Abstract
Focal adhesion kinase (FAK), a major cell adhesion-activated tyrosine kinase, has an important function in cell adhesion and migration. Here, we report a new signalling of FAK in regulating chromatin remodelling by its interaction with MBD2 (methyl CpG-binding protein 2), underlying FAK regulation of myogenin expression and muscle differentiation. FAK interacts with MBD2 in vitro, in myotubes, and in isolated muscle fibres. Such an interaction, increased in myotubes exposed to oxidative stress, enhances FAK nuclear localization. The nuclear FAK–MBD2 complexes alter heterochromatin reorganization and decrease MBD2 association with HDAC1 (histone deacetylase complex 1) and methyl CpG site in the myogenin promoter, thus, inducing myogenin expression. In line with this view are observations that blocking FAK nuclear localization by expressing dominant negative MBD2 or suppression of FAK expression by its miRNA in C2C12 cells attenuates myogenin induction and/or impairs muscle-terminal differentiation. Together, these results suggest an earlier unrecognized role of FAK in regulating chromatin remodelling that is important for myogenin expression and muscle-terminal differentiation, reveal a new mechanism of MBD2 regulation by FAK family tyrosine kinases, and provide a link between cell adhesion and chromatin remodelling.
Keywords: FAK, MBD2, muscle differentiation
Introduction
Cell adhesion to the extracellular matrix (ECM) factors/cues is an important cellular event in cell proliferation, growth, and differentiation (Dugina et al, 2001; Kang et al, 2004). Focal adhesion kinase (FAK), a major cytoplasmic tyrosine kinase activated on cell-ECM adhesion, has important functions in mediating cell adhesion signalling (Guan and Shalloway, 1992; Hanks et al, 2003; Parsons, 2003; Zhao et al, 2003; Mitra et al, 2005). Knocking-out FAK in mice leads to early embryonic lethal (Ilic et al, 1995). Fibroblasts derived from FAK knock-out mice exhibit reduced cell motility and increased focal adhesions, showing a role of FAK in regulating focal adhesion turnover and cell migration (Ilic et al, 1995). FAK has a crucial function not only in fibroblastic cell migration, but also in neuronal axon path finding (Li et al, 2004; Liu et al, 2004; Ren et al, 2004) and cardiac vascular function (Eble et al, 2000; Taylor et al, 2000; Peng et al, 2006). However, exactly how FAK regulates/coordinates multiple cellular processes (e.g. cell migration, survival, and differentiation) remains largely unknown.
Our studies presented in this paper have pointed to a role of MBD2 (methyl CpG-binding protein 2) in FAK signalling. MBD2 belongs to a family of MBD domain containing proteins, including MBD1, MBD2, MBD3, MBD4, and MeCP2. They associate with heterochromatin in the nuclei by their interaction with methylated DNA at CpG islands, and appear to ‘read' and ‘translate' the DNA methylation signal into transcriptional repression at least partially by recruiting silencing complexes and histone deacetylases, thereby stabilizing and consolidating the heterochromatic state (Bird and Wolffe, 1999; Leonhardt and Cardoso, 2000). MBD2 is the methyl-binding component of the MeCP1 complex, which contains the histone deacetylase (HDAC) proteins HDAC1 (histone deacetylase complex 1), HDAC2, and the RbAp46 and RbAp48 proteins (also known as RBBP7 and RBBP4), allowing MBD2 to target HDAC/chromatin remodelling activity to methylated templates (Bird and Wolffe, 1999; Leonhardt and Cardoso, 2000). MBD2 can also act synergistically with other proteins; for example, the zinc-finger protein MIZF interacts with MBD2 and acts as an HDAC-dependent transcriptional repressor (Sekimata et al, 2001).
In this paper, we provide evidence that FAK interacts with MBD2. This interaction is mediated through the C-terminal region (FAT, focal adhesion-targeting domain) of FAK and the N-terminal region (GC-rich region and MBD domain) of MBD2. The interaction leads to FAK translocation into the nucleus. The nuclear FAK–MBD2 complexes, increased in myoblasts/myotubes exposure to oxidative stress (H2O2), cause altered heterochromatin organization and decreased MBD2 association with HDAC1 and methyl CpG site in the myogenin promoter, thus, inducing myogenin expression. Inhibition of FAK nuclear translocation by expressing dominant negative MBD2 or suppression of FAK expression by its miRNA in C2C12 cells reduces myogenin induction and impairs muscle-terminal differentiation. Knock-down FAK expression in mouse skeletal muscle by in vivo eletroporation also decreases myogenin expression and muscle fibre thickness. Taken together, these results suggest an earlier unrecognized role of FAK in regulating heterochromatin remodelling and muscle-terminal differentiation, and reveal a new mechanism of MBD2 regulation by FAK family tyrosine kinases.
Results
FAK and PYK2 interaction with MBD2
We searched for binding proteins for FAK family kinases with the hypothesis that these proteins may mediate or regulate FAK/PYK2 signalling/functions. MBD2 was identified by a yeast two-hybrid screening using PYK2 C-terminal domain as bait.
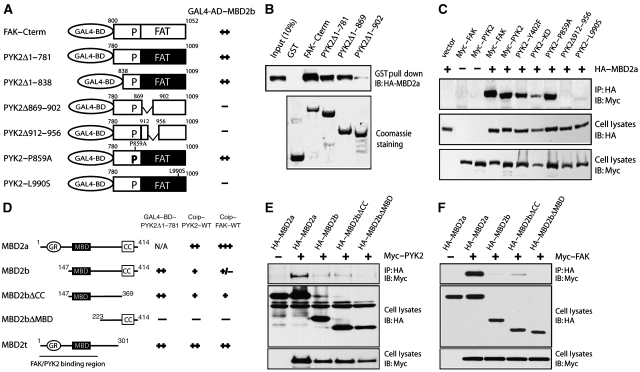
To identify the region in PYK2 that interacts with MBD2, we characterized several PYK2 mutants. The bait contains an FAT domain and a proline-rich sequence. We thus tested whether these two domains are involved in interacting with MBD2. As shown in Figure 1A, PYK2 constructs with the intact FAT domain interacted with MBD2, indicating the sufficiency of this domain in binding to MBD2. Partial deletions of the FAT domain (PYK2-869–902 and PYK2-912–956) or point mutation of the FAT domain (PYK2–L990S) abolished the interaction, suggesting the dependence of MBD2 interaction on FAT domain (Figure 1A). As the FAT domain is highly homologous between PYK2 and FAK, MBD2 indeed interacted with FAK, in addition to PYK2 (Figure 1A). These results show that both PYK2 and FAK interact with MBD2 through their FAT domains in yeast.
Figure 1.
FAK and PYK2 interaction with MBD2. (A) Requirement of the FAT domain of FAK/PYK2 for binding to MBD2 in yeast. Yeast cells were co-transformed with indicated constructs, seeded in Leu−/Try−/His− plates, and scored as follows for β-gal activity: (−) no blue after 24 h and (++) blue within 4 h. (B) MBD2 interaction with PYK2/FAK FAT domain in vitro. HEK 293 cells expressing HA-tagged MBD2 were lysed and resulting lysates were incubated with indicated GST-fusion proteins (∼5 μg) immobilized on beads. Bound proteins were probed with anti-HA antibodies (top). GST-fusion proteins were revealed by Coomassie staining (bottom). (C) Co-immunoprecipitation of MBD2 with FAK/PYK2 in a manner dependent on the FAT domain. HEK 293 cells were transiently transfected with indicated plasmids. Cell lysates were incubated with anti-HA antibodies to immunoprecipitate MBD2 complexes, which were resolved on SDS–PAGE and immunoblotted with antibodies against Myc. HA-tagged MBD2 and Myc-tagged FAK/PYK2 in lysates was revealed by immunoblotting with anti-HA and anti-Myc antibodies, respectively. (D) Requirement of the MBD domain for MBD2 binding to PYK2 in yeast. The assay was performed as described in (A). Co-immunoprecipitation (coip) data obtained from (E) and (F) were also summarized: (−) no detectable association, (+) weak, and (++) or (+++) strong, association of MBD2 with FAK/PYK2. (E, F) Co-immunoprecipitation of MBD2 with PYK2 (E) and FAK (F) in a manner dependent on GC-rich and MBD domains. HEK 293 cells expressing indicated plasmids were lysed and resulting lysates were incubated with anti-HA antibodies (for MBD2 and its mutants). Bound proteins were probed with anti-Myc antibodies (for PYK2 or FAK) (top). MBD2, its mutants, and PYK2/FAK in lysates were revealed by immunoblotting with anti-HA or anti-Myc antibodies, respectively.
The PYK2/FAK–MBD2 interaction occurs not only in yeast cells, but also in vitro pull-down assays using purified proteins (Figure 1B). Lysates of cells expressing HA–MBD2 was incubated with various GST–FAK/PYK2 fusion proteins immobilized on agarose beads. GST alone did not precipitate with MBD2 (Figure 1B). In contrast, GST-fusion proteins containing the FAT domain of FAK or PYK2 pulled down MBD2 (Figure 1B).
To determine whether FAK/PYK2 interacts with MBD2 in mammalian cells, we examined their association in HEK 293 cells transfected with HA-tagged MBD2 and Myc-tagged FAK/PYK2. As shown in Figure 1C, FAK/PYK2 was detected in immunoprecipitates of MBD2, suggesting that FAK and PYK2 bind to MBD2 in mammalian cells. This interaction seemed to be MBD2 specific, as MBD3, a MBD2-related protein (Hendrich et al, 2001), failed to associate with FAK/PYK2 in HEK 293 cells co-expressing these proteins (data not shown). Taken together, these results show an interaction of MBD2 with FAK and PYK2 in yeast, in vitro, and in mammalian-expression cells.
We next determined the region in MBD2 necessary for the interaction. Three splice variants, MBD2a, MBD2b, and MBD2t, have been reported (Hendrich and Bird, 1998; Jiang et al, 2004). MBD2a protein contains a GC-rich region, MBD, and a coil-coil domain (Figure 1D) (Jiang et al, 2004). MBD2b is a shorter isoform lacking the N-terminal 140 amino acids because of the usage of a different initiation codon (Figure 1D) (Hendrich and Bird, 1998; Jiang et al, 2004). MBD2t, a testis-specific isoform, lacks the C-terminal 113 amino acids (Figure 1D). To determine the region in MBD2 that is necessary for the interaction, co-immunoprecipitation assays were carried out. It is shown that MBD2a seemed to associate with FAK with much stronger affinity than those with MBD2b (Figure 1E and F). These data suggest that both GC-rich region and the MBD domain of MBD2a seemed to be required for its interaction with FAK/PYK2.
FAK translocation to the nuclei and interaction with MBD2 in myotubes and in muscle
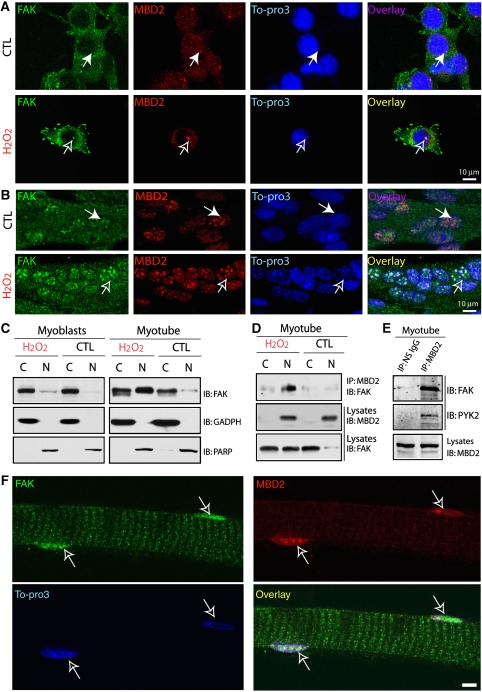
A perplex for FAK interaction with MBD2 is that FAK is thought to be a cytoplasmic tyrosine kinase predominantly distributed either at focal adhesions or in the cytoplasm, whereas MBD2 is mainly localized at the nuclei (Jiang et al, 2004). To address this issue, we examined their distribution in C2C12 cells, a well-established model of muscle differentiation. C2C12 cells remain as proliferative and undifferentiated myoblasts when cultured in growth medium (containing 20% fetal bovine serum), but differentiate into multi-nucleated myotubes when cultured in fusion medium (FM) (containing 4% horse serum). In myoblasts, FAK was predominantly localized at focal adhesions or in the cytoplasm, whereas MBD2 was distributed in the nuclei (Figure 2A). It was unable to observe obvious FAK co-localization with MBD2 (Figure 2A). On treatment with H2O2 (∼50 μM for 1 h), an oxidative stress stimulus that activates FAK (data not shown), a weak FAK co-localization with MBD2 at the peri-nuclei of myoblasts was observed (Figure 2A). FAK co-localization with MBD2 in the nuclei of myotubes was detectable, though it was partially co-localized (Figure 2B). Interestingly, it was more obviously detected in myotubes exposed to H2O2 stimulation (Figure 2B). Such nuclear punctate co-staining of FAK and MBD2 seemed to be nicely associated with Topro3-labelled heterochromatin (Figure 2B), different from that of myoblasts exposure to H2O2 (Figure 2A). FAK immunofluorescence seemed to be specific, as C2C12 myoblasts expressing miRNA–FAK, which efficiently and specifically suppressed FAK expression (Supplementary Figure 1A), blocked the immunostaining (Supplementary Figure 1B). Together, these results suggest that FAK not only resides in the cytoplasm or focal adhesions, but can also be present in the nuclei of myotubes, which seemed to be enhanced by oxidative stress stimulation (e.g. H2O2). In support of this notion was the observations that FAK was detected in the nuclear fractions of myotubes that were further increased by exposure to H2O2 (Figure 2C), and that FAK was co-precipitated with MBD2 immunocomplexes from myotubes and myotubes stimulated with H2O2 (Figure 2D). Finally, FAK–MBD2 co-localization was also observed in the nuclei of isolated muscle fibres (Figure 2F).
Figure 2.
Endogenous FAK co-localization and interaction with MBD2 in the nuclei of myotubes exposed to H2O2 and in the nuclei of isolated muscle fibres. (A, B) C2C12 myoblasts (A) and myotubes (B, cells were incubated with FM for 4 days) were treated with or without H2O2 (∼50 μM) for 1 h. Cells were then fixed with methanol and co-immunostained with FAK and MBD2 antibodies. In (A), monoclonal anti-PY397 antibody (green) and polyclonal rabbit anti-MBD2 antibody (red) were used. In (B), polyclonal rabbit anti-FAK antibody (green) and polyclonal sheep anti-MBD2 antibody (red) were shown. Open arrows indicate co-localization of FAK with MBD2, and filled arrows indicate non-co-localization. Bars, 10 μm. (C) Myoblasts and myotubes treated with or without H2O2 (∼50 μM, 1 h) were harvested and isolated into nuclear (N) and cytoplasmic (C) fractions with the CellLytic Nuclear Extraction Kit (Sigma). Lysates were subjected to western blot analysis using indicated antibodies. (D, E) Co-immunoprecipitation of FAK with MBD2 in myotubes exposed to control or H2O2. Lysates isolated from the nuclear (N), cytoplasmic (C) fractions, or total lysates (E) of myotubes exposed with or without H2O2 were subjected for immunoprecipitation using anti-MBD2 antibodies. The resulting lysates were immunoblotted with indicated antibodies. (F) Co-immunostaining analysis of FAK and MBD2 in isolated muscle fibres. Confocal images were shown. Open arrows indicate the nuclei of the muscle fibre. Bar, 10 μm.
Role of FAK–MBD2 interaction in FAK nuclear localization in myotubes and muscle-terminal differentiation
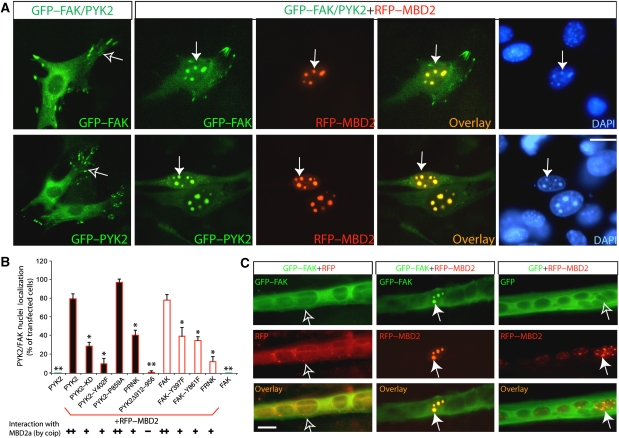
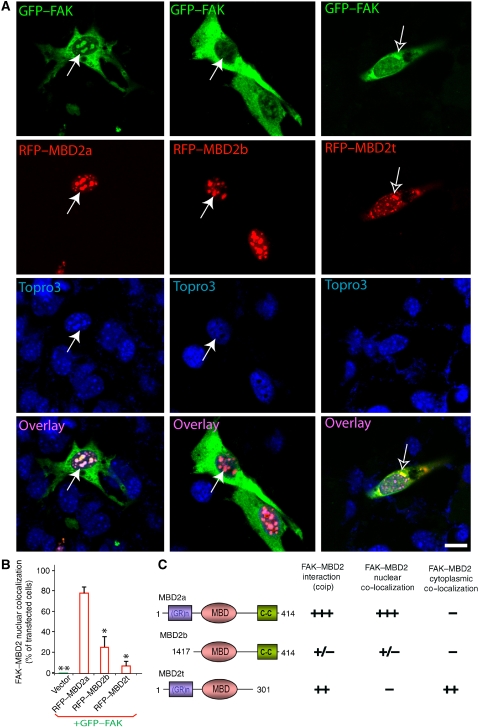
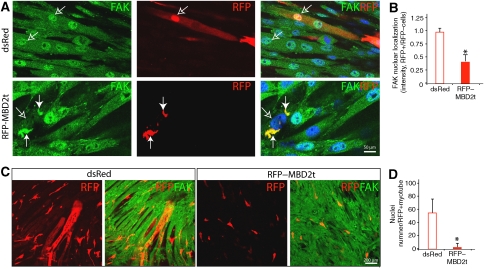
We next determined whether FAK–MBD2 interaction is required for FAK nuclear localization in myoblasts and/or myotubes. Myoblasts were transfected with GFP-tagged FAK with or without RFP-tagged MBD2a (RFP–MBD2a). When transfected alone, GFP–FAK was predominantly distributed at focal adhesions or cytoplasm (Figure 3A), and RFP–MBD2 was localized as punctate clusters in the nuclei (data not shown), consistent with the earlier reports (Jiang et al, 2004). However, co-expression with RFP–MBD2 caused a reduced distribution of GFP–FAK at focal adhesions and an increased co-localization of FAK with MBD2 in the nuclei (Figure 3A). This FAK nuclear localization had the following characteristics. First, the nuclei localization of FAK seemed to be regulated by FAK tyrosine phosphorylation (Figure 3B). In comparison with wild-type control, less nuclear localization was observed with FAK mutants that either lack autophosphorylation site (e.g. FAK–Y397F) or are catalytically inactive (FRNK) (Figure 3B), consistent with the results for a reduced co-immunoprecipitation between FRNK and MBD2 (data not shown). Second, MBD2-mediated FAK nuclear localization was not only observed in myoblasts, but also in myotubes (Figure 3C). Third, FAK nuclear localization was dependent on the interaction between MBD2 and FAK. FAK mutants unable to interact with MBD2 remained in the cytoplasm (Figure 3B), and MBD3 or MBD2 mutants unable to interact with FAK (MBD2b) did not alter FAK distribution (Figure 4). MBD2t, a testis-specific isoform missing the C-terminal coil-coil domain, was able to interact and co-localize with FAK (Figure 4). However, their nuclear localization was reduced in C2C12 myoblasts (Figure 4). In myotubes expressing MBD2t, the nuclear localization of endogenous FAK was also dramatically reduced (Figure 5A and B). Instead, both MBD2t and FAK were co-localized as clusters at peri-nuclear regions (Figure 5A). Of interest to note is that muscle differentiation was also significantly attenuated in cells expressing MBD2t (Figure 5C and D), suggesting a dominant negative effect by MBD2t, and implicating the importance of FAK nuclear localization or MBD2 for muscle differentiation.
Figure 3.
Exogenous FAK/PYK2 co-localization with MBD2a in the nuclei of C2C12 myoblasts and myotubes in a manner dependent on their interaction. C2C12 myoblasts (A) and myotubes (C) were co-transfected with indicated plasmids. Transfected cells were fixed and examined for their fluorescence. GFP, RFP, their overlay, and DAPI images are shown. Bars, 20 μm. Filled arrows indicate the nuclear aggregates containing RFP–MBD2 or GFP–FAK/PYK2, and open arrows indicate cytoplasmic/focal adhesion distribution of GFP–FAK/PYK2 when it was not co-expressed with RFP–MBD2. (B) Quantification analysis of nuclear localization of PYK2, FAK, and their mutants in the absence or presence of RFP–MBD2 expression. The percentage of cells with FAK/PYK2 co-localization with MBD2 in the nuclei over the total number of transfected cells was illustrated. *P<0.05 and **P<0.01, significant difference from PYK2/FAK co-expressing with RFP–MBD2 (t-test).
Figure 4.
Effects of MBD2 isoforms on their co-localization with FAK in myoblasts. (A) C2C12 myoblasts were co-transfected GFP–FAK with indicated MBD2 plasmids. Transfected cells were fixed and examined for their fluorescence. GFP, RFP, their overlay, and DAPI images are shown. Bars, 20 μm. Filled arrows indicate the nuclear aggregates containing RFP–MBD2 or GFP–FAK. (B) Quantification analysis of data from (A). *P<0.05 and **P<0.01, significant difference from the control (RFP–MBD2a). (C) Illustration of the domains in MBD2 isoforms and summary of the data regarding MBD2 interaction and co-localization with FAK. +++, strong interaction/co-localization and +/−, weak/no interaction or co-localization.
Figure 5.
Inhibition of FAK nuclear translocation and muscle differentiation in C2C12 cells expressing MBD2t. (A, C) C2C12 myoblasts were transfected with dsRed or RFP–MBD2t. Transfected cells were switched into FM to induce myotube formation. Cells were then fixed and immunostained with indicated antibodies. Confocal images are shown. Filled arrows indicate co-localization of expressed RFP–MBD2t with endogenous FAK at the peripheral of the nuclei. Open arrows indicate nuclear staining. Lower magnification of RFP and FAK-labelled myotube images are shown in (C). (B, D) Quantitative analysis of data from (A) and (C). *P<0.01, significant difference from cells expressing control dsRed.
FAK in muscle-terminal differentiation and in maintenance of muscle fibres in culture and in vivo
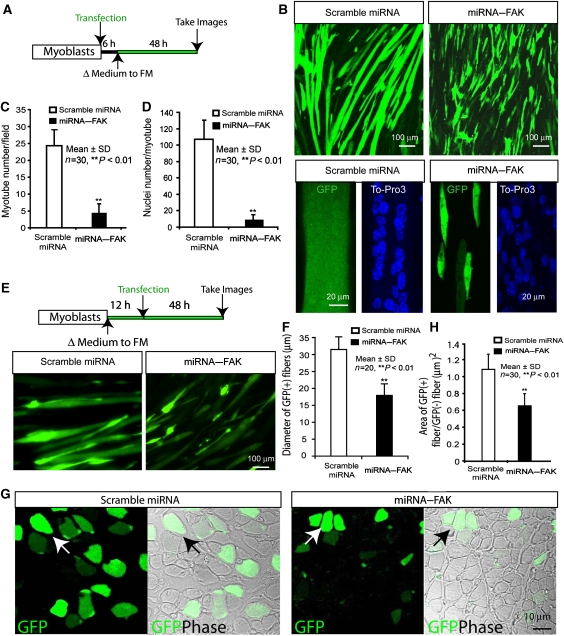
We further investigated the effect of FAK suppression by their miRNAs on myotube differentiation in C2C12 cells. The miRNA interference constructs of FAK were generated and tested for effects on FAK expression. Transfection of miRNA–FAK-1 and -2, but not miRNA–FAK-3 or control (a scrambled miRNA), inhibited expression of co-transfected FAK in HEK293 cells (Supplementary Figure 1A) and endogenous FAK in C2C12 myoblasts (Supplementary Figure 1B). miRNA–FAK-2 was more potent than miRNA–FAK-1 (Supplementary Figure 1A) and was subsequently used for further studies. The miRNA plasmids were transfected in myoblast culture, which was then cultured in the FM for myotube differentiation (Figure 6A). Transfection with scrambled miRNA seemed to have little effect on myotube formation (Figure 6B–D). However, significant numbers of C2C12 myoblasts expressing miRNA–FAK remained partially differentiated (fused myotubes with reduced numbers of nuclei per myotube and reduced size in morphology), indicating a defect of muscle differentiation when FAK expression is suppressed in myoblast culture (Figure 6A–D), supporting the notion that FAK may be required for muscle differentiation/maturation in vitro.
Figure 6.
Requirement of FAK expression for muscle-terminal differentiation and maintenance of muscle fibres in culture and in vivo. (A) Illustration of a protocol to suppress FAK expression in C2C12 myoblasts before their differentiation. In this protocol, C2C12 myoblasts were transfected with miRNAs; 6 h after transfection, transfected cells were switched into FM to induce myotube formation. Data using this protocol were shown in (B–D). (B) GFP-labelled myotube images are shown, which were obtained from the protocol of (I) described above. Higher magnification of GFP and DAPI-labelled myotube images are shown in bottom panels. (C, D) Quantitative analysis of data from (B). Myotube number per field (C) and nuclei number per myotube (D) were counted. *P<0.01, significant difference from cells expressing control miRNA. (E) Illustration of a protocol to suppress FAK expression in C2C12 cells after differentiation programme was induced. GFP-labelled myotube images are shown, which were obtained from this protocol. (F) Quantitative analysis of data from (E). Myotube diameter per myotube were measured. *P<0.01, significant difference from cells expressing control miRNA. (G) GFP and DIC images of muscle fibres expressing scramble miRNA and miRNA–FAK. In vivo eletroporation was carried out as described in Materials and method section. Cross sections of muscle fibres were fixed and examined. Arrows indicate muscle fibres expressing GFP. Bar, 10 μm. (H) Quantification analysis of data from (F). The ratio of area of GFP positive over GFP negative fibres was presented. **P<0.05, significant difference from cells expressing scramble miRNA (t-test).
We then investigated the role of FAK in muscle differentiation/maturation in myotube culture and in vivo. To this end, the miRNA plasmids were transfected into partially differentiated myotubes as illustrated in Figure 6E. Transfection with either scrambled miRNA or miRNA–FAK seemed to have little effect on myotube formation (Figure 6E). However, the fused myotubes with reduced size/diameter in morphology was observed in myotubes expressing miRNA–FAK (Figure 6E and F), indicating a defect of muscle fibre maintenance when FAK expression is suppressed at later stage of muscle differentiation. To further test this notion, miRNA–FAK was expressed in tibia anterior muscles of postnatal day 4 mouse by electroporation. Seven days after electroporation, muscle fibres were dissected and examined by immunohistochemical analysis. As shown in Figure 6G, muscle fibres expressing scrambled miRNA (GFP is an indicator of expressed plasmid) appeared to be normal. In contrast, fibres expressing miRNA–FAK exhibited ‘atrophy'-like phenotypes: reduced thickness/GFP-labelled area of each muscle fibre (Figure 6G and H). Taken together, these results support the view that FAK is not only required for muscle-terminal differentiation in culture, but also has a function in maintenance of muscle fibres in myotube culture and in vivo.
Reorganization of MBD2-containing heterochromatin by FAK interaction with MBD2
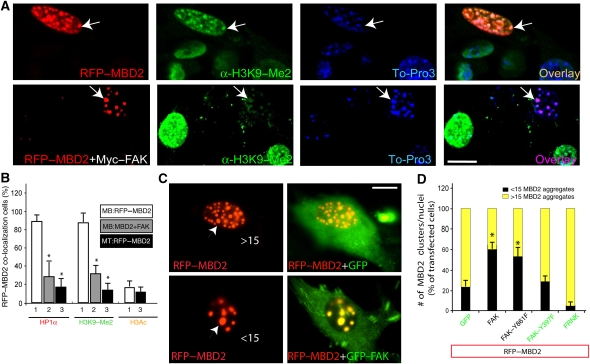
To investigate mechanisms underlying FAK regulation of muscle-terminal differentiation, we examined whether MBD2-containing heterochromatin foci, a repressive chromatin condition that prevents gene transcription (Berger and Bird, 2005; Fatemi and Wade, 2006), are regulated by its interaction with FAK. C2C12 cells expressing RFP–MBD2 alone or with Myc–FAK were immunostained with antibodies against heterochromatin protein 1α (HP1α) and dimethylation of histone 3 at lysine 9 (H3K9-me2), both markers of heterochromatin foci (Maison and Almouzni, 2004; Fuchs et al, 2006). Expression of RFP–MBD2 in myoblasts led to accumulation of HP1 and H3K9-me2 that were co-localized with RFP–MBD2 (Figure 7A), confirming the association of RFP–MBD2 with heterochromatin foci and suggesting a role of MBD2 in promoting the formation of such heterochromatin foci (Figure 7A). Consistently, the MBD2-containing heterochromatin foci did not stain with antibodies against acetylated H3 (H3K9/14ac) nor dimethylated histone 3 at lysine 4 (H3K4-me2), both markers of euchromatin (data not shown).
Figure 7.
Modulation of MBD2-containing heterochromatin foci by co-expression of MBD2a and FAK in myoblasts. C2C12 myoblasts (A, C) were transfected with RFP–MBD2 alone or co-transfected with Myc–FAK. Transfected cells were fixed and immunostained with indicated antibodies. Confocal images were shown. Bars, 10 μm. Arrows indicate the RFP–MBD2-expressing cells. (B) Quantification analysis of data from (A). The percentage of cells with RFP–MBD2 co-localization with HP1α, H3K9-me2, or H3Ac in the chromatin foci over the total number of RFP–MBD2-expressing cells was illustrated. 1, C2C12 myoblasts expressing RFP–MBD2 alone; 2, C2C12 myoblasts expressing RFP–MBD2 plus Myc–FAK; and 3, C2C12 myotube (incubation with FM, 48 h) expressing RFP–MBD2 alone. *P<0.01, significant difference from myoblasts expressing RFP–MBD2 alone (t-test). (C) Modulation of MBD2-containing chromatin size and number by co-expression of FAK. C2C12 myoblasts expressing RFP–MBD2+GFP or RFP–MBD2+GFP–FAK. Bar, 10 μm. Arrow head indicates RFP–MBD2 cluster. >15, or <15, represents numbers of RFP–MBD2 clusters per nuclei. (D) Quantification analysis of data from (C). The percentage of the RFP–MBD2 expressing cells with <15 MBD2 clusters/nuclei was presented as black column. *P<0.01, significant difference from myoblasts expressing RFP–MBD2 alone (t-test).
We next examined whether MBD2-containing foci in myoblasts is regulated by co-expression of FAK. As shown in Figure 7A and B, MBD2-induced nuclear H3K9-Me2/HP1α accumulation in myoblasts was significantly attenuated when FAK was co-expressed, suggesting an inhibitory role of FAK on MBD2's effects on heterochromatin foci. Furthermore, MBD2-containing foci were notably fewer in myoblasts co-expressing FAK as compared with cells expressing MBD2 alone (Figure 7C). A total of 75% of MBD2 expressing myoblasts contained >15 clusters/nucleus (Figure 7C and D); however, 55% of nuclei in myoblasts co-expressing FAK/PYK2 contained fewer than 15 foci (Figure 7C and D). Concomitantly, the size of MBD2-containing foci increased in cells co-expressing FAK (Figure 7C). Similar phenomenon was observed in myotubes co-expressing MBD2 with FAK (data not shown). This effect required FAK interaction with MBD2 and their tyrosine phosphorylation because it was not observed with FAK mutants that fail to interact with MBD2 or contain a tyrosine phosphorylation mutation (e.g. FAK–Y397F) (Figure 7D). Together, these results suggest a regulatory role of FAK in remodelling of MBD2-containing heterochromatin foci.
FAK inhibition of MBD2 binding to the methyl CpG site of myogenin promoter and to HDAC1 and induction of myogenin expression
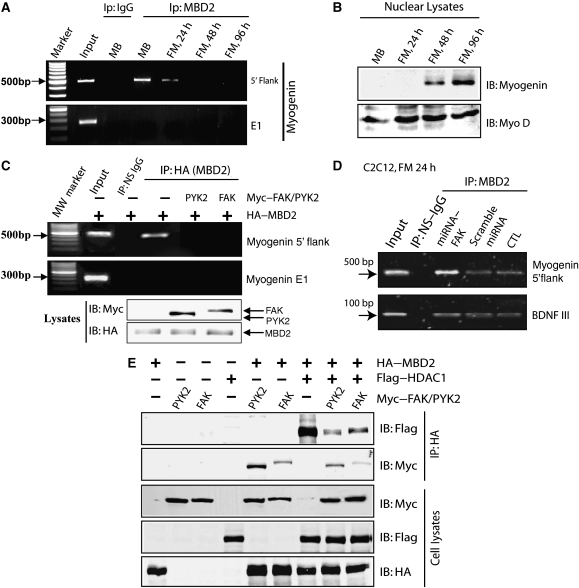
MBD2 is believed to be an important linker of methylated DNA with the nucleosome remodelling and histone deacetylation complex (NuRD, also known as Mi-2), an essential complex for chromatin remodelling (Ng et al, 1999; Zhang et al, 1999). To assess the role of FAK or PYK2 in regulating MBD2 function, we examined whether FAK or PYK2 regulates MBD2 binding to methyl CpG. The methyl CpG in the promoter of myogenin, an important myogenic factor, has been found to correlate with myogenin expression during muscle differentiation (Scarpa et al, 1996; Lucarelli et al, 2001). We thus assessed MBD2 binding with myogenin promoter by chromatin immunoprecipitation (CHIP) assay. Two regions of the myogenin gene, the 5′-flank region of the promoter and the exon 1 (E1), were examined for MBD2 binding. Both contain CCGG site; however, the CCGG site in 5′-flank region, but not in E1, is reported to be methylated (Scarpa et al, 1996; Lucarelli et al, 2001). Indeed, MBD2 was associated with the 5′-flank region of myogenin, but not E1 region (Figure 8A). Such a binding was decreased during myotube differentiation (Figure 8A), strongly correlating with myogenin expression (Figure 8B), consistent with the earlier reports (Scarpa et al, 1996; Lucarelli et al, 2001). We then examined the effect of FAK/PYK2 on this event by CHIP assay. Remarkably, co-expression of FAK or PYK2 decreased MBD2 binding to the 5′-flank region of myogenin (Figure 8C). Suppression of FAK expression in C2C12 cells by its miRNA enhanced MBD2 binding to the 5′-flank region of myogenin (Figure 8D). BDNF is expressed in muscle cells and its promoter III is reported to contain an MBD-binding site (Chen et al, 2003; Klose and Bird, 2003). However, suppression or over expression of FAK seemed to have no obvious effect on this event (Figure 8D and data not shown). These results suggest that MBD2 is capable to associate with methyl CpG site of myogenin promoter, which is inhibited on co-expression of FAK or PYK2, and enhanced by suppression of FAK expression.
Figure 8.
FAK/PYK2 suppression of MBD2 binding to myogenin promoter and to HDAC1. (A) Reduced MBD2 binding to methyl CpG site of myogenin during C2C12 differentiation by CHIP assay analysis. C2C12 cell differentiation and CHIP assays were performed as described in Materials and methods. Precipitates without antibody (input) or with indicated antibodies were used as template for PCR. PCR products were resolved on 1.5% agarose gel. (B) Increased nuclear myogenin expression during C2C12 differentiation by western blot analyses. Nuclear fractions of differentiated C2C12 cells were isolated with CelLytic NuCLEAR Extraction Kit (Sigma, St Louis, MO). Lysates were then subjected to western blot analysis by antibodies against myogenin and Myo D. (C) FAK/PYK2 suppression of MBD2 binding to methyl CpG site of myogenin promotor by CHIP analysis. C2C12 cells were transiently transfected HA–MBD2 with or without Myc-tagged PYK2/FAK. CHIP assay was performed as described in (A). (D) Increase of MBD2 binding to methyl CpG site of myogenin promoter, but not BDNF promoter III, in C2C12 cells expressing miRNA–FAK. C2C12 cells were transiently transfected with scramble miRNA or miRNA–FAK. After incubation with the FM for 24 h, GFP-expression cells were purified by FACS sorting. Cells were then subjected to CHIP analysis as described in (A). (E) FAK/PYK2 reduction of MBD2 binding to HDAC1 by co-immunoprecipitation analysis. HEK 293 cells expressing indicated plasmids were lysed and resulting lysates were incubated with anti-HA antibodies (for MBD2). Bound proteins were probed with anti-Flag antibody (for HDAC1) and anti-Myc antibodies (for PYK2 or FAK). MBD2, HDAC1, and PYK2/FAK in lysates were revealed by immunoblotting with anti-HA, anti-Flag, or anti-Myc antibodies, respectively.
In addition to binding to methylated DNA, MBD2 is able to associate with HDAC1/2 (Ng et al, 1999). Such an association is believed to be important for MBD2-mediated chromatin remodelling and transcriptional repression. We thus examined whether FAK/PYK2 regulates MBD2 binding to HDAC1. To this end, HA–MBD2 and Flag–HDAC1 were co-transfected with or without Myc–FAK/PYK2 in HEK 293 cells. MBD2 and its associated proteins were isolated by immunoprecipitation using anti-HA antibodies. As shown in Figure 8E, MBD–HDAC1 interaction was reduced in cells co-expressing FAK or PYK2. Co-expression of HDAC1 also reduced MBD2 binding to FAK or PYK2 (Figure 8E). These results suggest a potential competition for MBD2 interaction with HDAC1 and FAK/PYK2. Note that FAK, PYK2, and HDAC1 could bind to the same region (e.g. MBD domain) of MBD2, implying a potential mechanism for the competition.
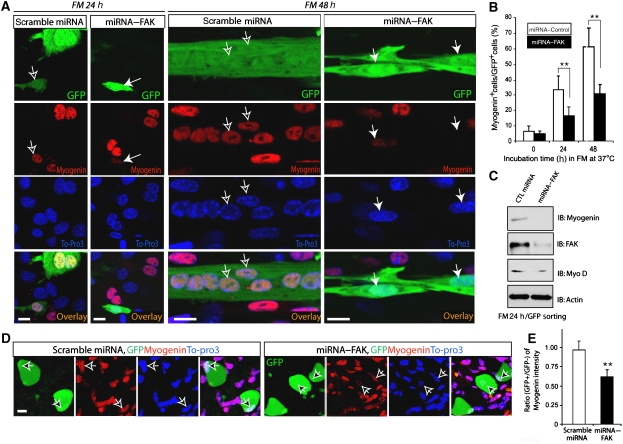
FAK reduction of MBD2 binding to methyl CpG site of myogenin and to HDAC1 by FAK suggests a role of FAK–MBD2 interaction in regulating myogenin expression. To test this notion, we examined myogenin expression in C2C12 cells suppressing FAK expression by its miRNA during myotube differentiation. Although immunofluorescence analysis showed an induction of myogenin expression during myotube differentiation in C2C12 cells expressing scrambled miRNA, suppression of FAK expression in C2C12 cells by miRNA–FAK reduced such induction (Figure 9A and B). Consistently, western blot analysis of lysates from GFP (marker for miRNA expression) positive C2C12 cells (by Facs GFP sorting analysis) showed a reduced myogenin, but not Myo D, in cells expressing miRNA–FAK (Figure 9C), and immunohistochemical analysis of mouse skeletal muscles showed a reduced myogenin staining in muscle fibres expressing miRNA–FAK, but not scramble miRNA, by in vivo electroporation (Figure 9D and E). Taken together, these results suggest a requirement of FAK for myogenin induction during myotube differentiation in vitro and in vivo.
Figure 9.
Requirement of FAK expression for myogenin induction during muscle differentiation in culture and in vivo. (A) Immunostaining analysis of myogenin expression in differentiating C2C12 cells expressing miRNA–FAK. C2C12 cells transiently transfected with scramble miRNA or miRNA–FAK (indicated by GFP) were incubated with FM for 24 or 48 h. Fixed cells were immunostained using antibody against myogenin (red) and To-Pro3 (marker for nuclei, blue). Confocal images were shown. Bars, 10 μm. Filled arrows indicate miRNA–FAK expressing cells and open arrows indicate control miRNA expressing cells. (B) Quantification analysis of data from (A). The number of cells with positive staining of GFP and myogenin in the nuclei were counted and divided by the total number of GFP positive cells. **P<0.05, significant difference from cells expressing scramble miRNA (t-test). (C) Western blot analysis of myogenin expression in differentiating C2C12 cells expressing miRNA–FAK. C2C12 cells transiently transfected with miRNA–FAK or control miRNA were incubated with FM for 24 h to allow myogenin induction. GFP expressing cells were then isolated by Facs sorting and their lysates were subjected to western blot analysis using indicated antibodies. (D) Immunohistochemical staining of muscle fibres expressing scramble miRNA or miRNA–FAK (indicated by GFP) by in vivo eletroporation. In vivo eletroporation was carried out as described in the Materials and method section. Cross sections of muscle fibres were fixed and immunostained with indicated antibodies. Arrows indicate muscle fibres expressing GFP. Bars, 10 μm. (E) Quantification analysis of data from (D). The ratio of myogenin fluorescence intensity of GFP positive over GFP negative fibres was presented. **P<0.05, significant difference from cells expressing scramble miRNA (t-test).
As the interaction of FAK–MBD2, that was increased in myotubes in response to H2O2, seemed to have a function in the induction of myogenin expression, we thus examined whether H2O2 also regulates myogenin expression and muscle differentiation. Exposure of low level of H2O2 (<100 μM) at myotube culture seemed to enhance myotube size (Supplementary Figure 2A). This effect was observed when C2C12 myotubes were exposed to H2O2 after the differentiation programme is established, but not before the differentiation (data not shown). Of note is that myogenin expression was increased in response to H2O2 (Supplementary Figure 2B). The induction was abolished in myotubes suppressing FAK expression (Supplementary Figure 2C), suggesting a requirement of FAK in this event.
Discussion
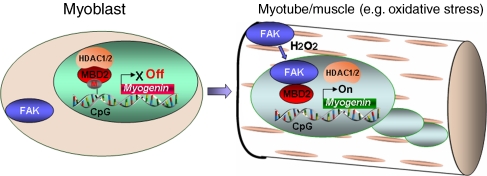
We provide evidence for a role of FAK in chromatin remodelling, myogenin induction, and muscle differentiation by their interaction with MBD2. The interaction of MBD2 with FAK, increased in myoblasts/myotubes exposure to oxidative stress stimulation, leads to an increased FAK nuclear or peri-nuclear localization. FAK interaction with MBD2 seems to reorganize MBD2-based heterochromatin foci, possibly as a consequence of decreased MBD2 binding to the methyl CpG and to HDAC. These results lead us to propose a working hypothesis depicted in Figure 10. According to this model, in myoblasts, MBD2-containing complex that does not include FAK seems to be involved in the formation of heterochromatin foci, providing a local environment to repress expression of differentiation-associated genes, such as myogenin. During differentiation or in response to oxidative stress stimulation (e.g. H2O2), FAK goes into the nuclei and forms a complex with MBD2, leading to the disruption of the MBD2-centred NuRD complex and activation of differentiation-associated genes including myogenin, thus, promoting muscle-terminal differentiation.
Figure 10.
A working hypothesis. In myoblasts, MBD2-containing complex that does not include FAK seems to be involved in the formation of heterochromatin foci, providing a local environment to repress expression of differentiation-associated genes, such as myogenin. During differentiation or in response to oxidative stress stimulation (e.g. H2O2), FAK goes into the nuclei and forms a complex with MBD2, leading to the disruption of the MBD2-centred NuRD complex and activation of differentiation-associated genes (e.g. myogenin), thus, promoting muscle-terminal differentiation.
FAK, a well-known cytoplasmic tyrosine kinases activated on cell-ECM adhesion, mediates cell adhesion signalling in the regulation of cell migration, survival, and differentiation (Guan and Shalloway, 1992; Hanks et al, 2003; Parsons, 2003; Zhao et al, 2003; Mitra et al, 2005). Whether they function in the nuclei or what functions they have in the nuclei remains largely unclear. Several lines of evidence suggest that FAK may regulate cell cycle and gene transcription at the nuclei. Serine 722, but not tyrosine 397, phosphorylated FAK, is localized at the nuclei in hypertrophic myocardium (Yi et al, 2003). Through KLF8, a transcriptional factor, FAK regulates cyclin D1 and cell cycle progression (Zhao et al, 2003). PYK2, an FAK-related tyrosine kinase, can also be translocated into the nuclei on depolarization in cultured neurons/PC12 cells or in cultured hippocampal slices (Faure et al, 2007). Together, these reports suggest that although FAK or PYK2 is a resident at the focal adhesions or cytoplasm, they, under specific circumstances, translocate to the nuclei and have a function in the control of nuclear functions, such as regulation of gene expression and cell cycle. Exactly how do FAK or PYK2 translocates into the nuclei and regulates gene expression and cell cycle remains unclear. It has been reported that FAK N-terminal FERM domain contains an NLS (nuclear localization signal) (Lim et al, 2008), and that FAK nuclear translocation seems to be highly regulated, depending on its protein conformation. We speculate that MBD2 binding to the FAK FAT domain may change the conformation of FAK, preventing FAK targeting to focal adhesions, exposing FAK N-terminal NLS, thus, increasing FAK nuclear translocation. In addition, MBD2 binding to FAK at the nuclei may also prevent FAK export from nucleus, maintaining FAK localization at the nuclei. These studies provide novel insights into FAK/PYK2 translocation and function in the nuclei, implicating a novel link for coordination between cell adhesion and gene expression at the nuclei.
MBD2, as the methyl-binding component of the MeCP1 complex, is believed to target HDAC/chromatin remodelling activity to methylated templates, thus, behaving as a linker of transcriptional repression with the methylated templates that are associated with heterochromatin (Ng et al, 1999; El-Osta and Wolffe, 2000; Wade, 2001; Berger and Bird, 2005). Besides the possible repressor action that MBD2 may exert, other features of MBD2, which could help us to understand the effects of its over expression on myogenin expression and muscle differentiation, are needed to be considered. A correlation among FAK–MBD2 interaction, reduced MBD2 binding to myogenin promoter, and myogenin induction during muscle differentiation were observed. This is in line with the earlier report for a correlation of a reduced myogenin DNA methylation and increased myogenin expression during muscle differentiation (Lucarelli et al, 2001). Does MBD2–FAK interaction in the nuclei lead to demethylation of myogenin promotor? Interestingly, MBD2 is an enzyme capable of actively demethylating DNA (Bhattacharya et al, 1999; Detich et al, 2002, 2003; Patra et al, 2002). However, MBD2 is believed to act as a transcriptional repressor (Ng et al, 1999; El-Osta and Wolffe, 2000; Wade, 2001; Berger and Bird, 2005). Both features may coexist in the same enzyme to coordinate the expression of some promoters and the inactivation of others. We speculate that MBD2–HDAC complex that regulates chromatin remodelling associated with transcriptional repression may be regulated under specific circumstances when FAK/PYK2 associates with MBD2. FAK/PYK2–MBD2 interaction may displace HDAC1/2's binding with MBD2 and attenuate MBD2's suppressor activity. On the other hand, FAK/PYK2–MBD2 interaction may also induce MBD2's demethylase activity (Bhattacharya et al, 1999; Detich et al, 2002), thus, reducing methylation of myogenin promoter and increasing its transcription. However, the latter speculation needs additional experiments to support in future studies.
Muscle cell differentiation involves the fusion of myoblasts into multi-nucleated myotubes, a process regulated by the activation of myogenic basic helix-loop-helix factors (e.g. MyoD, Myf5, myogenin, and MRF4) (Lassar et al, 1989; Olson, 1992, 1993; Rudnicki and Jaenisch, 1995; Perry and Rudnick, 2000; Pownall et al, 2002; Berkes and Tapscott, 2005). Myogenin is believed to be crucial for the expression of genes associated with muscle differentiation, whereas MyoD and Myf5 are required for commitment to or initiation of the myogenesis (Venuti et al, 1995; Knapp et al, 2006). Our results, that myogenin is induced under oxidative stress by the FAK–MBD2 pathway, are in line with this view, supporting a role of myogenin not only in muscle differentiation, but also in the maintenance of muscle fibres. It is noteworthy that muscle differentiation seems to be associated with chromatin remodelling (Mejat et al, 2005). During muscle differentiation, formation of large clusters of heterochromatin, characterized by high levels of DNA methylation, specifically methylated forms of histone H3, and deacetylated histone H4, is observed (Brero et al, 2005). This event correlates with the over expression of the MBD proteins, MeCP2 or MBD2, in muscle cells (Brero et al, 2005), corroborating with our observation that increased FAK–MBD2 complex leads to the formation of large clusters of heterochromatin. This FAK–MBD2 complex thus may regulate expression of multiple genes, in addition to myogenin, which remained to be further investigated.
It is also of interest to note that muscle differentiation is regulated by oxidative stress stimulation. There is an increasing evidence that oxidative stress (e.g. H2O2) seems to mediate dual functions on myogenesis, depending on its dose and time of treatment. Not merely as a damaging agent (Rando et al, 1998; Langen et al, 2002; Escobedo et al, 2004; Zaccagnini et al, 2007), it also acts as a useful signalling molecule to regulate growth, differentiation, proliferation, and apoptosis, at least within the physiological concentration (Ji, 2007). When higher dose of H2O2 (e.g. >100 μM) is applied before differentiation induction in C2C12 cells, but not after the differentiation programme, it blocks myogenin expression and myogenic differentiation (Rando et al, 1998; Langen et al, 2002; Escobedo et al, 2004; Zaccagnini et al, 2007) (Supplementary Figure 3A). However, exposure of low level of H2O2 can enhance muscle differentiation and function (Ji, 2007). This view is consistent with our observations that C2C12 myotubes exposed to H2O2 (<100 μM) after the differentiation programme is established, but not before the differentiation, lead to FAK translocation to the nuclei, and modulates myogenin expression and muscle-terminal differentiation (Supplementary Figure 3). The physiological source of H2O2 that stimulates FAK nuclear activity in myotubes remains unclear. However, we speculate that increased metabolic activity during muscle contraction could produce low levels of oxidants and that H2O2-induced FAK/PYK2–MBD2 complex at the nuclei may also be involved in regulating oxidative stress-induced chromatin remodelling and many gene expression (e.g. anti-oxidant genes).
Together, our results suggest that FAK translocates into nuclei under specific circumstances (e.g. exposure to oxidative stress of muscle cells), in which it forms a complex with MBD2, attenuates MBD2's transcriptional repressor activity, activates gene (e.g. myogenin) expression, and promotes muscle-terminal differentiation. These results suggest an earlier unrecognized mechanism underlying FAK/PYK2 regulation of gene expression, and reveal a new mechanism of MBD2 regulation by FAK/PYK2 tyrosine kinases.
Materials and methods
Reagents
mAbs were purchased from Santa Cruz Biotechnology, Inc (anti-Myc), Sigma-Aldrich (anti-Flag and anti-vinculin), and Transduction Laboratories (anti-PYK2, anti-FAK, and anti-PY397). pAbs were obtained from Upstate Biotechnology (anti-PYK2, anti-FAK, anti-H3K4-me2, anti-H3K9-me2, and anti-acetyl-H3), Biosource International (anti-PY397 and anti-PY402), and Abcam Inc (anti-HP1α, anti-myogenin, and anti-MBD2). The cDNA encoding mouse MBD2 was purchased from the American Type Culture Collection. The plasmids encoding RFP–MBD2 and myc–MBD3 were provided by Dr Gerd P Pfeifer (Beckman Research Institute of the City of Hope, Duarte, CA) (Jiang et al, 2002, 2004).
Yeast two-hybrid studies
The PYK2 COOH terminus (amino-acid residues 781–1009) subcloned into pGBT10 (pGBT10–PYK2Δ1-781) was used as bait to screen mouse brain cDNA libraries fused to the GAL4 transcriptional activation domain (Gal4-AD) as described earlier (Ren et al, 2001; Wang et al, 2003). Briefly, the Y190 yeast strain was first transformed with pGBT10–PYK2Δ1–781, and subsequently with the mouse brain cDNA library. Positive clones were screened out on plates lacking leucine, tryptophan, and histidine with 30 mM 3-aminotriazole and by filter assays for β-galactosidase activity. Plasmid DNA was purified from the His+ β-gal+ colonies and was re-transformed into yeast with different bait vectors to determine specificity. To characterize binding between PYK2/FAK and MBD2b, plasmids encoding FAK, PYK2, MBD2b, or their mutants were co-transformed into yeast Y190; interactions were characterized by growth and by β-galactosidase activity (Ren et al, 2001, 2004; Wang et al, 2003).
Expression vectors
The cDNAs of FAK and PYK2 were subcloned into mammalian-expression vectors downstream of an Myc epitope tag (MEQKLISEEDL) as described earlier (Xiong and Parsons, 1997). The cDNAs of FAK, PYK2, and their mutants were also subcloned into mammalian-expression vector (pEGFP-C1) fused with GFP. The cDNAs encoding MBD2 or its mutants were amplified by PCR, and subcloned into mammalian-expression vectors downstream of an HA epitope tag (MYPYDVPDYAGS) under the control of the CMV promoter (Xie et al, 2005). The plasmid encoding dsRed–MBD2 was kindly provided by Dr Gerd P Pfeifer (Jiang et al, 2004; Jin et al, 2005). The cDNA encoding HDAC1 was also amplified by PCR, and subcloned into expression vector downstream of a Flag epitope tag (MDYKDDDDKGP) (Wang et al, 2003).
miRNA-expression vectors that suppress expression of FAK, PYK2, or MBD2 were generated by the BLOCK-iT Lentiviral miR RNA Expression System (Invitrogen) as described earlier (Zhu et al, 2007). Briefly, based on mouse FAK, PYK2, and MBD2 sequence analysis by a program provided by Invitrogen, three regions for each gene were picked and cloned into pcDNA6.2–GW/EmGFP–miR expression vector to yield pcDNA6.2–GW/EmGFP–miRNA–FAK/PYK2/MBD2. They were co-transfected with FAK/PYK2/MBD2 expression construct into HEK 293 cells to identify effective clones based on their ability to suppress FAK/PYK2/MBD2 expression by western blot analysis. Their efficiency in suppressing specific gene expression was further confirmed by immunostaining. The sequences for the miRNA–FAK, PYK2, and MBD2 constructs are as follows:
(1) miRNA–FAK:
FAK-1763-T: 5′-TGCTGATCATTTGAAGACACCAGAACGTTTTGGCCACTGAC TGACGTTCTGGTCTTCAAATGAT-3′
FAK-1763-B: 5′-CCTGATCATTTGAAGACCAGAACGTCAGTCAGTGGCCAA AACGTTCTGGTGTCTTCAAATGATC-3′
(2) miRNA–PYK2:
PYK2-1761-T: 5′-TGCTGTAAGGATACAGTTCCATGACGGTTTTGGCCACTG ACTGACCGTCATGGCTGTATCCTTA-3′
PYK2-1761-B: 5′-CCTGTAAGGATACAGCCATGACGGTCAGTCAGTG GCCAAAACCGTCATGGAACTGTATCCTTAC-3′
(3) miRNA–MBD2:
MBD2-1137-T: 5′-TGCTGAGATGTGTTAAGCCAAACAGCGTTTT GGCCACTGACTGACGCTGTTTGTTAACACATCT-3′
MBD2-1137-B: 5′-CCTGAGATGTGTTAACAAACAGCGTCAGTCAG TGGCCAAAACGCTGTTTGGCTTAACACATCTC-3′
Cell culture, transfection, and myotube differentiation
HEK 293 cells were maintained in DMEM supplemented with 10% fetal calf serum, 100 Units/ml penicillin G, and streptomycin (GIBCO). Cells were plated at a density of 106 cells per 10-cm culture dish and allowed to grow for 12 h before transfection using the calcium phosphate precipitation method (Ren et al, 2004). Thirty-six hours after transfection, cells were lysed in modified RIPA buffer (50 mM Tris–HCl, pH 7.4, 150 mM sodium chloride, 1% NP40, 0.25% sodium-deoxycholate, and proteinase inhibitors) (Ren et al, 2001). Lysates were subjected to immunoprecipitation or immunoblotting.
C2C12 cells were maintained as described earlier (Kim et al, 2005). Myoblasts (80% confluency) were transiently transfected with indicated plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Twenty-four hours after transfection, myoblasts were switched to the DM.
Protein–protein interaction assays
Immunoprecipitation was carried out as described earlier (Ren et al, 2001). Cell lysates (500 μg of protein) were incubated with the indicated antibodies (1–2 μg) at 4°C for 1 h in a final volume of 1 ml modified RIPA buffer with constant rocking. After the addition of protein A/G-agarose beads, the reactions were incubated at 4°C for 1 h. Immune complexes were resolved by SDS–PAGE and subjected to immunoblotting. GST pull-down assay was carried out as described earlier (Ren et al, 2001). Transiently transfected HEK 293 cells were lysed in the modified RIPA buffer. Cell lysates were pre-cleared with GST immobilized on glutathione-sepharose 4B (Pharmacia, Piscataway, NJ), and then incubated with the indicated GST-fusion proteins (2–5 μg) immobilized on glutathione-sepharose beads at 4°C for 1 h with constant rocking. The beads were washed three times with modified RIPA buffer, and bound proteins were resolved by SDS–PAGE and subjected to immunoblotting.
In addition to coimmunoprecipiation assay, pull-down assay was used to examine protein–protein interaction as described earlier (Ren et al, 2001; Wang et al, 2003). Expressed HA–MBD2 proteins were incubated with bead-immobilized GST–FAK, PYK2, or PYK2 mutants, respectively, in the binding buffer (25 mM HEPES, pH 7.5, 1 mM DTT, 0.5% Triton X-100, and 150 mM NaCl for 2 h at 4°C on a rotator). After washing with PBST, bound MBD2 proteins were resolved on SDS–PAGE and detected by western blot analysis using anti-HA antibody.
Immunofluorescence analysis
C2C12 myoblasts or myotubes were fixed for immunostaining as described earlier (Kim et al, 2005; Zhu et al, 2007). Briefly, cells were fixed with 4% paraformaldehyde or −20°C methanol for 20 min, permeated, blocked with 2% goat serum, and incubated with indicated antibodies. Double-labelled immunostaining was done with appropriate fluorochrome-conjugated secondary antibodies. Images were taken on a Zeiss fluorescence microscope at × 63.
ChIP assay
CHIP assays were carried out as described earlier (Kim et al, 2005). C2C12 cells (myoblasts and myotubes) were incubated with 1% formaldehyde in PBS at room temperature for 30 min. The cross-linking reaction was stopped by the addition of glycine to a final concentration of 0.125 M. Eluted DNA was used as template in PCR for the amplification of the mouse myogenin gene with the following primers as described earlier (Lucarelli et al, 2001)—for the 5′-flanking region (499 bp): the forward primer, 5′-TGGAGTGGTCCTGATGTGGTAGTGG-3′ (nt 1022–1046), and the backward primer, 5′-ACCCAGAGATAAATATAGCCAACGC-3′ (nt 1496–1520); for E1 (326 bp): the forward primer, as above, and the backward primer, 5′-GCGCTCAATGTACTGGATGGCG-3′ (nt 1990–2011).
In vivo electroporation and immunohistochemical analysis
In vivo electroporation was performed as described earlier (Aihara and Miyazaki, 1998) with modification. Briefly, 4-day-old C57BL/6J mice were anesthetized with isoflurane. Plasmid encoding miRNA against FAK (miRNA–FAK1763) was injected into the tibialis anterior (TA) muscles of three mice (5 μg DNA in 10 μl TE buffer). The contra-lateral control muscle was injected with a vector encoding the scrambled miRNA. A pair of electrode needles with a 5-mm gap was inserted into the muscle to encompass the DNA injection sites, and electric pulses were delivered using an electric pulse generator (ECM830; BTX, San Diego, CA). Five pulses followed by five more pulses of the opposite polarity of 50 V each were administered to each injection site at a rate of one pulse per second, each pulse lasting for 50 ms.
Seven days after injection, mice were killed and the TA muscles were fixed in cold 4% paraformaldehyde-PBS for 24 h. Serial sections (15 μm thick) were sliced and placed on slide glasses. Samples were then fixed and immunostained with anti-myogenin antibody (F5D, mouse monoclonal antibody). GFP (green) indicates the expression of the miRNA–FAK plasmid or scrambled miRNA plasmid.
Supplementary Material
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Review Process File
Acknowledgments
This work was supported in part by grants from NIH (LM and WCX). We are grateful to Dr Gerd P Pfeifer for valuable constructs and reagents.
References
- Aihara H, Miyazaki J (1998) Gene transfer into muscle by electroporation in vivo. Nat Biotechnol 16: 867–870 [DOI] [PubMed] [Google Scholar]
- Berger J, Bird A (2005) Role of MBD2 in gene regulation and tumorigenesis. Biochem Soc Trans 33: 1537–1540 [DOI] [PubMed] [Google Scholar]
- Berkes CA, Tapscott SJ (2005) MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol 16: 585–595 [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M (1999) A mammalian protein with specific demethylase activity for mCpG DNA. Nature 397: 579–583 [DOI] [PubMed] [Google Scholar]
- Bird AP, Wolffe AP (1999) Methylation-induced repression—belts, braces, and chromatin. Cell 99: 451–454 [DOI] [PubMed] [Google Scholar]
- Brero A, Easwaran HP, Nowak D, Grunewald I, Cremer T, Leonhardt H, Cardoso MC (2005) Methyl CpG-binding proteins induce large-scale chromatin reorganization during terminal differentiation. J Cell Biol 169: 733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302: 885–889 [DOI] [PubMed] [Google Scholar]
- Detich N, Bovenzi V, Szyf M (2003) Valproate induces replication-independent active DNA demethylation. J Biol Chem 278: 27586–27592 [DOI] [PubMed] [Google Scholar]
- Detich N, Theberge J, Szyf M (2002) Promoter-specific activation and demethylation by MBD2/demethylase. J Biol Chem 277: 35791–35794 [DOI] [PubMed] [Google Scholar]
- Dugina V, Fontao L, Chaponnier C, Vasiliev J, Gabbiani G (2001) Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J Cell Sci 114: 3285–3296 [DOI] [PubMed] [Google Scholar]
- Eble DM, Strait JB, Govindarajan G, Lou J, Byron KL, Samarel AM (2000) Endothelin-induced cardiac myocyte hypertrophy: role for focal adhesion kinase. Am J Physiol Heart Circ Physiol 278: H1695–H1707 [DOI] [PubMed] [Google Scholar]
- El-Osta A, Wolffe AP (2000) DNA methylation and histone deacetylation in the control of gene expression: basic biochemistry to human development and disease. Gene Expr 9: 63–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J, Pucci AM, Koh TJ (2004) HSP25 protects skeletal muscle cells against oxidative stress. Free Radic Biol Med 37: 1455–1462 [DOI] [PubMed] [Google Scholar]
- Fatemi M, Wade PA (2006) MBD family proteins: reading the epigenetic code. J Cell Sci 119: 3033–3037 [DOI] [PubMed] [Google Scholar]
- Faure C, Corvol JC, Toutant M, Valjent E, Hvalby O, Jensen V, El Messari S, Corsi JM, Kadare G, Girault JA (2007) Calcineurin is essential for depolarization-induced nuclear translocation and tyrosine phosphorylation of PYK2 in neurons. J Cell Sci 120: 3034–3044 [DOI] [PubMed] [Google Scholar]
- Fuchs J, Demidov D, Houben A, Schubert I (2006) Chromosomal histone modification patterns—from conservation to diversity. Trends Plant Sci 11: 199–208 [DOI] [PubMed] [Google Scholar]
- Guan JL, Shalloway D (1992) Regulation of pp125FAK both by cellular adhesion and by oncogenic transformation. Nature 358: 690–692 [DOI] [PubMed] [Google Scholar]
- Hanks SK, Ryzhova L, Shin NY, Brabek J (2003) Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci 8: d982–d996 [DOI] [PubMed] [Google Scholar]
- Hendrich B, Bird A (1998) Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol Cell Biol 18: 6538–6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrich B, Guy J, Ramsahoye B, Wilson VA, Bird A (2001) Closely related proteins MBD2 and MBD3 play distinctive but interacting roles in mouse development. Genes Dev 15: 710–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T (1995) Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 377: 539–544 [DOI] [PubMed] [Google Scholar]
- Ji LL (2007) Antioxidant signaling in skeletal muscle: a brief review. Exp Gerontol 42: 582–593 [DOI] [PubMed] [Google Scholar]
- Jiang CL, Jin SG, Lee DH, Lan ZJ, Xu X, O'Connor TR, Szabo PE, Mann JR, Cooney AJ, Pfeifer GP (2002) MBD3L1 and MBD3L2, two new proteins homologous to the methyl-CpG-binding proteins MBD2 and MBD3: characterization of MBD3L1 as a testis-specific transcriptional repressor. Genomics 80: 621–629 [DOI] [PubMed] [Google Scholar]
- Jiang CL, Jin SG, Pfeifer GP (2004) MBD3L1 is a transcriptional repressor that interacts with methyl-CpG-binding protein 2 (MBD2) and components of the NuRD complex. J Biol Chem 279: 52456–52464 [DOI] [PubMed] [Google Scholar]
- Jin SG, Jiang CL, Rauch T, Li H, Pfeifer GP (2005) MBD3L2 interacts with MBD3 and components of the NuRD complex and can oppose MBD2-MeCP1-mediated methylation silencing. J Biol Chem 280: 12700–12709 [DOI] [PubMed] [Google Scholar]
- Kang JS, Yi MJ, Zhang W, Feinleib JL, Cole F, Krauss RS (2004) Netrins and neogenin promote myotube formation. J Cell Biol 167: 493–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Xiong WC, Mei L (2005) Inhibition of MuSK expression by CREB interacting with a CRE-like element and MyoD. Mol Cell Biol 25: 5329–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose R, Bird A (2003) Molecular biology. MeCP2 repression goes nonglobal. Science 302: 793–795 [DOI] [PubMed] [Google Scholar]
- Knapp JR, Davie JK, Myer A, Meadows E, Olson EN, Klein WH (2006) Loss of myogenin in postnatal life leads to normal skeletal muscle but reduced body size. Development 133: 601–610 [DOI] [PubMed] [Google Scholar]
- Langen RC, Schols AM, Kelders MC, Van Der Velden JL, Wouters EF, Janssen-Heininger YM (2002) Tumor necrosis factor-alpha inhibits myogenesis through redox-dependent and -independent pathways. Am J Physiol Cell Physiol 283: C714–C721 [DOI] [PubMed] [Google Scholar]
- Lassar AB, Buskin JN, Lockshon D, Davis RL, Apone S, Hauschka SD, Weintraub H (1989) MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell 58: 823–831 [DOI] [PubMed] [Google Scholar]
- Leonhardt H, Cardoso MC (2000) DNA methylation, nuclear structure, gene expression and cancer. J Cell Biochem Suppl 35: 78–83 [DOI] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL (2004) Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat Neurosci 7: 1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D (2008) Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell 29: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, Gorski J, Jones KR, Reichardt LF, Wu J, Rao Y (2004) Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci 7: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli M, Fuso A, Strom R, Scarpa S (2001) The dynamics of myogenin site-specific demethylation is strongly correlated with its expression and with muscle differentiation. J Biol Chem 276: 7500–7506 [DOI] [PubMed] [Google Scholar]
- Maison C, Almouzni G (2004) HP1 and the dynamics of heterochromatin maintenance. Nat Rev Mol Cell Biol 5: 296–304 [DOI] [PubMed] [Google Scholar]
- Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L (2005) Histone deacetylase 9 couples neuronal activity to muscle chromatin acetylation and gene expression. Nat Neurosci 8: 313–321 [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6: 56–68 [DOI] [PubMed] [Google Scholar]
- Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, Tempst P, Reinberg D, Bird A (1999) MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet 23: 58–61 [DOI] [PubMed] [Google Scholar]
- Olson E (1992) Activation of muscle-specific transcription by myogenic helix-loop-helix proteins. Symp Soc Exp Biol 46: 331–341 [PubMed] [Google Scholar]
- Olson EN (1993) Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol 7: 1369–1378 [DOI] [PubMed] [Google Scholar]
- Parsons JT (2003) Focal adhesion kinase: the first ten years. J Cell Sci 116: 1409–1416 [DOI] [PubMed] [Google Scholar]
- Patra SK, Patra A, Zhao H, Dahiya R (2002) DNA methyltransferase and demethylase in human prostate cancer. Mol Carcinog 33: 163–171 [DOI] [PubMed] [Google Scholar]
- Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, Ji G, Cheng L, Yang Q, Kotlikoff MI, Chen J, Chien K, Gu H, Guan JL (2006) Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest 116: 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RL, Rudnick MA (2000) Molecular mechanisms regulating myogenic determination and differentiation. Front Biosci 5: D750–D767 [DOI] [PubMed] [Google Scholar]
- Pownall ME, Gustafsson MK, Emerson CP Jr (2002) Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol 18: 747–783 [DOI] [PubMed] [Google Scholar]
- Rando TA, Disatnik MH, Yu Y, Franco A (1998) Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul Disord 8: 14–21 [DOI] [PubMed] [Google Scholar]
- Ren XR, Du QS, Huang YZ, Ao SZ, Mei L, Xiong WC (2001) Regulation of CDC42 GTPase by proline-rich tyrosine kinase 2 interacting with PSGAP, a novel pleckstrin homology and Src homology 3 domain containing rhoGAP protein. J Cell Biol 152: 971–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XR, Ming GL, Xie Y, Hong Y, Sun DM, Zhao ZQ, Feng Z, Wang Q, Shim S, Chen ZF, Song HJ, Mei L, Xiong WC (2004) Focal adhesion kinase in netrin-1 signaling. Nat Neurosci 7: 1204–1212 [DOI] [PubMed] [Google Scholar]
- Rudnicki MA, Jaenisch R (1995) The MyoD family of transcription factors and skeletal myogenesis. Bioessays 17: 203–209 [DOI] [PubMed] [Google Scholar]
- Scarpa S, Lucarelli M, Palitti F, Carotti D, Strom R (1996) Simultaneous myogenin expression and overall DNA hypomethylation promote in vitro myoblast differentiation. Cell Growth Differ 7: 1051–1058 [PubMed] [Google Scholar]
- Sekimata M, Takahashi A, Murakami-Sekimata A, Homma Y (2001) Involvement of a novel zinc finger protein, MIZF, in transcriptional repression by interacting with a methyl-CpG-binding protein, MBD2. J Biol Chem 276: 42632–42638 [DOI] [PubMed] [Google Scholar]
- Taylor JM, Rovin JD, Parsons JT (2000) A role for focal adhesion kinase in phenylephrine-induced hypertrophy of rat ventricular cardiomyocytes. J Biol Chem 275: 19250–19257 [DOI] [PubMed] [Google Scholar]
- Venuti JM, Morris JH, Vivian JL, Olson EN, Klein WH (1995) Myogenin is required for late but not early aspects of myogenesis during mouse development. J Cell Biol 128: 563–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade PA (2001) Methyl CpG-binding proteins and transcriptional repression. Bioessays 23: 1131–1137 [DOI] [PubMed] [Google Scholar]
- Wang Q, Xie Y, Du QS, Wu XJ, Feng X, Mei L, McDonald JM, Xiong WC (2003) Regulation of the formation of osteoclastic actin rings by proline-rich tyrosine kinase 2 interacting with gelsolin. J Cell Biol 160: 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ding YQ, Hong Y, Feng Z, Navarre S, Xi CX, Zhu XJ, Wang CL, Ackerman SL, Kozlowski D, Mei L, Xiong WC (2005) Phosphatidylinositol transfer protein-alpha in netrin-1-induced PLC signalling and neurite outgrowth. Nat Cell Biol 7: 1124–1132 [DOI] [PubMed] [Google Scholar]
- Xiong W, Parsons JT (1997) Induction of apoptosis after expression of PYK2, a tyrosine kinase structurally related to focal adhesion kinase. J Cell Biol 139: 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi XP, Wang X, Gerdes AM, Li F (2003) Subcellular redistribution of focal adhesion kinase and its related nonkinase in hypertrophic myocardium. Hypertension 41: 1317–1323 [DOI] [PubMed] [Google Scholar]
- Zaccagnini G, Martelli F, Magenta A, Cencioni C, Fasanaro P, Nicoletti C, Biglioli P, Pelicci PG, Capogrossi MC (2007) p66(ShcA) and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hind limb ischemia. J Biol Chem 282: 31453–31459 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D (1999) Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev 13: 1924–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL (2003) Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell 11: 1503–1515 [DOI] [PubMed] [Google Scholar]
- Zhu XJ, Wang CZ, Dai PG, Xie Y, Song NN, Liu Y, Du QS, Mei L, Ding YQ, Xiong WC (2007) Myosin X regulates netrin receptors and functions in axonal path-finding. Nat Cell Biol 9: 184–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Review Process File