Abstract
Childhood cancer survivors are at risk for medical and psychosocial late effects as a result of their cancer and its therapy. Promotion of healthy lifestyle behaviors and provision of regular risk-based medical care and surveillance may modify the evolution of these late effects. This manuscript summarizes publications from the Childhood Cancer Survivor Study (CCSS) that have examined health behaviors, risk-based health care, and interventions to promote healthy lifestyle practices. Long-term survivors use tobacco and alcohol and have inactive lifestyles at higher rates than is ideal given their increased risk of cardiac, pulmonary, and metabolic late effects. Nearly 90% of survivors report receiving some form of medical care. However, only 18% report medical visits related to their prior cancer that include discussion or ordering of screening tests or counseling on how to reduce the specific risks arising from their cancer. One low-cost, peer-driven intervention trial has been successful in improving smoking cessation within the CCSS cohort. On the basis of data from CCSS investigations, several trials to promote improved medical surveillance among high-risk groups within the cohort are underway. Despite their long-term risks, many survivors of childhood cancer engage in risky health behaviors and do not receive adequate risk-based medical care.
INTRODUCTION
Other articles in this issue of the Journal of Clinical Oncology describe in detail the increased risk of serious morbidity,1 premature mortality,2,3 and diminished quality of life and health status4 among long-term survivors of childhood cancer. Depending on their treatment exposures, survivors may be at increased risk of ischemic coronary artery disease, cerebrovascular disease, diabetes, hypertension, dyslipidemia, renal insufficiency, second and subsequent malignancies, and life-threatening infections.1 Importantly, the risk and severity of these and other outcomes are potentially modifiable by preventive strategies that encourage healthy lifestyle behaviors, specialized surveillance and screening, and risk management. The following three examples of the management of Hodgkin's lymphoma and acute lymphoblastic leukemia (ALL) survivors illustrate this concept of preventive health strategies among pediatric cancer survivors.
Hodgkin's lymphoma survivors treated with chest irradiation have an increased risk of lung cancer.5–7 Tobacco use increases this risk by more than 20-fold.6 Successful smoking prevention and cessation strategies among survivors in their childhood, adolescent, and young adult years can decrease the risk of this prevalent and highly morbid cancer of adulthood, while also decreasing the development and progression of atherosclerosis and other second cancers.
Women treated with chest irradiation for a childhood cancer have a significantly increased risk of breast cancer at a young age.8,9 As in the general population, breast cancer outcomes among childhood cancer survivors are strongly associated with their stage at diagnosis.10–12 Thus breast cancer surveillance with annual mammography and breast magnetic resonance imaging (MRI) is recommended to detect early breast cancer and improve survival.13
Lastly, survivors of ALL, depending on their treatment exposures and era of therapy, have an increased risk of many different conditions, including osteoporosis,14,15 obesity,16,17 insulin resistance,18,19 cardiovascular and cerebrovascular disease,20,21 and chronic hepatitis C.22,23 Importantly, each of these conditions can be positively affected by healthy lifestyle practices (eg, avoiding tobacco use and excessive alcohol consumption, eating a low-fat diet with appropriate amounts of calcium and Vitamin D, and maintaining a physically active lifestyle) and periodic health care with risk-based surveillance.
Thus Childhood Cancer Survivor Study (CCSS) investigators have devoted much effort to determining the prevalence and predictors of various risky health behaviors and the health care utilization patterns of childhood cancer survivors. The goal of such investigation is to develop and test theoretically based interventions aimed at reducing risky behaviors and enhancing the practice of healthy behaviors and risk-based health care in vulnerable survivors. In the following sections, we summarize findings from published CCSS studies focusing on these three topics: health behaviors, medical care, and interventions to promote healthy living.
Most of the information regarding these outcomes is from the baseline (administered to most participants from 1994 to 1998) and the 2000 and 2003 follow-up CCSS surveys (hereafter referred to as baseline, 2000, and 2003 surveys). In addition, ancillary studies led by Emmons et al that have been conducted through the CCSS are included. With each topic, we have included a section discussing limitations and future directions of study. To illustrate particular observations, we have included previously unpublished tables and figures that include data from different surveys or time points.
HEALTH BEHAVIORS
Tobacco Use
It is well known that smoking harms nearly every organ of the body24 and significantly increases the risk of serious morbidity25 and mortality26 from multiple cancers, ischemic heart disease, cerebrovascular disease, and pulmonary disease among individuals in the general population. Indeed, smoking is the most harmful health behavior associated with preventable causes of death and diminished quality of life.24 Among childhood cancer survivors, smoking potentiates the organ damage associated with many different treatment exposures, including irradiation to the head, neck, chest, abdomen, or pelvis and chemotherapy with pulmonary toxic agents (eg, bleomycin, carmustine, and lomustine). Thus, the single most important risky health behavior to address among childhood cancer survivors is tobacco use.
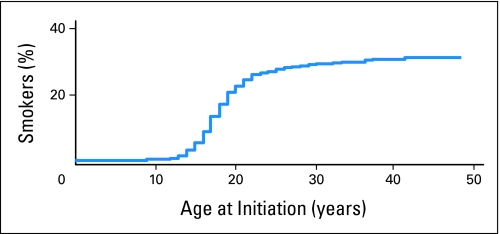
In the baseline survey, 9,709 adult survivors, age 18 years or older, were queried about their use of tobacco. Among this large cohort, 28% reported having smoked at least 100 cigarettes in their lifetime (Fig 1).27 Seventeen percent of survivors (19% of males, 15% of females) reported current cigarette smoking. In 1999, contemporary with the baseline survey, 23.5% of the US adult population reported current smoking (27.5% for adults between the ages of 18 and 44 years).28 The actuarial estimated incidence of initiating smoking within the CCSS was 32% by 40 years of age. The frequency of smoking initiation was significantly lower among survivors compared with that of the general population (observed to expected ratio, 0.72; 95% CI, 0.69 to 0.75). Additionally, 11% reported using tobacco products other than cigarettes (almost all by men, with < 1% of women reporting use). Among men, 10% reported currently using cigars, 6% reported currently using chewing tobacco, 3% reported currently using snuff, and 2% reported currently using pipes.
Fig 1.
Actuarial estimates of proportion of individuals who smoked by age at initiation.27
The prevalence of cigarette smoking was significantly lower among black female survivors (10%) and Hispanic female survivors (10%) compared with non-Hispanic white survivors (17%).29 Minority survivors' smoking prevalence was also lower than that of blacks (23%) and Hispanics (23%) in the general population.30
Among the respondents to the baseline survey, the average reported rate of smoking was 14 cigarettes a day (median, 13; range, one to 60).27 A multivariate model of smoking initiation identified lower educational attainment, having an annual household income of less than $20,000, being nonblack, not having cranial radiation, not having pulmonary toxic treatment, and being older than 10 years at cancer diagnosis as risk factors for smoking initiation. Current cigarette use did not differ substantially by treatment exposures known to be associated with cardiac and/or pulmonary complications (eg, bleomycin, carmustine, lomustine, anthracyclines, or radiation to chest or spine27; Table 1 lists risky health behaviors by cardiac and pulmonary toxic treatment exposures.) Alarmingly, survivors at greatest risk do not alter their tobacco use accordingly.
Table 1.
Risky Health Behaviors and Medical Care Among Survivors of Childhood Cancer by Various Types of Cancer Therapy Associated With an Increased Risk of Long-Term Morbidity
| Cancer Therapy | Risky Health Behaviors* (%) |
Medical Care† (%) |
|||||
|---|---|---|---|---|---|---|---|
| Current Smoker | Risky Drinking | Physically Inactive | No Medical Care | General Medical Care | General Survivor-Focused Care | Risk-Based Survivor-Focused Care | |
| Total cohort | 14.3 | 14.5 | 23.9 | 11.2 | 57.3 | 13.7 | 17.8 |
| Chest radiation therapy | |||||||
| Yes | 13.0 | 14.0 | 20.8 | 7.9 | 49.3 | 14.7 | 28.0 |
| No | 14.8 | 14.8 | 24.9 | 12.0 | 59.4 | 13.3 | 15.3 |
| Anthracyclines ≥ 300 mg/m2 | |||||||
| Yes | 12.1 | 14.1 | 23.0 | 11.3 | 55.4 | 13.1 | 20.2 |
| No | 14.7 | 14.7 | 24.1 | 11.1 | 57.8 | 13.7 | 17.3 |
| Pulmonary toxic therapy‡ | |||||||
| Yes | 11.6 | 12.9 | 25.8 | 8.4 | 51.9 | 15.5 | 24.1 |
| No | 14.7 | 14.9 | 23.6 | 11.5 | 58.0 | 13.4 | 17.0 |
| Alkylating agent therapy | |||||||
| Yes | 12.4 | 14.4 | 22.9 | 10.6 | 52.2 | 14.6 | 22.5 |
| No | 15.0 | 14.9 | 24.5 | 11.5 | 59.5 | 13.1 | 15.9 |
Includes only survivors 18 years or older who responded; current smoker and physically inactive sample size is 6,244 participants who responded to the 2003 Childhood Cancer Survivor Study (CCSS) survey; risky drinking sample size is 8,988 participants who responded to the baseline CCSS survey.
Includes survivors of any age who completed the 2003 CCSS survey; sample size is 8,522 participants.
Pulmonary toxic therapies include any of the following: bleomycin, busulfan, carmustine, lomustine.
A follow-up study was conducted among the 796 smokers identified in the baseline survey.31 More than half of these participants reported that a majority of people in their social networks were also smokers. Other factors associated with smoking rate in the final multivariate model included older age, lower levels of education, no support for quitting, and higher psychological distress. Cancer-related variables (including diagnosis, age at diagnosis, treatment modality) were not significant. These survivors smoked despite having high perceived vulnerability to health problems resulting from smoking because of their previous treatment for cancer, with only 12% perceiving a low or slightly increased risk for smoking-related illnesses.
Smoking Cessation
Quit attempts were fairly common among smokers identified in the baseline survey, with 41% reporting a quit attempt in the previous 2 years.27 Survivors reported moderate readiness to quit, with 18% of current smokers in precontemplation, 43% in contemplation, and 39% in preparation. Even though many participants were contemplating quitting, confidence (self-efficacy) in their ability to quit was low.
Participants who were male, diagnosed with cancer at a younger age, received a lot of support for quitting, and had higher perceived vulnerability for smoking-related health problems had higher self-efficacy for quitting. Those who made more quit attempts were younger, reported a lot of encouragement from family/friends for quitting, saw themselves as more vulnerable to smoking-related illnesses, and had social networks that were comprised by at least half nonsmokers.31 Younger age (< 3 years) at cancer diagnosis was associated with an increased likelihood of quitting smoking.27 A multivariate model of factors associated with decreased smoking cessation included being younger than 14 years at smoking initiation, not having graduated high school, and having received cranial radiation therapy.
Alcohol Use
Excessive alcohol consumption increases the risk for a number of diseases, including oropharyngeal, esophageal, breast, and liver cancer; depression; epilepsy; hypertension; stroke; osteoporosis; and liver cirrhosis.32–34 Several groups of childhood cancer survivors may be at increased risk for conditions that would be further exacerbated by excessive alcohol consumption, including those with chronic hepatitis C acquired from transfusions with blood products (before the advent of hepatitis C screening in 1993), patients with hepatic steatosis after total-body or cranial irradiation, patients with an anthracycline-related cardiomyopathy, and those with liver dysfunction after moderate to high-dose abdominal irradiation.
Alcohol use was assessed among 10,398 adult survivors in the CCSS cohort at baseline.35 Three primary outcomes were investigated: (1) current alcohol consumption (use in the past year); (2) risky drinking, defined as more than three drinks per day or seven drinks per week for women and more than four drinks per day or 14 drinks per week for men; and (3) heavy drinking, defined as five or more drinks per day for women and six or more drinks per day for men at least once a month in the past year. Seventy-three percent of CCSS survivors reported they had consumed alcohol in the past year. Approximately 16% reported risky drinking and 8% reported heavy drinking. Risk factors for risky and heavy drinking were similar and included being young, male, having less than a high school education, and initiating drinking at a young age.35 After controlling for age, sex, race/ethnicity, education, and age at first drinking, the risk factors associated with heavy drinking among cancer survivors included fair or poor self-assessed health, depression, anxiety, somatization, activity limitations, and cancer-related fears and uncertainty. Protective factors (lower rates of heavy drinking) included treatment with intrathecal methotrexate, cranial radiation, and diagnosis during late adolescence (age 15 to 21 years).35 Cancer diagnosis during this time period may interrupt exposure to negative peer interactions, such as experimentation with drinking, and thereby may explain this protective factor of age at diagnosis.
Black and Hispanic survivors engaged in significantly less heavy drinking than non-Hispanic white survivors.29 Black survivors were also significantly less likely to report heavy drinking than white and Hispanic survivors.
Tobacco and Other Risky Health Behaviors
In an assessment of a subpopulation of 796 CCSS survivors who were enrolled in a smoking cessation trial, the prevalence of five behavioral risk factors (physical inactivity, excess consumption of alcohol or red meat, not taking a daily vitamin, lack of health care) was examined. Approximately 31% of the sample engaged in zero or one health-risk behavior in addition to smoking, 63% engaged in two or three additional risk behaviors, and 6% engaged in four or five.36 There were positive linear relationships between number of risk factors and smoking rate and nicotine dependence. Not surprisingly, 8.1% of the current smokers also reported drinking more alcohol than is recommended (risky drinking). This group also tended to smoke more heavily than those who were not risky drinkers.
Physical Activity
Regular moderate-intensity physical activity has been demonstrated to be protective against osteoporosis,37 hypertension,38 noninsulin-dependent diabetes mellitus,39 cardiovascular disease,40,41 and all-cause mortality42,43 in the general population. Adequate levels of physical activity are particularly important for childhood ALL survivors, who are often at increased risk for each of these health conditions.2,3,14–16,18,21 The 2003 survey included a seven-item instrument from the Behavior Risk Factor Surveillance System questionnaire regarding physical activity in the past week. Two primary outcomes were assessed: not meeting the United States Centers for Disease Control and Prevention recommendation of at least 30 minutes of moderate-intensity physical activity on five or more days per week or at least 20 minutes of vigorous-intensity physical activity on three days or more per week; and physical inactivity, defined as no leisure-time physical activity in the month before completing the survey. Among 2,684 adult survivors of childhood ALL, 53% did not meet the Centers for Disease Control and Prevention recommendation for physical activity, and 23% reported being physically inactive, significantly higher than in the general population (20.3%).44 Cranial radiotherapy was associated with both adverse outcomes. It is well known that cranial radiotherapy is also associated with obesity16,17 and that obesity is strongly associated with physical inactivity.45 Thus to determine whether the physical inactivity among ALL survivors was simply a result of obesity (or vice versa), multivariate models that controlled for body mass index were assessed and demonstrated that survivors treated with cranial radiotherapy were less likely to be physically active independent of their body mass index. This suggests that additional mechanisms, such as decreased muscle mass and strength and impairment of balance and postural control, may affect levels of physical activity.
Among 541 current cigarette smokers from the entire CCSS cohort, 29% spent less than 150 minutes per week engaged in moderate-intensity physical activity.36 Those who were not physically active also reported feeling less confident in their ability to refrain from smoking in challenging situations. To identify other groups at risk of physical inactivity, we are currently assessing physical activity levels, on the basis of responses to the 2003 survey, among the remainder of the cohort.
Limitations and Future Directions of CCSS Research Among Health Behaviors
Given the increased risk of cardiac, pulmonary, and metabolic late effects, CCSS studies have demonstrated that long-term childhood cancer survivors in the cohort are using tobacco at concerning rates. These findings, coupled with the high levels of physical inactivity reported in the CCSS population, highlight the urgency of research in the area of health behavior interventions. As illustrated in Table 1 and described above, tobacco and alcohol use did not vary by key treatment exposures, indicating that there is little or no relationship between risk and behavior in this group. Through semiannual newsletters, the CCSS cohort is routinely informed of the importance of knowing their treatment exposures and their long-term health risks. Therefore, the findings that behavior and risk are not significantly related in the cohort are disappointing, although not surprising. There is a large body of published literature that suggests that knowledge and risk are not necessarily sufficient conditions for motivating change. For example, a majority of smokers in the CCSS reported high perceived vulnerability to smoking-related illnesses, yet continued to smoke. The published CCSS studies have been limited predominantly to individual-level factors, but more research is needed to understand the interpersonal, community, and organizational influences that are associated with health behaviors among survivors. For example, socioeconomic status was a key predictor of smoking status in both the CCSS population and a similar cohort of survivors in Britain.46 This parallels extensive data in the general population suggesting that socially derived factors may be as important or may operate in concert with individual-level factors to influence risk.47
Although our initial assessments of health behaviors have generally focused on a single behavior, several current CCSS studies are attempting to determine the prevalence and predictors of survivors with multiple risky health behaviors. It is clear that we have much to understand about what promotes positive health behaviors and what facilitates engagement in less healthy behaviors among adult survivors of pediatric cancer. It is also evident that given the fairly high rates of tobacco use, risky and heavy alcohol use, and physical inactivity, more attention should be given to childhood cancer survivors' lifestyle behaviors when they are seen for routine medical care or risk-based long-term follow-up. The longitudinal nature of the CCSS will allow us to assess the temporal ordering of risk factors for less healthy behaviors, assess the relationship of cigarette smoking and other risky health behaviors to serious morbidity and mortality among cancer survivors, and evaluate the impact and durability of targeted interventions.
MEDICAL CARE
Risk-Based Care and Cancer Screening Practices
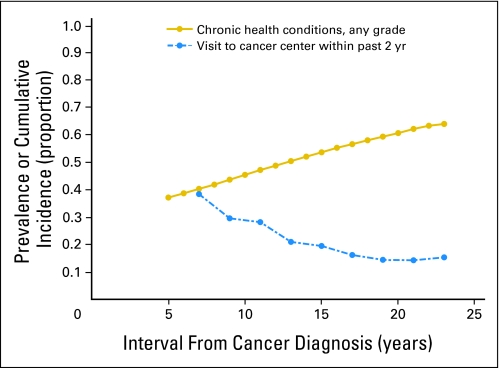
The Institute of Medicine strongly recommends that all childhood cancer survivors have regular medical care that is adapted to the specific risks that arise from their previous cancer and its therapy, genetic predispositions, lifestyle, and any comorbid health conditions.48 Such risk-based care requires that every survivor have an individualized plan for periodic medical assessments and surveillance tests. Two CCSS publications have examined the medical care reported by adult survivors of childhood cancer. The first presented data from the baseline survey.49 The 9,434 respondents reported on four types of medical care received in the preceding 2 years. These categories were not mutually exclusive. Eighty-seven percent reported general or nonspecific contact with a health care provider, 71% reported a general physical examination, 42% reported a cancer-related medical visit, and 19% reported a medical visit to a cancer center. This analysis generated four primary findings: almost 90% of survivors reported some contact with the medical system; the likelihood of a general physical examination or a cancer-related medical visit decreased as survivors' age and time from diagnosis increased; less than 20% of survivors were seen regularly in a cancer center; and most survivors did not report care related to their prior cancer. Figure 2 displays the cumulative incidence of any chronic health condition among survivors1 and the percent of survivors with a visit to a cancer center in the preceding 2 years49 by the interval from the cancer diagnosis to time of baseline enrollment. As the cumulative incidence of chronic health conditions, such as heart disease and second cancers, increases, the likelihood of a survivor being actively observed in a cancer center decreases.
Fig 2.
Percentage of survivors with a visit to a cancer center in the past 2 years and cumulative incidence of any chronic condition by years since cancer diagnosis.
Subsequent to the baseline survey, the concept of risk-based care was refined by two seminal reports on cancer survivorship from the Institute of Medicine48,50 and the publication of expert consensus guidelines for ongoing surveillance of survivors.51,52 Accordingly, the 2003 survey examined risk-based medical care in greater detail. The medical care received by 8,522 survivors during the preceding 2 years was classified hierarchically into four mutually exclusive categories: 11% reported no medical care, 57% reported general medical care (a medical visit unrelated to their prior cancer), 14% reported general survivor-focused medical care (a medical visit related to their prior cancer), and 18% reported risk-based, survivor-focused medical care (a medical visit related to their prior cancer in which screening tests were discussed or ordered or the survivor was counseled on how to reduce his/her specific risks).53 Consistent with the baseline study, most survivors (89%) reported some contact with the medical system; however, fewer than one third reported an encounter related to their prior cancer, and fewer than one of five survivors reported a visit in which they discussed ways to reduce their risks. A concerning trend is evident when comparing data from the baseline survey with that from the 2003 survey: although the risk of developing a late effect of therapy increases as survivors grow older,1 the frequency of cancer-related medical visits (42% v 32%) and of visits to a cancer center (19% v 15%) decreased. In essence, as risk increases, risk-based care decreases.
The observation that most survivors do not receive appropriate risk-based medical care is supported by the low rates of recommended surveillance tests to detect late effects before they become clinically evident. Among the participants who completed the 2003 survey and who were at increased risk for developing cardiomyopathy or breast cancer as a result of their therapy, only 511 (28%) of 1,810 participants and 169 (41%) of 414 participants had undergone a recommended echocardiogram or mammogram, respectively, within the preceding 2 years.53 Among female respondents on the baseline survey, only 62% reported a clinical breast examination in the preceding year.54 Frequencies of breast self-examination (27%) and testicular self-examination (17%) were similarly low. Although the efficacy of self-examination in the general population has been questioned,55,56 these low rates in cancer survivors (in 1994 through 1995) are further evidence of the poor uptake of risk-based care strategies. Because participants in the CCSS study have access to the newsletters, CCSS Web site for questions, and further research studies, the CCSS data probably overestimate the risk-based care received by childhood cancer survivors in general.
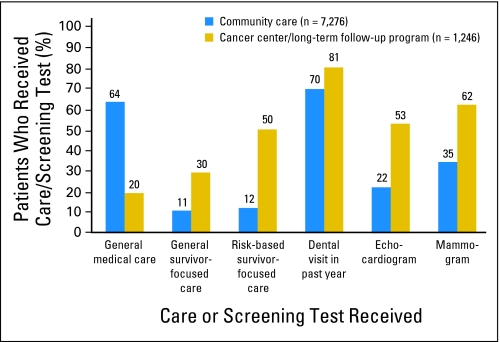
Ideally, the receipt of risk-based medical care should not be contingent on whether survivors receive their ongoing care at a cancer center or in their community from a primary care provider. In fact, among survivors who reported some form of medical care in the 2 years preceding the 2003 survey, fewer than 15% had been seen in a cancer center.53 Unfortunately, data from the CCSS cohort suggest that those patients who are seen by a primary care clinician are less likely than those who are seen at a cancer center to receive an indicated echocardiogram (22% v 53%) or mammogram (35% v 62%; Fig 3). Although 50% of survivors seen at a cancer center reported risk-based, survivor-focused care (the highest level of care on the hierarchy), only 12% of those seen in the community reported such care. Because most primary care physicians will see few, if any, childhood cancer survivors in their practice, their unfamiliarity with the specific health risks faced by this population is a major barrier to appropriate survivor care.57 However, it is unlikely that cancer survivor programs will be able to accommodate the growing population of adult survivors of childhood cancer. Improvements in risk-based care will require providing primary care clinicians with the necessary resources (including information about their patients' prior treatment, long-term risks, recommended screening practices, and bidirectional communication with the cancer center) to follow this population. Most importantly, survivors must be familiarized with their own risks and empowered to advocate for risk-based care.
Fig 3.
Levels of medical care, dental care, and indicated screening practices (in high-risk groups) by location of care.
Several subgroups of patients are particularly vulnerable to receiving inadequate or no medical care (Table 2). Of the 11% of patients in the CCSS cohort without health insurance, 29% reported having received no medical care in the preceding 2 years.53 In contrast, only 9% of survivors with health insurance had not received medical care in the same period. Other groups at risk of receiving no care included male survivors and survivors with household incomes less than $40,000 per year. Among survivors who did report some form of medical care, black survivors, the uninsured, and survivors who were older at the time of interview were less likely to have received risk-based, survivor-focused care. As might be expected, survivors who have already developed sequelae of their cancer therapy (such as pain, anxiety, or a severe or life-threatening chronic condition) are more likely to report having received risk-based, survivor-focused care. In contrast, it seems that many asymptomatic survivors who are at risk of serious morbidity are not receiving the recommended surveillance. Survivors' poor knowledge of their prior therapy is probably a major contributor to such inadequate care. For example, in a cross-sectional survey of 635 adult CCSS participants, only 33% of those survivors who had received doxorubicin and 8% of those who had received daunorubicin recalled receiving an anthracycline agent.58 This limited knowledge about anthracycline exposure may contribute to the poor compliance with recommended echocardiography to detect subclinical cardiac dysfunction arising from treatment with these agents. Furthermore, survivors who have been exposed to an anthracycline (without chest radiation) are no more likely than those survivors without cardiac risk factors to report risk-based survivor-focused care (Table 1). For these patients, opportunities to modify lifestyle to mitigate cardiac risk or to intervene if subclinical cardiac dysfunction is detected are lost frequently.
Table 2.
Characteristics of the Study Cohort and Their Medical Care, Dental Care, and Selected Screening Practices
| Characteristic | Medical Care in Preceding 2 Years (%)* |
Dental Care (%): Dental Visit in Past Year (n = 6,079) | Screening in Preceding 2 Years (%) |
||||
|---|---|---|---|---|---|---|---|
| No Medical Care (n = 953) | General Medical Care (n = 4,882) | General Survivor-Focused Care (n = 1,166) | Risk-Based Survivor-Focused Care (n = 1,521) | Indicated† Echocardiogram (n = 511 of 1,810) | Indicated† Mammogram (n = 169 of 414) | ||
| Age at diagnosis, years | |||||||
| Mean | 7.5 | 8.1 | 8.3 | 8.8 | 8.3 | 9.8 | 12.5 |
| Standard deviation | 5.5 | 5.8 | 5.9 | 6.1 | 6.0 | 6.2 | 5.7 |
| Age at interview, years | |||||||
| Mean | 30.9 | 31.8 | 31.4 | 32.2 | 31.9 | 33.5 | 38.1 |
| Standard deviation | 7.1 | 7.5 | 7.8 | 8.1 | 7.8 | 8.3 | 7.4 |
| Sex | |||||||
| Male | 15.6 | 55.8 | 12.7 | 15.9 | 67.5 | 26.1 | NA |
| Female | 6.7 | 58.8 | 14.7 | 19.8 | 75.2 | 30.6 | 40.8 |
| Race/ethnicity | |||||||
| White, non-Hispanic | 10.6 | 57.6 | 14.1 | 17.8 | 71.8 | 27.9 | 40.9 |
| Hispanic | 11.1 | 57.0 | 9.6 | 22.2 | 73.3 | 45.7 | 62.5 |
| Black | 18.6 | 61.5 | 9.1 | 10.8 | 55.8 | 27.5 | 71.4 |
| Other | 14.5 | 53.7 | 12.2 | 19.7 | 70.9 | 27.8 | 28.6 |
| Annual household income | |||||||
| < $40,000 | 14.5 | 55.7 | 13.9 | 15.9 | 60.1 | 23.9 | 29.7 |
| $40,000-$79,000 | 8.6 | 59.4 | 13.5 | 18.5 | 75.7 | 29.2 | 41.9 |
| ≥ $80,000 | 7.8 | 59.4 | 12.5 | 20.2 | 82.5 | 31.5 | 49.2 |
| Educational attainment | |||||||
| < High school | 12.2 | 51.9 | 16.8 | 19.2 | 63.0 | 25.9 | 33.3 |
| High school graduate | 14.0 | 55.5 | 14.0 | 16.5 | 66.5 | 26.0 | 36.7 |
| College graduate | 7.8 | 60.0 | 12.9 | 19.3 | 77.9 | 30.3 | 43.6 |
| Health insurance status | |||||||
| No, United States | 28.5 | 51.3 | 10.4 | 9.8 | 46.8 | 12.8 | 26.7 |
| Yes, United States | 8.8 | 58.5 | 13.8 | 18.8 | 74.3 | 30.9 | 41.9 |
| Canadian resident | 9.3 | 53.0 | 17.5 | 20.2 | 77.6 | 23.6 | 42.9 |
| Poor emotional health | |||||||
| No | 11.4 | 58.0 | 13.2 | 17.4 | 71.9 | 27.5 | 40.1 |
| Yes | 9.0 | 49.0 | 19.1 | 22.9 | 65.1 | 37.5 | 47.5 |
| Cancer-related anxiety | |||||||
| None, a small amount | 11.5 | 58.5 | 13.1 | 17.0 | 71.5 | 26.8 | 39.4 |
| Moderate, a lot, extreme | 7.6 | 45.6 | 20.1 | 26.8 | 69.2 | 41.0 | 50.0 |
| Cancer-related pain | |||||||
| None, a small amount | 11.6 | 58.8 | 12.7 | 16.9 | 71.7 | 27.2 | 40.9 |
| Moderate, a lot, extreme | 6.6 | 39.9 | 24.5 | 29.0 | 66.7 | 36.3 | 40.5 |
| Poor physical health | |||||||
| No | 11.3 | 59.7 | 12.3 | 16.7 | 72.5 | 27.1 | 42.3 |
| Yes | 10.9 | 49.6 | 18.0 | 21.5 | 67.6 | 31.3 | 35.8 |
| Chronic disease status‡ | |||||||
| Grade 0, 1, 2 | 12.0 | 60.3 | 12.1 | 15.6 | 70.9 | 26.8 | 40.7 |
| Grade 3, 4 | 8.6 | 48.2 | 18.4 | 24.8 | 72.7 | 31.2 | 41.1 |
Percentages are calculated by row.
Screening tests were indicated by age and treatment with chest irradiation (mammogram) and/or anthracyclines (echocardiogram).
Grade 0, 1, 2: either no chronic condition (grade 0) or at least one grade 1 (mild) or grade 2 (moderate) chronic condition; grade 3, 4: at least one grade 3 (severe) or grade 4 (life-threatening or disabling) chronic condition.
Dental Care
At least 30% of cancer survivors will develop dental abnormalities, with a particularly high prevalence in patients diagnosed before the age of 5 years and those who were treated with cranial radiation therapy (CRT).59–62 Thus survivors require regular dental care so that dental problems are detected and treated expeditiously. The Children's Oncology Group (COG) Long-Term Follow-Up Guidelines recommend that all survivors exposed to chemotherapy or radiation have a dental checkup every 6 months,63 consistent with recommendations for the general population. The CCSS examined the dental care received by 9,434 adult survivors and a comparison group of 3,858 siblings.64 Only 60% of survivors had seen a dentist within the preceding year, with a further 23% having seen a dentist in the preceding 1 to 2 years. This did not differ from the frequency reported by their siblings, despite the increased risk of dental problems in survivors. Lack of health insurance, black ethnicity, and lack of a college education all predicted the absence of an annual dental visit—similar risk factors have been shown to predict decreased compliance with regular preventive dental care in the general population.65 Despite the increased risk of dental abnormalities in patients who receive CRT, compliance with dental visits was no higher in women who had received CRT compared with those who had not received CRT, although men treated with CRT were more likely to have seen a dentist. Overall, compliance with recommended dental surveillance is suboptimal, consistent with the deficiencies in risk-based medical care noted above.
Complementary and Alternative Medicine Therapy
Many adults and children with cancer report using complementary and alternative medicine (CAM) therapy to alleviate symptoms, enhance well-being, improve quality of life, or treat the malignancy.66–68 Little research has focused on whether the prevalent use of CAM continues in the survivor population. The CCSS assessed CAM use in the 2000 survey among 9,984 survivors and a comparison group of 2,474 siblings.69 Overall, 39% of survivors reported the use of at least one CAM therapy in the preceding year. Surprisingly, this did not differ from the frequency reported by their siblings (41%; P = .75) or the frequency reported in the general United States population.70–72 Survivors who were female, nonblack, older, or who had a college education were more likely to report CAM use, as were survivors who reported increased pain, psychological distress, or a major medical morbidity. Herbal remedies, massage/bodywork, and chiropractic manipulation were the three most common CAM modalities reported by survivors.
Limitations and Future Directions of CCSS Research Regarding Health Care
The CCSS has relied on data generated from patient self-report to estimate the medical care received by survivors. Self-report has been shown to be a valid measure of certain health care encounters (eg, some types of dental care73) and surveillance tests (eg, mammography74,75). However, the validity of patient reports of risk-based care has not been established, particularly when the assessment of that care relies on a patient's impression of the intent of his or her health care practitioner during a medical visit. For example, the CCSS surveys have inquired whether medical visits are related to each patient's previous cancer. It is possible that clinicians adapt their history, physical examination, or ordering of tests to the risks arising from the prior malignancy without the patient's awareness. Administrative database linkage, which couples cancer registry data with national health care or insurance data, provides an alternative approach to assessing the care received by cancer survivors.76 This approach has been used to assess survivor care in the Nordic countries77,78 and is currently being used to assess care in two cohorts of survivors in Canada. Similar methodology can be applied to Medicare79 and HMO databases in the United States. Such data is free from the selection and recall bias that affects studies based on patient self-report. However, it is restricted to the types of information routinely stored in the cancer registries and other databases. Here too, the purpose of a survivor's visit to his or her physician cannot always be deduced.
Future CCSS studies will assess the relationship of various outcomes (eg, mortality, chronic disease, quality of life) and health care utilization patterns. In particular, we are interested in determining whether certain patterns of health care are associated with reduced morbidity and mortality and maintenance of quality of life. Through longitudinal measures, data will be available to assess whether screening or surveillance patterns are associated with a reduction in morbidity. For example, for children treated with anthracycline chemotherapy, the COG Long-Term Follow-Up Guidelines recommend surveillance with a periodic echocardiogram, with frequency based on age at exposure, cumulative dose of anthracycline, and chest irradiation. Longitudinal data collected through the CCSS will provide the opportunity to determine whether a regular pattern of surveillance with an echocardiogram is associated with a lower incidence of congestive heart failure and cardiac-related mortality. Lastly, CCSS investigators are beginning to collaborate with health economists to determine the cost of health care of survivors.
INTERVENTIONS TO PROMOTE HEALTHY LIVING
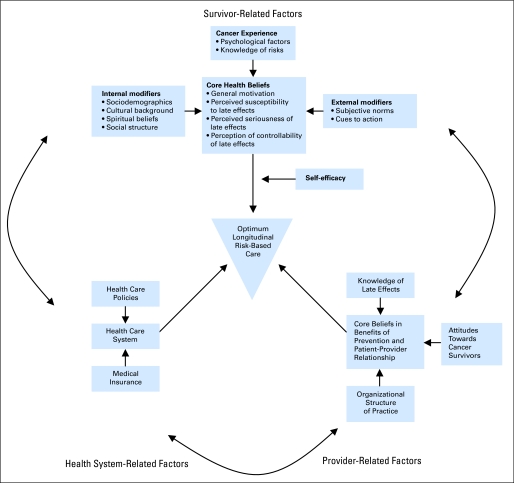
The CCSS has added substantially to the understanding of the health and health care of long-term survivors of childhood cancer. We have described a paradigm of cancer survivorship that is distinct from the traditional models of chronic disease.80 Once cured of their primary disease, most children with cancer enjoy a period of relative health during their adolescent years, with morbidity only developing many years later. Many survivors have unhealthy lifestyles that can be expected to further contribute to their health risks as they age. Most survivors are not observed at a cancer center, are not receiving recommended risk-based health care or surveillance, are unaware of their risks, and are observed by health care providers who are, understandably, unfamiliar with this population. These factors should be considered when developing interventions aimed at encouraging healthy lifestyles and risk-based health care. Figure 4 illustrates some of the key relationships.81
Fig 4.
Factors associated with optimum risk-based care. Adapted with permission.81
Emmons et al82 completed the first intervention study through CCSS that aimed to promote a healthy lifestyle (smoking cessation). The Partnership for Health study enrolled 796 participants from the CCSS cohort who identified themselves as smokers. The goals of the intervention were to address survivor-related factors associated with optimal health promotion, enhance self-efficacy and social support, increase knowledge about the health risks of smoking, reduce barriers to quitting, help participants set goals, and provide feedback regarding behavior change. Participants were randomly assigned to either a self-help intervention or a peer-counseling intervention. Self-help participants received a letter from the study physicians highlighting the importance of smoking cessation to reduce the risk of secondary cancers and a cessation manual. In the peer-counseling intervention, each survivor was assigned a trained childhood cancer survivor as a counselor. Up to six telephone calls were provided in a 7-month period, along with tailored and targeted materials and free nicotine replacement therapy. Follow-up assessments, including the primary outcome measure of smoking status, were conducted 8 and 12 months after the baseline survey for both groups of participants.
Results of the Partnership for Health study revealed that 15% of all participants had quit smoking at the initial 8-month follow-up. The smoking quit rate in the peer-counseling group was statistically significantly higher than in the self-help group at both the 8-month (17% v 9%; P < .01) and 12-month follow-up evaluations (15% v 9%; P < 0.01).82 Controlling for baseline self-efficacy and depression, participants in the peer-counseling intervention group were twice as likely to quit smoking by the 12-month follow-up than those in the self-help group (odds ratio, 1.99; 95% CI, 1.27 to 3.14). The total cost of the intervention in the peer-delivered group was approximately $300 per participant compared with $1.25 in the control group. Thus a relatively low-intensity and low-cost intervention resulted in a high-impact behavioral modification, namely, smoking cessation. The Partnership for Health study also demonstrated the ability to successfully conduct a large-scale behavioral intervention study through the CCSS and serves as a model for future health behavior intervention studies.
To our knowledge, there are no published studies that promote risk-based care and surveillance among vulnerable childhood cancer survivors. The CCSS has designed three such studies that are aimed to promote breast cancer screening among women treated with chest irradiation, cardiovascular screening among survivors treated with cardiotoxic therapy, and skin protection and early skin cancer detection among survivors treated with irradiation. Pending external funding, we anticipate conducting these trials in the near future.
There is much more opportunity for study in this area. Over time, the CCSS has developed an infrastructure capable of supporting the rigorous testing of interventions, has an extensive track record of successful collaborations with independent investigators, and has served as a resource for numerous investigator-initiated, externally supported studies. With the current expansion of the CCSS to include long-term childhood cancer survivors diagnosed from 1987 to 1999, we will have access to a cohort of survivors whose ages span from childhood to the late 50s, with a diversity of race and ethnicity, geographical locale, socioeconomic strata, and interval from cancer diagnosis. There are few comparable resources that offer such an established infrastructure and a diverse cohort of survivors to allow the completion of adequately powered intervention trials aimed at encouraging healthy lifestyle behaviors and promoting risk-based health care.
As noted in the introduction of this special issue, the CCSS is an open resource available to investigators at non-CCSS institutions. We strongly encourage CCSS and non-CCSS investigators to collaborate with us in developing scientifically rigorous intervention studies. Potential topics of study include promoting a healthy diet (including adequate calcium intake) and physical activity and avoiding excessive alcohol consumption. These healthy habits, in addition to avoidance or cessation of smoking, can be targeted individually, or several habits can be targeted in the same intervention. Similarly, there is much opportunity to promote risk-based care and recommended screening and surveillance. This might include study of the transition of adolescent or young adult survivors from the treating institution to their primary care physician and the testing of a shared care model. Recognizing that most primary care physicians have only a few childhood cancer survivors in their practice, it is highly unlikely that traditional methods of continuing medical education will provide enough detail for primary care clinicians to follow their cancer survivors. Instead, study through the CCSS offers the opportunity to test various methods of providing patient-specific education to clinicians. Similarly, little attention has been given to integrating insurance companies or other health payors into the promotion of risk-based care among this population. When designing such trials, it is important to understand that these interventions will need to be delivered at a distance, because most adult survivors of childhood cancer are not being observed actively by their treating institution. However, this is also true of most cancer survivors in North America, and thus the interventions are more likely to be generalizable in comparison with high-intensity and controlled trials within single institutions. Finally, the CCSS has demonstrated that risky health behaviors and poor compliance with recommended medical and dental care are influenced frequently by social factors such as income and education. Similar risk factors prevail in the general population. Thus future interventions targeted at decreasing smoking or alcohol use, increasing levels of physical activity, or improving compliance with guidelines for risk-based care might be more effective if they address the conditions that lead to social disadvantage.47
SUMMARY
Despite their long-term risk of morbidity and mortality, many childhood cancer survivors engage in risky health behaviors and do not receive regular risk-based medical care. The CCSS has described the prevalence and predictors of risky health behaviors and medical care utilization. Future studies will take advantage of the CCSS cohort to evaluate interventions targeted at modifying health behaviors and improving compliance with recommended risk-based medical care.
Footnotes
Supported by Grant No. U24 CA55727 (L.L.R.) from the National Cancer Institute, Bethesda, MD, with additional support provided to St Jude Children's Research Hospital by the American Lebanese Syrian Associated Charities (ALSAC).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Manuscript writing: Paul C. Nathan, Jennifer S. Ford, Tara O. Henderson, Melissa M. Hudson, Karen M. Emmons, Jacqueline N. Casillas, E. Anne Lown, Kirsten K. Ness, Kevin C. Oeffinger
REFERENCES
- 1.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 2.Mertens AC, Yasui Y, Neglia JP, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol. 2001;19:3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 3.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA. 2003;290:1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow AJ, Schoemaker MJ, Allerton R, et al. Lung cancer after Hodgkin's disease: A nested case-control study of the relation to treatment. J Clin Oncol. 2001;19:1610–1618. doi: 10.1200/JCO.2001.19.6.1610. [DOI] [PubMed] [Google Scholar]
- 6.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 7.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiotherapy and smoking in lung cancer following Hodgkin's disease. J Natl Cancer Inst. 1995;87:1530–1537. doi: 10.1093/jnci/87.20.1530. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 10.Cutuli B, Borel C, Dhermain F, et al. Breast cancer occurred after treatment for Hodgkin's disease: Analysis of 133 cases. Radiother Oncol. 2001;59:247–255. doi: 10.1016/s0167-8140(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 11.Wolden SL, Hancock SL, Carlson RW, et al. Management of breast cancer after Hodgkin's disease. J Clin Oncol. 2000;18:765–772. doi: 10.1200/JCO.2000.18.4.765. [DOI] [PubMed] [Google Scholar]
- 12.Yahalom J, Petrek JA, Biddinger PW, et al. Breast cancer in patients irradiated for Hodgkin's disease: A clinical and pathologic analysis of 45 events in 37 patients. J Clin Oncol. 1992;10:1674–1681. doi: 10.1200/JCO.1992.10.11.1674. [DOI] [PubMed] [Google Scholar]
- 13.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 14.Brennan BM, Rahim A, Adams JA, et al. Reduced bone mineral density in young adults following cure of acute lymphoblastic leukaemia in childhood. Br J Cancer. 1999;79:1859–1863. doi: 10.1038/sj.bjc.6690296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies JH, Evans BA, Jenney ME, et al. Skeletal morbidity in childhood acute lymphoblastic leukaemia. Clin Endocrinol (Oxf) 2005;63:1–9. doi: 10.1111/j.1365-2265.2005.02263.x. [DOI] [PubMed] [Google Scholar]
- 16.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26:4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oeffinger KC, Mertens AC, Sklar CA, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 18.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 19.Link K, Moell C, Garwicz S, et al. Growth hormone deficiency predicts cardiovascular risk in young adults treated for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 2004;89:5003–5012. doi: 10.1210/jc.2004-0126. [DOI] [PubMed] [Google Scholar]
- 20.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:5277–5282. doi: 10.1200/JCO.2006.07.2884. [DOI] [PubMed] [Google Scholar]
- 21.Oeffinger KC. Are survivors of acute lymphoblastic leukemia (ALL) at increased risk of cardiovascular disease? Pediatr Blood Cancer. 2008;50:462–467. doi: 10.1002/pbc.21410. discussion 468. [DOI] [PubMed] [Google Scholar]
- 22.Castellino S, Lensing S, Riely C, et al. The epidemiology of chronic hepatitis C infection in survivors of childhood cancer: An update of the St Jude Children's Research Hospital hepatitis C seropositive cohort. Blood. 2004;103:2460–2466. doi: 10.1182/blood-2003-07-2565. [DOI] [PubMed] [Google Scholar]
- 23.Locasciulli A, Testa M, Pontisso P, et al. Prevalence and natural history of hepatitis C infection in patients cured of childhood leukemia. Blood. 1997;90:4628–4633. [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. The health consequences of smoking: A report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 25.Cigarette smoking-attributable morbidity: United States, 2000. MMWR Morb Mortal Wkly Rep. 2003;52:842–844. [PubMed] [Google Scholar]
- 26.Annual smoking-attributable mortality, years of potential life lost, and productivity losses: United States, 1997-2001. MMWR Morb Mortal Wkly Rep. 2005;54:625–628. [PubMed] [Google Scholar]
- 27.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: A report from the childhood cancer survivor study. J Clin Oncol. 2002;20:1608–1616. doi: 10.1200/JCO.2002.20.6.1608. [DOI] [PubMed] [Google Scholar]
- 28.Cigarette smoking among adults: United States, 1999. MMWR Morb Mortal Wkly Rep. 2001;50:869–873. [PubMed] [Google Scholar]
- 29.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. J Clin Oncol. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 1995. [Google Scholar]
- 31.Emmons KM, Butterfield RM, Puleo E, et al. Smoking among participants in the childhood cancer survivors cohort: The Partnership for Health Study. J Clin Oncol. 2003;21:189–196. doi: 10.1200/JCO.2003.06.130. [DOI] [PubMed] [Google Scholar]
- 32.Rehm J, Room R, Graham K, et al. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: An overview. Addiction. 2003;98:1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thakker KD. An overview of health risks and benefits of alcohol consumption. Alcohol Clin Exp Res. 1998;22:285S–298S. doi: 10.1097/00000374-199807001-00003. [DOI] [PubMed] [Google Scholar]
- 35.Lown EA, Goldsby R, Mertens AC, et al. Alcohol consumption patterns and risk factors among childhood cancer survivors compared to siblings and general population peers. Addiction. 2008;103:1139–1148. doi: 10.1111/j.1360-0443.2008.02242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butterfield RM, Park ER, Puleo E, et al. Multiple risk behaviors among smokers in the childhood cancer survivors study cohort. Psychooncology. 2004;13:619–629. doi: 10.1002/pon.764. [DOI] [PubMed] [Google Scholar]
- 37.Wolff I, van Croonenborg JJ, Kemper HC, et al. The effect of exercise training programs on bone mass: A meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9:1–12. doi: 10.1007/s001980050109. [DOI] [PubMed] [Google Scholar]
- 38.Whelton SP, Chin A, Xin X, et al. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. doi: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 39.Helmrich SP, Ragland DR, Leung RW, et al. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 40.Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294:2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- 41.Hu FB, Willett WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 42.Kujala UM, Kaprio J, Sarna S, et al. Relationship of leisure-time physical activity and mortality: The Finnish twin cohort. JAMA. 1998;279:440–444. doi: 10.1001/jama.279.6.440. [DOI] [PubMed] [Google Scholar]
- 43.Paffenbarger RS, Jr, Hyde RT, Wing AL, et al. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 44.Florin TA, Fryer GE, Miyoshi T, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16:1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 45.Barlow CE, Kohl HW, 3rd, Gibbons LW, et al. Physical fitness, mortality and obesity. Int J Obes Relat Metab Disord. 1995;19(suppl 4):S41–S44. [PubMed] [Google Scholar]
- 46.Frobisher C, Winter DL, Lancashire ER, et al. Extent of smoking and age at initiation of smoking among adult survivors of childhood cancer in Britain. J Natl Cancer Inst. 2008;100:1068–1081. doi: 10.1093/jnci/djn210. [DOI] [PubMed] [Google Scholar]
- 47.Emmons K. Smoking among childhood cancer survivors: We can do better. J Natl Cancer Inst. 2008;100:1048–1049. doi: 10.1093/jnci/djn242. [DOI] [PubMed] [Google Scholar]
- 48.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 49.Oeffinger KC, Mertens AC, Hudson MM, et al. Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Fam Med. 2004;2:61–70. doi: 10.1370/afm.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2005. [Google Scholar]
- 51.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 52.United Kingdom Children's Cancer Study Group. Therapy-based long-term follow-up: Practice statement. http://ukccsg.org/public/followup/PracticeStatement/LTFU-full.pdf.
- 53.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeazel MW, Oeffinger KC, Gurney JG, et al. The cancer screening practices of adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2004;100:631–640. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]
- 55.Buetow SA. Testicular cancer: To screen or not to screen? J Med Screen. 1996;3:3–6. doi: 10.1177/096914139600300103. [DOI] [PubMed] [Google Scholar]
- 56.Humphrey LL, Helfand M, Chan BK, et al. Breast cancer screening: A summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 57.Mertens AC, Cotter KL, Foster BM, et al. Improving health care for adult survivors of childhood cancer: Recommendations from a delphi panel of health policy experts. Health Policy. 2004;69:169–178. doi: 10.1016/j.healthpol.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Kadan-Lottick NS, Robison LL, Gurney JG, et al. Childhood cancer survivors' knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA. 2002;287:1832–1839. doi: 10.1001/jama.287.14.1832. [DOI] [PubMed] [Google Scholar]
- 59.Kaste SC, Hopkins KP, Bowman LC. Dental abnormalities in long-term survivors of head and neck rhabdomyosarcoma. Med Pediatr Oncol. 1995;25:96–101. doi: 10.1002/mpo.2950250209. [DOI] [PubMed] [Google Scholar]
- 60.Kaste SC, Hopkins KP, Bowman LC, et al. Dental abnormalities in children treated for neuroblastoma. Med Pediatr Oncol. 1998;30:22–27. doi: 10.1002/(sici)1096-911x(199801)30:1<22::aid-mpo8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 61.Kaste SC, Hopkins KP, Jones D, et al. Dental abnormalities in children treated for acute lymphoblastic leukemia. Leukemia. 1997;11:792–796. doi: 10.1038/sj.leu.2400670. [DOI] [PubMed] [Google Scholar]
- 62.Maguire A, Welbury RR. Long-term effects of antineoplastic chemotherapy and radiotherapy on dental development. Dent Update. 1996;23:188–194. [PubMed] [Google Scholar]
- 63.Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http://www.survivorshipguidelines.org.
- 64.Yeazel MW, Gurney JG, Oeffinger KC, et al. An examination of the dental utilization practices of adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Public Health Dent. 2004;64:50–54. doi: 10.1111/j.1752-7325.2004.tb02726.x. [DOI] [PubMed] [Google Scholar]
- 65.Goodman HS, Manski MC, Williams JN, et al. An analysis of preventive dental visits by provider type, 1996. J Am Dent Assoc. 2005;136:221–228. doi: 10.14219/jada.archive.2005.0147. [DOI] [PubMed] [Google Scholar]
- 66.Kelly KM. Complementary and alternative medicines for use in supportive care in pediatric cancer. Support Care Cancer. 2007;15:457–460. doi: 10.1007/s00520-006-0162-2. [DOI] [PubMed] [Google Scholar]
- 67.Monti DA, Yang J. Complementary medicine in chronic cancer care. Semin Oncol. 2005;32:225–231. doi: 10.1053/j.seminoncol.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 68.Weiger WA, Smith M, Boon H, et al. Advising patients who seek complementary and alternative medical therapies for cancer. Ann Intern Med. 2002;137:889–903. doi: 10.7326/0003-4819-137-11-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 69.Mertens AC, Sencer S, Myers CD, et al. Complementary and alternative therapy use in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2008;50:90–97. doi: 10.1002/pbc.21177. [DOI] [PubMed] [Google Scholar]
- 70.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: Results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 71.Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States: Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–252. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 72.Kessler RC, Davis RB, Foster DF, et al. Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med. 2001;135:262–268. doi: 10.7326/0003-4819-135-4-200108210-00011. [DOI] [PubMed] [Google Scholar]
- 73.Gilbert GH, Rose JS, Shelton BJ. A prospective study of the validity of self-reported use of specific types of dental services. Public Health Rep. 2003;118:18–26. doi: 10.1016/S0033-3549(04)50213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King ES, Rimer BK, Trock B, et al. How valid are mammography self-reports? Am J Public Health. 1990;80:1386–1388. doi: 10.2105/ajph.80.11.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puleo E, Zapka JG, Goins KV, et al. Recommendations for care related to follow-up of abnormal cancer screening tests: Accuracy of patient report. Eval Health Prof. 2005;28:310–327. doi: 10.1177/0163278705278273. [DOI] [PubMed] [Google Scholar]
- 76.Cooper GS, Schultz L, Simpkins J, et al. The utility of administrative data for measuring adherence to cancer surveillance care guidelines. Med Care. 2007;45:66–72. doi: 10.1097/01.mlr.0000241107.15133.54. [DOI] [PubMed] [Google Scholar]
- 77.Nord C, Ganz PA, Aziz N, et al. Follow-up of long-term cancer survivors in the Nordic countries. Acta Oncol. 2007;46:433–440. doi: 10.1080/02841860701203552. [DOI] [PubMed] [Google Scholar]
- 78.Ross L, Johansen C, Dalton SO, et al. Psychiatric hospitalizations among survivors of cancer in childhood or adolescence. N Engl J Med. 2003;349:650–657. doi: 10.1056/NEJMoa022672. [DOI] [PubMed] [Google Scholar]
- 79.Snyder CF, Earle CC, Herbert RJ, et al. Preventive care for colorectal cancer survivors: A 5-year longitudinal study. J Clin Oncol. 2008;26:1073–1079. doi: 10.1200/JCO.2007.11.9859. [DOI] [PubMed] [Google Scholar]
- 80.Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. JAMA. 2007;297:2762–2764. doi: 10.1001/jama.297.24.2762. [DOI] [PubMed] [Google Scholar]
- 81.Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27:143–167. doi: 10.1016/s0147-0272(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 82.Emmons KM, Puleo E, Park E, et al. Peer-delivered smoking counseling for childhood cancer survivors increases rate of cessation: The partnership for health study. J Clin Oncol. 2005;23:6516–6523. doi: 10.1200/JCO.2005.07.048. [DOI] [PubMed] [Google Scholar]