Abstract
Background
The most common cause of idiopathic nephrotic syndrome in children and younger adults is the minimal change nephrotic syndrome (MCNS). In the elderly MCNS is relatively uncommon. Over the last decade some reports suggest a rare but possible association with the administration of various vaccines.
Case presentation
A 82-year old Caucasian female presented with pronounced nephrotic syndrome (proteinuria of 7.1 g/d, hypoproteinemia of 47 g/l). About six weeks prior to admission, she had received a combination vaccination for tetanus, diphtheria and poliomyelitis as a booster-vaccination from her general practitioner. The renal biopsy revealed typical minimal change lesions. She responded well to the initiated steroid treatment. As through physical examination as well as extensive laboratory and imaging studies did neither find any evidence for malignancies nor infections we suggest that the minimal change nephrotic syndrome in this patient might be related to the activation of the immune system triggered by the vaccination.
Conclusion
Our case as well as previous anecdotal reports suggests that vaccination and the resulting stimulations of the immune system might cause MCNS and other severe immune-reactions. Increased awareness in that regard might help to expand the database of those cases.
Background
The most common cause of idiopathic nephrotic syndrome in children and younger adults is the minimal change nephrotic syndrome (MCNS). In the elderly MCNS is relatively uncommon. If present it can be associated with several conditions as malignancies [1], viral infections, allergies [2] and various drugs. Over the last ten years four anecdotal reports linking MCNS to vaccinations against hepatitis [3-5] pneumococcus [6] and influenca [7] have been published. Here we report the first case of MCNS following a combined tetanus-diphteria-poliomyelitis-vaccination and also summarize all previous published reports on MCNS after vaccination.
Case presentation
An 82 year old Caucasian female was admitted to our hospital in April 2008 with pitting edema of her legs arms and back. Her past medical history was significant for an appendectomy at the age of 29 years, a hyster- and ovariectomy at the age of 50 years, as well as status post varicose stripping, bilateral knee arthrosis, hypercholesterolemia and a reported diphtheria infection as a child. No history of allergic diseases or malignoma was present. Her regular medication consisted of low dose acetylsalicylic acid (100 mg/d), simvastatine (5 mg/d) and occasionally diclofenac (about 3 times a year, last time four months prior to admission). The outpatient nephrologist started medical therapy with spironolactone (25 mg) and furosemide (up to 400 mg/d) three days prior to admission. Laboratory data from her routine outpatient visits showed no proteinuria as well as normal renal function until January 2007.
On January 17th 2008, the patient received a combined intramuscular vaccination for tetanus, diphtheria and poliomyelitis (REVAXIS, Sanofi Pasteur MSD GmbH, Leimen, Germany), as her last vaccination for tetanus had been performed in April 1998. The vaccination was composed of cleaned tetanus toxoid, cleaned diphtheria toxoid, inactive poliomyelitis virus Typ 1–3, aluminiumhydroxide, 2-Phenoxyethanol, formaldehyde, medium 199 (mixture of aminoacids, minerals, vitamins, polysorbat 80) and trace amounts of neomycin, streptomycin and polymyxin B. No local vaccination reaction occurred after injection. At the end of February slight leg edema occurred. The general practitioner performed echocardiography for suspected heart failure. As the echocardiography revealed only mild aortic-valve-insufficiency and mild mitral valve-insufficiancy, with an intact overall contractility this diagnosis was dismissed. Meanwhile the edema worsened constantly, impressively indicated by an increase in bodyweight from 72 kg to 84 kg in one month. Moreover, the previous normotensive patient became hypertensive with blood pressure of 186/101 mmHg. Urinary dip-stick analysis now showed massive (+++) proteinuria on which the patient was transferred to a nephrologist. The 24 hours collected urine showed massive unselective proteinuria (12 g/day), therapy with spironolactone and furosemide failed to alleviate the edematous state. The urine sediment revealed multiple erythrocyte cylinders on which the patient was transferred to our tertiary care hospital for further nephrological evaluation and treatment. Upon admission physical examination was significant for general edema and multiple localised makulopapulous exanthema of the feet and legs. Vital parameters were unremarkable. The chest radiograph showed no signs of pleural effusions or malignancies. Laboratory tests revealed unselective nephrotic proteinuria (7.13 g/d), hypoproteinemia (47 g/l) and hypercholesterolemia (605 mg/dl). Serum creatinine was 74 μmol/l. The 24 hour collected urine revealed a reduced creatinine-clearance of 46 ml/min. Urinary sediment showed mild microhaematuria (5–10 erythrocytes/μl) and a few granulated and hyaline cylinders but no signs of an active sediment. Analysis for antinuclear antibodies, ANCA, antibodies against double-strain-DNS, anti-GBM-antibodies and Hepatitis B and C were negative or within normal limits. The kidneys were of normal size but showed bilateral mild enhancement in the abdominal ultrasound. Further diagnostic workup showed no evidence of malignancies.
Renal biopsy was performed and showed typical minimal change lesion without evidence of focal and segmental glomerulosclerosis (Figure 1). Three of the 22 glomerula showed globalsclerosis, minimal tubulo-atrophie and interstitial fibrosis (< 5% of the cortex). Further immunological staining excluded mesangial and glomerular IgM, IgA, IgG, C3 or fibrin/fibrinogen deposits.
Figure 1.
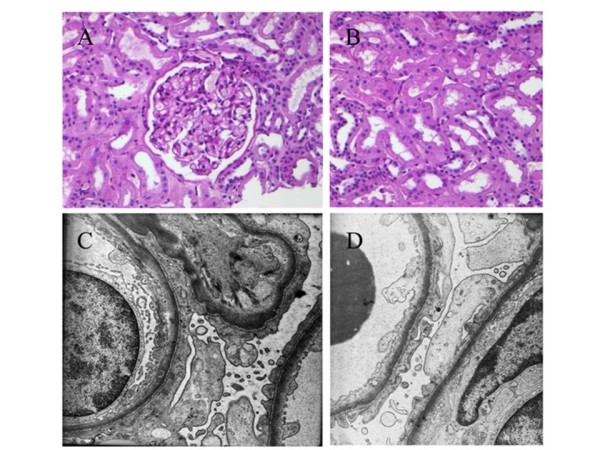
Light-microscopy of a representative Glomerulum and the tubulus-interstitium (A, B) and ultrastructure of a glomerulum with missing podocytes and thin basements without deposits (C, D).
After minimal change diagnosis was assured by biopsy, therapy with steroids (1 mg/kg body weight/day) and an ACE-inhibitor (ramipril 5 mg/d) was initiated. After ten days of therapy with steroids (75 mg/d) with concomitant oral phenprocourmon the nephrotic range proteinuria reduced to 1.17 g/24 h (Figure 2). On the last follow-up in the end of April 2009 serum-creatinine fell to 62 μmol/l, the blood pressure normalized and proteinuria was not detectable in a 24 h urine collection.
Figure 2.
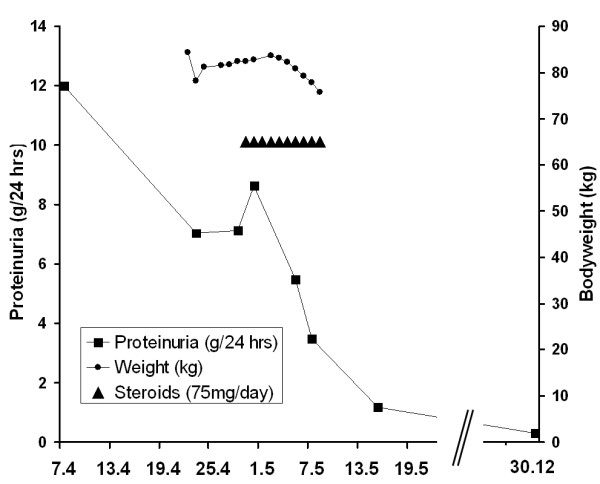
Clinical course of the patient.
Methods
For this paper we searched PubMed for relevant articles using the following medical subject headings: "minimal change nephropathy," "vaccination," through Feburary 1, 2009. We supplemented the search by scanning references of selected articles identified by this search.
Conclusion
Minimal change nephrotic syndrome (MCNS) is a major cause of nephrotic syndrome in both children and younger adults. Although the exact underlying cause of MCNS is not fully understood a burgeoning body of evidence suggests that systemic T cell dysfunction results in the production of a glomerular permeability factor. This circulating factor directly induces foot process fusion resulting in severe alteration of the glomerular filter system and resulting in marked proteinuria. The clinical and histopathological findings in our patient as well as the course of the disease were typical for MCNS. This syndrome is rarely seen in elderly patients (3/million/year) [8]. The fact that our patient did not exhibit any other condition known to be associated with MCNS at the time of presentation nor on follow up, suggests that the vaccination might have caused MCNS in our patient.
To date six reports of MCNS after vaccination had been published, including our report (Table 1). The first published evidence for a nephrotic syndrome following vaccination is given in an article from Chamberlain et. al in 1966 [9]. A woman suffered a nephrotic syndrome 10 days after smallpox vaccination. Biopsy and later necropsy was performed showing slight basement thickening making MCGN an unlikely diagnosis. In all presented cases the patients suffered a nephrotic syndrome shortly after vaccination of different sera (Tetanus-diphteria-poliomyelitis; Pneumococcus; Influenza; Hepatitis B). In 4 cases diagnosis was assured by biopsy, except one patient all others were treated with steroids. In three of the published cases follow-up phase was negative concerning proteinuria. Our patient reported the occasional use of diclofenac. Non-steroidal-anti-rheumatic-drugs (NSAID's) are well known to cause MCGN as Galesic et al have recently published [10]. In our case this cause is unlikely because the patient denied the use of any NSAID four month prior to the admission.
Table 1.
Summary of MCNS following vaccination in literature
| Vaccination against | Tetanus-diphteria-poliomyelitis-vaccination | Pneumococcus [6] | Influenza [7] | Hepatitis B [3] |
Hepatitis B [4] |
Hepatitis B [5] |
|---|---|---|---|---|---|---|
| Age [years] | 82 | 67 | 65 | 3 | 40 | 4 |
| Gender | female | female | female | male | female | male |
| Baseline creatinine | 76 μmol/l | no data | normal | 44 μmol/l | normal | no data |
| Peak creatinine | 138 μmol/l | 274 μmol/l | 158 μmol/l | no data | no data | no data |
| Baseline proteinuria | negative in dip-stick | past history unremarkable | no data | past history unremarkable | past history unremarkable | past history unremarkable |
| Peak proteinuria | 12 g/day | 10.4 g/day | 13.2 g/day | 24.8 g/day | 8 g/day | 1.25 g/day |
| Vaccination to onset of symptoms | 4 weeks | 4 months | 4 days | 17 days | after 2nd inoculation | 8 days |
| Biopsy | typical minimal change lesion (MCL) | MCL and mild interstitial nephritis | typical minimal change lesion (MCL) | not indicated | minimal change nephropathy | not indicated |
| Treatment | Steroids 1 mg/kg bw ACE-inhibitor | 750 mg Steroids for 3 days; followed by 40 mg/day | None specific | Steroids 2 mg/kg bw | Steroids (12 mg every other day) | Steroids 2 mg/kg bw |
| Renal function/follow up | 80 μmol/l 6 months after diagnosis |
Urinary protein neg. after one year; 15 mg Steroids/day | Clearacnce 95 ml/min after one year | no data | no data | Complete remission |
On admission in our hospital the patient complained of multiples makulopapulous exanthema on her legs which shortly occurred after the ambulatory start of the diuretic therapy with furosemide. As furosemide is known to cause skin exanthema [11] and the lesions disappeared after discontinuation of the loop-diuretic, we attribute the skin lesions to the furosemide administration as an allergic drug exanthema and not as an erythema exsudativum multiforme as a sign for an infection. The allergic origin is supported by the fact that was a lag period between the clinical signs of the nephritic syndrome and a temporal association between the begin of the furosemid therapy and the begin of the exanthema.
In our patient the time of onset of the nephrotic syndrome after the vaccination correlates with the reported time at which seroprotective antibody-levels are confirmed after vaccination with REVAXIS (28 days after injection) [12,13]. The time until the onset of symptoms varies in all described cases cases from days to months, which could be explained by the different time it took for the respective vaccine to trigger an immune response. Nephrotic syndrome occurred after the second administration of the vaccine which suggests that the last administration has boosted a pre-existing immune response from the first vaccination. Similar findings are described in a case from Floege et al. [19].
Another serious reaction after vaccination on renal function is described in several cases. Santoro reported a Lupus nephritis as an uncommon complication following a hepatitis B vaccination [14]. In another case from Poland, a 17-year-old girl suffered a necrotizing glomerulonephritis after a vaccination against influenza, which underlines the hypothesis of the activation of the immune-system after vaccination [15].
Should we than stop vaccinations, at least for children with nephrotic syndrome? In a study assessing the relapse rate of 54 children with known steroidsensitive nephrotic syndrome showed no evidence that meningococcal C conjugate vaccine (MCCV) triggered relapses of nephrotic syndrome [16]. This is in contrast to findings by Abeyagunawardena and colleagues who advised to carefully consider the vaccination of children with steroid-sensitive nephrotic syndrome. In their cohort of 106 children with nephrotic syndrome showed a significantly higher relapse rate after MCCV-vaccination with a relative incidence of 1.52 (95% CI 1·10–2·11) comparing 12 months pre- and post-vaccination [17]. This is in line with a another study showing a nephrotic syndrome relapse rate of 9% after vaccination against viral hepatitis B in children [18]. In summary, various vaccinations are associated with a higher relapse of steroid-sensitive nephrotic syndrome, yet the benefit for the preventive medicine prevails the risks of triggering a relapse these patients. The currently available data do in our view not allow giving any firm recommendation concerning the use vaccines patients with nephrotic syndrome. In the general population the benefit of various vaccines has been clearly documented as an effective way of primary prevention of diseases. Extremely rare side effects of vaccinations, like MCNS, can by no means outweigh the benefits of vaccinations.
It is beyond the scope of a case report to elucidate the possible pathophysiology between the immune response after vaccination in MCNS. Still one could hypothesize that under certain preconditions, the immune response after vaccination influences the cytosceleton of podocytes, leading to proteinuria. Indeed, recent publications showed that the podocyte is not only heavily equipped with cytokine receptors but is also a target of immunosuppressive drugs like cyclosporin. Hence, podocyte integrity could, under certain preconditions, be altered in the context of a stimulated immune system after vaccination.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CC, JS, CC and JTK were the treating physicians of the patient reported. VB performed the evaluation of the renal biopsy. All of the authors have participated in the discussion and in writing of the submitted manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Christian Clajus, Email: clajus.christian@mh-hannover.de.
Janine Spiegel, Email: Spiegel.Janine@mh-hannover.de.
Verena Bröcker, Email: Broecker.Verena@mh-hannover.de.
Christos Chatzikyrkou, Email: Chatzikyrkou.Christos@mh-hannover.de.
Jan T Kielstein, Email: kielstein@yahoo.com.
Acknowledgements
Written consent was obtained from the patient for publication of this study.
References
- Eagen JW. Glomerulopathies of neoplasia. Kidney Int. 1977;11:297–303. doi: 10.1038/ki.1977.47. [DOI] [PubMed] [Google Scholar]
- Sandberg DH, Bernstein CW, McIntosh RM, Carr R, Strauss J. Severe steroid-responsive nephrosis associated with hypersensitivity. Lancet. 1977;1:388–391. doi: 10.1016/S0140-6736(77)92603-4. [DOI] [PubMed] [Google Scholar]
- Ozdemir S, Bakkaloglu A, Oran O. Nephrotic syndrome associated with recombinant hepatitis B vaccination: a causal relationship or just a mere association? Nephrol Dial Transplant. 1998;13:1888–1889. doi: 10.1093/ndt/13.7.1889b. [DOI] [PubMed] [Google Scholar]
- Macario F, Freitas L, Correia J, Campos M, Marques A. Nephrotic syndrome after recombinant hepatitis B vaccine. Clin Nephrol. 1995;43:349. [PubMed] [Google Scholar]
- Islek I, Cengiz K, Cakir M, Kucukoduk S. Nephrotic syndrome following hepatitis B vaccination. Pediatr Nephrol. 2000;14:89–90. [PubMed] [Google Scholar]
- Kikuchi Y, Imakiire T, Hyodo T, Higashi K, Henmi N, Suzuki S, Miura S. Minimal change nephrotic syndrome, lymphadenopathy and hyperimmunoglobulinemia after immunization with a pneumococcal vaccine. Clin Nephrol. 2002;58:68–72. doi: 10.5414/cnp58068. [DOI] [PubMed] [Google Scholar]
- Kielstein JT, Termuhlen L, Sohn J, Kliem V. Minimal change nephrotic syndrome in a 65-year-old patient following influenza vaccination. Clin Nephrol. 2000;54:246–248. [PubMed] [Google Scholar]
- Sharpstone P, Ogg CS, Cameron. Nephrotic syndrome due to primary renal disease in adults: I. Survey of incidence in South-east England. Br Med J. 1969;2:533–535. doi: 10.1136/bmj.2.5656.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain MJ, Pringle A, Wrong OM. Oliguric renal failure in the nephrotic syndrome. Q J Med. 1966;35:215–235. [PubMed] [Google Scholar]
- Galesic K, Ljubanovic D, Bulimbasic S, Racic I. Minimal change disease and acute tubular necrosis caused by diclofenac. Nephrology (Carlton) 2008;13:87–88. doi: 10.1111/j.1440-1797.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- Thestrup-Pedersen K. Adverse reactions in the skin from anti-hypertensive drugs. Dan Med Bull. 1987;34(Suppl 1):3–5. [PubMed] [Google Scholar]
- Laroche P, Barrand M, Wood SC, Van HK, Lang J, Harzer E, Hessel L. The immunogenicity and safety of a new combined diphtheria, tetanus and poliomyelitis booster vaccine (Td-eIPV) Infection. 1999;27:49–56. doi: 10.1007/BF02565175. [DOI] [PubMed] [Google Scholar]
- Stojanov S, Liese JG, Bendjenana H, Harzer E, Barrand M, Jow S, Dupuy M, Belohradsky BH. Immunogenicity and safety of a trivalent tetanus, low dose diphtheria, inactivated poliomyelitis booster compared with a standard tetanus, low dose diphtheria booster at six to nine years of age. Munich Vaccine Study Group. Pediatr Infect Dis J. 2000;19:516–521. doi: 10.1097/00006454-200006000-00005. [DOI] [PubMed] [Google Scholar]
- Santoro D, Stella M, Montalto G, Castellino S. Lupus nephritis after hepatitis B vaccination: an uncommon complication. Clin Nephrol. 2007;67:61–63. doi: 10.5414/cnp67061. [DOI] [PubMed] [Google Scholar]
- Hyla-Klekot L, Kucharska G, Cieslak W. [Necrotizing glomerulonephritis in decursu vasculitis after vaccination against influenza] Pol Merkur Lekarski. 2005;19:75–77. [PubMed] [Google Scholar]
- Taylor B, Andrews N, Stowe J, Hamidi-Manesh L, Miller E. No increased risk of relapse after meningococcal C conjugate vaccine in nephrotic syndrome. Arch Dis Child. 2007;92:887–889. doi: 10.1136/adc.2006.105247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeyagunawardena AS, Goldblatt D, Andrews N, Trompeter RS. Risk of relapse after meningococcal C conjugate vaccine in nephrotic syndrome. Lancet. 2003;362:449–450. doi: 10.1016/S0140-6736(03)14072-X. [DOI] [PubMed] [Google Scholar]
- Szajner-Milart I, Zajaczkowska M, Zinkiewicz Z, Borzecka H, Majewski M. Efficacy of vaccination against viral hepatitis type B in children with the nephrotic syndrome. Lancet. 2003;58:402–408. [PubMed] [Google Scholar]
- Floege J, Lonnemann G, Stichtenoth DO, Koch KM, Brunkhorst R. Minimal change nephrotic syndrome in a 74-year-old patient following parenteral administration of sheep cells. Nephrol Dial Transplant. 1998;13:1484–1488. doi: 10.1093/ndt/13.6.1484. [DOI] [PubMed] [Google Scholar]


