Abstract
Purpose
To review the reports of subsequent neoplasms (SNs) in the Childhood Cancer Survivor Study (CCSS) cohort that were made through January 1, 2006, and published before July 31, 2008, and to discuss the host-, disease-, and therapy-related risk factors associated with SNs.
Patients and Methods
SNs were ascertained by survivor self-reports and subsequently confirmed by pathology findings or medical record review. Cumulative incidence of SNs and standardized incidence ratios for second malignant neoplasms (SMNs) were calculated. The impact of host-, disease-, and therapy-related risk factors was evaluated by Poisson regression.
Results
Among 14,358 cohort members, 730 reported 802 SMNs (excluding nonmelanoma skin cancers). This represents a 2.3-fold increase in the number of SMNs over that reported in the first comprehensive analysis of SMNs in the CCSS cohort, which was done 7 years ago. In addition, 66 cases of meningioma and 1,007 cases of nonmelanoma skin cancer were diagnosed. The 30-year cumulative incidence of SMNs was 9.3% and that of nonmelanoma skin cancer was 6.9%. Risk of SNs remains elevated for more than 20 years of follow-up for all primary childhood cancer diagnoses. In multivariate analyses, risks differ by SN subtype, but include radiotherapy, age at diagnosis, sex, family history of cancer, and primary childhood cancer diagnosis. Female survivors whose primary childhood cancer diagnosis was Hodgkin's lymphoma or sarcoma and who received radiotherapy are at particularly increased risk. Analyses of risk associated with radiotherapy demonstrated different dose-response curves for specific SNs.
Conclusion
Childhood cancer survivors are at a substantial and increasing risk for SNs, including nonmelanoma skin cancer and meningiomas. Health care professionals should understand the magnitude of these risks to provide individuals with appropriate counseling and follow-up.
INTRODUCTION
Beginning with reports from the Late Effects Study Group almost 30 years ago, investigators have studied cohorts of childhood cancer survivors in an effort to estimate the incidence and understand the risk factors of the development of what some consider the most serious late complication of disease and treatment—second malignant neoplasms (SMNs).1–4 The Childhood Cancer Survivor Study (CCSS) publications concerning SMNs have involved the largest cohort of relatively long-term survivors, and because of the extensive medical review of cases and controls, we have been able to analyze the relations between anticancer treatment and SMN development. In addition to facilitating focused medical follow-up care and surveillance of the ever-growing population of childhood cancer survivors, this knowledge can increase our understanding of carcinogenic processes in general. Because most children have had limited exposure to known environmental carcinogens and can be expected to live for many years after anticancer treatment, they provide an opportunity to gain knowledge about the mechanisms by which certain chemotherapeutic agents and radiation can lead to neoplastic changes.
Although we now know that childhood cancer survivors are likely to experience more cancers as adults than are individuals of similar age in the general population as a result of host-related factors (eg, deletion of the RB1 gene, which causes heritable retinoblastoma) and that certain aspects of anticancer treatment increase the risk of SMNs, our knowledge is constrained by the limited follow-up period (25 to 30 years) of the existing survivor cohorts. Not until there are large enough numbers of survivors in their fourth and fifth decades of life will we learn how normal aging processes and the “natural” increase of cancer in the general population influence the development of SMNs in survivors of childhood cancer.
The CCSS has the ability to continue investigating this aging population and to understand the factors that increase their risk of subsequent neoplasms (SNs). Here we summarize the knowledge gained about the incidence and characteristics of second cancers in individuals who were diagnosed with childhood cancer between 1970 and 1986 and report the most prevalent SNs that occur in this cohort before they reach 21 years of age and have survived their primary disease for at least 5 years.
PATIENTS AND METHODS
The CCSS Cohort
A complete description of the methods involved in CCSS case ascertainment can be found in this volume and elsewhere.5 Briefly, the CCSS consists of a well-characterized cohort of childhood cancer survivors that was constructed to evaluate hypotheses related to long-term health-related outcomes.6 Eligibility included treatment at one of the collaborating institutions between January 1, 1970, and December 31, 1986, for leukemia, Hodgkin's lymphoma (HL), non-Hodgkin's lymphoma (NHL), neuroblastoma, soft tissue sarcoma, bone cancer, CNS cancer, or Wilms tumor; age younger than 21 years at diagnosis of primary cancer; and survival for at least 5 years after diagnosis. The human subjects committees at each institution approved the protocol.
There were 20,626 survivors eligible for the CCSS. Of those, 3,058 could not be located, and 3,205 refused participation; thus 14,363 survivors were enrolled. Patients who survived at least 5 years after diagnosis and subsequently died are included in the cohort. Analyses of SNs in this population used all or part of the cohort.
Cancer Treatment Information
Therapeutic exposures were ascertained through abstraction of medical and radiation therapy records of each participant by using a standardized protocol. For patients who received radiation therapy, the full therapy records were copied and sent to the Radiation Dosimetry Center to allow for detailed dosimetry in selected studies. For external-beam treatments, the following details were abstracted from the records: dates of the beginning and end of each course of radiation therapy, type of machine, energy of radiation, anatomic location of treatment fields, blocking used (if applicable), and radiation dose delivered to the tumor. For brachytherapy treatments, the following information was abstracted: the treatment time, anatomic location of radiation sources, and dose delivered to the tumor. For each patient in a study, we estimated the absorbed dose at the organ or site of interest for the at-risk time period appropriate to the study outcome. Dose calculations were based on a three-dimensional mathematical phantom using measurements in a water phantom.7
Definition and Ascertainment of Subsequent Neoplasms
SNs included three exclusive subsets: SMNs, nonmelanoma skin cancer (NMSC), and meningioma. SMNs included only malignancies with a /3 behavior code following the morphology code of the United States Surveillance, Epidemiology, and End Results (SEER) Program. All SNs were initially ascertained through self-report questionnaires completed by the individual or a proxy for survivors who had died or were younger than 18 years at the time of initial contact. In a small subset of cases, the SN was first identified by reviewing the death certificate. When an event was identified, that section of the questionnaire (or other relevant data) was reviewed by one of the authors (J.N.), who determined whether an investigation should take place. Information indicating that the event was recurrence of the original cancer was not investigated. The process favored finding all possible new malignancies, so relatively few reports were discounted. Investigation proceeded by locating the pathology records that matched the information provided by the participant. If necessary, the slides from the original tumor and possible new cancer were reviewed to determine whether a new primary had occurred. After the pathologist (S.H.) reviewed the reports or slides, the case was classified as “new malignancy” or “other.” Other categories included recurrence of previous disease, metastases of previous disease, benign tumor, or benign process. Benign meningiomas and in situ cancers were also tabulated. For those cases without an available pathology report, medical records were obtained and reviewed. NMSCs were initially defined by survivor self-report of a history of any skin cancer, including basal cell carcinoma and squamous cell carcinoma.
Statistical Analyses
Cumulative incidence estimates of SNs were calculated using time from 5 years after childhood cancer diagnosis to first occurrence of SN. Death was treated as a competing risk event, and data were censored at the date of last contact.8 SEs of cumulative incidence estimates were calculated and used to evaluate 95% CIs.9
Risks of SMNs were calculated using standardized incidence ratios (SIRs) and excess absolute risk (EAR). The SIR was calculated as the ratio of observed malignancies to expected malignancies by using SEER age, sex, and race-specific rates for the expected number (SEER). All multiple SMNs were counted in the numerator of SIRs. The EAR was determined by subtracting the expected number of malignancies from the observed number, dividing the difference by the person-years of follow-up, and multiplying by 1,000. Meningiomas and NMSCs were not included in the SIR or EAR calculations.
Relative risk (RR) of developing an SN was estimated for each of the host characteristics and therapeutic exposures by using a Poisson multivariable regression model that used age as the time scale.10,11 Person-years of follow-up were calculated as the minimum time to SMN, death, or last contact. Conditional logistic regression was used to evaluate the excess RR (ERR = RR − 1) as a function of radiation dose in selected case-control studies.12
RESULTS
Characteristics of the Cohort and Subsequent Neoplasms
As of January 1, 2006, 802 SNs and SMNs, excluding NMSC, have been diagnosed in 730 CCSS participants. Four survivors had three SMNs, 64 survivors had two SMNs, and 662 survivors had one SMN. There were 1,007 cases of NMSC among 493 survivors, of whom 430 had NMSC without another SMN. Sixty-six individuals have had a meningioma. The characteristics of the CCSS cohort with regard to SMNs or NMSC are shown in Table 1. Patients with an SMN or NMSC were older at the time of their childhood cancer diagnosis, and most were survivors of HL who were treated with radiotherapy.
Table 1.
Demographics of the CCSS Cohort and Subsets Based on the Development of Subsequent Neoplasms
| Characteristic | CCSS Cohort |
Cohort Subsets Based on SNs |
||||||
|---|---|---|---|---|---|---|---|---|
| SMN |
NMSC Only |
No SN |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 14,358 | 730 | 430 | 13,198 | ||||
| Age at diagnosis, years | ||||||||
| Mean | 8.3 | 11.0 | 11.8 | 8.0 | ||||
| Median | 6.8 | 12.1 | 12.9 | 6.4 | ||||
| Sex | ||||||||
| Male | 7,714 | 53.7 | 287 | 39.3 | 218 | 50.7 | 7,209 | 54.6 |
| Female | 6,644 | 46.3 | 443 | 60.7 | 212 | 49.3 | 5,989 | 45.4 |
| Race | ||||||||
| White | 12,396 | 86.3 | 665 | 91.1 | 413 | 96.0 | 11,318 | 85.8 |
| Black | 694 | 4.8 | 23 | 3.2 | 1 | 0.2 | 670 | 5.1 |
| Other | 1,217 | 8.5 | 39 | 5.3 | 13 | 3.0 | 1,165 | 8.8 |
| Unknown | 51 | 0.4 | 3 | 0.4 | 3 | 0.7 | 45 | 0.3 |
| Primary diagnosis | ||||||||
| Leukemia | 4,829 | 33.6 | 152 | 20.8 | 138 | 32.1 | 4,539 | 34.4 |
| CNS tumor | 1,877 | 13.1 | 68 | 9.3 | 40 | 9.3 | 1,769 | 13.4 |
| HL | 1,927 | 13.4 | 247 | 33.8 | 163 | 37.9 | 1,517 | 11.5 |
| NHL | 1,081 | 7.5 | 43 | 5.9 | 27 | 6.3 | 1,011 | 7.7 |
| Wilms tumor | 1,256 | 8.7 | 33 | 4.5 | 13 | 3.0 | 1,210 | 9.2 |
| Neuroblastoma | 955 | 6.7 | 33 | 4.5 | 6 | 1.4 | 916 | 6.9 |
| Soft tissue sarcoma | 1,245 | 8.7 | 80 | 11.0 | 23 | 5.3 | 1,142 | 8.7 |
| Bone cancer | 1,188 | 8.3 | 74 | 10.1 | 20 | 4.7 | 1,094 | 8.3 |
| Chemotherapy | 12,575 | 669 | 395 | 11,511 | ||||
| Alkylating agents | 6,545 | 52.0 | 393 | 58.7 | 195 | 49.4 | 5,957 | 51.8 |
| Anthracyclines | 4,966 | 39.5 | 255 | 38.1 | 108 | 27.3 | 4,603 | 40.0 |
| Epipodophyllotoxins | 990 | 7.9 | 35 | 5.2 | 21 | 5.3 | 934 | 8.1 |
| Platinum agents | 666 | 5.3 | 26 | 3.9 | 6 | 1.5 | 634 | 5.5 |
| Radiation therapy | 8,412 | 67.0 | 550 | 82.3 | 367 | 92.7 | 7,495 | 65.2 |
| Splenectomy | 1,230 | 9.8 | 173 | 25.9 | 123 | 31.2 | 934 | 8.1 |
Abbreviations: CCSS, Childhood Cancer Survivor Study; SMN, second malignant neoplasm; NMSC, nonmelanoma skin cancer; SN, subsequent neoplasm; HL, Hodgkin's lymphoma; NHL, non-Hodgkin's lymphoma.
The associations between the original and second cancer diagnoses are shown in Table 2. SMNs of the breast and gastrointestinal tract most commonly followed a primary diagnosis of HL. Second thyroid cancers were most common in leukemia and HL survivors, as were skin cancers (melanoma and NMSC). Second sarcomas predominantly occurred among survivors of primary soft tissue sarcoma or HL. Second hematopoietic cancers most commonly followed a primary diagnosis of HL or leukemia. Second malignancies of the CNS most commonly occurred after original diagnoses of a CNS tumor or leukemia. This represents a 2.3-fold increase over the 314 SMNs that occurred in 298 survivors as of January 1, 2000, as identified during the first comprehensive analysis of these events in the CCSS cohort.13 As of January 1, 2003, 213 individuals reported one or more NMSCs,14 compared with 493 in this more recent analysis. In addition to the comprehensive analyses of SMNs12 and NMSC,14 eight other detailed analyses of SNs have been completed by the CCSS,15–22 and the impact of SNs has been considered with respect to chronic disease burden,23 mortality,21 and family history of cancer24 or included in analyses of overall outcomes in primary childhood cancer diagnostic groups.25,26
Table 2.
Primary and Second Malignant Neoplasms in the CCSS Cohort
| Primary Diagnosis | No. of Patients | Second Malignant Neoplasms That Occurred in CCSS Survivors (n) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | Thyroid Cancer | CNS Tumors | Sarcoma | Bone | Leukemia | Melanoma | Lymphoma | GI Carcinomas | Other | NMSC | ||
| HL | 247 | 94 | 36 | 7 | 19 | 6 | 14 | 11 | 14 | 14 | 32 | 163 |
| Leukemia | 152 | 16 | 23 | 45 | 4 | 4 | 9 | 11 | 10 | 2 | 28 | 138 |
| Soft tissue sarcoma | 80 | 10 | 7 | 3 | 18 | 12 | 3 | 6 | 2 | 2 | 17 | 23 |
| Bone cancer | 74 | 21 | 9 | 3 | 5 | 9 | 6 | 5 | 1 | 7 | 8 | 20 |
| CNS tumor | 68 | 3 | 12 | 18 | 6 | 5 | 3 | 4 | 4 | 2 | 11 | 40 |
| NHL | 43 | 6 | 7 | 4 | 2 | 5 | 2 | 2 | 4 | 2 | 9 | 27 |
| Neuroblastoma | 33 | 2 | 8 | 1 | 4 | 0 | 4 | 0 | 1 | 0 | 13 | 6 |
| Wilms tumor | 33 | 5 | 2 | 0 | 7 | 5 | 2 | 3 | 0 | 4 | 5 | 13 |
| Total | 1,160 | 157 | 104 | 81 | 65 | 46 | 43 | 42 | 36 | 33 | 123 | 430 |
Abbreviations: CCSS, Childhood Cancer Survivor Study; NMSC, nonmelanoma skin cancer; HL, Hodgkin's lymphoma; NHL, non-Hodgkin's lymphoma.
Cumulative Incidence of Subsequent Neoplasms
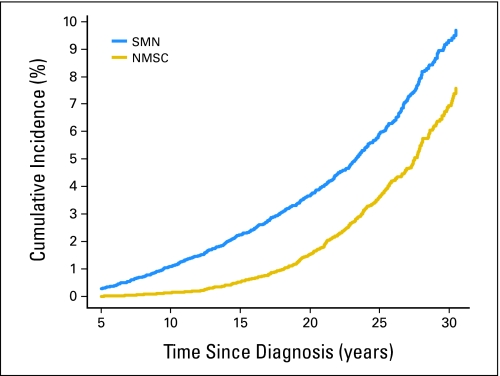
As of January 2000, the 20-year cumulative incidence of SMNs, excluding NMSC, was 3.2%.13 In the ensuing 7 years, the 30-year cumulative incidence for SMN was 9.3%, and that of NMSC was 6.9% (Fig 1). Other analyses of specific primary and secondary cancer diagnoses done during the past 7 years have illustrated the contributions to these overall cumulative incidence estimates. In 4,151 survivors of acute lymphoblastic leukemia, the 25-year cumulative incidence of any second neoplasm was 5.2%.26 In 272 survivors of acute myeloid leukemia, the 20-year cumulative incidence of all SMNs was 1.7%.25 In 1,074 survivors of NHL, the 30-year cumulative incidence of solid cancers was 4%; if meningioma and myelodysplastic syndrome were included, the cumulative incidence of neoplasms in NHL survivors increased to nearly 12%.15 Among 108 survivors who experienced secondary sarcomas, the 30-year cumulative incidence was 1.1% and highest among survivors of primary sarcoma (2.2%) or HL (1.9%).16 In an analysis of female breast cancer, the cumulative incidence at an attained age of 40 years was highest (12.9%) for survivors of HL treated with radiotherapy.22 By far, the largest number of breast cancer cases (65 of 95) occurred among women whose primary diagnosis was HL.
Fig 1.
Cumulative incidence of second malignant neoplasms (SMNs) and nonmelanoma skin cancer (NMSC) in childhood cancer survivors. At the 30-year follow-up, the cumulative incidence of SMNs and NMSC continues to increase with time since 5 years after diagnosis of primary childhood cancer.
As of January 1, 2003, 213 CCSS participants had reported NMSC. Of those, 99 (46%) had multiple occurrences. For basal cell carcinoma, the incidence rate was analyzed with respect to attained age and was 168.2, 1449.3, and 3785.9 per 100,000 person-years for survivors younger than 35 years, 35 to 44 years, and 45 to 54 years, respectively.14
Risk of SMNs in the CCSS Cohort Compared With That in the General Population
In an aggregate, overall analysis of SMNs, the SIR was 6.4 as of January 2000. Excesses of secondary malignancies were observed among all primary childhood cancer diagnostic groups compared with that seen in the general population. The largest risk excesses were observed for breast cancers (SIR = 16.2), bone cancers (SIR = 19.1), and thyroid cancers (SIR = 11.3).13 With ongoing continuous observation of this cohort, subsequent analyses have been conducted for several primary diagnostic groups. For survivors of leukemia and NHL, risk remained elevated with an additional 5 years of follow-up, despite increased age of the CCSS and general populations. In the subsequent analysis of acute myeloid leukemia survivors, the SIR was 3.5,25 and for acute lymphoblastic leukemia survivors, the SIR was 5.026; both values were similar to that for all leukemias (SIR = 5.7) in the original analysis.13 For survivors of NHL, the risk has also remained stable with ongoing follow-up, and they remain at elevated risk of leukemia and cancers of the breast, thyroid, oral cavity and pharynx, bones and joints, brain and nervous system, and urinary bladder.15 Solid cancer risk is higher among female survivors than among male survivors, and the SIR remains elevated for all survivors aged 5 to 39 years.
The risk of sarcoma after an initial childhood cancer was nine-fold greater than that in the general population (SIR = 9.0; 95% CI, 7.4 to 10.9) and was particularly elevated after a primary soft tissue sarcoma (SIR = 24.7), bone cancer (SIR = 10.6), HL (SIR = 11.7), or renal tumor (SIR = 14.6).16 Risk of sarcoma was higher in those who received radiotherapy, but the SIR also was significantly elevated among those who did not.
Risk of subsequent CNS gliomas remained elevated with ongoing follow-up (SIR = 8.7). Those younger than 5 years at the time of their childhood cancer diagnosis were at the highest risk (SIR = 14.5), as were those treated with either combined chemoradiotherapy (SIR = 12.9) or radiotherapy alone (SIR = 11.6). As suggested by the increased risk associated with radiotherapy, survivors of leukemia (SIR = 16.9) or primary CNS tumors (SIR = 14.2) were at the highest risk of subsequent CNS gliomas.17 The risk of meningiomas continued to increase with time from radiation therapy.
An analysis of secondary breast cancer among female survivors in the cohort demonstrated a markedly increased risk for several subgroups.22 Risk was highest for those treated with chest radiotherapy (SIR = 24.7), such as survivors of HL, NHL, sarcoma, or Wilms tumor. In addition, survivors of sarcoma who did not receive radiotherapy were at increased risk for bone sarcoma (SIR = 6.7) and soft tissue sarcoma (SIR = 7.6). Women with a family history of breast cancer also were at increased risk (SIR = 2.7).
Risk of carcinomas (other than those of breast, thyroid, or skin) was four-fold higher than that expected (95% CI, 3.1 to 5.1).19 An elevated RR appeared 5 to 10 years after the initial cancer (SIR = 5.3) and decreased with time thereafter, though it remained significantly elevated after 20 years (SIR = 2.8). The most common sites of the carcinomas were head and neck (mostly parotid gland), gastrointestinal tract, female genitourinary tract, and kidney. Radiation treatment was associated with increased risk of carcinoma at all sites, with the exception of the genitourinary tract, but it was most pronounced for head and neck carcinomas (SIR = 18.5 for patients who received radiotherapy versus SIR = 2.3 for those who did not). Risk was elevated for all primary childhood diagnostic groups in the cohort, but it was highest for survivors of neuroblastoma (SIR = 24.2) or soft tissue sarcoma (SIR = 6.2).
Risk Factor Analyses for SNs
Overall, female survivors were at greater risk than male survivors for the occurrence of any SMN (RR = 1.64). Age at the time of original cancer diagnosis was also an important predictor of risk (ie, children younger at diagnosis are at increased overall risk of SMNs and specific risk of thyroid and CNS SMNs). Exposure to increased doses of alkylating agents, anthracyclines, and epipodophyllotoxins was also associated with an increased risk of any SMN.
Among survivors of NHL, mediastinal site of lymphoma, female sex, radiotherapy with breast or thyroid in the field, and use of cytarabine for primary lymphoma treatment were independent risk factors for SMN.15 In an analysis of secondary sarcomas, a Cox multivariate regression model found the following factors to be associated with increased risk of secondary sarcoma: primary diagnosis of HL, CNS or kidney tumor, sarcoma, radiotherapy, higher doses of anthracyclines or alkylating agents, and history of another SMN.16 Family histories of breast cancer or thyroid disease were independently associated with increased risk of subsequent breast cancer, and pelvic radiotherapy was protective, with a 40% reduction in risk.22 White race, oldest age at diagnosis, longest time since diagnosis, family history of skin cancer, primary diagnosis of HL, and radiotherapy each independently increased the risk of NMSC14 (data not shown).
Role of Radiotherapy in SNs
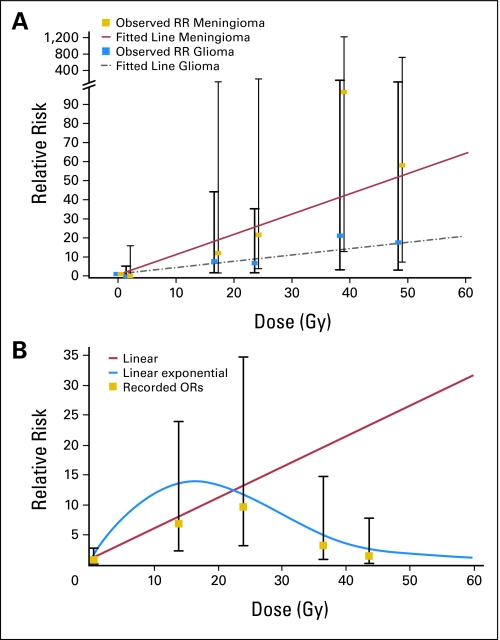
Radiotherapy has been repeatedly reported to increase the risk for SMNs in the CCSS cohort. As a result of meticulous collection of radiation therapy records, we have been able to examine the risk of SMN by radiation dose for several SMN types. Our nested case-control study of 69 cases of subsequent thyroid cancer and 265 matched controls without thyroid cancer revealed an increased risk of thyroid cancer with radiation doses as high as 29 Gy (odds ratio [OR] = 9.8; 95% CI, 3.2 to 34.8) but a decrease in the risk of secondary thyroid cancer at doses greater than 30 Gy (Fig 2A).20 This finding is consistent with a cell-killing effect. Radiobiologically based statistical models were applied to describe the dose response and estimate the ERR, which was 1.3/Gy at doses less than 6 Gy with a decrease of 0.2% per unit dose squared with increasing dose.18 Dose response was most pronounced with age younger than 10 years at diagnosis. Chemotherapy exposure was not associated with risk of subsequent thyroid cancer in this study.
Fig 2.
Dose-response relations between radiotherapy dose and relative risk (RR) of second neoplasms. (A) Subsequent neoplasms of the CNS were classified as meningioma (yellow squares) or glioma (blue squares). Fitted lines were calculated by Poisson regression and indicated positive relations between radiotherapy dose and higher RR of both tumors.16 (B) The RR of subsequent thyroid cancer seemed to be associated with radiotherapy doses less than 29 Gy.19 OR, odds ratio.
In an analysis of subsequent CNS neoplasms, radiation exposure was associated with increased risk of subsequent glioma (OR = 6.8) and meningioma (OR = 9.9). As compared with what was noted with subsequent thyroid cancers, here the dose response for ERR was linear for glioma (ERR/Gy = 0.33) and meningioma (ERR/Gy = 1.06; Fig 2B). The ERR/Gy for glioma was highest for those younger than 5 years at the time of primary childhood cancer diagnosis.17
Genetic Predisposition Toward Second Neoplasms
To investigate the possibility that second neoplasms are a component of a family cancer syndrome, we analyzed the family history of cancer among the siblings of cohort survivors in whom second neoplasms developed. Overall, the siblings had an increased risk of cancer compared with that of the general population (SIR = 1.5). Compared with the cancer risk of relatives of CCSS probands without SMN, that in siblings (SIR = 2.4) and offspring (SIR = 15.0) of CSSS probands with SMN was elevated.24
Individual susceptibility to subsequent malignancy varies; thus we collected genomic DNA to study the variability in the capacity to repair DNA damage caused by radiation therapy or to detoxify chemotherapy agents. In an initial analysis, we investigated the association between SMN risk and polymorphisms in the XRCC1 gene, which is involved in DNA repair, and in glutathione-S-transferase M1 (GSTM1) and glutathione-S-transferase T1 (GSTT1), both of which may metabolize some drugs. Individuals lacking GSTM1 but not GSTT1 were at a small increased risk of SMN (OR = 1.5). No increased risk of secondary thyroid cancer was associated with polymorphisms in either gene or with the arginine/glutamine polymorphism at codon 399 in the XRCC1 gene.27
Impact of SMNs on the Burden of Chronic Disease
An analysis and comparison of chronic health conditions of 10,397 adult cohort members (ie, those older than 18 years) with those of 3,034 siblings was conducted. Overall, the survivors had a 3.3-fold increased risk of a chronic health condition and an 8.2-fold increased risk of a life-threatening health condition. Excluding NMSC, the risk of SMN was 14.8-fold higher in survivors than in siblings: 2.4% of survivors experienced an SMN, but only 0.3% of siblings experienced a malignancy.23
Impact of SMNs on Mortality in the Cohort
In a recent analysis of the mortality of this large cohort, 2,831 5-year survivors (13%) who enrolled in the CCSS subsequently died.21 Death certificate information was available for deaths occurring between January 1, 1979, and December 31, 2002. The leading cause of death was recurrence of primary disease in 67% of the members. Death was attributable to SMNs in 18.6%. At 20-year follow-up, death due to SNs exceeded that due to all other causes of death. Exposure to radiation therapy was cited as the most frequent predisposing factor of SNs, but a few cases were attributable to chemotherapy or genetic predisposition.
DISCUSSION
Since our original analysis 7 years ago,13 the number of SMNs has increased 2.3 fold, from 314 SMNs in 298 individuals to 802 SMNs in 730 individuals. We can now estimate a 30-year cumulative incidence of 9.3% compared with the 20-year incidence of 3.2%. Although the cohort has aged over this period, the SIRs might be expected to decrease with increased likelihood of cancer in the general age-matched population. However, this has not come to pass. SIRs remain elevated in the CCSS cohort. These data highlight the increasing incidence of SNs with continued follow-up of the CCSS cohort and the need for ongoing surveillance of survivors. The lifetime risk and the period of excess risk for SNs is still not known.
Although survivors of HL comprised only 13.4% of the cohort, they experienced 33.8% of the reported SMNs and 37.9% of the NMSCs. For the SMNs, this is largely driven by breast cancer, with 94 (59.9%) of 157 second breast cancers in the CCSS occurring in HL survivors. This finding is consistent with reports by others.28,29 However, the significant proportion of NMSC among HL survivors has not been previously reported. As this study's cohort was derived from patients surviving 5 years or more, it is not surprising that leukemia was not a common SMN and occurred in only 43 individuals.
Although rarely fatal, NMSC is an important late effect of childhood cancer therapy and an important public health issue. In the CCSS, the largest proportion of NMSC occurred in survivors of HL (37.9%), leukemia (32.1%), and CNS tumors (9.3%). The SEER Program does not collect incidence data on NMSC; thus we have a limited ability to track overall NMSC rates and trends in the United States and to compare the results of the CCSS with that of the general population. However, the CCSS rates of NMSC exceed those reported in a population-based study done in New Hampshire.30 The locations of basal cell carcinomas observed in the subjects of the New Hampshire study were also quite different from those in the CCSS group, which reflect the sites of radiotherapy. In the New Hampshire group, basal cell carcinomas were reported as head/neck (68%), back/chest (21%), or extremity (11%), and in the CCSS group, head/neck (43%), back/chest (51%), or extremity (3%). Similarly high rates of NMSC have been identified in survivors of hematopoietic stem-cell transplantation, in whom the risk of basal cell carcinoma is increased by total-body irradiation during childhood, and that of basal cell and squamous cell carcinomas is increased by graft-versus-host disease.31
Survivors of bone cancers or soft-tissue sarcomas comprise 17% of the CCSS cohort and 21% of all survivors who experienced SMNs, 20% of which were breast cancer. Risk of cancer was increased among siblings of sarcoma survivors and those of survivors with SMNs.24 Risk of breast cancer was increased among sarcoma survivors not treated with radiotherapy,22 and risk of secondary sarcoma was increased in primary sarcoma survivors and those with a family history of cancer.16 This is consistent with data showing an association between soft tissue sarcoma and breast cancer or sarcomas as SMNs in patients or close relatives32 and suggests the need for further study of the interactions between genetic predisposition, disease, and treatment. The collection of genomic DNA from the CCSS cohort has provided an opportunity to investigate genetic susceptibility to subsequent cancers. Of particular interest is the demonstration of a strong association between exposure to radiation therapy and the high risk of SNs. Molecular epidemiology studies will be necessary to address the role of genetics in the observed interindividual variation in risk of subsequent cancers and will be possible only with continuing follow-up of this cohort.
Radiotherapy increased the risk of SNs in our analyses, which is consistent with previous reports in which radiation dose responses have been demonstrated.33–35 Detailed information about radiotherapy exposures has been collected by CCSS investigators to enable dosimetric analyses of organs of interest. The thyroid gland is highly susceptible to the carcinogenic effects of ionizing radiation, but the dose-response relations had not previously been well characterized over a wide range of doses. Our study is one of the first to demonstrate a reduction in radiation-related risk of SNs associated with treatment of solid tumors with high-doses of radiation.18,20 We also detected a linear relation between the radiation dose received during treatment for childhood cancer and the relative risk of subsequent gliomas or meningiomas.17
During the past 35 years, pediatric chemotherapy and radiotherapy protocols have been modified to better balance toxicity and efficacy. Therefore, it will be important to observe patients treated during the latter treatment era (eg, after the mid-1980s) of this cohort, as well as those treated on contemporary protocols for many more years, to determine whether the risk of SNs decreases with changes in chemotherapy and radiotherapy (ie, lower doses, smaller volumes, and improvements in techniques). During the interim, it is important that the childhood cancer survivors treated between 1970 and 1986 and their health care providers are aware of the risk of SNs over a prolonged follow-up period. This cohort is still young (median age, 34 years), and cancer in their age group in the general population is low. Our data suggest the need for targeted surveillance as this cohort ages. Specific cancer screening at younger ages than that recommended for the general population, regular physical examinations, and patient education are essential components of the follow-up for all childhood cancer survivors.
Footnotes
Supported by Grant No. U24 CA55727 (L.L.R.) from the National Cancer Institute, Bethesada, MD, with additional support provided to St. Jude Children's Research Hospital, Memphis, TN, by the American Lebanese Syrian Associated Charities (ALSAC).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Anna T. Meadows, Debra L. Friedman
Manuscript writing: Anna T. Meadows, Debra L. Friedman, Joseph P. Neglia, Ann C. Mertens, Sarah S. Donaldson, Marilyn Stovall, Sue Hammond, Yutaka Yasui, Peter D. Inskip
Final approval of manuscript: Anna T. Meadows
REFERENCES
- 1.Meadows AT, D'Angio GJ, Evans AE, et al. Oncogenesis and other late effects of cancer treatment in children. Radiology. 1975;114:175–180. doi: 10.1148/114.1.175. [DOI] [PubMed] [Google Scholar]
- 2.Meadows AT, D'Angio GJ, Mike V, et al. Patterns of second malignant neoplasms in children. Cancer. 1977;40:1903–1911. doi: 10.1002/1097-0142(197710)40:4+<1903::aid-cncr2820400822>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Tucker MA, Meadows AT, Boice JD, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 4.Olsen JH, Garwicz S, Hertz H, et al. Second malignant neoplasms after cancer in childhood or adolescence: Nordic Society of Paediatric Haematology and Oncology Association of the Nordic Cancer Registries. BMJ. 1993;307:1030–1036. doi: 10.1136/bmj.307.6911.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 6.St Jude Children's Research Hospital. Childhood Cancer Survivor Study. http://www.stjude.org/ccss.
- 7.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 8.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Marubini E, Valsecchi MG. Analyzing Survival Data from Clinical Trials and Observational Studies. Chichester, United Kingdom: Wiley; 1995. [Google Scholar]
- 10.Breslow NE, Day NE. Statistical Methods in Cancer Research: Volume II. The Design and Analysis of Cohort Studies. Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 11.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 12.Preston DL, Lubin JH, Pierce DA, et al. Epicure Users Guide. Seattle, WA: Hirosoft International Corporation; 1993. [Google Scholar]
- 13.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five year survivors of childhood cancer: Report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93:618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 14.Perkins JL, Liu Y, Mitby PA, et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2005;23:3733–3741. doi: 10.1200/JCO.2005.06.237. [DOI] [PubMed] [Google Scholar]
- 15.Bluhm EC, Ronckers C, Hayashi RJ, et al. Cause-specific mortality and second cancer incidence after non-Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2008;111:4014–4021. doi: 10.1182/blood-2007-08-106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: A report from the Childhood Cancer Survivor study. J Natl Cancer Inst. 2007;99:300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 18.Ronckers CM, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: A detailed evaluation of radiation dose response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 19.Bassal M, Mertens AC, Taylor L, et al. The risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 20.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): A nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 21.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among five-year survivors of childhood and adolescent cancer diagnosed between 1970-1986: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 23.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 24.Friedman DL, Kadan-Lottick NS, Whitton J, et al. Increased risk of cancer among siblings of long-term childhood cancer survivors: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2005;14:1922–1927. doi: 10.1158/1055-9965.EPI-05-0066. [DOI] [PubMed] [Google Scholar]
- 25.Mulrooney DA, Dover DC, Li S, et al. Twenty years follow-up among survivors of childhood and young adult acute myeloid leukemia: A report from the childhood cancer survivor study. Cancer. 2008;112:2071–2079. doi: 10.1002/cncr.23405. [DOI] [PubMed] [Google Scholar]
- 26.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood. 2008;111:5515–5523. doi: 10.1182/blood-2007-10-117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertens AC, Mitby PA, Radloff G, et al. XRCC1 and glutathione-S-transferase gene polymorphisms and susceptibility to radiotherapy-related malignancies in survivors of Hodgkin disease: A report from the Childhood Cancer Survivor Study. Cancer. 2004;101:1463–1472. doi: 10.1002/cncr.20520. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Maule M, Scelo G, Pastore G, et al. Risk of second malignant neoplasms after childhood leukemia and lymphoma: An international study. J Natl Cancer Inst. 2007;99:790–800. doi: 10.1093/jnci/djk180. [DOI] [PubMed] [Google Scholar]
- 30.Karagas MR, Greenberg ER, Spencer SK, et al. Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. Int J Cancer. 1999;81:555–559. doi: 10.1002/(sici)1097-0215(19990517)81:4<555::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 31.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 32.Strong LC, Stine M, Norsted TL. Cancer in survivors of childhood soft tissue sarcoma and their relatives. J Natl Cancer Inst. 1987;79:1213–1220. [PubMed] [Google Scholar]
- 33.Bhatia S, Sather HN, Pabustan OB, et al. Low incidence of second neoplasms among children diagnosed with acute lymphoblastic leukemia after 1983. Blood. 2002;99:4257–4264. doi: 10.1182/blood.v99.12.4257. [DOI] [PubMed] [Google Scholar]
- 34.Garwicz S, Anderson H, Olsen JH, et al. Second malignant neoplasms after cancer in childhood and adolescence: A population-based case-control study in the 5 Nordic countries—The Nordic Society for Pediatric Hematology and Oncology. The Association of the Nordic Cancer Registries. Int J Cancer. 2000;88:672–678. doi: 10.1002/1097-0215(20001115)88:4<672::aid-ijc24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 35.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]