Abstract
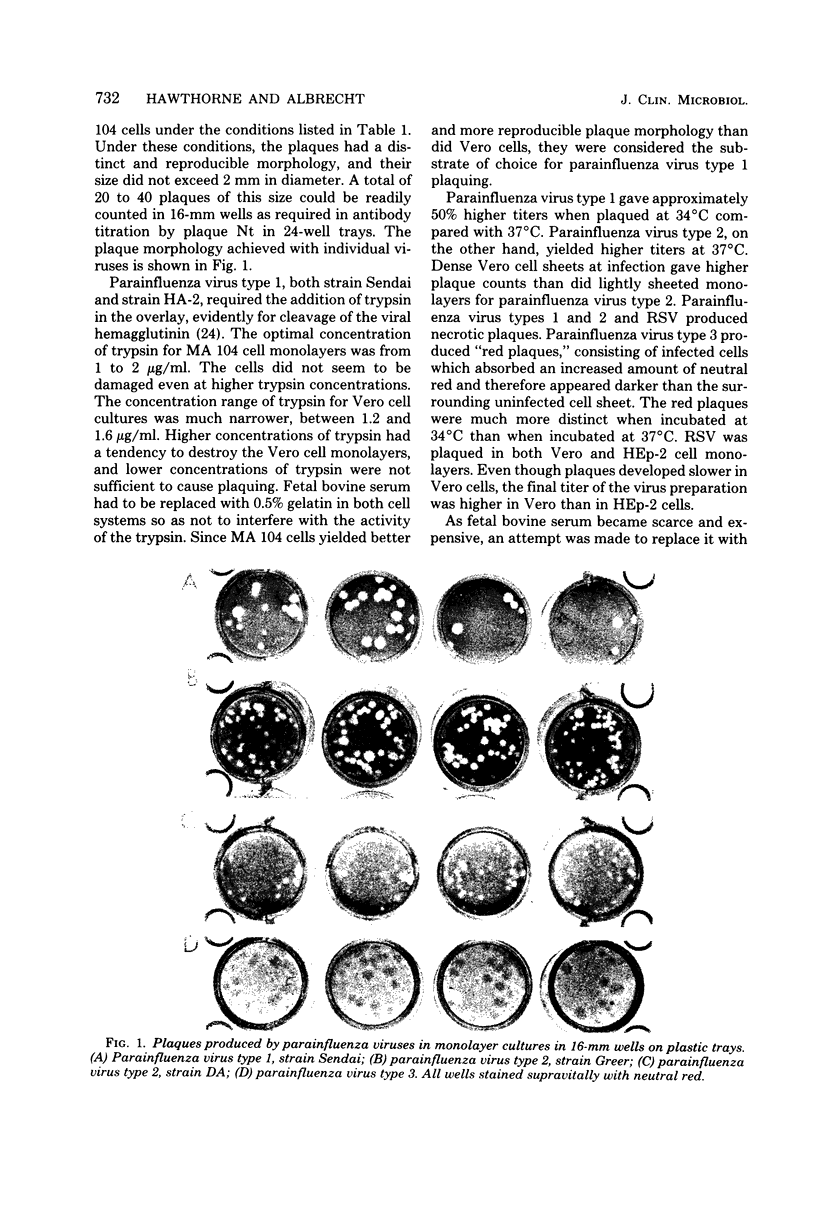
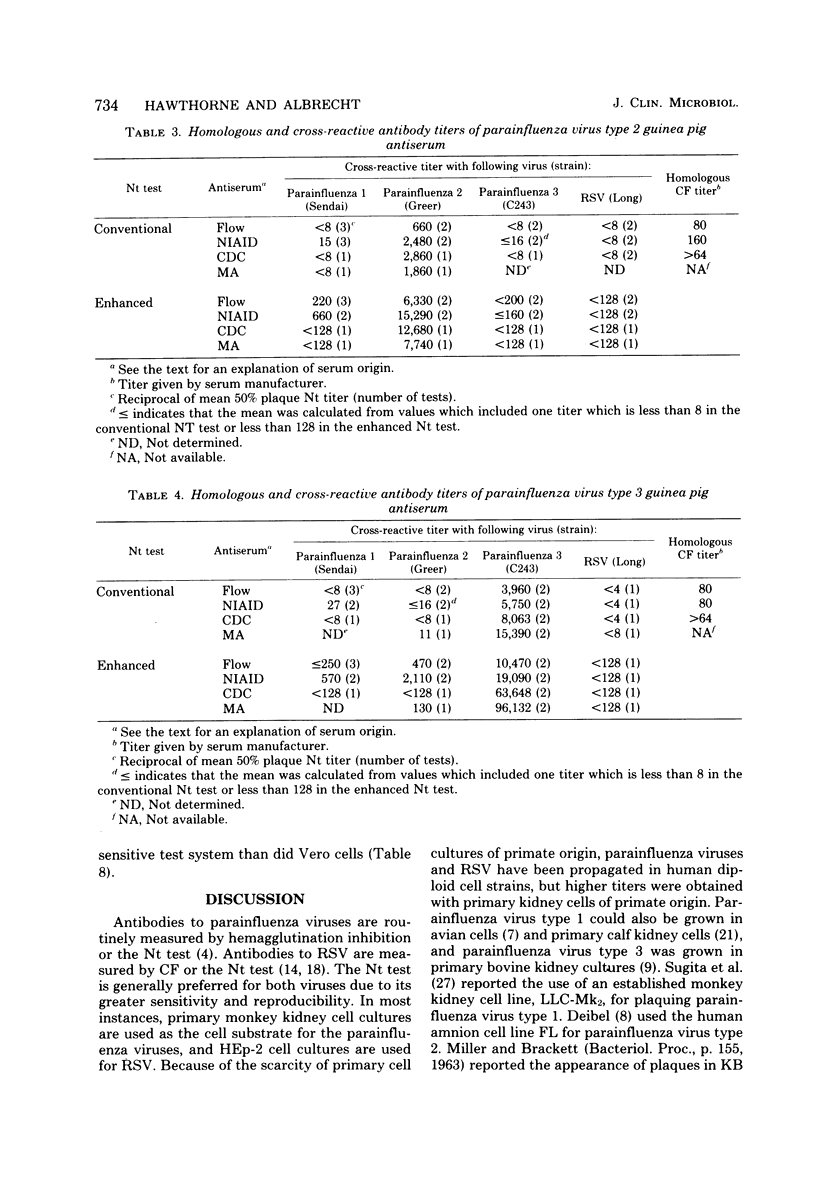
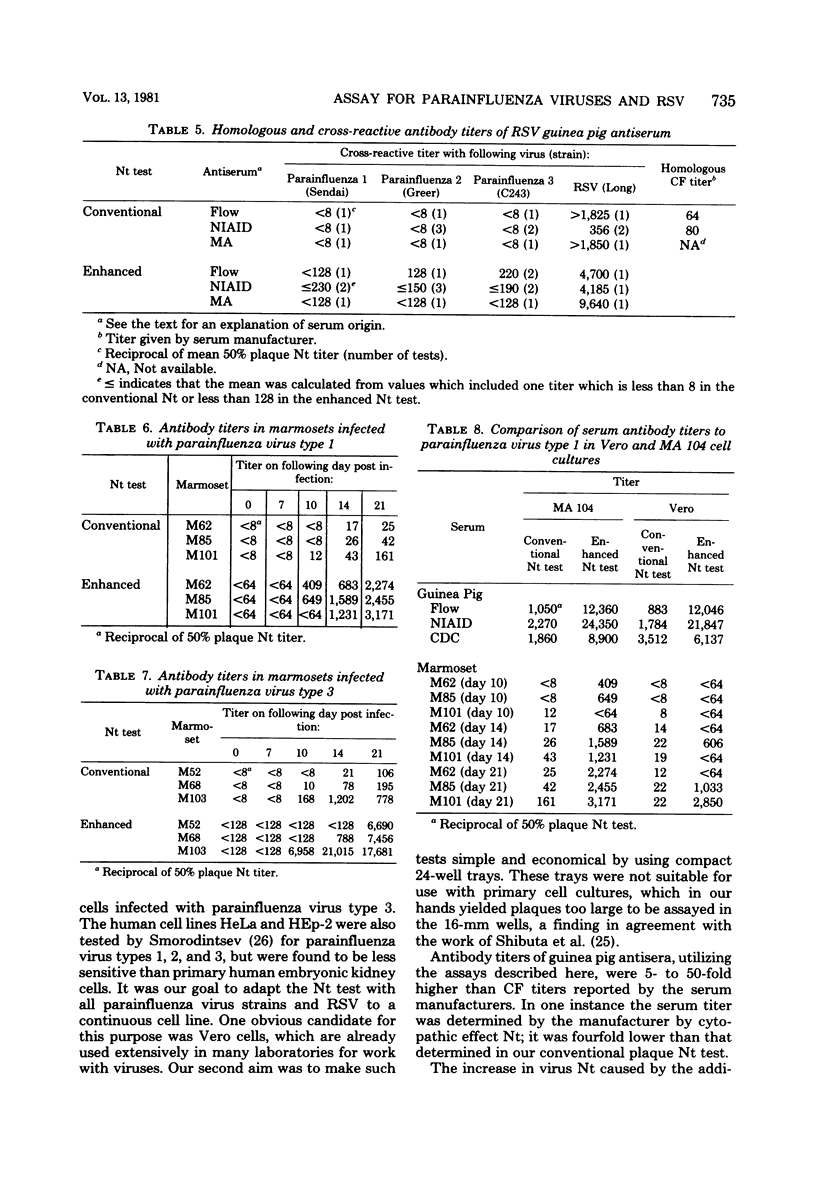
A sensitive plaque neutralization assay for parainfluenza virus types 1, 2, and 3 and respiratory syncytial virus was developed in Vero and MA 104 cell cultures. The tests were performed in semimicrotoiter trays containing 24 wells, 16 mm in diameter. Parainfluenza virus type 1 formed plaques in Vero and MA 104 cells only when trypsin was added to the overlay medium. Plaquing of parainfluenza virus type 1 was more sensitive and technically reproducible in MA 104 cells than in Vero cells. Parainfluenza virus types 2 and 3 and respiratory syncytial virus readily formed plaques in Vero cells. Plaques with all viruses were necrotic in character, except for plaques produced by parainfluenza virus type 3, which appeared red due to an increased uptake of neutral red by infected cells. Different conditions for plaquing of the four viruses had to be used to obtain plaques of suitable size. Antibody titers of commercially prepared guinea pig typing sera were 5- to 50-fold higher by the plaque neutralization test than by complement fixation. The addition of guinea pig immunoglobulin G antiglobulin to the serum-virus mixtures enhanced the conventional neutralization test 5- to 10-fold. The sensitivity and specificity of the plaque neutralization test was also determined with sera of marmosets experimentally infected with parainfluenza virus types 1 and 3. The generally low postinfection titers could be enhanced, on the average, 40-fold by using human immunoglobulin G antiglobulin in the neutralization test. A low degree of cross-reactivity was shown between parainfluenza virus types 1 and 3 both in the conventional neutralization test and in the anti-immunoglobulin enhanced neutralization test.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMEIDA J., CINADER B., HOWATSON A. THE STRUCTURE OF ANTIGEN-ANTIBODY COMPLEXES. A STUDY BY ELECTRON MICROSCOPY. J Exp Med. 1963 Sep 1;118:327–340. doi: 10.1084/jem.118.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht P., Lorenz D., Klutch M. J., Vickers J. H., Ennis F. A. Fatal measles infection in marmosets pathogenesis and prophylaxis. Infect Immun. 1980 Mar;27(3):969–978. doi: 10.1128/iai.27.3.969-978.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H., COOK K., ANDREWS B. E., BELL J. A., REICHELDERFER T., KAPIKIAN A. Z., MASTROTA F. M., HUEBNER R. J. Newly recognized myxoviruses from children with respiratory disease. N Engl J Med. 1958 Jan 30;258(5):207–213. doi: 10.1056/NEJM195801302580502. [DOI] [PubMed] [Google Scholar]
- CHANOCK R., ROIZMAN B., MYERS R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg. 1957 Nov;66(3):281–290. doi: 10.1093/oxfordjournals.aje.a119901. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. Plaque formation in monolayers of FL cells infected with the hemadsorption type 1 virus. Virology. 1959 Jun;8(2):262–263. doi: 10.1016/0042-6822(59)90010-8. [DOI] [PubMed] [Google Scholar]
- DINTER Z., HERMODSSON S., BAKOS K. Studies on variants of a bovine strain of parainfluenza 3 virus. 1. Isolation and growth characteristics. Acta Pathol Microbiol Scand. 1960;49:485–492. doi: 10.1111/j.1699-0463.1960.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- Dreizin R. S., Ponomareva T. I., Rapoport R. I., Petrova E. I. Respiratory syncytial virus infection of diploid cell strains. Acta Virol. 1967 Nov;11(6):533–537. [PubMed] [Google Scholar]
- Gengozian N., Salter B. L., Basford N. L., Kateley J. R. Characterization of the antibody response of the marmoset to sheep red blood cells. Clin Exp Immunol. 1976 Mar;23(3):525–535. [PMC free article] [PubMed] [Google Scholar]
- HULL R. N., MINNER J. R., SMITH J. W. New viral agents recovered from tissue cultures of monkey kidney cells. I. Origin and properties of cytopathogenic agents S.V.1, S.V.2, S.V.4, S.V.5, S.V.6, S.V.11, S.V.12 and S.V.15. Am J Hyg. 1956 Mar;63(2):204–215. doi: 10.1093/oxfordjournals.aje.a119804. [DOI] [PubMed] [Google Scholar]
- JOHNSON K. M., CHANOCK R. M., COOK M. K., HUEBNER R. J. Studies of a new human hemadsorption virus. I. Isolation, properties and characterization. Am J Hyg. 1960 Jan;71:81–92. doi: 10.1093/oxfordjournals.aje.a120092. [DOI] [PubMed] [Google Scholar]
- JOHNSON K. M., CHANOCK R. M., RIFKIND D., KRAVETZ H. M., KNIGHT V. Respiratory syncytial virus. IV. Correlation of virus shedding, serologic response, and illness in adult volunteers. JAMA. 1961 May 27;176:663–667. [PubMed] [Google Scholar]
- KAPIKIAN A. Z., BELL J. A., MASTROTA F. M., JOHNSON K. M., HUEBNER R. J., CHANOCK R. M. An outbreak of febrile illness and pneumonia associated with respiratory syncytial virus infection. Am J Hyg. 1961 Nov;74:234–248. doi: 10.1093/oxfordjournals.aje.a120216. [DOI] [PubMed] [Google Scholar]
- KISCH A. L., JOHNSON K. M. A plaque assay for respiratory syncytial virus. Proc Soc Exp Biol Med. 1963 Mar;112:583–589. doi: 10.3181/00379727-112-28111. [DOI] [PubMed] [Google Scholar]
- KRAVETZ H. M., KNIGHT V., CHANOCK R. M., MORRIS J. A., JOHNSON K. M., RIFKIND D., UTZ J. P. Respiratory syncytial virus. III. Production of illness and clinical observations in adult volunteers. JAMA. 1961 May 27;176:657–663. [PubMed] [Google Scholar]
- KUROYA M., ISHIDA N. Newborn virus pneumonitis (type Sendai). II. The isolation of a new virus possessing hemagglutinin activity. Yokohama Med Bull. 1953 Aug;4(4):217–233. [PubMed] [Google Scholar]
- Luczak M., Korbecki M. Comparative studies on susceptibility of established cell lines to parainfluenza 3 virus. Acta Virol. 1970 Jul;14(4):279–284. [PubMed] [Google Scholar]
- NAGATA I., MAENO K., YOSHII S., MATSUMOTO T. PLAQUE FORMATION BY HVJ IN CALF KIDNEY CELLS. (BRIEF REPORT). Arch Gesamte Virusforsch. 1965;15:257–259. doi: 10.1007/BF01257738. [DOI] [PubMed] [Google Scholar]
- SMORODINTSEV A. A., Jr Experiences with the isolation and propagation of parainfluenza viruses. Acta Virol. 1962 Jul;6:338–346. [PubMed] [Google Scholar]
- Sato H., Albrecht P., Hicks J. T., Meyer B. C., Ennis F. A. Sensitive neutralization test for virus antibody. 1. Mumps antibody. Arch Virol. 1978;58(4):301–311. doi: 10.1007/BF01317822. [DOI] [PubMed] [Google Scholar]
- Sato H., Albrecht P., Krugman S., Ennis F. A. Sensitive neutralization test for rubella antibody. J Clin Microbiol. 1979 Feb;9(2):259–265. doi: 10.1128/jcm.9.2.259-265.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Akami M., Matumoto M. Plaque formation by sendai virus of parainfluenza virus group, type 1 on monkey, calf kidney and chick embryo cell monolayers. Jpn J Microbiol. 1971 Mar;15(2):175–183. doi: 10.1111/j.1348-0421.1971.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Sugita K., Maru M., Sato K. A sensitive plaque assay for Sendai virus in an established line of monkey kidney cells. Jpn J Microbiol. 1974 May;18(3):262–264. doi: 10.1111/j.1348-0421.1974.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Svehag S. E. Formation and dissociation of virus-antibody complexes with special reference to the neutralization process. Prog Med Virol. 1968;10:1–63. [PubMed] [Google Scholar]



