Abstract
Host gender has previously been identified as a determining factor in the resolution of Trichuris muris infection in mice lacking Interleukin-4 (IL-4KO BALB/c). Worm expulsion in these mice is delayed, but occurs in females. In this study we were able to demonstrate delayed expulsion occurs at day 26 post infection and is associated with the production of the key Th2 associated cytokine IL-13 by both CD4+ T cells and an auxiliary DX5+ Natural Killer (NK) cell source - as well as a concurrent reduction in pro-inflammatory cytokines. NK cell number was comparably increased in both sexes, but NK cells from male mice were found to express higher levels of the chemokine receptor CXCR3. Depletion of CD4+ T cells completely prevented parasite expulsion, whereas loss of NK cells resulted in a mild, but significant delay. Furthermore, IL-18 - a cytokine with the capacity to enhance both Th1 and Th2 responses - was found to be dispensable for worm expulsion in female mice but was a key factor for the suppression of the Th2 response in male IL-4KO mice. In contrast neutralisation of IFN-γ resulted in a complete restoration of typical WT BALB/c expulsion kinetics. This study sheds further light on the role of accessory NK cells in supplementing the IL-13 driven immune response when normal Th2 immunity is disrupted, and further identifies host gender as a key factor in determining the generation of ‘NK help’.
Keywords: Natural Killer Cells, Parasitic-Helminth, Cytokines, Comparative Immunology, Th1/Th2 cells
INTRODUCTION
Multiple factors determine the ability of a host to generate a protective type 2 response in response to infection with the gut nematode Trichuris muris. Genetic components - including the haplotype and strain of the mouse - are directly correlated with the host’s ability to generate a Th2 response and expel worms from the intestinal niche (1). Resolution of T. muris infection is typically characterised by the production of type 2 cytokines such as IL-4 and IL-13 which subsequently results in intestinal goblet cell hyperplasia and an increased turnover of the intestinal epithelium mediating parasite loss (2). Previous studies with BALB/c mice deficient in IL-4 demonstrated the ability of these mice to mount an effective immune response against T. muris due to a compensatory increase in IL-13, whereas mice lacking IL-13 were rendered susceptible to chronic infection (3). Interestingly however, this compensatory mechanism was only present in female KO mice of this strain - and not their male counterparts - suggesting, an important influence of host sex on the development of Th2 responses to gastrointestinal nematodes (4). Gender differences have previously been noted in other murine models of parasitic disease with a prominent female bias towards resistance in nematode infections, including Nippostrongylus brasiliensis and Trichinella spiralis (reviewed in (5)).
The immune response to T. muris was found to be impaired in female IL-4KO BALB/c mice as expulsion was significantly delayed beyond day 21 post infection (p.i.) and was associated with high IFN-γ production whereas, WT BALB/c mice had few remaining worms (4). It has become clear that upon disruption of normal CD4+ T cell mediated Th2 responses other cell types may be induced to produce type 2 cytokines in order to compensate for, or supplement, the insufficient response. For example, T. muris expulsion can be mediated via IL-13 derived from both CD4+ T cell and DX5+ NK cells in mice where B7 co-stimulation is abrogated, dependent on neutralisation of subsequent IFN-γ production (6, 7). These findings are in line with reports that NK cells are stimulated to produce IL-13 by IL-2 in the absence of IFN-γ (8). Intraepithelial NK cells have also previously been identified as an important source of IL-13 in driving intestinal pathology during T. spiralis infection (9) and IL-13 producing NK-T cells are responsible for pathology in a model of Ulcerative Colitis and the development of allergen induced airway hyperreactivity (10, 11).
In this study we demonstrate a gender-biased expulsion of T. muris, which occurs in a delayed manner at 26 days p.i. and is characterised by a dramatic increase in IL-13 production and concurrent decrease in the pro-inflammatory cytokines TNF-α and IL-6 in the presence of high levels of IFN-γ. The delayed immune response in resistant female IL-4KO animals was accounted for by a late increase in IL-13 derived from both CD4+ T cells and DX5+ NK cells, but was absent from male counterparts. Equivalent expansion of NK cells was detected in both male and female IL-4KO mice but male NK cells expressed higher levels of the IFN-γ associated chemokine receptor CXCR3 and IFN-γ mRNA ex vivo, despite comparable levels of this cytokine in both sexes following antigen restimulation. Depletion of both NK cells and CD4+ cells suggested only T cells are essential for resistance to chronic infections in these mice, although NK depletion delayed parasite expulsion significantly in comparison to controls. Interestingly, a delay in worm expulsion was also noted in male WT BALB/c mice compared to female WT BALB/c, although both fully expelled worms prior to IL-4KO mice. In addition male WT mice also demonstrated increased IL-13+ NK cell numbers in the draining lymph node upon infection.
IL-18 is a cytokine with pleiotropic effects that can stimulate the production of IL-13 by NK cells and T cells in combination with IL-2 - and has been linked to resistance to T. muris (6, 12). In contrast studies from our laboratory have previously demonstrated a repressive effect of IL-18 on IL-13 production and Th2 immunity during T. muris infection, suggesting a classical role of IL-18 as a potent inducer of Th1 responses (13, 14). In line with this, IL-18 has been shown to be essential for priming of NK cells and for IFN-γ mRNA translation and protein production following IL-12 stimulation (15). Interestingly, polymorphisms in the IL-18 receptor signalling complex gene IL18RAP have recently been shown to be strongly associated with the evolutionary selection pressure driven by macroparasites, such as helminths, identifying IL-18 signalling as a key factor in determining susceptibility to helminth infections (16). In this study IL-18 was dispensable for resistance in female IL-4KO mice but surprisingly was responsible for the suppression of IL-13 responses in male IL-4KO mice. Critically however, only neutralisation of IFN-γ was sufficient to fully restore optimal WT BALB/c expulsion kinetics in mice lacking IL-4. Thus, taken together the data presented here further implicates NK cells as an auxiliary source of IL-13 in sub-optimal or disrupted Th2 immune responses and identifies an important role for IL-18 in a novel gender biased IL-4KO mouse model.
MATERIALS AND METHODS
Mice
IL-4KO BALB/c mice and WT BALB/c control mice were originally obtained from Dr. N. Noben-Trauth, NIH, USA (17) and subsequently bred and maintained under specific pathogen free conditions in the animal facilities of the University of Manchester, UK. Mice were used for infections between the ages of 6 and 8 weeks and each experimental group contained 5 mice unless indicated. All experiments were performed under the regulations of the Home Office Scientific Procedures Act (1986).
Parasite maintenance and infection
Adult T. muris was recovered from the intestines of immunodeficient SCID mice and embryonated eggs generated as previously described (18). Briefly, the caecum and proximal colon were removed and adult worms extracted into RPMI 1640 media (Invitrogen) and incubated for 4 hours at 37°C. The eggs were removed via centrifugation and allowed to embryonate at RT for 8 weeks prior to use. Supernatant containing Excretory/Secretory antigen (E/S) was concentrated using a Centriprep YM-10 (Amicon, Gloucester, UK) and dialysed into PBS. Antigen concentration was determined using a Nanodrop (Nanodrop technologies). Mice were infected via oral gavage with 100-200 infective T. muris eggs and worm burdens were assessed at multiple time points p.i. via manual extraction of worms from the caecum and proximal colon after longitudinal dissection.
Lymphocyte restimulation and cytokine analysis
Mesenteric lymph nodes (MLNs) were removed from infected and naive mice and single cell suspensions were resuspended and plated at 5 × 106 cells/ml in RPMI 1640 supplemented with 10% FCS, 2mM L-glutamine, 100U/ml penicillin and 100μg/ml Streptomycin (Invitrogen). Cultures were re-stimulated with 50μg of T. muris E/S for 24 hours at 37°C / 5% CO2. Cells were subsequently removed via centrifugation and supernatants stored at -20°C until further use. The concentrations of IFN-γ, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13 and TNF-α were measured via Cytokine Bead Assay kit (CBA, BD Biosciences) following the manufacturer’s instructions and assessed using a BD FACSCalibur. IL-17A was measured via sandwich ELISA using commercially available paired antibodies (BD Biosciences).
Antibody analysis
Parasite specific IgG1 and IgG2a were assessed via ELISA. Briefly, Immunlon IV plates (Thermo lifesciences) were coated at 4°C overnight with 5μg/ml of T. muris E/S in carbonate/bicarbonate buffer, pH 9.6. Plates were subsequently blocked to prevent non-specific binding with 3% BSA in 0.05% Tween 20 PBS and sera from mice were added to wells in twofold dilutions. Parasite specific antibodies were detected using biotinylated rat anti-mouse IgG1 (Serotec) or biotinylated rat anti-mouse IgG2a (BD Biosciences). Plates were developed with TMB and the O.D. at 450nm, with reference to 570nm, was measured.
Histological Analysis
Sections of caecum were removed and stored for 12 hours in 4% Neutral Buffered Formalin prior to processing and embedding in paraffin wax. Tissue sections of 4μm were cut and dewaxed using citroclear and dehydrated prior to staining with Periodic Acid and Schiff’s reagent to visualise goblet cells. Sections were mounted and the number of goblet cells per 20 crypt forming units (CFU) was counted for each section. Crypt lengths were measured and calculated using Image J software (NIH).
Isolation of intraepithelial lymphocytes (IELs)
The caecum and intestine were flushed with ice cold Ca2+-free HBSS with 2% FCS, cut longitudinally and trimmed of fat and Peyer’s patches and subsequently cut into 3cm sections. Tissue sections were incubated in HBSS supplemented with 1mM DTT and 0.8M EDTA at 37°C for 30 minutes with vigorous shaking. Supernatant containing epithelium was then removed and remaining tissue incubated for a further 30 minutes with DTT and EDTA. Cells from five mice were pooled and passed through a 40μm strainer prior to resuspension in RPMI 1640 media containing 25mM HEPES for use in flow cytometry or cell sorting.
Flow cytometry and cell sorting
CD4+ T cells and DX5+ NK cells were isolated via staining of pooled mesenteric lymph node cells from 5 mice. Cell were blocked for non specific binding via incubation with anti-Fc block (anti CD16/32, BD Biosciences) for 10 minutes on ice and subsequently stained with anti CD3ε FITC, anti DX5 PE, anti CD4 PercP and anti MHC class II APC (BD Biosciences) for 30 minutes on ice. Cells were washed and resuspended in RPMI 1640 supplemented with 25mM HEPES and CD4+ T cells (CD3+ CD4+) and NK cells (CD3- DX5+) were isolated using a BD FACS DIVA cell sorter. All MHC class II positive cells were gated out of the total cell population prior to sorting to prevent contamination with Antigen Presenting Cells . Epithelial cells and intraepithelial lymphocytes (IELs) were identified and sorted via staining with the lectin UEA-1 (Vector) and anti CD45 PE antibody (BD Biosciences), respectively. Following sorting isolated cells were analysed for purity using a BD FACSCalibur and were consistently found to be over 95% pure. Sorted cells were then placed directly into TRIzol reagent (Invitrogen) for RNA extraction. DX5+ NK cell phenotype was assessed via staining with anti IL-2Rβ FITC (BD biosciences) or with purified rat anti-mouse CXCR3 (R&D Systems) followed by biotinylated mouse anti-rat IgG2a and Streptavidin APC (BD Biosciences).
RNA extraction and Real Time PCR
RNA was extracted from sorted cells stored in TRIzol reagent via Chloroform and Isopropanol precipitation and reverse transcribed using ImProm-II RT (Promega). Real time PCR reactions were performed using the following primers to determine the levels of HPRT (Sense 5′ GCGTCGTGATTAGTGATGATGAAC 3′ / Antisense 5′ GAGCAAGTCTTTTCAGTCCTGTCCA 3′), IL-13 (Sense 5′ AGGAGCTTATTGAGGAGCTGAAGCA 3′ / Antisense 5′ TGGAGATGTTGGTCAGGGAATCCA 3′), IFN-γ (Sense 5′ GGCCATCAGCAACAACATAAGCGT 3′ / Antisense 5′ TGGGTTGTTGACCTCAAACTTGGC 3′) and CXCL10 (Sense 5′ ATGAGGGCCATAGGGAAGCTTGAA 3′ / Antisense 5′ CCGGATTCAGACATCTCTGCTCAT 3′). Products were amplified using SYBR green master mix (Finnzymes) with 0.1μg cDNA and 50pmol of primers. All reactions were performed with the following cycle conditions: 40 cycles of 94°C 10 seconds, 60°C 20 seconds and 72°C for 15 seconds. Melting curves were checked to confirm specific gene amplification and relative expression determined compared to house keeping genes and control sample via the comparative 2-ΔΔC(t) method.
In vivo depletion of cells and cytokines
CD4+ T cells were depleted from IL-4KO BALB/c mice via intraperitoneal (i.p.) administration of 500μg anti-CD4 mAb (YTS 169) every 2 days beginning on the day of infection until termination. Control animals received 500μg of total Rabbit Ig (Sigma Aldrich) as an isotype control. NK cells were depleted via intravenous injection of 750μg anti-asialo GM1 antibody (Cedarlane laboratories) at days 10, 14, 18 and 22 post infection. Depletion of cells in treated groups was confirmed via flow cytometry and immunohistochemistry of caecal tissue (data not shown). IFN-γ was neutralised via i.p. treatment with 500μg anti-IFN-γ (XMG 1.6) every 2 days from infection until termination - control mice received 500μg of rat IgG isotype control (GL113). IL-18 was neutralised via i.p. injection of 750μg mouse anti-mouse IL-18 (SK11 3AE - kindly donated by Prof. Irmgard Förster, University of Düsseldorf), or mice were treated with technical grade mouse Ig (Sigma Aldrich) every 4 day from infection until termination.
Statistics
Statistical significance for the data presented, representative of three independent experiments unless indicated, was calculated using a Kruskal Wallis ANOVA followed by a Dunn’s post hoc test. Values of p=<0.05 were considered significant.
RESULTS
Female WT and IL-4KO BALB/c mice show increased resistance to T. muris in comparison to male equivalents
We have previously reported a profound gender difference in the expulsion of T. muris in IL-4KO BALB/c mice (4). In order to define the underlying mechanisms we infected WT and IL-4KO BALB/c male and female mice with 150 infective T. muris eggs and assessed worm burdens and immune responses at key time points post infection. Figure 1A confirms comparable establishment of T. muris in the caecum and proximal colon of male and female WT and IL-4KO mice 14 days p.i. Furthermore, 21 days p.i. IL-4KO mice retained full worm burdens, confirming a lack of expulsion in these mice at this time point as previously described (4). In line with previous reports female WT BALB/c mice had almost completely expelled their worm burdens by this time point. Surprisingly however, male WT mice still retained high numbers of worms at this time point indicating expulsion in male WTs is delayed in comparison to females beyond day 21, with expulsion complete by day 26 p.i. WT BALB/c mice worm expulsion was associated with a strong Th2 restricted response as frequently reported (19) (data not shown). Female IL-4KO BALB/c mice were previously demonstrated to expel T. muris between day 21 and 35 p.i., whereas, male IL-4KOs are unable to expel the worms and develop a chronic adult infection (full worm burdens retained at day 35 p.i. - data not shown). Expulsion in female KO mice occurs at approximately day 26 p.i. (Fig 1A) and is associated a marked increase in the type 2 cytokines IL-9 and IL-13 and reductions in the pro-inflammatory cytokines TNF-α and IL-6, while IFN-γ and IL-10 levels remained comparable to males in these mice (Fig 1B). Expulsion in female KO mice was also associated with an increase in intestinal goblet cell number, previously reported to be driven by IL-13 (Fig 1C) (20). No significant differences in crypt length, associated with increased IFN-γ driven epithelial cell proliferation, were detected between male and female mice at this time (Fig 1C)(21).
Figure 1.
Female IL-4KO BALB/c mice develop a Th2 immune response leading to the expulsion of T. muris whereas male mice develop chronic nematode infections. A) Male and female mice from WT and IL-4KO BALB/c received 150 infective T. muris eggs and worm burdens in the caecum and large intestine assessed at day 14, 21 and 26 post infection B) The concentrations of Th2 (IL-9, IL-13 and IL-10) and Th1/pro-inflammatory (IFN-γ, TNF-α and IL-6) cytokines were measured in supernatants of mesenteric lymph node cells isolated from infected IL-4KO mice after 26 days and re-stimulated for 24 hours with T. muris E/S antigen C) Caecal histology including goblet cell hyperplasia and crypt length was measured at day 26 post infection in IL-4KO mice of both sex following PAS staining, scale bar = 50μm. Naiive values are indicated in red and represent the mean value for male and female naïve mice which did not differ significantly from one another. * - p≤0.05, ** - p ≤0.01, *** - p≤0.001.
NK cells provide an additional source of IL-13 during delayed expulsion
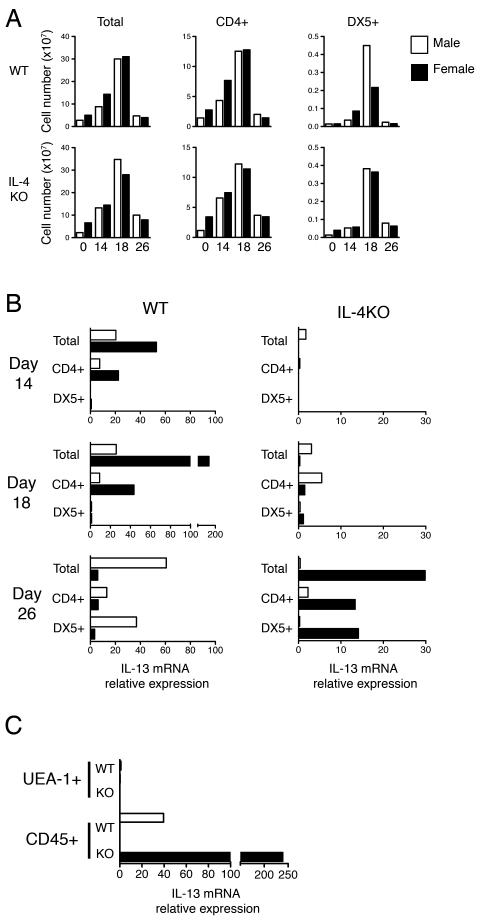
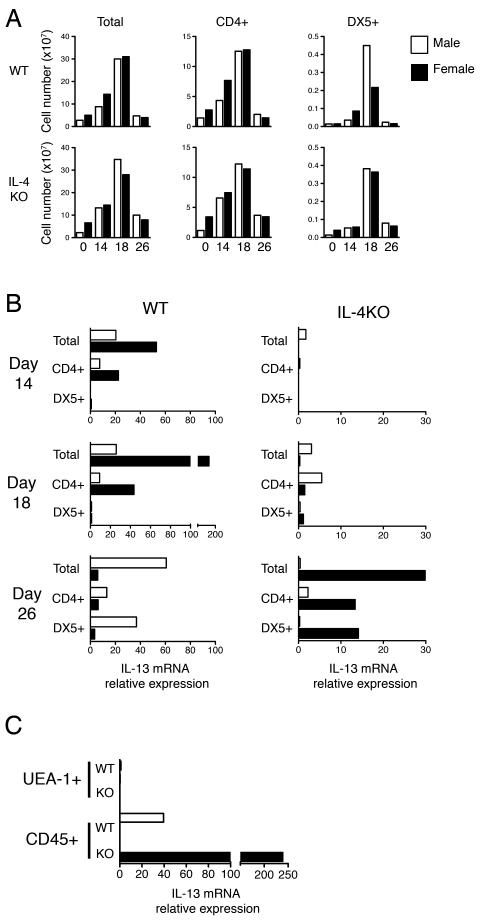
IL-13 is a principal cytokine in mediating T. muris expulsion (2). Depending on circumstances NK and NK-T cells are able to produce the type 2 cytokine IL-13 and contribute to a Th2 response (8-11, 22). Previous studies have reported IL-13 production from NK cells following ablation of B7 co-stimulation in female BALB/c mice - resulting in a loss of resistance to T. muris - which manifested only following subsequent neutralisation of the resulting high IFN-γ levels (6, 7). In order to determine whether NK cells also contribute IL-13 during delayed expulsion in the gender biased IL-4KO model, mice were infected with T. muris and MLNCs were pooled (n=5) and sorted via flow cytometry in order to obtain purified CD4+ T cell and DX5+ NK cells. Total ex vivo lymph node numbers were comparable between groups over the course of infection, with peak cellularity observed at day 18 p.i. irrespective of expulsion kinetics (Fig 2A). Similarly, CD4+ T cell numbers were found to increase during infection, before returning to naive levels by day 26 p.i. (Fig 2A). Intriguingly, NK cell numbers were found to dramatically increase at day 18 p.i. in both male and female IL-4KO MLNs, as well as in male but not female WT lymph nodes (Fig 2A). The relative expression of IL-13 mRNA in male and female WT and IL-4KO BALB/c mice over the course of T. muris infection is shown in Figure 2B. At day 14 p.i. IL-13 mRNA was only detectable in the WT BALB/c mice, in line with rapid expulsion kinetics in WT mice compared to mice lacking IL-4. Female WT mice had a large increase in total IL-13 mRNA at day 18 p.i., coinciding with worm expulsion, which was derived only from the CD4+ T cell population and not DX5+ cells (Fig 2B). Surprisingly, expression in male WTs was distinctly lower at this time point. In contrast, only a low expression of IL-13 mRNA was detected in IL-4KO mice at day 18 p.i. At day 26 p.i. high expression of IL-13 mRNA was detected in total lymphocytes of female IL-4KO mice, derived from both the CD4+ T cell and DX5+ NK cell populations, whereas, male IL-4KO mice produced only negligible amounts of IL-13 mRNA in line with their inability to generate type 2 cytokines in response to T. muris. Interestingly, male WT BALB/c at this same time point demonstrated a comparably high expression of IL-13 mRNA, also detectable in both CD4+ T cells and DX5+ NK cells (Fig 2B). In order to confirm the gender specific IL-13 expression at day 26 p.i. at the site of host:parasite interaction we isolated the infected caecal and colonic tissue and separated epithelial cells and intraepithelial lymphocytes via their respective expression of the lectin UEA-1 or lymphocyte marker CD45. In agreement with data from the MLN, IL-13 mRNA was only detectable in male WT and female IL-4KO BALB/c at this time point and was restricted to the intraepithelial lymphocyte population (Fig 2C).
Figure 2.
Natural Killer Cells provide an additonal source of IL-13 during delayed expulsion of T. muris. A) The total lymphocyte number of mesenteric lymph nodes and the relative numbers of CD4+ T cells and DX5+ NK cells were calculated after normalising cell percentages derived via flow cytometry to total cell counts. Cell numbers of total cells and respective subpopulations of naive and pooled (n=5) T. muris infected lymph nodes 14, 18 and 26 days p.i. from male and female WT or IL-4KO BALB/c mice are indicated B) The relative expression of IL-13 mRNA transcripts in the pooled MLNs was determined ex vivo via real time PCR using the 2-ΔC(t) method. IL-13 expression was determined relative to the housekeeping gene HPRT and to that of naive mice of the same sex. C) IL-13 expression was determined in UEA-1+ epithelial cells and CD45+ lymphocyte populations isolated from the pooled caecums of infected mice at day 26 p.i. via EDTA/DTT treatment and Percoll gradient centrifugation. Data is representative of two independent experiments.
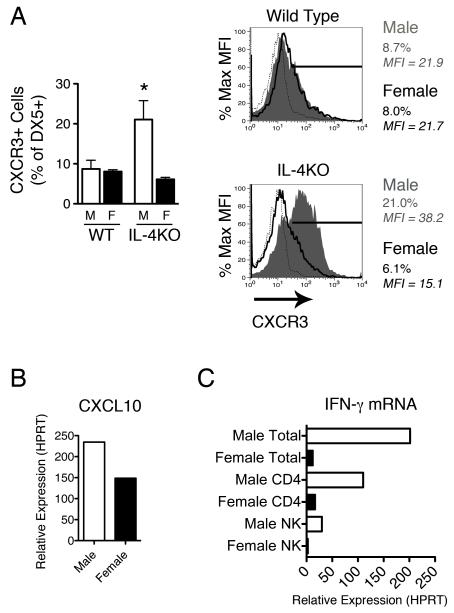
NK cells from male IL-4KO mice are more responsive to CXCL10
As NK cell numbers in the draining lymph node were comparable in both sexes of IL-4KO mice at day 18 p.i.. yet only female NK cells synthesised detectable IL-13 mRNA we hypothesised that “programming” of NK cells during this expansion may differ between the sexes. In order to address this the phenotype of NK cell markers was assessed via flow cytometry. DX5 cells from both male and female mice were IL-2Rβ+, suggesting these cells represent NK cells and not Basophils, which have previously been reported to be negative for this receptor chain (23) (data not shown). Of the panel of markers investigated NK cells derived from the two sexes of IL-4KO mice were found to differ only in their expression of CXCR3, the receptor for the IFN-γ induced chemokine CXCL10. Few NK cells from WT mice and female IL-4KO BALB/c mice expressed this marker (∼10%), whereas, NK cells derived from male IL-4KO mice intensely expressed CXCR3 on 25 ± 4.7% of cells (Fig 3A), suggesting NK cells from male IL-4KO BALB/c may be more responsive to CXCL10. CXCL10 is an IFN-γ induced chemokine stimulated during the onset of Th1 responses and previously found to inhibit IL-13 mediated expulsion of T. muris (2, 24). CXCL10 expression in total MLNCs was found to be increased compared to naïve mice in both male and female IL-4KO mice at the crucial day 28 time point (Fig 3B), with higher expression of this chemokine in males (average 234 fold) than females (average 149 fold). Despite comparable production of IFN-γ protein at this time point in response to antigen restimulation, we assessed whether this increase in CXCL10 responsiveness on NK cells resulted in an increase in the proportion of NK cells expressing IFN-γ mRNA ex vivo. MLNCs derived from male mice at day 28 p.i. had high levels of IFN-γ mRNA, whereas expression was almost undetectable in females (Fig 3C). Increased ex vivo expression of IFN-γ at the mRNA level was detected in both CD4+ T cells (110 fold) and NK cells (30 fold increase), suggesting a proportion of NK cells derived from male mice are activated in a classical IFN-γ+ manner.
Figure 3.
Natural Killer cells derived from male IL-4KO mice express higher levels of the CXCL10 receptor (CXCR3). A) Mesenteric lymph node cells (n=4) were extracted from the MLNs of male and female WT and IL-4KO BALB/c mice at day 18 p.i. and stained for the NK marker DX5 and the chemokine receptor CXCR3. DX5+ cells were gated and the percentage of DX5+ CXCR3+ cells assessed (top panel). Plots indicate isotype control (dotted line), female (blue line) and male (red fill) MFI and percentage of DX5+ CXCR3+ cells. Representative FACs plots from each group are shown (bottom panel) B) The relative expression of CXCL10 was assessed in pooled total MLNCs (n=4) from male and female IL-4KO mice at day 28 p.i. in comparison to naïve controls via real time PCR following normalisation to HPRT. C) Expression of IFN-γ mRNA in total MLNCs and sorted CD4+ T cell and DX5+ NK cell populations was assessed in male and female IL-4KO mice at day 28 p.i. * - p≤0.05
Expulsion of T. muris from IL-4KO BALB/c mice requires CD4+ T cells, but not NK cells
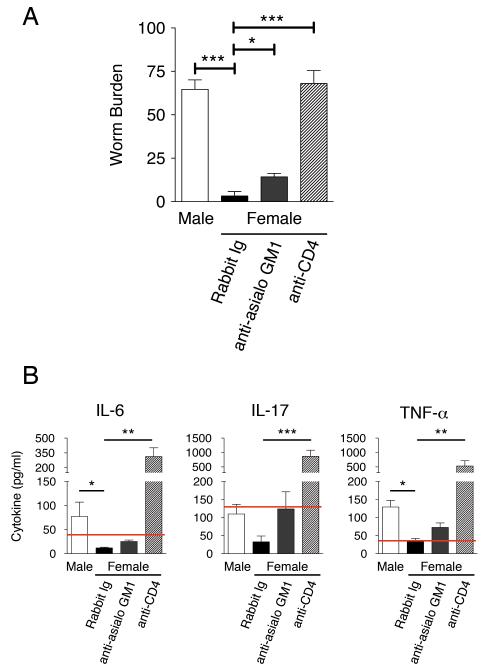
In order to address the relative importance of CD4+ T cell and NK cell derived IL-13 these cells were depleted via treatment with either anti-CD4 mAb or anti asialo-GM1. After 28 days female IL-4KO mice that received the control Rabbit Ig expelled their worm burdens as per usual, whereas control male IL-4KO mice retained high parasite numbers (Fig 4A). Depletion of CD4+ T cells resulted in a failure to expel worms from the lumen in females in a comparable manner to susceptible male KOs. Depletion of CD4+ T cells in female mice also led to an enhanced production of the pro-inflammatory cytokines TNF-α, IL-6 and IL-17A (Fig 4B). Conversely, treatment with anti-asialo GM1 antibody resulted in a mild, but significant, increase in the retained worm numbers in comparison to controls, suggesting NK cells contribute to, but are not essential for, expulsion in these mice (Fig 4A).
Figure 4.
CD4+ T cells, but not asialo-GM1+ cells, are essential for IL-13 driven immunity in female IL-4KO BALB/c mice. IL-4KO BALB/c mice females were injected with Control Rabbit Ig, anti-asialo GM1 or anti CD4 antibodies to neutralise NK and CD4+ T cells, respectively. A) Worm burdens of susceptible male IL-4KO control mice and female IL-4KO mice were assessed after treatment at day 28 p.i. (n=5) B) Levels of pro-inflammatory cytokines in E/S restimulated MLNC cultures were measured via CBA assay (IL-6 and TNF-α) and ELISA (IL-17) from culture restimulations of MLNCs, * - p≤0.05, ** - p ≤0.01, *** - p≤0.001.
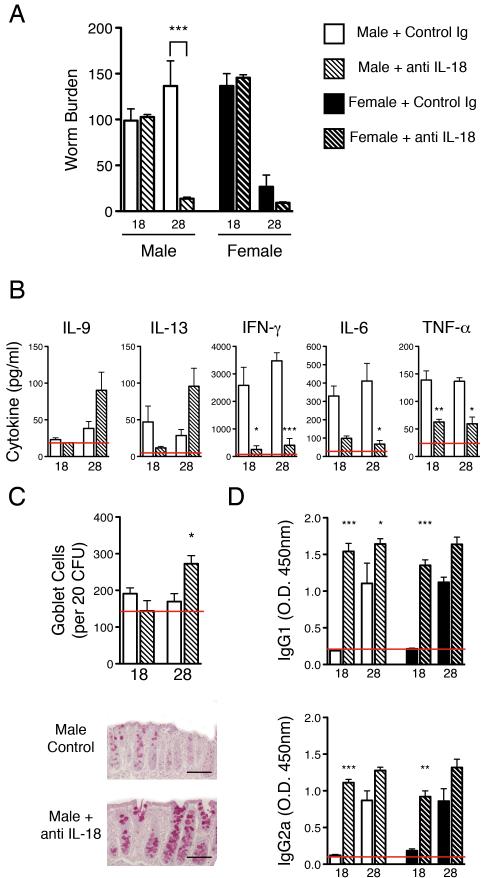
IL-18 is not essential for IL-13 mediated resistance in female IL-4KO mice but suppresses worm expulsion in male IL-4KOs
The cytokine IL-18 has been shown to enhance both Th1 and Th2 associated cytokines depending on the associated cytokine milieu. We have previously reported an increase in production of IL-18 and its receptor (IL-18R), as well as the pro-cytokine cleaving enzyme necessary for IL-18 maturation (Caspase 1), in mice susceptible to T. muris infection (13). Conversely, IL-18 has also been recently reported to enhance IL-13 production in an IL-4 independent manner (25). Furthermore, a key role for this cytokine has been suggested in enhancing IL-13 production and mediating expulsion of T. muris (6). It was thus investigated whether IL-18 is also necessary for the novel gender biased induction of CD4+ and accessory DX5+ cell derived IL-13. Figure 5A shows worm expulsion in female IL-4KO BALB/c mice is unaffected by neutralisation of IL-18, suggesting the mechanisms of IL-13 production in these mice is not dependent on this cytokine. Surprisingly however, neutralisation of this cytokine in male IL-4KO mice led to a restoration of worm expulsion, which occurred at day 28 p.i., comparable with that of females (Fig 5A). Worm expulsion in WT BALB/c mice was unaffected by neutralisation of IL-18, irrespective of host gender (data not shown). Restoration of worm expulsion in male IL-4KOs was associated with a trend toward increased production of IL-9 and IL-13 and a statistically significant reduction of TNF-α, IL-6 and IFN-γ (Fig 5B). The decrease in pro-inflammatory cytokines following neutralisation of IL-18 was also mirrored in female mice at day 18 p.i. suggesting IL-18 may also enhance these cytokines in resistant females (data not shown). Histological analysis confirmed an increase in IL-13 driven goblet cell hyperplasia at day 28 p.i. in male IL-4KOs following IL-18 neutralisation (Fig 5C). Finally, neutralisation of this cytokine in both sexes of IL-4KO significantly increased the early production of parasite specific IgG1 and IgG2a antibody, suggesting an effect of this cytokine on both Th1 and Th2 associated cytokine driven antibody class switching (Fig 5D).
Figure 5.
Neutralisation of IL-18 restores resistance in male IL-4KO comparable with female IL-4KO mice. A) Male and female IL-4KO BALB/c mice received anti-IL-18 or the control Ig and worm burdens were assessed in the caecum and proximal colon at day 18 and 28 p.i. B) The concentration of Th2 (IL-9 and IL-13), Th1 (IFN-γ) and pro-inflammatory cytokines (IL-6 and TNF-α) in E/S restimulated MLNC supernatants and C) goblet cell number were assessed following treatment with anti-IL-18 - scale bar = 50μm. D) Levels of parasite specific IgG1 (top panel) and IgG2a (bottom panel) in the sera were assessed via antigen specific ELISA, * - p≤0.05, ** - p ≤0.01, *** - p≤0.001.
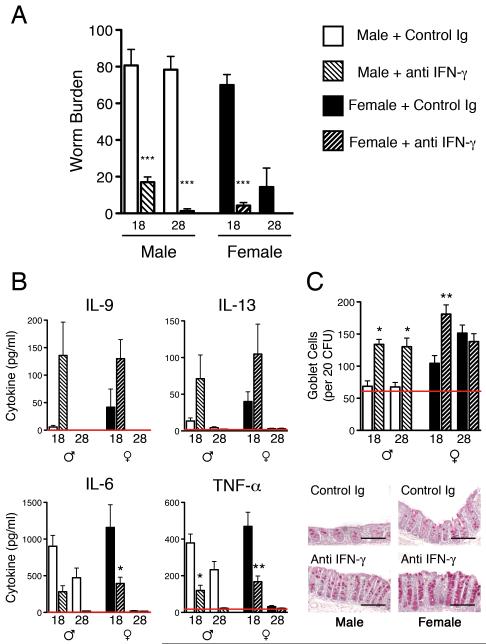
IFN-γ is responsible for delayed expulsion kinetics in IL-4KO mice
IL-4KO BALB/c mice produce large amounts of the Th1 associated cytokine IFN-γ in response to T. muris irrespective of the ultimate resolution of infection. As IFN-γ is a potent suppressor of Th2 responses, and as IL-18 neutralisation of susceptible male IL-4KO BALB/c restored T. muris expulsion only in a delayed manner, it was hypothesised that IFN-γ is the ‘master suppressor’ of normal expulsion kinetics in these mice. Figure 6A confirms that following neutralisation of IFN-γ both male and female IL-4KO BALB/c mice demonstrate normal expulsion kinetics comparable with other WT resistant strains of mice, with full expulsion occurring between day 18 and day 21 p.i. Treatment with anti-IFN-γ also restored normal Th2 responses at day 18 p.i., associated with increased IL-9 and IL-13, and reduced pro-inflammatory cytokines (Fig 6B). Enhancement of expulsion kinetics in both male and female mice correlated with an earlier increase in goblet cell hyperplasia, which was significantly increased over the corresponding control mice (Fig 6C).
Figure 6.
IFN-γ neutralisation restores normal expulsion kinetics in IL-4KO BALB/c mice. A) Male and female IL-4KO BALB/c mice received anti IFN-γ monoclonal antibody and worm burdens were assessed on day 18 and 28 p.i. B) Concentrations of Th2 and pro-inflammatory cytokines in E/S restimulated MLNC culture supernatants were assessed via CBA assay and C) the number of goblet cells per 20 crypt forming units were counted in the caecum of control and treated infected mice, scale bar = 50μm. * - p≤0.05, ** - p ≤0.01, *** - p≤0.001.
DISCUSSION
Resolution of gastrointestinal nematode infection has been relatively well characterised and it is clear that the development of a Th2 immune response, and particularly the production of IL-13, is essential for the expulsion of many helminths - including T. muris. One question still to be fully addressed is how the host immune system copes when normal Th2 immunity is lesioned or suboptimal. In line with previous data we were also able to demonstrate the onset of IL-13 driven Th2 immunity in response to parasite infection in the absence of the classical type 2 cytokine IL-4 (4, 26-29). There are many factors which can influence and mediate IL-13 production and here we shed further light on the generation and role of accessory IL-13+ NK cells, in circumstances of sub-optimal Th2 responses immunity, by using a novel gender biased, IL-4 independent model (summarised in Figure 7). This model is of particular interest in that the development and regulation of Th2 immunity to T. muris in these mice is subject to a striking gender bias. Host gender is an important factor in determining immunity to parasitic infections (reviewed in (5, 30)) and the majority of infections show a bias towards increased resistance in female mice (4, 5). Host gender has been shown to be an important determining factor in the development of an adaptive immune response and expulsion of T. muris, particularly in mice with disruptions in important Th2 associated cytokines. Thus, female mice lacking IL-4 or TNF receptor subunits have been shown to be resistant to T. muris whereas, their male counterparts are not (4, 31). As well as confirming the female bias of IL-4KO BALB/c mice we also noted a surprising influence of host sex on the kinetics of a Th2 response in WT mice - as male WT BALB/c mice demonstrated delayed IL-13 production and consequently significantly delayed worm expulsion in comparison to females (Fig 1A and 2B), a finding also previously observed in C57BL/6 WT mice (31). These differences are likely to be directly linked to the interaction of sex hormones and the immune system as initial data suggests androgens may influence the development of Th2 responses to T. muris in male IL-4KO mice (Hepworth and Grencis, Manuscript In Preparation).
Figure 7.
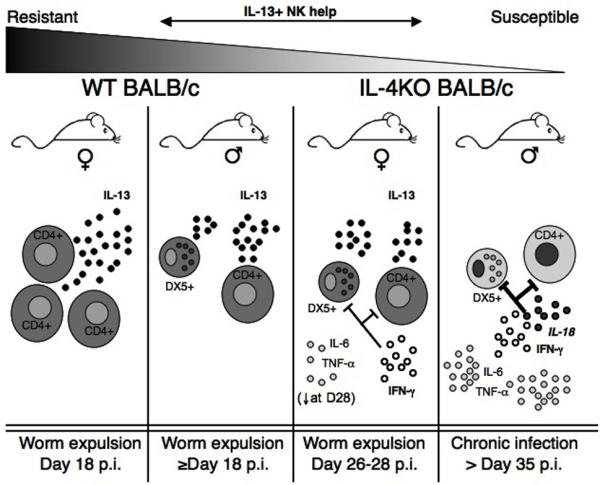
Host gender highlights a role for IL-13+ NK cells during the ‘gradient’ of resistance to T. muris in WT and IL-4KO BALB/c mice. Female WT mice are highly resistant to T. muris infection and expel the parasite through a fast and efficient CD4+ restricted Th2 response. Female IL-4KO mice are also resistant to T. muris despite high levels of IFN-γ, but expulsion of adult worms is delayed up to 10 days in comparison to WT females. This delayed worm expulsion is associated with both IL-13+ T cells and NK cells and a concurrent reduction of pro-inflammatory cytokine levels at day 28 p.i. Neutralisation of IFN-γ in female IL-4KO mice resulted in restored expulsion kinetics comparable with WT mice. Surprisingly, male WT mice also exhibited a delayed expulsion and also demonstrated accessory IL-13+ NK ‘help’. Conversely, Male IL-4KO BALB/c mice are completely susceptible to chronic helminth infection associated with high levels of IFN-γ, IL-6 and TNF-α. Resistance in these mice could be restored with delayed kinetics following depletion of IL-18 or fully via neutralisation of IFN-γ. This data suggests a complex interplay between host gender and the development of a successful immune responses in normal or disrupted environments.
We were able to clearly demonstrate an important contribution of DX5+ NK cells in providing an additional source of IL-13 during the IL-4 independent Th2 response and identified an important correlation with host sex. Specifically, female IL-4KO BALB/c mice, but not male equivalents expelled T. muris - associated with IL-13 production from both T cells and NK cells which was “delayed” until approximately day 26 p.i., therefore providing evidence for a role of a typically innate cell in aiding and supplementing the development and action of a delayed adaptive response. The data presented here suggests NK cells are stimulated to produce IL-13 alongside T cells in situations where the Th2 response is sub-optimal or delayed. Interestingly, male WT mice also suffered a delay in the onset of expulsion and also had detectable IL-13 mRNA in the DX5+ cell population whereas in ‘optimal’ expelling females IL-13 production was restricted to CD4+ T cells. Furthermore, delayed expulsion was mirrored in CD45+ cells at the site of infection. This data is supportive of previous work demonstrating the importance of NK cell derived IL-13 in the intraepithelial lymphocyte population during T. spiralis infection (9).
An increase in NK cell numbers was noted in both male and female IL-4KO BALB/c mice, as well as in male WT mice, 18 days p.i. in the MLN. However, as male IL-4KO BALB/c mice did not produce IL-13 during the infection we hypothesised NK cells from male IL-4KO mice may have received different “programming” to that of male WT and female IL-4KO mice. Over 80% of NK cells - irrespective of gender and host background - were found to express IL-2Rβ necessary for NK cell activation and proliferation (32) (data not shown), thus, differentiating them from reported type 2 cytokine producing basophils which also express DX5 but lack expression of this receptor (23). Interestingly significant differences were found in the expression of the chemokine receptor CXCR3 on NK cells which was expressed at higher levels on male IL-4KO NK cells in comparison to females. Expression of CXCR3 on NK cells has previously been demonstrated to occur as a result of enhanced IFN-γ and Th1 cell dependent interactions (33, 34). Furthermore, the ligand for this receptor, CXCL10, is important in the IFN-γ driven suppression of intestinal epithelial turnover which prevents expulsion of T. muris, hinting at an intriguing link between these two mechanisms (2). Levels of this chemokine were found to be high in both male and female IL-4KO mice, although males expressed markedly higher levels. Furthermore, despite seemingly comparable levels of IFN-γ protein following antigen restimulation at day 28p.i. male NK cells were observed to express increased levels of IFN-γ mRNA ex vivo, which was only mildly increased over naïve controls in females at this time. The increase in CXCR3 expression in males could be related to the continuous high levels of pro-inflammatory cytokines in these mice as TNF-α and/or IL-18 can synergise with IFN-γ to enhance expression of CXCR3 and its ligands (35-37). Further studies to address differences in other important markers of NK activation such as NKG2D and CD94 could also prove informative (38).
IL-18 is a pleiotropic cytokine that can enhance both type 1 and type 2 cytokine production depending on the cytokine milieu. IL-18 precursor is constitutively produced and secreted following enzymatic cleavage, and is mainly derived from monocytes, dendritic cells and intestinal mucosal cells (39-42). IL-18 exerts its effects predominantly on T cells and macrophages and is typically associated with the induction of IFN-γ production (43). We previously reported a repressive effect of IL-18 on IL-13 during T. muris infection and observed an increase in active IL-18 and receptor expression in susceptible mice disrupted in Th2 immunity (13). However, recent studies determined IL-18 can stimulate T cell derived IL-13 in the absence of IL-4 (25). Furthermore, it has previously been demonstrated that NK cells are also stimulated to produce IL-13 by IL-18 in combination with IL-2 (12, 15, 25, 44), a mechanism which is tightly regulated by IFN-γ (8). In line with these results two studies from Gause et al elegantly demonstrated enhancement of IL-13 in T. muris infected mice following ablation of B7 costimulation which required co-neutralisation of IFN-γ (7). IL-13 production in B7/IFN-γ neutralised mice was shown to be dependent upon IL-18, and found to be derived from both NK and T cell sources (6). In contrast to these recent studies we found neutralisation of IL-18 in female IL-4KO mice did not effect worm expulsion and IL-13 production, which occurred as usual approximately 26 days after infection and in the presence of high levels of IFN-γ. Surprisingly, however, neutralisation of IL-18 restored worm expulsion in previously susceptible male IL-4KO BALB/c mice, restoring delayed kinetics comparable to resistant females and reflected by an increase in IL-9 and IL-13 production and a decrease in pro-inflammatory cytokines. Thus, these findings demonstrate IL-18 can act to directly suppress worm expulsion in the absence of IL-4, and that this action is gender dependent.
Worm expulsion in IL-4KO mice consistently occurred in the presence of high levels of the Th1 cytokine IFN-γ. IFN-γ was found to be crucial for the suppression of optimal Th2 immunity in this model as neutralisation of this cytokine resulted in the complete restoration of WT BALB/c expulsion kinetics (with worm expulsion occurring at 18 days p.i.) in both male and female IL-4KO BALB/c mice. However, as we have previously demonstrated an IFN-γ independent role of IL-18 in suppressing Th2 immunity to T. muris and T. spiralis it is not yet clear whether these two cytokines suppress resistance via similar or disparate mechanisms in this system (13, 45).
Susceptibility to T. muris was also consistently correlated with the pro-inflammatory cytokines IL-6 and TNF-α in this gender-biased model of immunity. IL-4KO BALB/c mice, but not WT control littermates, of both sex produced high levels of IL-6 and TNF-α in response to T. muris and worm expulsion in female mice at day 26-28 p.i. was directly correlated not only with an increase in the resistance mediating cytokines IL-9 and IL-13 (2, 46), but also with a significant decrease in production of these pro-inflammatory cytokines from MLN cells (Fig 1B, Fig 5B and Fig 6B). This data suggests the decrease in pro-inflammatory cytokines is a consistent hallmark of worm expulsion in IL-4KO mice, with similar reductions seen upon restoration of worm expulsion following administration of anti-IFN-γ or anti-IL-18 mAbs (Fig 5B and Fig 6B). Indeed similar sex differences in TNF-α production have been noted in other parasitic infections including T. gondii (47), and of particular interest, it has also been shown that disruption of TNF receptor (p55 or p75) signalling results in a strong female sex bias towards resistance to T. muris (31). Furthermore, following CD4 T cell depletion we observed an increase in pro-inflammatory cytokines, including IL-17. IL-17 is critical for the development of intestinal inflammation in mouse models of colitis (48), and is dramatically increased in T. muris infected mice following disruption of epithelial cell signalling (49), resulting in a loss of Th2 immunity. The major source of IL-17A is Th17 cells, however, other cells such as CD4-γδ T cells can also produce IL-17 (50, 51). As these cells would not be depleted by anti-CD4 treatment one possibility is that following depletion IL-17 is produced by these or other cell types, contributing to the pro-inflammatory environment observed. Interestingly, recent reports suggest RELM-β - a goblet-cell-derived cysteine rich secreted protein which is highly induced during T. muris infection - may directly stimulate production of IL-6 and TNF-α, as well as directly enhance the IL-23-IL-17 axis, via interactions with lamina propria macrophages (52, 53).
In summary the findings presented here provide new and important insight in regards to the auxiliary mechanisms that compensate for sub-optimal Th2 responses as well as the complex interplay between host gender and the immune response against parasitic nematodes. These data have considerable implications for circumstances where the Th2 compartment is lesioned, and strongly implicate host sex as an important factor in the generation of IL-13 producing accessory cells. We have also identified IL-18 as a key factor in regulating this gender bias in Th2 immunity towards T. muris and thus, suggest that the interactions between IL-18 and the enhancement of type 2 cytokines may be regulated by other factors, such as host sex hormones. Further studies are needed in order to determine the exact mechanisms that form the basis of this gender difference and which control the recruitment and activation of accessory cells in the generation of IL-4 independent and sub optimal Th2 immunity.
ACKNOWLEDGEMENTS
The authors would like to thank Neil Humphreys and Michael Jackson (Life Sciences cell sorting facility, University of Manchester), Prof. Irmgard Förster for anti IL-18 hybridomas and those who contributed helpful feedback for the manuscript.
The work included in this manuscript was supported by funding from the Medical Research Council U.K. and the Wellcome Trust
REFERENCES
- 1.Else KJ, Wakelin D, Wassom DL, Hauda KM. The Influence of Genes Mapping within the Major Histocompatibility Complex on Resistance to Trichuris-Muris Infections in Mice. Parasitology. 1990;101:61–67. doi: 10.1017/s0031182000079762. [DOI] [PubMed] [Google Scholar]
- 2.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft AJ, McKenzie ANJ, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. Journal of Immunology. 1998;160:3453–3461. [PubMed] [Google Scholar]
- 4.Bancroft AJ, Artis D, Donaldson DD, Sypek JP, Grencis RK. Gastrointestinal nematode expulsion in IL-4 knockout mice is IL-13 dependent. European journal of immunology. 2000;30:2083–2091. doi: 10.1002/1521-4141(200007)30:7<2083::AID-IMMU2083>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite immunology. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Liu Z, Whitmire J, Alem F, Hamed H, Pesce J, Urban JF, Jr., Gause WC. IL-18 stimulates IL-13-mediated IFN-gamma-sensitive host resistance in vivo. European journal of immunology. 2006;36:1187–1198. doi: 10.1002/eji.200535668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban J, Fang H, Liu Q, Ekkens MJ, Chen SJ, Nguyen D, Mitro V, Donaldson DD, Byrd C, Peach R, Morris SC, Finkelman FD, Schopf L, Gause WC. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive. J Immunol. 2000;164:4250–4256. doi: 10.4049/jimmunol.164.8.4250. [DOI] [PubMed] [Google Scholar]
- 8.Hoshino T, Winkler-Pickett RT, Mason AT, Ortaldo JR, Young HA. IL-13 production by NK cells: IL-13-producing NK and T cells are present in vivo in the absence of IFN-gamma. J Immunol. 1999;162:51–59. [PubMed] [Google Scholar]
- 9.McDermott JR, Humphreys NE, Forman SP, Donaldson DD, Grencis RK. Intraepithelial NK cell-derived IL-13 induces intestinal pathology associated with nematode infection. J Immunol. 2005;175:3207–3213. doi: 10.4049/jimmunol.175.5.3207. [DOI] [PubMed] [Google Scholar]
- 10.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 11.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature medicine. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino T, Wiltrout RH, Young HA. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–5077. [PubMed] [Google Scholar]
- 13.Helmby H, Takeda K, Akira S, Grencis RK. Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. Journal of Experimental Medicine. 2001;194:355–364. doi: 10.1084/jem.194.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 15.Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, Bresolin N, Sironi M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med. 2009 doi: 10.1084/jem.20082779. jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noben-Trauth N, Kohler G, Burki K, Ledermann B. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 1996;5:487–491. doi: 10.1007/BF01980214. [DOI] [PubMed] [Google Scholar]
- 18.Wakelin D. The development of the early larval stages of Trichuris muris in the albino laboratory mouse. J Helminthol. 1969;43:427–436. doi: 10.1017/s0022149x00004995. [DOI] [PubMed] [Google Scholar]
- 19.Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- 20.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 21.Artis D, Potten CS, Else KJ, Finkelman FD, Grencis RK. Trichuris muris: Host intestinal epithelial cell hyperproliferation during chronic infection is regulated by interferon-gamma. Experimental Parasitology. 1999;92:144–153. doi: 10.1006/expr.1999.4407. [DOI] [PubMed] [Google Scholar]
- 22.Katsumoto T, Kimura M, Yamashita M, Hosokawa H, Hashimoto K, Hasegawa A, Omori M, Miyamoto T, Taniguchi M, Nakayama T. STAT6-dependent differentiation and production of IL-5 and IL-13 in murine NK2 cells. J Immunol. 2004;173:4967–4975. doi: 10.4049/jimmunol.173.8.4967. [DOI] [PubMed] [Google Scholar]
- 23.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr., Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. The Journal of experimental medicine. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luster AD, Ravetch JV. Genomic characterization of a gamma-interferon-inducible gene (IP-10) and identification of an interferon-inducible hypersensitive site. Mol Cell Biol. 1987;7:3723–3731. doi: 10.1128/mcb.7.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu D, Trajkovic V, Hunter D, Leung BP, Schulz K, Gracie JA, McInnes IB, Liew FY. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. European journal of immunology. 2000;30:3147–3156. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, McKenzie AN, Teixeira MM, Liew FY, Xu D. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 27.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nature immunology. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 28.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. The Journal of experimental medicine. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteyne P, Renauld JC, Van Broeck J, Dunne DW, Brombacher F, Coutelier JP. IL-4-independent regulation of in vivo IL-9 expression. J Immunol. 1997;159:2616–2623. [PubMed] [Google Scholar]
- 30.Alexander J, Stimson WH. Sex-Hormones and the Course of Parasitic Infection. Parasitology Today. 1988;4:189–193. [Google Scholar]
- 31.Hayes KS, Bancroft AJ, Grencis RK. The role of TNF-alpha in Trichuris muris infection I: influence of TNF-alpha receptor usage, gender and IL-13. Parasite immunology. 2007;29:575–582. doi: 10.1111/j.1365-3024.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 32.Umehara H, Bloom ET. The IL-2 receptor beta subunit is absolutely required for mediating the IL-2-induced activation of NK activity and proliferative activity of human large granular lymphocytes. Immunology. 1990;70:111–115. [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature immunology. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 34.Wald O, Weiss ID, Wald H, Shoham H, Bar-Shavit Y, Beider K, Galun E, Weiss L, Flaishon L, Shachar I, Nagler A, Lu B, Gerard C, Gao JL, Mishani E, Farber J, Peled A. IFN-gamma acts on T cells to induce NK cell mobilization and accumulation in target organs. J Immunol. 2006;176:4716–4729. doi: 10.4049/jimmunol.176.8.4716. [DOI] [PubMed] [Google Scholar]
- 35.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154:5235–5244. [PubMed] [Google Scholar]
- 36.Coma G, Pena R, Blanco J, Rosell A, Borras FE, Este JA, Clotet B, Ruiz L, Parkhouse RM, Bofill M. Treatment of monocytes with interleukin (IL)-12 plus IL-18 stimulates survival, differentiation and the production of CXC chemokine ligands (CXCL)8, CXCL9 and CXCL10. Clinical and experimental immunology. 2006;145:535–544. doi: 10.1111/j.1365-2249.2006.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanda N, Shimizu T, Tada Y, Watanabe S. IL-18 enhances IFN-gamma-induced production of CXCL9, CXCL10, and CXCL11 in human keratinocytes. European journal of immunology. 2007;37:338–350. doi: 10.1002/eji.200636420. [DOI] [PubMed] [Google Scholar]
- 38.Ho EL, Heusel JW, Brown MG, Matsumoto K, Scalzo AA, Yokoyama WM. Murine Nkg2d and Cd94 are clustered within the natural killer complex and are expressed independently in natural killer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6320–6325. doi: 10.1073/pnas.95.11.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97–106. [PubMed] [Google Scholar]
- 40.Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr., Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- 41.Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk AH. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. European journal of immunology. 1998;28:3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 43.Dinarello CA, Fantuzzi G. Interleukin-18 and host defense against infection. Journal of Infectious Diseases. 2003;187:S370–S384. doi: 10.1086/374751. [DOI] [PubMed] [Google Scholar]
- 44.Chakir H, Lemay AM, Webb JR. Cytokine expression by murine DX5+ cells in response to IL-12, IL-18, or the combination of IL-12 and IL-18. Cell Immunol. 2001;212:71–81. doi: 10.1006/cimm.2001.1844. [DOI] [PubMed] [Google Scholar]
- 45.Helmby H, Grencis RK. IL-18 regulates intestinal mastocytosis and Th2 cytokine production independently of IFN-gamma during Trichinella spiralis infection. Journal of Immunology. 2002;169:2553–2560. doi: 10.4049/jimmunol.169.5.2553. [DOI] [PubMed] [Google Scholar]
- 46.Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:767–772. doi: 10.1073/pnas.97.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts CW, Cruickshank SM, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory bowel diseases. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 49.Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR, Eckmann L, Karin M, Artis D. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 50.Ley K, Smith E, Stark MA. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunologic research. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 51.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nair MG, Guild KJ, Du Y, Zaph C, Yancopoulos GD, Valenzuela DM, Murphy A, Stevens S, Karow M, Artis D. Goblet cell-derived resistin-like molecule beta augments CD4+ T cell production of IFN-gamma and infection-induced intestinal inflammation. J Immunol. 2008;181:4709–4715. doi: 10.4049/jimmunol.181.7.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]