Abstract
Dense Deposit Disease (DDD), or membranoproliferative glomerulonephritis type II, is a rare renal disease characterized by dense deposits in the mesangium and along the glomerular basement membranes that can be seen by electron microscopy. Although these deposits contain complement factor C3, as determined by immunofluorescence microscopy, their precise composition remains unknown. To address this question, we used mass spectrometry to identify the proteins in laser microdissected glomeruli isolated from paraffin-embedded tissue of eight confirmed cases of DDD. Compared to glomeruli from five control patients, we found that all of the glomeruli from patients with DDD contain components of the alternative pathway and terminal complement complex. Factor C9 was uniformly present as well as the two fluid-phase regulators of terminal complement complex clusterin and vitronectin. In contrast, in nine patients with immune complex–mediated membranoproliferative glomerulonephritis, glomerular samples contained mainly immunoglobulins and complement factors C3 and C4. Our study shows that in addition to fluid-phase dysregulation of the alternative pathway, soluble components of the terminal complement complex contribute to glomerular lesions found in DDD.
Keywords: alternative and terminal complement pathways, Dense Deposit Disease, Factor H–related protein I, kidney
Dense Deposit Disease (DDD, also known as membranoproliferative glomerulonephritis type II) is characterized by thickening of glomerular basement membranes (GBMs) by dense intramembranous deposits. The deposits are pathognomic of this type of glomerulonephritis. They stain for C3 but not immunoglobulins on immunofluorescence microscopy, but otherwise lack substructure even by electron microscopy, which resolves their presence in the mesangium and along GBMs.1,2
DDD is the result of dysregulation of the alternative pathway (AP) of the complement cascade and secondary persistent complement activation.3,4 This abnormal state is reflected in the serum of affected patients by low C3 levels and detectable C3 degradation products.5 One mechanism of AP dysregulation in DDD is associated with the presence of C3 nephritic factor, an autoantibody that stabilizes C3 convertase leading to C3 consumption and persistent AP activation.6,7 DDD can also develop as a result of deficiency of, or mutations in factor H, an essential serum protein for AP control.3,4,8,9
Although immunofluorescence microscopy has confirmed the presence of C3 in the dense deposits of DDD, their composition otherwise remains unknown. In this study, we have identified several additional proteins in the glomeruli of patients with DDD by using laser capture microdissection followed by liquid chromatography and mass spectrometry (LCMS).
RESULTS
Patient characteristics and renal biopsy results
Renal biopsies from eight patients ranging in age from 11 to 49 years and diagnosed with DDD were selected for this study. Nephrotic range proteinuria was present in three of seven patients; in one patient, the extent of proteinuria was not known. Five patients had stable renal function. Complement factors C3 and C4 were low in most cases (Table 1).
Table 1.
Clinical features and laboratory values of Dense Deposit Disease patients
| Patient no. |
Age and sex | Proteinuria (24 h) |
Serum creatinine (mg/100 ml) |
C3 (70–150 mg/100 ml) | C4 (14–40 mg/100 ml) | Urinalysis |
|---|---|---|---|---|---|---|
| 1 | 11 years, male | 4.8 g | 0.5 | 12 | 32 | 3+ protein, 1+ blood |
| 2 | 15 years, female | — | 0.9 | — | — | 3+ protein, 3+ blood |
| 3 | 41 years, male | 1.4 g | 3.6 | 21 | 108 | 3+ protein, 2+ blood |
| 4 | 15 years, male | 0.500 g | 1.0 | 19 | — | 4+ blood |
| 5 | 18 years, female | 7.7 g | 0.7 | 13 | 22 | 3+ protein, 2+ blood |
| 6 | 25 years, male | 0.966 g | 0.8 | — | — | 3+ protein, 2+ blood |
| 7 | 20 years, male | 2.7 g | 3.3 | 68 | 24 | 3+ protein, 2+ blood |
| 8 | 49 years, female | 7 g | 3.5 | 6 | 22 | 3+ protein, 1+ blood |
Renal biopsy revealed a membranoproliferative pattern of injury on light microscopy (Table 2). The glomeruli were enlarged and showed mesangial expansion with increased mesangial cellularity. Segmental endocapillary proliferation was noted. The glomerular capillary walls were thickened, and many loops showed cellular interposition and new basement membrane formation resulting in double contours. Six biopsies showed secondary focal and segmental glomerulosclerosis. Four biopsies o showed no or minimal tubulointerstitial scarring (<10%); two cases showed moderate tubulointerstitial scarring (approximately 20–25%); and two cases showed extensive tubulointerstitial scarring (>50%).
Table 2.
Renal biopsy results of Dense Deposit Disease patients
| Patient no. | Glomerular pathology | Secondary findings | Crescents/necrosis | Interstitial scarring |
|---|---|---|---|---|
| 1 | Membranoproliferative glomerulonephritis | FSGS | None | 10% |
| 2 | Membranoproliferative glomerulonephritis | FSGS | None | 10% |
| 3 | Membranoproliferative glomerulonephritis | FSGS | None | 20% |
| 4 | Membranoproliferative glomerulonephritis | ATN | None | 25% |
| 5 | Membranoproliferative glomerulonephritis | FSGS | None | 0 |
| 6 | Membranoproliferative glomerulonephritis | — | None | 0 |
| 7 | Membranoproliferative glomerulonephritis | FSGS | None | 60% |
| 8 | Membranoproliferative glomerulonephritis | FSGS | None | 60% |
ATN, acute tubular necrosis; FSGS, focal and segmental glomerulosclerosis.
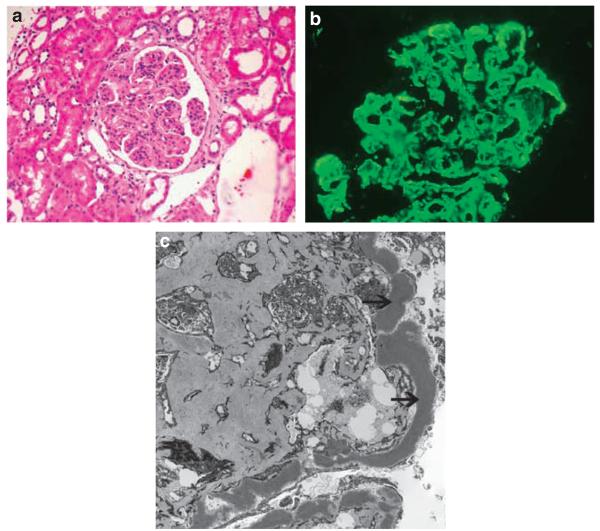
All cases showed C3 deposition in the mesangium and along the glomerular capillary walls on immunofluorescence microscopy, while staining for IgG, IgM, IgA, kappa, and lambda light chains was negative. Electron microscopy showed the typical electron dense deposits in the mesangium and along the GBMs. Figure 1 shows representative light microscopy (Figure 1a), immunofluorescence (Figure 1b), and electron microscopy (Figure 1c) findings.
Figure 1. Representative light, immunofluorescence, and electron microscopy in a case of Dense Deposit Disease.
(a) Periodic acid schiff (PAS)–stained section showing a membranoproliferative glomerulonephritis pattern of Dense Deposit Disease; (b) Immunofluorescence microscopy with C3 deposition in the mesangium and along glomerular capillary walls; (c) Electron microscopy resolving dense deposits in the mesangium and along the glomerular basement membranes (arrows).
Control tissue was obtained from five cases of Day 0 transplant protocol biopsies. All cases were from living donors. The glomeruli and interstitium were well preserved and there was no significant scarring.
Laser microdissection of glomeruli
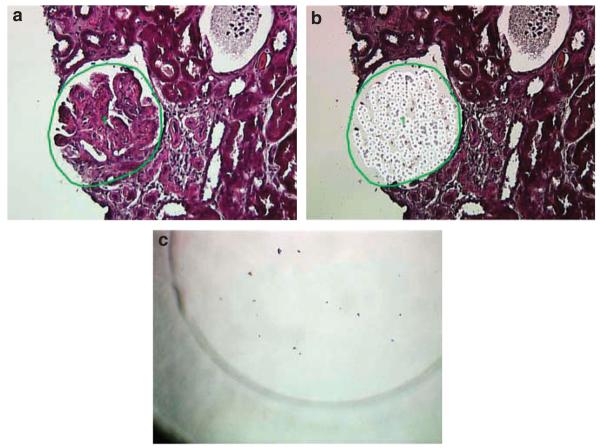
Preserved glomeruli (2 glomeruli per slide, 2 slides per case) were microdissected. Although more cases were available, the presence of extensive global glomerulosclerosis precluded their use. Only cases with a membranoproliferative pattern of injury were selected for LCMS studies. A representative glomerulus is shown in Figure 2. Figure 2a shows the marked glomerulus to be microdissected, and 2b shows the vacant area in the glomerulus after microdissection. Each small circle in the vacant area in the glomerulus represents one laser shot used to transfer the cut tissue into the tube cap. Figure 2c shows fragments of the glomerulus in the microcentrifuge tube caps.
Figure 2. Laser microdissection of a glomerulus.
(a) Glomerulus to be microdissected; (b) Vacant space on slide following microdissection; (c) Fragments of the microdissected glomerulus in the microcentrifuge tube cap.
Protein identification via mass spectrometry
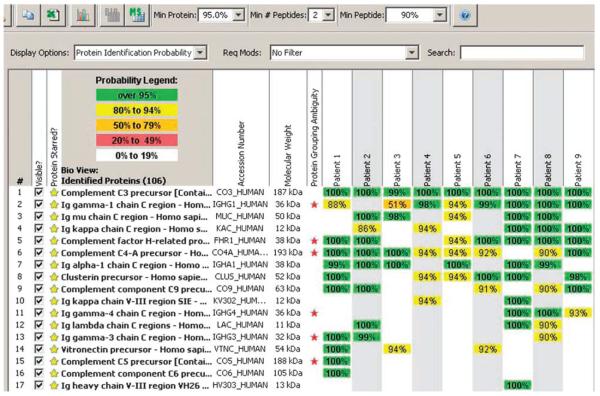
A typical Scaffold readout following LCMS from Case 4 is shown in Figure 3a. Forty-five proteins were identified in the microdissection sample (40–60 proteins are typically identified per microdissection sample). Several proteins of interest are highlighted with a yellow star. Complement precursors to C3, C5, C8α, and C9 were present with 100% probability, as were complement factor H-related protein 1 (FHR1), vitronectin, and apolipoprotein E (apoE). The first 2 columns (highlighted in green) show the samples in duplicate (microdissection 1 and microdissection 2). Immunostain PID004S1 represents microdissection 1, and immunostain PID004S2 represents microdissection 2. The next 2 columns represent blanks post and pre run. They typically do not contain any proteins but may show a little carryover (in this case C3a and FHR1). A typical protein identification pattern is shown in Figure 3b. In Case 4, there is a 6-peptide match for precursor to C5.
Figure 3. Protein identification by LCMS.
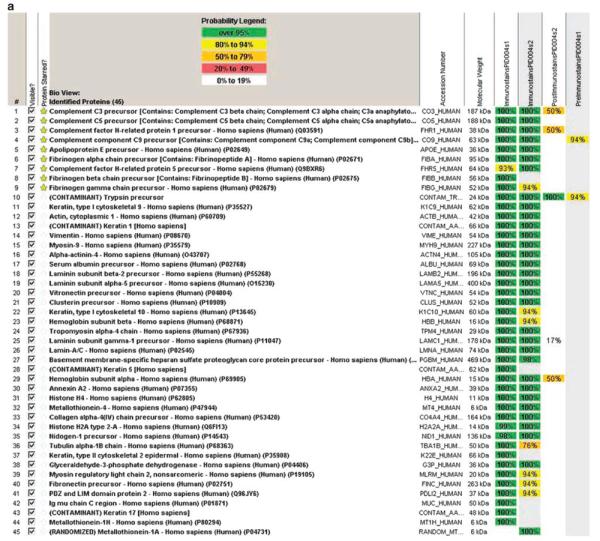

(a) Representative scaffold readout of glomerular proteins for Case 4; (b) C5a identification by peptide analysis; (c) Scaffold readout of proteins of interest in all eight cases of dense deposit disease and one control sample.
In the Scaffold readout from all eight cases of DDD, we identified components of the AP and terminal complement complex (TCC) in nearly all dissected glomeruli. C9 was uniformly present, as were clusterin and vitronectin, two fluid-phase regulators of the TCC. FHR1 was seen in all DDD glomeruli and FHR5, another member of the factor H-related gene family, was seen in two. ApoE was also always identified (Table 3). Protein identification by LCMS of normal glomeruli did not reveal any of the above listed proteins in any case. A representative control and all DDD readouts are shown in Figure 3c.
Table 3.
DDD versus IC-MPGN LCMS findings
| Pathology Protein |
DDD (n=8) |
IC-MPGN (n=9) |
||
|---|---|---|---|---|
| >95%a | >80% | 95% | >80% | |
| C3 | 8 | 8 | 6 | 7 |
| C4 | 3 | 3 | 5 | 8 |
| C5 | 7 | 7 | 1 | 1 |
| C6 | 4 | 6 | 1 | 1 |
| C7 | 1 | 4 | 0 | 0 |
| C8α | 3 | 5 | 0 | 0 |
| C8β | 2 | 7 | 0 | 0 |
| C8γ | 2 | 5 | 0 | 0 |
| C9 | 8 | 8 | 3 | 5 |
| Immunoglobulin | 0 | 0 | 9 | 9 |
| FHR1 | 8 | 8 | 6 | 7 |
| FHR5 | 1 | 2 | 0 | 0 |
| Clusterin | 8 | 8 | 4 | 6 |
| Vitronectin | 8 | 8 | 1 | 3 |
| Apolipoprotein E | 8 | 8 | 0 | 0 |
DDD, dense deposit disease; IC-MPGN, immune complex-mediated membranopro-liferative glomerulonephritis; LCMS, liquid chromatography and mass spectrometry; FHR, factor H-related protein.
Protein prediction probability.
Immune complex-mediated membranoproliferative glomerulonephritis
For comparison, we completed LCMS protein identification on nine cases of immune complex-mediated membranoproliferative glomerulonephritis (IC-MPGN). Included were biopsies of idiopathic MPGN type I and MPGN secondary to hepatitis B or C, autoimmune diseases such as lupus, cryoglobulins, chronic bacterial infections, and monoclonal gammopathy of unknown significance. LCMS identified immunoglobulins in all cases although the type of immunoglobulin (IgG, IgA, IgM, Ig kappa light chains, Ig lambda light chains, Ig heavy chains) varied reflecting the different etiologies of IC-MPGN. C3 was the only complement factor seen in all cases, although C4 was present in eight cases with >80% certainty. FHR1 was the next most frequently identified protein. TCC complement factors and vitronectin were rarely identified, and apoE was never seen (Figure 4; Table 3).
Figure 4. Scaffold readout of proteins of interest for all nine cases of immune complex-mediated membranoproliferative glomerulonephritis (MPGN).
Clinical history is as follows: patient 1 was a case of hepatitis B; patient 2 had cryptogenic cirrhosis; patient 3 had an autoimmune disease with positive antinuclear antibodies and rheumatoid factor; patients 4 and 5 had no significant history and likely had idiopathic MPGN type I; patient 6 had monoclonal gammopathy of unknown significance; patient 7 had cryoglobulins and a questionable autoimmune disease; patient 8 likely had idiopathic MPGN type I; and patient 9 had chronic bacterial infections.
DISCUSSION
DDD is characterized by amorphous electron-dense deposits in the mesangium and along GBMs that stain for C3 by immunofluorescence microscopy,1,2 and as expected, C3 was present in glomeruli of all cases of DDD by LCMS. As compared with both normal controls and IC-MPGN glomeruli, DDD glomeruli had significantly more C5, C6, C7, C8 (α, β, and γ), vitronectin, and apoE (vitronectin—95% protein prediction, P<0.05; remainder—80% protein prediction probability, P<0.05). FHR5 was also only seen in DDD glomeruli.
Proteins present in both DDD and IC-MPGN glomeruli but absent in controls included FHR1, C4, clusterin, GBM-specific heparan sulfate proteoglycan core protein precursor, myosin 9, and some of the laminin subunits. Immunoglobulin was uniquely present only in IC-MPGN samples. Proteins found in all three types of glomeruli included keratins, vimentin, collagen, actin, and histones.
Complement factors in DDD glomeruli
The AP is an important arm of innate immunity. Low level auto-activity continuously occurs as a result of spontaneous hydrolysis of a reactive thiolester on the α-chain of C3. This process is known as ‘tick over’ and leads to the formation of C3[H2O], which binds factor B to form C3[H2O]B. After cleavage to C3[H2O]Bb by factor D, C3[H2O]Bb cleaves additional C3 molecules to C3a and C3b. The C3b generated by this process combines with factor B to form the AP C3 convertase, C3bBb.10,11
We detected both C3β and C3β chains in DDD glomeruli by LCMS, a finding consistent with the structure of C3 as single α and single β chains are joined by two disulfide bonds.12 It is not possible by LCMS to determine whether the detected peptides represent C3b or its breakdown products iC3b, C3dg, or C3c, as we are analyzing trypsin digests. Differentiating C3b from iC3b, C3dg, or C3c would be valuable, as it could provide an indication of the site of complement activation; however, factor B was not seen in any DDD glomeruli. Its absence is noteworthy because both the AP C3 convertase (C3bBb) and the C5 convertase ([C3b]Bb[C3b]) include factor B in their structures. These convertases form on surfaces where AP activity is occurring, suggesting that the major site of AP activity in DDD is not in the glomeruli themselves but rather in the fluid phase.
The importance of fluid-phase activation of the AP in the pathogenesis of DDD is supported by the work of Pickering and colleagues who showed that factor I activity is an absolute requirement for GBM C3 deposition in the Cfh −/− mouse.13 The Cfh−/− mouse typically develops glomerular hypercellularity, mesangial expansion, and thickening of the capillary walls as seen by light microscopy. Electron microscopy resolves the presence of subendothelial dense capillary wall deposits and immunofluorescence shows capillary wall and mesangial C3 staining.14 In the absence of factor I (Cfh−/−.Cfi−/− double mutant), capillary wall C3 staining is not seen although mesangial hypercellularity and C3 staining remain. This finding is consistent with the dysregulation of fluid-phase AP convertase activity and mesangial C3 production.13
DDD is frequently associated with the presence of C3 nephritic factor, which prolongs the half-life of C3 convertase by protecting it from factor H-mediated decay.6,7 Consumption of plasma C3 drives levels down and leads to the formation of iC3b, C3dg, and C3c in the circulation. Our findings and the work of Pickering and colleagues suggest that it is these breakdown products of C3 that become sequestered in the glomeruli.
We also found that TCC components C5, C6, C7, and C8 were specific for DDD. In the plasma, TCC activity is controlled by complement regulatory proteins like clusterin (SP-40, 40, cytolysis inhibitor, or ApoJ) and vitronectin (S protein),15–17 both of which were present in all DDD glomeruli. This observation is pertinent because fluid-phase C5 convertase activity in DDD generates C5b, which binds C6 and C7 to form C5b-7. Normally, the C5b-7 complex either inserts itself into a cell membrane or binds to a single molecule of clusterin and vitronectin to form a soluble C5b-7/clusterin/vitronectin complex. Once formed, soluble C5b-7/clusterin/vitronectin complexes cannot insert into a cell membrane; however, binding with C8 and C9 can occur to produce inactive but soluble membrane attack complex/clusterin/vitronectin (sMAC).15 Our data suggest that sMAC becomes sequestered in DDD glomeruli.
This observation implies that in addition to uncontrolled activity of the AP in the fluid phase, DDD is also characterized by excessive fluid-phase TCC production, with sMAC accumulation in glomeruli. sMAC activates the caspace pathway and apoptosis, and also causes transendothelial migration of polymorphonuclear leucocytes,18,19 properties that could accelerate glomerular injury. Supporting this possibility, Pickering et al.20 have shown that the DDD phenotype in Cfh−/−.C5−/− double mutant mice is characterized by lower mortality, decreased leucocyte infiltration, and a reduction in serum creatinine when compared with the phenotype observed in Cfh−/− mutants. Surprisingly, however, in Cfh−/−.C6−/− double mutants, there was no change in disease severity when compared with Cfh−/− mutants, a finding these investigators interpreted to mean that C5a is more important than the TCC in the pathogenesis of DDD. Alternatively, however, it is possible that some protein-protein interactions within sMAC occur in the absence of C6. In the fluid-phase, C6 and C7 mediate reversible binding to C5 and bound C6 is easily displaced by C7. Only the reaction between C5 and the C7 factor I modules are important for MAC assembly.21
Factor H-related proteins in DDD and IC-MPGN glomeruli
The most important fluid-phase regulator of the AP is factor H.10 This protein is composed of 20 short consensus repeats (SCRs), each of which approximates 60 amino acids, with neighboring SCRs joined by short 6–12 amino acid linkers. The SCRs differ in amino acid sequence, imparting to factor H multiple SCR-specific functions. Factor H-like 1, a splice variant of the factor H gene, consists of 7 SCRs that are identical to the first 7 N-terminal SCRs of factor H and a C-terminus that includes 4 additional amino acids encoded by a unique exon that includes a stop codon and unique untranslated 3′ end. Also included in the factor H family are five factor H-related proteins encoded by distinct genes (CFHR1-5).22
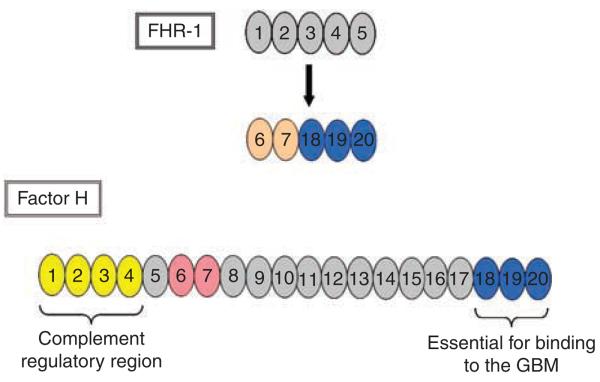
We detected FHR1 in all DDD glomeruli and seven of nine IC-MPGN glomeruli; FHR5 was present in two DDD glomeruli (>80% certainty). The first finding suggests that FHR1 may play a non-specific role in C3-associated glomerulonephritis. FHR1 is comprised of five SCRs, the last three of which are nearly identical to SCRs 18, 19, and 20 of factor H. (There is a two amino acid difference in SCR5 of FHR1.) This degree of similarity with SCRs 18, 19, and 20 of factor H is not seen in any other FRH protein.22 SCRs 1–2 of FHR-1 are most similar to SCRs 6 and 7 of factor H and, therefore, may have some endothelial cell and heparin binding affinity.23 Because FHR1 lacks SCRs with similarity to SCRs1–4 of factor H, it is not predicted to have major complement regulatory activity22 (Figure 5).
Figure 5. Comparison of the SCRs of factor H with those of FHR1.
FHR1 is comprised of five SCRs. The last three SCRs have nearly 100% identity with SCRs 18, 19, and 20 of factor H and are shown in blue. SCRs 1 and 2 of FHR1 have modest identity with SCRs 6 and 7 of factor H, hence the slightly different colors. SRC, short consensus repeat; FHR1, factor H-related protein 1; GBM, glomerular basement membrane.
The GBM lacks intrinsic regulators of AP activation and sequesters factor H in small quantities from the serum.24 The two terminal SCRs of factor H are essential for binding to the GBM25 and distinguishing between activator surfaces (those surfaces that activate the complement cascade) and nonactivator surfaces.26 Ferreira and colleagues demonstrated their importance by showing that a small recombinant protein composed of only SCRs 19 and 20 of factor H (rH19-20) inhibits regulatory activities of factor H toward cell-bound C3b, and by out-competing factor H for binding sites, causes aggressive complement-mediate lysis of nonactivating sheep erythrocytes.26 Even partial lysis of human erythrocytes was observed, which is significant as human erythrocytes have both CD55 and CD59 on their surface.
It is possible, therefore, that FHR1 may bind to the GBM in a variety of complement-mediated and immune complex-mediated glomerular diseases. Whether its presence is harmful, protective, or an epiphenomenon is unclear. The possible function of FHR5 in DDD glomeruli is not known, although mutations in this gene have been identified in a few affected patients.27
Apolipoprotein E
ApoE is a 299-amino acid polymorphic glycoprotein. Its three common alleles, ∈2, ∈3, and ∈4, code for E2, E3, and E4 to determine the phenotypes E2/E2, E3/E3, E4/E4, E2/E3, E2/E4, and E3/E4.28–31 The liver is the primary site of synthesis and in the circulation apoE plays a major role in lipoprotein clearance.32,33 An important secondary site of synthesis is the mesangial cell, and in the kidney apoE has an autocrine function to mediate uncontrolled mesangial expansion and to regulate heparan sulfate glycoprotein levels.34 The ∈3 allele is most effective in inhibiting mesangial cell proliferation.34 Although several studies have identified associations between apoE polymorphisms and nephropathy,35–38 the significance of the presence of apoE in DDD glomeruli has not been explored.
Conclusion
This study used LCMS to identify proteins in DDD glomeruli. Findings were compared with normal controls and to biopsies of IC-MPGN and suggest that fluid-phase dysregulation of both the AP and the TCC contributes to the glomerular pathology that is seen in DDD. ApoE was also uniquely seen in DDD glomeruli, although the significance of this finding is unclear. FHR1 was seen in both DDD and IC-MPGN glomeruli and may play a role in complement-mediated and immune-complex glomerulonephritis.
MATERIALS AND METHODS
Patients
Between January 2006 and September 2007, renal biopsies from eight patients demonstrating DDD were evaluated. These biopsies were cases sent to the Mayo Clinic for interpretation and diagnosis. Normal-appearing glomeruli from five cases of living-related Day 0 protocol transplant biopsies were used as control samples. We also analyzed renal biopsies from nine cases of IC-MPGN. The Institutional Review Board at the Mayo Clinic approved the use of these biopsies on the basis that identifying information on each patient would remain unknown.
Specimen preparation and microdissection
10 μm-thick sections of formalin-fixed paraffin-embedded tissues were placed on DIRECTOR™ slides (Expression Pathology, Gaithersburg, MD, USA) for laser microdissection. Sections were air-dried, melted, and deparaffinized, and an H&E stain was performed on all samples. Two glomeruli were photographed and then microdissected into 0.5 ml microcentrifuge tube caps containing 35 μl Tris/EDTA/0.002% Zwittergent 3–16 buffer using a Leica DM6000B Microdissection System. The method was repeated with a second slide and again two glomeruli were microdissected.
Glomeruli with global sclerosis or extensive segmental sclerosis were not selected for microdissection. Collected tissues were heated at 98°C for 90 min with occasional vortexing. Following 30 min of sonication in a waterbath, samples were reduced DTT, alkylated with iodoacetamide, digested overnight at 37° with 1.5 μl 1 ug/ml trypsin (Promega, Madison, WI, USA).
Protein identification via mass spectrometry
The trypsin-generated digests were used for protein identification by nano-flow liquid chromatography electrospray tandem mass spectrometry using a ThermoFinnigan LTQ Orbitrap Hybrid Mass Spectrometer (ThermoElectron Bremen, Germany) coupled to an Eksigent nanoLC-2D HPLC system (Eksigent, Dublin, CA, USA), as described previously.39
The peptide mixtures were injected onto a magic C18 packed 0.25 μl trap (Optimize Technologies) packed with Michrom 5 μm C18 plumbed into a 10-port valve. The peptides were then eluted onto a 75 μm × 10 cm column packed with 5 μm Michrom C18 and eluted with a 400 nl/min 60 min organic gradient (5–55% ACN in 0.1% formic acid).
The mass spectrometer was operated in the data-dependent mode to automatically switch between orbitrap-MS and iontrap-MS/MS (MS2) acquisition. Survey full scan MS spectra (from m/z 375–1800) were acquired in the orbitrap with resolution 60,000 at m/z 400 (after accumulation to a target value of 1,000,000 charges in the linear ion trap). The most intense ions (up to five, depending on signal intensity) were sequentially isolated for fragmentation in the linear ion trap using collisionally induced dissociation at a target value of 100,000 charges. The resulting fragment ions were recorded in the orbitrap with a resolution of 15,000 at m/z 400. For accurate mass measurements, the lock mass option was enabled in both MS and MS/MS mode, and the polydimethylcyclosiloxane ions generated in the electrospray process from ambient air (protonated (Si(CH3)2O))6; m/z _ 445.120025) were used for internal recalibration in real time.40 Target ions already selected for MS/ MS were dynamically excluded for 30 s. General mass spectrometric conditions were electrospray voltage, 2.2 kV; no sheath and auxiliary gas flow; ion transfer tube temperature, 200°C; collision gas pressure, 1.3 mT; normalized collision energy, 32% for MS2. Ion selection threshold was 100 counts for MS2. An activation q of 0.25 and activation time of 30 ms was applied for MS2 acquisitions.
Criteria for protein identification
An in-house developed workflow tool (SWiFT) was utilized to search ThermoFisher raw data files. This tool parses the raw data file to Sequest, Mascot, and X!Tandem search engines. The results are combined and assigned peptide and protein probability scores in Scaffold (version Scaffold-01_07_00, Proteome Software Inc., Portland, OR, USA). Peptide identifications were accepted if they could be established at greater than 90.0% probability as specified by the Peptide Prophet algorithm.41 Protein identifications were accepted if they could be established at greater than 95% probability and contained at least two identified peptide. Protein probabilities were assigned by the Protein Prophet algorithm.41,42
ACKNOWLEDGMENTS
This research was supported in part by NIH grant DK074409 to SS, PFZ, and RJHS.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 2.Smith RJH, Alexander J, Barlow PN, et al. Dense deposit disease focus group: new approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 4.Licht C, Heinen S, Jozsi M, et al. Deletion of Lys224 in regulatory domain 4 of factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kidney Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- 5.Schwertz R, Rother U, Anders D, et al. Complement analysis in children with idiopathic membranoproliferative glomerulonephritis: a long-term follow-up. Pediatr Allergy Immunol. 2001;12:166–172. doi: 10.1034/j.1399-3038.2001.012003166.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohi H, Watanabe S, Fujita T, et al. Detection of C3bBb-stabilizing activity (C3 nephritic factor) in the serum from patients with membranoproliferative glomerulonephritis. J Immunolog Methods. 1990;131:71–76. doi: 10.1016/0022-1759(90)90234-m. [DOI] [PubMed] [Google Scholar]
- 7.West CD, Witte DP, McAdams AJ. Composition of nephritic factor-generated glomerular deposits in membranoproliferative glomerulonephritis type 2. Am J Kid Dis. 2001;37:1120–1130. doi: 10.1053/ajkd.2001.24511. [DOI] [PubMed] [Google Scholar]
- 8.Abrera-Abeleda MA, Nishimura C, Smith JLH, et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease) J Med Genet. 2006;43:582–589. doi: 10.1136/jmg.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montes T, Goicoechea de Jorge E, Ramos R, et al. Genetic deficiency of complement factor H in a patient with age-related macular degeneration and membranoproliferative glomerulonephritis. Mol Immunol. 2008;45:2897–2904. doi: 10.1016/j.molimm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Zipfel PF, Heinen S, Jozsi M, et al. Complement and diseases: defective alternative pathway control results in kidney and eye diseases; Mol Immunol. 10th Meeting on Complement in Human Disease; 2006. pp. 97–106. [DOI] [PubMed] [Google Scholar]
- 11.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 12.Janssen BJ, Gros P. Structural insights into the central complement component C3. Mol Immunol. 2007;44:3–10. doi: 10.1016/j.molimm.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;11:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan AK, Moore TL. Presence of plasma complement regulatory proteins clusterin (Apo J) and vitronectin (S40) on circulating immune complexes (CIC) Clin Exp Immunol. 2006;145:398–406. doi: 10.1111/j.1365-2249.2006.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi NH, Nakano Y, Tobe T, et al. Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol. 1990;2:413–417. doi: 10.1093/intimm/2.5.413. [DOI] [PubMed] [Google Scholar]
- 17.Declerck PJ, De Mol M, Alessi MC. Purification and characterization of plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vibronectin) J Biol Chem. 1988;263:15454–15461. [PubMed] [Google Scholar]
- 18.Nauta AJ, Daha MR, van de Tijsma OWB, et al. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–792. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Dobrina A, Pausa M, Fischetti F, et al. Cytolytically inactive terminal complement complex causes transendothelial migration of polymorphonuclear leukocytes in vitro and in vivo. Blood. 2002;99:185–192. doi: 10.1182/blood.v99.1.185. [DOI] [PubMed] [Google Scholar]
- 20.Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonrphritis in factor H-deficient mice. Proc Natl Acad Sci USA. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thai Ct, Ogata RT. Recombinant C345 and factor I modules of complement components C5 and C7 inhibit C7 incorporation into the complement membrane attack complex. J Immunol. 2005;174:6227–6232. doi: 10.4049/jimmunol.174.10.6227. [DOI] [PubMed] [Google Scholar]
- 22.Zipfel P, Skerka C, Hellwage J, et al. Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans. 2002;30:971–978. doi: 10.1042/bst0300971. [DOI] [PubMed] [Google Scholar]
- 23.Skerka C, Lauer N, Weinberger AWA, et al. Defective complement control of FHL-1 and factor H (Y402H) in age-related macular degeneration. Mol Immunol. 2007;44:3398–3406. doi: 10.1016/j.molimm.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Licht C, Schlötzer-Schrehardt U, Kirschfink M, et al. MPGN II – genetically determined by defective complement regulation? Pediatr Nephrol. 2007;22:2–9. doi: 10.1007/s00467-006-0299-8. [DOI] [PubMed] [Google Scholar]
- 25.Cheng ZZ, Hellwage J, Seeberger H, et al. Comparison of surface recognition and C3b binding properties of mouse and human complement factor H. Mol Immunol. 2006;43:972–979. doi: 10.1016/j.molimm.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira VP, Herbert AP, Hocking HG, et al. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J Immunol. 2006;177:6308–6316. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 27.Abrera-Abeleda MA, Nishimura C, Smith JLH, et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease) J Med Genet. 2006;43:582–589. doi: 10.1136/jmg.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rall SC, Weigraber KH, Mahley RW. Human apolipoprotein E: the complete amino acid sequence. J Biol Chem. 1982;257:4171–4178. [PubMed] [Google Scholar]
- 29.Utermann G, Hees M, Steinmetz A. Polymorphism of apolipoprotein E and occurrence of dysbetalipoproteinaemia in man. Nature. 1977;269:604–607. doi: 10.1038/269604a0. [DOI] [PubMed] [Google Scholar]
- 30.Zannis VI, Breslow JL. Human very low-density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and post-translational modification. Biochemistry. 1981;20:1022–1041. doi: 10.1021/bi00507a059. [DOI] [PubMed] [Google Scholar]
- 31.Zannis VI, Breslow JL, Utermann G, et al. Proposed nomenclature of apo E isoproteins, apo E genotypes and phenotypes. J Lipid Res. 1982;23:911–914. [PubMed] [Google Scholar]
- 32.Aalto-Setälä K, Benlian P, Bowyer D, et al. Atherosclerosis; European Lipoprotein Club: report of the 20th annual conference; Tutzing. 8–11 September 1997; 1998. pp. 223–229. [DOI] [PubMed] [Google Scholar]
- 33.Weisgraber KH. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J Lipid Res. 1990;31:1503–1511. [PubMed] [Google Scholar]
- 34.Guangping C, Paka L, Kako Y, et al. A protective role for kidney apolipoprotein E. J Biol Chem. 2001;276:49142–49147. doi: 10.1074/jbc.M104879200. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury TA, Dyer PH, Kumar S, et al. Association of apolipoprotein epsilon2 allele with diabetic nephropathy in Caucasian subjects with IDDM. Diabetes. 1998;47:278–280. doi: 10.2337/diab.47.2.278. [DOI] [PubMed] [Google Scholar]
- 36.Werle E, Fiehn W, Hasslacher C. Apolipoprotein E polymorphism and renal function in German type 1 and type 2 diabetic patients. Diabetes Care. 1998;21:994–998. doi: 10.2337/diacare.21.6.994. [DOI] [PubMed] [Google Scholar]
- 37.Eto M, Horita K, Morikawa A, et al. Increased frequency of apolipoprotein epsilon 2 allele in non-insulin dependent diabetic (NIDDM) patients with nephropathy. Clin Genet. 1995;48:288–292. doi: 10.1111/j.1399-0004.1995.tb04111.x. [DOI] [PubMed] [Google Scholar]
- 38.Onuma T, Laffel LM, Angelico MC, et al. Apolipoprotein E genotypes and risk of diabetic nephropathy. J Am Soc Nephrol. 1996;7:1075–1078. doi: 10.1681/ASN.V771075. [DOI] [PubMed] [Google Scholar]
- 39.Olsen JV, de Godoy LMF, Li G, et al. Parts per million mass accuracy on an orbitrap mass spectrometer via lock mass injection into a C-trap 10.1074/mcp.T500030-MCP200. Mol Cell Proteomics. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- 40.Olsen JV, de Godoy LM, Li G, et al. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol Cell Proteomics. 2005;4:2010–2021. doi: 10.1074/mcp.T500030-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Keller A, Nesvizhskii AI, Kolker E, et al. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 42.Nesvizhskii AI, Keller A, Kolker E, et al. A statistical model for identifying proteins by tandem mass spectrometry. Ana Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]