJ, et al. Carcinoma of the supraglottic larynx: Treatment results with radiotherapy alone or with planned neck dissection. Head Neck. 2002;24:456–467. doi: 10.1002/hed.10069. [DOI] [PubMed] [Google Scholar]
- 21.Frata P, Cellai E, Magrini SM, et al. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. II. The case of T2N0 disease. Int J Radiat Oncol Biol Phys. 2005;63:1387–1394. doi: 10.1016/j.ijrobp.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2005;23:8636–8645. doi: 10.1200/JCO.2004.00.1990. [DOI] [PubMed] [Google Scholar]
- 24.Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 25.Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 26.Colevas AD, Busse PM, Norris CM, et al. Induction chemotherapy with docetaxel, cisplatin, fluorouracil, and leucovorin for squamous cell carcinoma of the head and neck: A phase I/II trial. J Clin Oncol. 1998;16:1331–1339. doi: 10.1200/JCO.1998.16.4.1331. [DOI] [PubMed] [Google Scholar]
- 27.Posner MR, Glisson B, Frenette G, et al. Multicenter phase I-II trial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol. 2001;19:1096–1104. doi: 10.1200/JCO.2001.19.4.1096. [DOI] [PubMed] [Google Scholar]
- 28.Holsinger FC. Swing of the pendulum: Optimizing functional outcomes in larynx cancer. Curr Oncol Rep. 2008;10:170–175. doi: 10.1007/s11912-008-0026-7. [DOI] [PubMed] [Google Scholar]
- 29.Potti A, Mukherjee S, Petersen R, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570–580. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 30.Varmus H. The new era in cancer research. Science. 2006;312:1162–1165. doi: 10.1126/science.1126758. [DOI] [PubMed] [Google Scholar]