Abstract
Nicotinic acetylcholine receptors (nAChRs) are homo- or heteropentameric ligand-gated ion channels mediating excitatory neurotransmission and muscle activation. Regulation of nAChR subunit assembly and transfer of correctly assembled pentamers to the cell surface is only partially understood. Here, we characterize an ER transmembrane (TM) protein complex that influences nAChR cell-surface expression and functional properties in Caenorhabditis elegans muscle. Loss of either type I TM protein, NRA-2 or NRA-4 (nicotinic receptor associated), affects two different types of muscle nAChRs and causes in vivo resistance to cholinergic agonists. Sensitivity to subtype-specific agonists of these nAChRs is altered differently, as demonstrated by whole-cell voltage-clamp of dissected adult muscle, when applying exogenous agonists or after photo-evoked, channelrhodopsin-2 (ChR2) mediated acetylcholine (ACh) release, as well as in single-channel recordings in cultured embryonic muscle. These data suggest that nAChRs desensitize faster in nra-2 mutants. Cell-surface expression of different subunits of the ‘levamisole-sensitive' nAChR (L-AChR) is differentially affected in the absence of NRA-2 or NRA-4, suggesting that they control nAChR subunit composition or allow only certain receptor assemblies to leave the ER.
Keywords: channelrhodopsin-2, nAChR biogenesis, Nicalin, NOMO, single-channel properties
Introduction
Nicotinic acetylcholine receptors (nAChRs) are homo- or heteropentamers composed of α- and non-α-subunits, which mediate fast synaptic transmission in neurons and muscles (Changeux and Edelstein, 2005). The agonist binds at the interface between an α-subunit and either another α- or a non-α-subunit (Chiara and Cohen, 1997). Two or three acetylcholine (ACh) molecules need to bind for maximal activation (Karlin, 2002; Rayes et al, 2009); thus, functional properties of nAChRs are affected by the number of α-subunits, and the presence of particular subunits in the pentamer. In vertebrates, α-, β-, δ-, γ- and ɛ-subunits are found in muscle, and nAChRs are of α2βδγ or α2βδɛ composition, depending on the developmental stage (Mishina et al, 1986); in neurons, 9 α- and 3 β-subunits form α5- or α2β3-type receptors. The nAChR subunit repertoire of Caenorhabditis elegans is even more complex: its genome encodes 29 confirmed nAChR subunits (Jones et al, 2007), of which at least seven are expressed in muscle, based on microarray profiling and biochemical purification (Gottschalk et al, 2005; Touroutine et al, 2005; Fox et al, 2007). However, expression of additional nAChRs in muscle was demonstrated (Treinin et al, 1998).
Regulating nAChR subunit composition is an important way to fine-tune cholinergic signalling. Subunit combinations can be predetermined by cell-specific expression, and many potential assembly intermediates may be unstable due to incompatible subunit interfaces. In vertebrate neurons, a vast variety of nAChRs could be generated; however, only few combinations were detected experimentally (Gotti et al, 2007). Out of the 208 possible combinations of vertebrate muscle nAChR subunits, only one is found in mature muscle. To some extent, this is explained by sequence-specific interactions within the N-terminal, as well as the first transmembrane (TM) domains, according to different models (Gu et al, 1991; Kreienkamp et al, 1995; Wang et al, 1996; Keller and Taylor, 1999; Wanamaker et al, 2003). HSP70 chaperones and the ER quality control assist in nAChR assembly (Blount and Merlie, 1991; Keller et al, 1996, 1998; Keller and Taylor, 1999). The ER-resident TM protein RIC-3 and the Golgi-associated protein UNC-50 are also required for efficient nAChR assembly, maturation or trafficking from the ER and beyond (Halevi et al, 2002; Eimer et al, 2007), and 14-3-3 proteins further assist nAChRs in leaving the ER (Jeanclos et al, 2001). Immature assemblies and single subunits are retained in the ER, as they expose retention motifs in the first TM helix, which are masked only on closed pentamer formation (Wang et al, 2002). Yet, no factors are known that select particular subunits for incorporation into mature receptors, particularly in cells expressing many different nAChR subunits. It is further unknown whether there is active sorting that allows only particular nAChRs to exit the ER.
The C. elegans ‘levamisole-sensitive' nAChR (L-AChR) is expressed in muscle cells, but some of its subunits are also found in neurons. Genetic screens based on levamisole-induced paralysis defined three essential subunits: UNC-38, UNC-63 (both α-subunits) and UNC-29 (non-α; Lewis et al, 1987; Fleming et al, 1997; Culetto et al, 2004). Additional L-AChR subunits, LEV-8 (α) and LEV-1 (non-α), are considered non-essential as their loss confers weak levamisole resistance (Lewis et al, 1987; Culetto et al, 2004; Towers et al, 2005). Co-expression of these five subunits in Xenopus oocytes, together with essential L-AChR biogenesis factors, RIC-3, UNC-50 and UNC-74, sufficed to constitute levamisole-activated currents (Boulin et al, 2008). An electrophysiologically defined ‘nicotine-sensitive' N-AChR contributes to ACh currents at neuromuscular junctions (NMJs). This apparently homopentameric receptor consists of ACR-16 subunits (Francis et al, 2005; Touroutine et al, 2005).
To define proteins contributing to L-AChR function, we previously purified the L-AChR by tandem affinity purification and identified co-purified proteins by mass spectrometry (Gottschalk et al, 2005). In addition to the five genetically identified L-AChR subunits, we found two more α-subunits, ACR-8 and ACR-12. Although ACR-12 is expressed in neurons only, ACR-8 is expressed in body wall muscle cells. Thus, seven nAChR subunits are implicated in L-AChR function in vivo, suggesting that L-AChRs may represent a mixed population of pentamers with variable subunit composition, and/or that their composition could depend on the particular cell. Non-nAChR proteins that co-purified with the L-AChR were screened for effects on the in vivo sensitivity to cholinergic agonists (Gottschalk et al, 2005). Among proteins causing reduced agonist sensitivity was the product of gene T05F1.1, subsequently termed nra-2.
Here, we show that NRA-2, in complex with a second protein, NRA-4, acts in the ER to affect functional properties and subunit composition of L-AChRs expressed at synapses. Electrophysiological properties of L- and N-AChRs are altered in nra-2 and nra-4 mutants, as well as single-channel L-AChR properties in embryonic muscle, consistent with faster desensitization of L-AChRs. Synaptic expression of UNC-29 and, particularly, UNC-38 subunits are characteristically altered in nra-2 and nra-4 mutants. Mutations in acr-8 suppress nra-2 phenotypes, and synaptic expression of ACR-8 is increased in nra-2 mutants, uncovering a reciprocal regulation of UNC-38 versus ACR-8 α-subunit incorporation into synaptic nAChRs by NRA-2. Thus, NRA-2 and NRA-4 affect L-AChR properties by altering subunit composition and/or the relative abundance of particular L-AChR subtypes at the synapse.
Results
NRA-2 and NRA-4 are type I TM proteins associated with L-AChRs
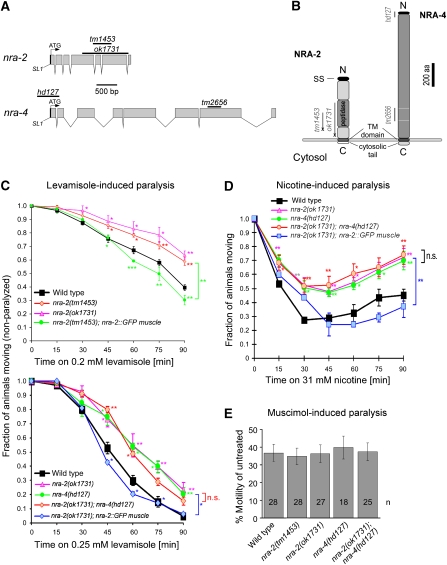
NRA-2 is a type I TM protein, consisting of a 518 amino acid (aa) luminal domain and an 18 aa cytosolic tail (Figure 1A and B), and contains a peptidase domain, likely inactive, as certain amino acids are non-conserved (Supplementary Figure 1). NRA-2 resembles vertebrate Nicalin (nicastrin-like protein; Supplementary Figures 2 and 3). Nicastrins are subunits of the integral membrane peptidase γ-secretase (Yu et al, 2000). Nicalin, which is not part of γ-secretase, antagonizes TGFβ signalling in an ill-defined manner, acting in complex with a second type I TM protein, termed NOMO (nodal modulator) in the ER (Haffner et al, 2004, 2007). Nicalin and NOMO were shown to stabilize each other in this complex. Interestingly, the C. elegans homologue of NOMO (gene C02E11.1; Figure 1A; Supplementary Figures 4 and 5), was among the proteins we co-purified with the L-AChR (Gottschalk et al, 2005). We termed this protein NRA-4. NRA-4 has a 1068 aa luminal domain, a 30 aa cytosolic tail and no motifs suggesting a function (Figure 1B). Both nra-2 and nra-4 produce only single-splice variants, based on published ESTs (www.wormbase.org) and sequencing of full-length cDNAs obtained from Y Kohara. Deletion alleles of nra-2 (tm1453 and ok1731) and nra-4 (hd127 and tm2656) were obtained for further study (Figure 1; Supplementary Figures 2 and 4).
Figure 1.
Cholinergic agonist-induced phenotypes are altered in nra-2 and nra-4 mutants, and rescued by muscle-specific expression. (A) The nra-2 and nra-4 genes, as annotated in www.wormbase.org, were confirmed by sequencing cDNAs kindly provided by Y Kohara. Sequences deleted in the alleles used are indicated by bars. (B) The nra-2 and nra-4 genes encode predicted type I TM proteins with signal sequences (SS), thus they are expected to be synthesized into the ER lumen, exposing a short C-terminal cytosolic tail. Deletion/insertion alleles tm1453 and ok1731 truncate NRA-2, bringing stop codons (X) in frame. nra-4(hd127) removes part of the promoter and exon I including SS and start codon and tm2656 is a predicted in-frame deletion. (C, D) Paralysis time-course of wild-type and mutant animals exposed to 0.2 or 0.25 mM levamisole (C) or 31 mM nicotine (D). The fraction of non-paralyzed animals was counted every 15 min. Experiments were repeated 3–7 times (30 animals tested each time), data represent mean±s.e.m., statistically significant differences to wild type are indicated (*P<0.05; **P<0.01; ***P<0.001). Brackets indicate overall significant differences between genotypes, if they were different for at least three time points. (E) Swimming cycles of animals immersed for 1 h in M9 buffer with 8 mM muscimol, a GABAAR agonist, were normalized to swimming cycles of untreated control animals.
Mutants in nra-2(ok1731) were slightly uncoordinated, and nra-2(ok1731) and nra-4(tm2656) mutants showed reduced brood size (data not shown). The nra-2 alleles truncate the NRA-2 protein C-terminal, leaving only 294 (tm1453) or 212 (ok1731) aa of the luminal domain (Supplementary Figure 6). Alleles of nra-4 delete N- (hd127) or C-terminal (tm2656) sequences. hd127 is predicted to remove 183 nt of the promoter and the first 48 aa, including a leader sequence (Figure 1; Supplementary Figure 6). As the second exon, unaffected by hd127, begins with an ATG, a protein without leader sequence could be made. RT–PCR analysis confirmed the presence of an nra-4 transcript lacking exon 1 in hd127 mutants (data not shown). However, it is unclear whether the truncated promoter expresses in the same tissues as the full-length promoter, or whether any functional protein is made in this mutant. The nra-4(tm2656) allele removes aa 816–920 of the luminal domain in-frame, leaving TM domain and cytosolic tail intact (Figure 1; Supplementary Figure 6). As most assays used in this work showed no phenotypes of nra-4(tm2656), we consider it at most a reduction-of-function allele (see Supplementary Figure 7 for a summary of experiments involving nra-4(tm2656)).
NRA-2 and NRA-4 affect in vivo sensitivity to cholinergic, but not GABAergic agonists, and act cell autonomously in muscle
We tested the nra-2 and nra-4 mutants in paralysis assays for altered in vivo sensitivity to cholinergic agonists (nicotine and levamisole), and to aldicarb, an ACh-esterase inhibitor that causes ACh accumulation in the synaptic cleft. Both alleles of nra-2 as well as nra-4(hd127) caused mild resistance to either drug, indicating reduced activity of muscle nAChRs (Figure 1C and D; Supplementary Figure 8). The paralysis phenotypes could be reversed by expression of the nra-2 cDNA in muscle only (using pmyo-3), and nra-4 under its own promoter, in the respective mutants (Figure 1C and D; Supplementary Figure 9A). Thus, at least NRA-2 acts cell autonomously in muscle. Double mutants of nra-2 and nra-4 (and double RNAi; data not shown) showed no exacerbation of the single-mutant effects in paralysis assays, indicating that NRA-2 and NRA-4 act in the same pathway.
To test whether NRA-2 and NRA-4 generally affect ligand-gated ion channels at the NMJ, we assayed function of the inhibitory GABAA receptor. Swimming behaviour was analysed in the presence of muscimol, a GABAAR agonist that slows down swimming rate. Muscimol sensitivity was unaffected in nra-2, nra-4 or nra-2; nra-4 double mutants, indicating that nra-2 and nra-4 do not act on GABAARs (Figure 1E).
Human Nicalin partially functions in C. elegans, likely independent of TGFβ signalling
The Nicalin/NOMO ER protein complex was shown to act in signalling through the nodal TGFβ pathway, but a potential function in vertebrate nAChR biology was not investigated (Haffner et al, 2004). We thus asked whether human Nicalin could rescue nra-2 cholinergic phenotypes. Human Nicalin cDNA, fused to GFP, was expressed in muscle cells of nra-2(ok1731) mutants, which caused partial rescue of levamisole and nicotine resistance phenotypes (Supplementary Figure 9B), indicating potential conservation of an nAChR-associated function of Nicalin. However, transgenic animals were small, slightly uncoordinated, and Nicalin∷GFP partially aggregated (Supplementary Figure 9C), possibly preventing full rescue.
As nra-2 and nra-4 mutants may affect cholinergic signalling indirectly through TGFβ pathways, we tested mutants in these pathways for cholinergic phenotypes. C. elegans has five TGFβ ligands (Savage-Dunn, 2005): two are of unknown function, DAF-7 controls the dauer larval state (Ren et al, 1996), whereas DBL-1 affects body size (Suzuki et al, 1999) and GABA signalling at the NMJ (Vashlishan et al, 2008), neither of which is altered in nra-2 or nra-4 mutants. UNC-129 affects dorsoventral axon guidance of some motor neurons, and could thus affect the NMJ (Colavita et al, 1998). We analysed levamisole and nicotine paralysis in the mutants unc-129(ev554), dbl-1(wk70), daf-7(e1372) and in TGFβ receptor mutants daf-1(m402) and sma-6(wk7) (Supplementary Figure 10A and B). dbl-1(wk70) and sma-6(wk7) animals were hypersensitive to nicotine and levamisole. For dbl-1, this was previously shown to be caused by a GABA signalling defect (Vashlishan et al, 2008). sma-6(wk7) mutants were sick and paralyzed immediately, likely indicating a cuticle defect. The other mutants had normal sensitivity to cholinergic agonists. Effects of nra-2 and nra-4 alleles on TGFβ signalling are most likely not causing the observed cholinergic defects, though we cannot completely rule out that the two TGFβ ligands of unknown function may affect NMJs.
NRA-2 and NRA-4 form a protein complex in the ER and co-localize with the L-AChR
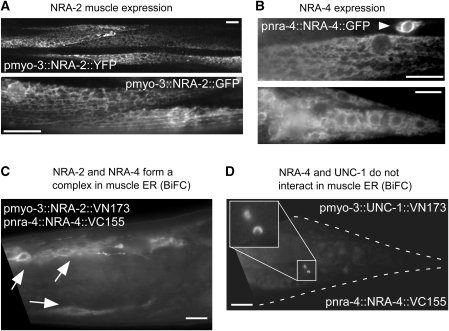
NRA-2 and NRA-4 may affect nAChR biogenesis and/or function either in the ER, in which the vertebrate homologues form a complex, in the Golgi, the secretory pathway or at synapses. To determine the site of action of these proteins, we analysed their subcellular localization using fluorescent proteins as tags. NRA-2∷GFP, NRA-2∷mCherry and NRA-4∷GFP showed a reticular pattern reminiscent of the ER in muscles (for NRA-2 and NRA-4; Figures 2 and 3) and other cells (for NRA-4∷GFP only; Figure 2B; pnra-4 and pnra-2 are active in muscles, neurons and other tissues; Supplementary Figure 11). NRA-2∷GFP also co-localized with an ER marker in HeLa cells (data not shown). To study whether NRA-2 and NRA-4 physically interact in vivo, we used bimolecular fluorescence complementation (BiFC; Chen et al, 2007; Shyu et al, 2008). Indeed, NRA-2 and NRA-4 interact within the ER membrane (Figure 2C), whereas NRA-4 and an unrelated control membrane protein, the stomatin UNC-1, do not (Figure 2D). Thus, NRA-2 and NRA-4 form a membrane protein complex in the ER of muscle cells, in which they may interact with nAChRs during biogenesis and assembly.
Figure 2.
NRA-2 and NRA-4 are expressed in the ER and interact in a complex. (A) NRA-2∷YFP (upper panel, single confocal plane) or NRA-2∷GFP (lower panel, epifluorescence) were expressed from the muscle-specific pmyo-3 promoter. Reticular expression, reminiscent of the ER was found. (B) NRA-4∷GFP was expressed from the endogenous pnra-4 promoter. Intracellular, reticular expression was observed in muscle cells (upper panel) and neurons (arrowhead), and in other tissues (lower panel: muscles, neurons and hypodermal cells in the tail). (C) NRA-2 and NRA-4 form a complex, as shown by bimolecular fluorescence complementation (BiFC). NRA-2 was fused to the VN173 fragment of Venus, and NRA-4 to the VC155 fragment. Fluorescence was restored in muscle ER (arrows point to muscle cell nuclei surrounded by ER), in which the two proteins were co-expressed. (D) NRA-4∷VC155 does not interact in the ER with the stomatin UNC-1∷VN173, expressed in muscle (a gift by ZW Wang). Occasionally, vesicular fluorescent structures were observed, possibly representing lysosomes in which the fusion proteins are degraded and in whose membranes their cytosolic tails (and Venus fragments) accumulate. Size bars are 10 μm.
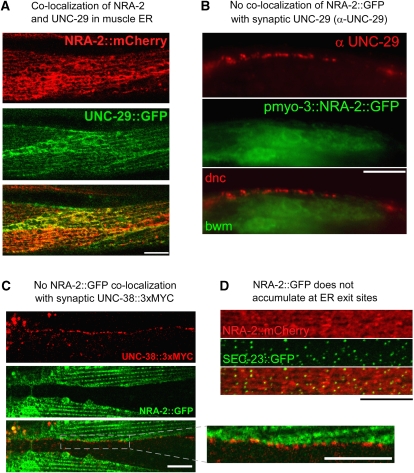
Figure 3.
NRA-2 co-localizes with L-AChR subunits in the ER, but not at synapses. (A) NRA-2∷mCherry (expressed from the pmyo-3 promoter) was co-expressed with the L-AChR subunit UNC-29∷GFP (expressed from punc-29) and co-localization was observed by confocal microscopy (single confocal plane of midbody muscle cells). (B) Endogenous UNC-29 protein was immunolabelled with specific antibodies in animals expressing NRA-2∷GFP in muscles (GFP fluorescence was preserved during fixation). Dorsal nerve cord (dnc) and adjacent muscle cells (bwm) are shown near the pharyngeal terminal bulb. No co-localization of NRA-2∷GFP and UNC-29 was apparent. (C) NRA-2∷GFP was co-expressed in muscle with epitope-tagged UNC-38∷3xMYC (expressed from punc-38). UNC-38, exposing the MYC tag on the cell surface, was labelled with Cy3-conjugated anti-MYC antibodies injected into the body cavity. The ventral nerve cord was imaged by confocal microscopy (single focal plane), showing punctate cell-surface L-AChR clusters that contain UNC-38. NRA-2∷GFP is adjacent to L-AChR clusters, but not co-localizing with them (inset: enlarged region). (D) SEC-23∷GFP, a COPII coat component that labels ER exit sites, and NRA-2∷mCherry were co-expressed in muscle and imaged by confocal microscopy. Puncta of SEC-23 accumulation contained also NRA-2; however, NRA-2 did not accumulate at these sites. Z-stack of confocal sections. Size bars are 10 μm.
Consistent with this idea, NRA-2∷mCherry and the L-AChR subunit UNC-29∷GFP largely co-localized in ER membranes (Figure 3A). Although L-AChR subunits are visible in the ER only when over-expressed (endogenous L-AChRs are only detectable at synapses; Figure 3B; Gally et al, 2004), a diffuse localization of nascent nAChRs in the ER is not unexpected. Several additional observations argue against direct interactions of NRA-2/NRA-4 with L-AChRs at synapses: (1) NRA-2∷GFP and NRA-4∷GFP did not accumulate at the plasma membrane or the tips of muscle arms, in which NMJ postsynaptic elements are found (Gottschalk et al, 2005; Gottschalk and Schafer, 2006; Eimer et al, 2007). (2) The endogenous L-AChR subunit UNC-29 does not co-localize with NRA-2∷GFP (Figure 3B). (3) NRA-2∷GFP does not co-localize with the synaptic UNC-38∷3xMYC L-AChR subunit (Figure 3C; the latter one immunolabelled at the cell surface, using fluorescent antibodies injected into the body cavity; Gottschalk et al, 2005; Gottschalk and Schafer, 2006; Eimer et al, 2007). (4) Minor amounts of cell-surface NRA-2 were detected with extracellular anti-HA antibody in animals expressing 3xHA∷NRA-2∷GFP, in clusters along muscle cell boundaries (Supplementary Figure 12), but this did not accumulate at nerve cords, in which synaptic L-AChRs are found. Cell-surface expression of 3xHA∷NRA-2∷GFP may be due to overexpression (its binding partner NRA-4 was not overexpressed). In sum, our observations do not support an interaction of NRA-2 with L-AChRs at synapses.
NRA-2 may interact with L-AChRs during assembly, or when they are sorted for ER exit. However, NRA-2∷mCherry and SEC-23∷GFP, a COPII coat component localizing to ER exit sites and secretory vesicles (Roberts et al, 2003) showed different localization patterns: SEC-23∷GFP was found in punctate intracellular clusters, whereas NRA-2∷mCherry was not enriched at these sites (Figure 3D). Thus, NRA-2 is likely not part of the ER exit machinery.
Cholinergic inward currents in muscle cells are reduced in nra-2 and nra-4 mutants
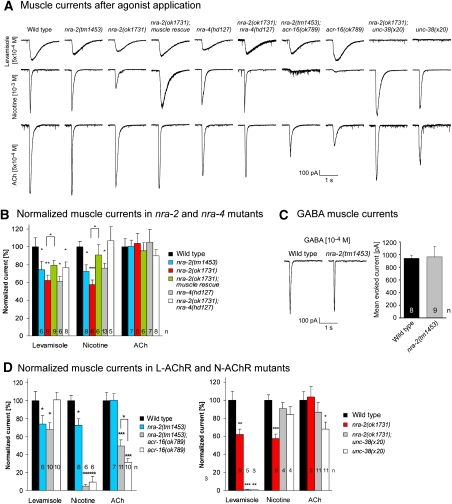
To directly measure nAChR and GABAAR function in muscle, we recorded postsynaptic currents (PSCs) evoked by pressure-applied ACh, levamisole, nicotine and GABA under whole-cell voltage-clamp (Supplementary Table 1; Richmond and Jorgensen, 1999; Francis et al, 2003; Richmond, 2006; Liewald et al, 2008). Levamisole- and nicotine-evoked PSCs were significantly reduced in both nra-2 mutants (ok1731: levamisole: 62±6%, normalized to wild type, P<0.01, t-test; nicotine: 57±5%, P<0.001; tm1453: levamisole: 74±9%, P<0.05; nicotine: 72±8%, P<0.05), as well as in nra-4(hd127) mutants (levamisole: 61±5%, P<0.05; nicotine: 76±6%, P<0.05), indicating that both L-AChRs and N-AChRs, were functionally compromised in these animals (Figure 4A and B). GABA-evoked PSCs were not affected (nra-2(tm1453): 103±17%; Figure 4C). Levamisole-induced PSCs in nra-2(ok1731); nra-4(hd127) double mutants were not further reduced than in single mutants, again indicating a function of NRA-2 and NRA-4 in the same pathway. Yet, nicotine-evoked PSCs were normal in these double mutants. Possibly, some nra-2 and nra-4 effects on L- and N-AChRs are allele specific, and such effects may be partly compensated in double mutants, for example, due to direct physical interactions of NRA-2 and NRA-4. Levamisole- and nicotine-induced PSCs in nra-2(ok1731) mutants were rescued by muscle-specific expression of NRA-2∷GFP (Figure 4A and B), confirming the cell-autonomous function of NRA-2.
Figure 4.
Whole-cell voltage-clamp analysis of muscle cells reveals altered nAChR function in nra-2 and nra-4 mutants. (A) Representative traces for levamisole- (top), nicotine- (middle) and ACh-evoked (bottom) muscle currents in wild-type animals and various mutants of nra-2, nra-4, L- and N-AChR subunits. (B) Normalized mean peak values of levamisole-, nicotine- and ACh-mediated muscle currents in wild-type animals and various nra-2 and nra-4 mutants, and nra-2(ok1731) animals rescued in muscle by NRA-2∷GFP expression. Only GFP-positive cells were patched. (C) Representative traces (left) and mean peak values (right) of GABA-mediated muscle currents were not altered in nra-2(tm1453) mutants, compared with wild type. (D) Normalized mean peak values of levamisole-, nicotine- and ACh-mediated muscle currents in wild-type animals, nra-2(tm1453 or ok1731) mutants as well as in mutants lacking the N-AChR (acr-16(ok789); left) or L-AChR (unc-38(x20); right), and respective double mutants. Displayed are means±s.e.m., statistically significant differences to the wild type are indicated (*P<0.05; **P<0.01; ***P<0.001), as well as the number of animals.
Short-term ACh sensitivity of L- and possibly N-AChRs is increased in nra-2 mutants
On the basis of agonist-evoked PSCs, both L- and N-AChRs are affected in nra-2 and nra-4 mutants. This is not seen in paralysis assays, as acr-16 mutants are not resistant to either agonist, in contrast to L-AChR mutants (Supplementary Figure 13), stressing differences between behavioural and electrophysiological phenotypes of L- versus N-AChR mutations. These could depend on the duration of agonist exposure, as L-AChRs desensitize much more slowly than N-AChRs. Surprisingly, PSCs in response to short-term ACh application in both nra-2 alleles, in nra-4(hd127) mutants and in several double-mutant combinations, were indistinguishable from the wild type (Figure 4A and B; Supplementary Figure 14). This was unexpected, as L- and N-AChRs are the only nAChRs contributing to cholinergic signalling at the NMJ (Richmond and Jorgensen, 1999; Francis et al, 2005; Touroutine et al, 2005).
Our findings indicated that sensitivity of the two nAChRs was altered in an agonist-specific manner, that is, reduced for levamisole and nicotine, but largely unaltered for ACh. As both nAChRs contribute to ACh PSCs, they could be differently affected for ACh sensitivity. To examine this, we assayed properties of each AChR individually, in acr-16(ok789) or unc-38(x20) mutants, in nra-2 or nra-4 backgrounds (Figure 4A and D; Supplementary Figure 14).
In acr-16(ok789); nra-2(tm1453) mutants, in which only the L-AChR contributes to PSCs, levamisole sensitivity was reduced as in nra-2(tm1453) mutants (68±7%, P<0.05), whereas nicotine sensitivity was abolished (5±2%, P<0.001), as in acr-16(ok789) single mutants (6±1%, P<0.001). However, ACh sensitivity in the acr-16; nra-2(tm1453) double mutants (50±7%, P<0.001, versus wild type) was significantly increased when compared with the acr-16 single mutant (32±5%, P<0.001, versus wild type; P<0.05, versus acr-16 mutant). This indicates that ACh sensitivity of the L-AChR was increased by nra-2(tm1453), even though sensitivity to the L-AChR-specific agonist levamisole was decreased. Thus, functional properties of the L-AChR may be altered in nra-2(tm1453) animals. In acr-16(ok789); nra-4(hd127) double mutants, ACh PSCs were not increased. These animals also had normal levamisole responses, but reduced nicotine responses (Supplementary Figure 14). This discrepancy to nra-2 mutant phenotypes may be due to allele-specific effects, or could point to different functions of the two proteins in the heteromeric complex.
Mean ACh sensitivity of the N-AChR was affected similarly when unc-38(x20) single mutants and nra-2(ok1731); unc-38(x20) double mutants were compared. Although ACh PSCs were significantly reduced in unc-38 mutants (68±8%, P<0.05), they were not significantly different from wild type (87±10%) in the nra-2; unc-38 double mutant, possibly suggesting that ACh sensitivity of also the N-AChR is increased by nra-2(ok1731). Interestingly, nicotine sensitivity of the N-AChR, which was significantly reduced in nra-2(ok1731) single mutants (57±5% of wild type, P<0.001), was not altered in nra-2(ok1731); unc-38(x20) double or unc-38(x20) single mutants (91±6% and 84±9% of wild type, respectively), possibly due to compensatory changes induced by lack of the L-AChR (Figure 4A and D). Although nra-2 effects were not as clear as for the L-AChR, our findings indicate that also N-AChR functional properties are altered in nra-2 mutants in an agonist-specific manner.
PSCs after prolonged optogenetic ACh release reveal altered nAChR desensitization in nra-2 mutants
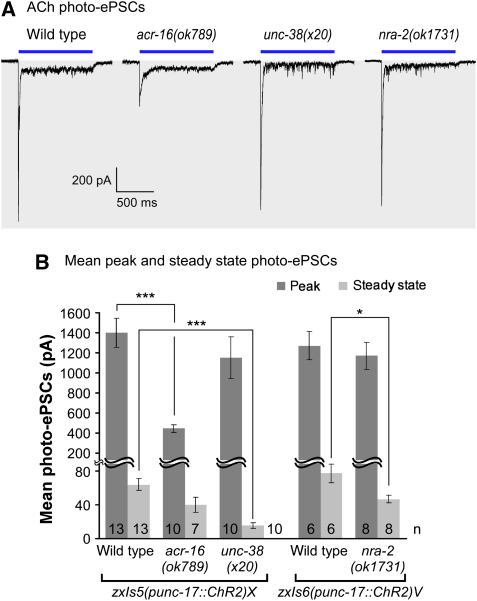
Somewhat contrasting our electrophysiological results, in which no reduction of acute ACh responses was seen in nra-2 mutants, these animals showed a slight resistance in aldicarb assays, in which endogenous ACh accumulates in the synaptic cleft (Supplementary Figure 8). This could indicate reduced postsynaptic AChR sensitivity, or reduced presynaptic ACh release. Aldicarb assays take 1–2 h, whereas the ‘puff' application of ACh in electrophysiological assays lasts only 70 ms, using non-physiological amounts of ACh, broadly sprayed over the muscle cell. Thus, long-term effects such as altered desensitization may cause different results in both types of experiments. To examine this, we used the light-gated cation channel channelrhodopsin-2 (ChR2) to stimulate ACh release at the NMJ, at endogenous levels, only at synapses, and for short or long durations (Liewald et al, 2008).
We photo-stimulated sustained release of ACh (1000 ms), which evokes large peak currents, followed by small steady-state currents that occur after nAChR desensitization. In acr-16 mutants (i.e. when only the L-AChR is present), we observed largely reduced peak, but unaltered steady-state currents. In unc-38(x20) mutants (N-AChR only), we observed reduced steady-state currents, and no major effects on peak currents (Figure 5). The differences in steady-state currents are likely explained by different rates of desensitization of the two nAChRs, and can thus help distinguishing which of the two nAChRs is affected. In nra-2(ok1731) mutants, we observed no significant differences in the peak photo-ePSCs (Figure 5), whereas steady-state currents were significantly smaller than in wild type. Our results rule out presynaptic defects, and indicate that alterations in the desensitization rate of L-AChRs may cause the slight aldicarb resistance of nra-2 mutants.
Figure 5.
Optogenetic analysis of ACh transmission in cholinergic and nra-2 mutants using channelrhodopsin-2 (ChR2). (A) Whole-cell voltage-clamp was used to record photo-ePSCs in animals expressing ChR2 in cholinergic motor neurons (punc-17 promoter), in response to a 1000 ms photo-stimulus, as described earlier (Liewald et al, 2008). Representative peak and steady-state currents were compared in wild type, acr-16(ok789), unc-38(x20) and nra-2(ok1731) mutants. Duration of light stimulus is indicated by a bar. (B) Mean peak and steady-state photo-ePSCs, obtained using two different integrated transgenes, as indicated. Displayed are mean currents±s.e.m., statistically significant differences to the wild type are indicated (t-test; *P<0.05; ***P<0.001), as is the number of animals used.
Single-channel L-AChR properties are altered in nra-2 mutant embryonic muscle
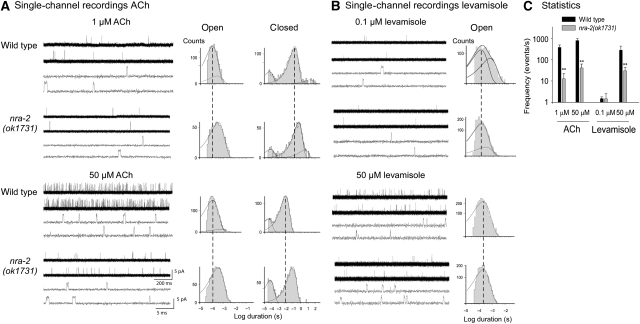
To assay L-AChR properties in more detail, we recorded single-channel currents from cell-attached patches of cultured embryonic muscle cells, which show activity of L- but not of N-AChRs (Rayes et al, 2007). We compared channels from wild type and nra-2(ok1731) mutants in the presence of different concentrations of ACh or levamisole.
Single-channel openings of about 3.5 pA activated by ACh or levamisole were detected in nra-2(ok1731) mutant muscle cells at −100 mV (Figure 6A and B). For both agonists, opening frequency increased with agonist concentration but was strongly reduced in nra-2(ok1731) mutants (Figure 6C), as observed in the closed time histogram by displacement of the main component to longer durations. This could reflect lower cell-surface density and/or altered open probability and/or increased desensitization of L-AChRs in the patch. Open time distributions of L-AChRs activated by 1 μM ACh in wild type cells are fitted by two exponential components (Rayes et al, 2007); duration of the main component (relative area <0.85) is 100±20 μs (Figure 6A). Significant changes in the open time distributions were observed in nra-2 mutants. For ACh, the mean open duration was three-fold longer than that of wild type L-AChRs; open time histograms showed a single component of 350±50 μs (Figure 6A). For channels activated by 0.1 μM levamisole, open time histograms are fitted by two components in wild type and nra-2 mutants. Yet, the mean open time of the slowest component was significantly briefer in levamisole-activated channels recorded from nra-2 mutant cells (τon=310±50 μs, relative area 0.1±0.08) with respect to wild type (τon=600±70 μs, relative area 0.35; Figure 6B). Higher levamisole concentrations produce open-channel block, which is observed as a reduction in the mean open time. The decreased frequency of opening events, increased closed times and decreased open durations of levamisole-activated L-AChRs from nra-2 mutants are in line with the reduced levamisole-induced PSCs in adult nra-2 mutants (Figure 4A and B). The comparison is not straightforward for ACh responses, as the increase in open duration but not the reduction in opening frequency supports increased ACh sensitivity. Yet, the reduced frequency may be explained by an increase in desensitization, consistent with the results from the optogenetic ACh release experiments. In sum, our single-channel recordings confirm that L-AChR functional properties differ significantly in nra-2 mutants, indicating agonist-specific kinetic changes of L-AChRs.
Figure 6.
Single-channel properties of the L-AChR in cell-attached patches of cultured embryonic muscle cells are altered in nra-2(ok1731) mutants. (A) Single-channel currents recorded from wild type and nra-2(ok1731) muscle cells in the presence of 1 μM (upper panel) and 50 μM (lower panel) ACh. Shown are representative traces (left) and open and closed time histograms (right). (B) Single-channel currents activated by 0.1 μM (upper panel) and 50 μM (lower panel) levamisole; representative traces (left) and open time histograms (right). (C) Frequency of channel openings in mutant and wild-type animals. Channel events were counted within the first minute of recording and plotted as events/s. Holding potential in all recordings was −100 mV. Displayed are means±s.d.
Contribution of ACR-8 and LEV-8 to L-AChR function in nra-2 and nra-4 mutants
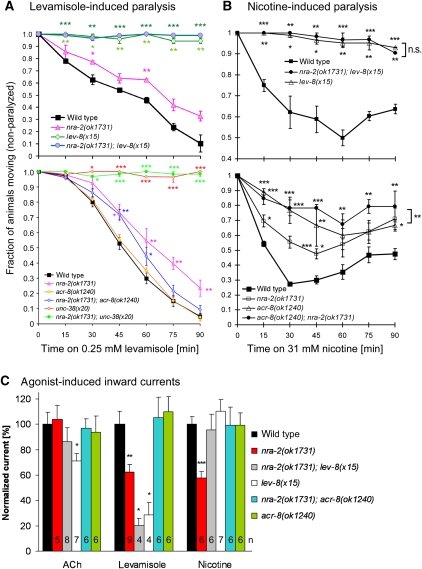
How do nra-2 and nra-4 mutations affect L-AChR properties? Receptor properties could be determined by posttranslational modifications, or by subunit composition of the pentamer. In purified L-AChRs, we identified seven subunits, more than the five present in any individual channel: ACR-8, ACR-12, UNC-63, UNC-38, UNC-29, LEV-8 and LEV-1 (Gottschalk et al, 2005). Co-expression of the latter five subunits reconstitutes levamisole-specific currents in Xenopus oocytes (Boulin et al, 2008). ACR-12 is expressed only in motor neurons, but a potential contribution of ACR-8, also expressed in muscle (Gottschalk et al, 2005), was not tested in oocytes. Different L-AChR populations with variable subunit content could exist, and the relative contribution of individual subunits to L-AChRs could be controlled by NRA-2/NRA-4. As essential subunits, UNC-38, UNC-63 and UNC-29 should be present in every L-AChR pentamer. Yet, receptors containing more than one of the essential subunits, for example, two copies of UNC-38, may exist, and one of them may be replaced with a non-essential α-subunit, LEV-8 and ACR-8, preserving function, but possibly altering functional properties. Thus, we investigated the contribution of ACR-8 and LEV-8 to NMJ function, in nra-2, lev-8 or acr-8 single mutants, or in combination.
Mutants lacking LEV-8 showed strong levamisole- and nicotine-resistance in paralysis assays, just like mutants in the essential subunit UNC-38 (Figure 7A and B); however, lev-8(x15) mutants were special in that only head and neck region of the animals were resistant. Consistent with our behavioural assays and a previous report (Towers et al, 2005), lev-8(x15) mutants showed largely reduced levamisole-induced PSCs (28±10%, P<0.05; Figure 7C), whereas nicotine PSCs were normal. Surprisingly, this was also the case in lev-8; nra-2 double mutants, even though nra-2(ok1731) mutants have reduced nicotine PSCs. Either, in the absence of NRA-2, LEV-8 assembles with ACR-16, thus explaining altered nicotine PSCs, or, as in unc-38(x20) mutants, N-AChRs undergo compensatory changes in lev-8 mutants.
Figure 7.
Contribution of essential and non-essential L-AChR subunits to cholinergic agonist sensitivity in nra-2(ok1731) mutants. (A, B) Paralysis assays (n=2–7; 30 animals each) in response to levamisole (A) and nicotine (B) of mutants in nra-2(ok1731), lev-8(x15), acr-8(ok1240) and unc-38(x20), and in double-mutant combinations as indicated. (C) Normalized mean peak values of ACh-, levamisole- and nicotine-induced muscle PSCs in wild-type animals, nra-2(ok1731) mutants, and mutants of the non-essential L-AChR α-subunits lev-8(x15) and acr-8(ok1240) as well as respective double mutants. Displayed are means±s.e.m., number of animals and significant differences to wild type (t-test; *P<0.05; **P<0.01; ***P<0.001) are indicated.
Mutants of acr-8(ok1240) showed no levamisole resistance, but a significant resistance to nicotine in paralysis assays, which was slightly elevated in the acr-8(ok1240); nra-2(ok1731) double mutants (Figure 7A and B). To our surprise, PSCs for ACh, levamisole, or nicotine did not differ between wild type, acr-8(ok1240) and acr-8(ok1240); nra-2(ok1731) double mutants, even though nra-2(ok1731) alone significantly reduces levamisole and nicotine PSCs (Figure 7C). Thus, the acr-8 mutation suppresses nra-2(ok1731) effects on L- and N-AChRs. This could be explained if NRA-2 prevents ACR-8 subunits from assembling with other subunits. In nra-2 mutants, ACR-8 could be integrated in L- and N-AChRs, thus altering their physiological properties, which cannot occur in acr-8 mutants.
Relative expression of individual L-AChR subunits is altered in nra-2 and nra-4 mutants
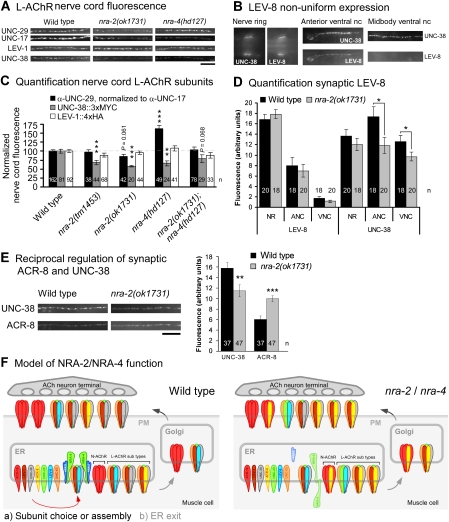
Our findings suggested that NRA-2/NRA-4 could affect nAChR properties by influencing the representation of particular subunits in the mature receptors. We thus probed synaptic expression of LEV-1 (4xHA tagged), UNC-38 (3xMYC tagged), LEV-8 (3xHA) and ACR-8 (6xHA) by antibody injection, and of UNC-29 by immunostaining relative to the presynaptic UNC-17 vesicular ACh transporter, in wild type, nra-2 and nra-4 mutants (Figure 8).
Figure 8.
Individual L-AChR subunit levels at postsynaptic elements vary in nra-2 and nra-4 mutants reciprocally. (A, B, E) Synaptic expression of different L-AChR subunits was analysed by quantitative fluorescence microscopy. Endogenous, postsynaptic UNC-29, as well as the presynaptic UNC-17 (vAChT), were immunolabelled with specific 1° and different fluorescent 2° antibodies, then UNC-29 fluorescence was normalized to UNC-17 and compared in the indicated mutants. Also, transgenic animals expressing epitope-tagged LEV-1 (4 HA tags), UNC-38 (3 MYC tags), LEV-8 (3 HA tags) or ACR-8 (6 HA tags) were injected into the body cavity with fluorescent tag-specific antibodies. Size bar: 10 μm. (B) LEV-8 is non-uniformly expressed in the nervous system, as compared with UNC-38. Shown is expression of both subunits in the nerve ring, and the anterior and midbody ventral nerve cords (nc). (C, D, E) Fluorescence in the ventral cord was quantified (as linescans, followed by background correction) either in fixed animals (UNC-29, UNC-17), or in live animals after a recovery period of >6 h (during which excess antibody is cleared from the extracellular fluid by scavenger cells). Shown is mean fluorescence±s.e.m. (normalized to wild type in C, arbitrary units in D and E), number of animals and significant differences to wild type are indicated (t-test; *P<0.05; **P<0.01; ***P<0.001). (F) Model of NRA-2/NRA-4 function. Left, NRA-2/NRA-4 either influence the choice of particular subunits (indicated by different colours) to be assembled into pentameric nAChRs, or they determine to which extent pentamers of particular subunit composition are allowed to leave the ER (less favoured, as no obvious accumulation of NRA-2 was seen at ER exit sites). ACR-16 N-AChRs and L-AChRs, rarely incorporating ACR-8 subunits (yellow) are preferably formed. Right, In the nra-2 or nra-4 mutants, nAChRs of other composition are found, for example, containing ACR-8 or UNC-29 subunits more often. Depending on the allele, NRA-2 and NRA-4 proteins could either be completely absent, not bound to ER membranes and secreted, or of inverted topology.
For UNC-38 (essential α-subunit), synaptic expression was significantly reduced in nra-2(tm1453 and ok1731), and in nra-4(hd127) animals. UNC-29 (essential non-α) was reduced in nra-2(ok1731) animals, and, intriguingly, significantly increased in nra-4(hd127) animals. LEV-1 cell-surface expression levels were not affected in either mutant (Figure 8A and C). Effects of NRA-2 on LEV-8 and ACR-8 synaptic expression were assayed relative to UNC-38. The synaptic expression pattern of LEV-8 was peculiar, and could explain our observations in paralysis assays (resistance in head and neck): we found the protein in L-AChR clusters in the nerve ring and the anterior ends of ventral and dorsal nerve cords, together with UNC-38 (largely co-expressed in the same synaptic clusters; Supplementary Figure 15). Interestingly, LEV-8 was almost not detectable in the rest of the body (Figure 8B); synaptic LEV-8 expression was not affected in the nra-2(ok1731) mutant (Figure 8D).
ACR-8 and UNC-38 expression was found in all regions of the nerve cords (Figure 8E); however, as we observed earlier, ACR-8 was present in many clusters that did not contain UNC-38, in addition to clusters in which both proteins were co-expressed (Supplementary Figure 15; Gottschalk et al, 2005). This indicates that ACR-8 is either found in (unknown) receptors that are different from the L-AChR, or that there are L-AChRs in which ACR-8 replaced UNC-38. In nra-2(ok1731) mutants, while synaptic UNC-38 levels were reduced, expression of ACR-8 was significantly increased (Figure 8E). Thus, NRA-2 function affects synaptic expression of UNC-38 and ACR-8 α-subunits reciprocally. In sum, NRA-2 and NRA-4 affect the relative composition of the synaptic L-AChR in a (subunit) gene-, and (nra-2/nra-4) allele-specific manner.
Discussion
In this work, we showed that NRA-2 and NRA-4, evolutionarily conserved type I TM proteins forming a protein complex in the ER, affect synaptic nAChR subunit composition in C. elegans. Mutants lacking these proteins exhibited moderate resistance to cholinergic but not GABAergic agonists, verifying that in muscle they affect nAChRs, and not GABAARs. The cholinergic deficits were accompanied by defects in agonist sensitivity in whole-cell voltage-clamp analyses: sensitivities of the L-AChR and the N-AChR to their ‘specific' agonists, levamisole and nicotine, were reduced, whereas sensitivity for short-term applied ACh was either unaffected (N-AChR) or increased (L-AChR). These effects may sum up such that overall ACh PSC are unaffected in nra-2 mutants; however, compensatory changes when both L- and N-AChRs are present in nra-2 mutants cannot be ruled out. Yet, optogenetic, prolonged application of ACh demonstrated increased desensitization of L-AChRs, even though N-AChRs were present. We further showed that in the absence of NRA-2 or NRA-4, the NMJ contained L-AChRs of different subunit composition, or altered relative amounts of different L-AChRs with specific subunit compositions, particularly UNC-38 and ACR-8. Thus, NRA-2 and NRA-4 either influence choice of subunits for assembly in the ER, or the extent to which particular pentamers are allowed to leave the ER and reach the NMJ (see model in Figure 8F).
Altered L-AChR single-channel properties in nra-2 mutants
Our conclusions are supported by analyses of single-channel properties of embryonic L-AChRs, which indicated changes in the functional properties of receptors in nra-2 mutants versus wild type, and which appeared to originate from changes in L-AChR subunit composition. The main kinetic change was an increase in the open duration of channels activated by ACh, and a decrease when levamisole was the agonist. These agonist-specific changes parallel the sensitivity changes observed in whole-cell experiments of adult muscle. However, correlating results from single-channel experiments with whole-cell currents is not trivial. For levamisole, both single channel and macroscopic currents showed decreased responses. Single-channel recordings of L-AChRs activated by ACh showed reduced frequency in the nra-2 mutant, whereas macroscopic currents were unaltered. One explanation is that desensitization is affected in the nra-2 mutant L-AChR. Single-channel recordings occur in the continuous presence of agonist, thus enhanced desensitization to ACh will appear as a decrease in single-channel opening frequency, as we observed, and in agreement with our results obtained after long-term photo-evoked ACh release, which uncovered increased desensitization of the L-AChR in nra-2 mutants. Another explanation for these differences is that embryonic, extrasynaptic L-AChRs are compared with synaptic adult L-AChRs, in which subunit composition may change during development, and interaction with additional proteins could occur, for example, LEV-10 (Gally et al, 2004). Also, more than one type of L-AChR may be present in adult muscle cells, though we only detect a single main functional population in embryonic cells. This L-AChR population is kinetically different in nra-2 mutants, likely due to altered subunit composition.
NRA-2 and NRA-4 affect subunit composition of synaptic nAChRs
The effects of nra-2 and nra-4 mutants on synaptic L-AChR subunit representation were subunit dependent. In particular, the α-subunit UNC-38 was reduced in these mutants, whereas the non-α-subunit UNC-29 was increased in nra-4(hd127) animals. The non-α-subunit LEV-1 was unaltered, as was the α-subunit LEV-8. In contrast, the α-subunit ACR-8 was increased in nra-2 mutants, and thus may compensate for the reduction in UNC-38 levels. This could explain the observed increase in short-term applied ACh sensitivity of L-AChRs, and the increased desensitization in long-term ACh application (either optically, or in response to aldicarb). In nra-4 mutants, in which UNC-38 is reduced and UNC-29 increased, fewer α-subunits, and thus fewer ACh-binding sites should be present in synaptic L-AChRs. This may explain why in nra-4; acr-16 double mutants compared with acr-16 single mutants ACh sensitivity of the L-AChR was not increased (Supplementary Figure 14). In this regard, we recently showed that channel activation rate and agonist sensitivity increase with the number of functional binding sites in homomeric Cys-loop receptors (Rayes et al, 2009). Yet, as we do not know the number of ACh-binding sites in the L-AChR or the number of bound agonist molecules required for maximal activation, ACh sensitivity in nra-2 or nra-4 mutants may mainly be affected by altering ACh-dependent desensitization rather than ACh binding, as suggested by optogenetic experiments and single-channel recordings.
Mode of action of NRA-2 and NRA-4 in L-AChR assembly
Our findings suggest that NRA-2/NRA-4 interact with L-AChRs in the ER. How do NRA-2 and NRA-4 influence L-AChR subunit composition? They may interact with RIC-3, an ER protein that affects biogenesis and/or trafficking of several types of nAChRs (Halevi et al, 2002; Gottschalk et al, 2005; Gottschalk and Schafer, 2006; Biala et al, 2009). We tested for possible genetic interactions between ric-3 and nra-2 by analysing swimming behaviour as an indirect measure for NMJ function. ric-3 mutants showed significantly less swimming cycles, which were further reduced in the ric-3; nra-2 double mutants (data not shown). Thus, RIC-3 and NRA-2 likely act in separate pathways.
An intriguing alternative is indicated by findings made for the NRA-2/Nicalin homologue Nicastrin: this γ-secretase component was implicated as ‘gate-keeper' of the intramembrane peptidase and regulates substrate access by binding their N-termini (Shah et al, 2005). NRA-2/NRA-4 could act as a ‘nucleation centre' for nAChR assembly and regulate inclusion of particular subunits during pentamer assembly. L-AChRs require essential subunits UNC-29, UNC-38 and UNC-63; remaining positions are occupied by non-essential subunits. NRA-2/NRA-4 could sort certain subunits into the pentamer, while excluding others, for example, ACR-8. Also N-AChR properties were altered in nra-2 and nra-4 mutants, which could be explained by NRA-2/NRA-4 ensuring that only ACR-16 is assembled. As nra-2 effects on N-AChRs were reversed by lev-8 and acr-8 mutations, these subunits may assemble with ACR-16 in the absence of NRA-2/NRA-4. Alternatively, NRA-2/NRA-4 could control which nAChR pentamer of particular composition is allowed to leave the ER. However, our observation that NRA-2/NRA-4 is not enriched at ER exit sites argues against this idea.
Evolutionary conservation and additional functions of NRA-2 and NRA-4
The nra-4 expression pattern was broad, extending beyond the neuromuscular system, and also the nra-2 promoter was active in tissues in addition to muscles and neurons. nra-2 and nra-4 mutants had reduced broodsize, indicating additional functions. Furthermore, these genes are conserved across all phyla, that is, also in species that do not express nAChRs (Supplementary Figures 2–5). Vertebrate homologues of NRA-2/NRA-4 (NOMO/Nicalin) antagonistically influence cell-surface signalling events through the nodal type of TGFβ ligands (Haffner et al, 2004, 2007), but how these signalling pathways are influenced by the ER proteins NOMO/Nicalin was not further investigated. Possibly, they may affect receptors for TGFβ-like ligands, that is, heterodimeric activin receptors. TGFβ receptors are antagonized by other membrane-associated co-receptors or inhibitors that bind to the complex (e.g. ‘Cripto'; Gray et al, 2003), and secretion or cell-surface expression of such antagonists could be influenced by ER-resident proteins. A role for TGFβ in Drosophila NMJ formation was shown (Rawson et al, 2003), thus we cannot rule out the possibility that NRA-2 and NRA-4 affect nAChRs indirectly through TGFβ pathways. Yet, though some mutants in TGFβ pathways we tested showed increased levamisole or nicotine sensitivity, the effects are likely indirect (e.g. through GABA signalling for dbl-1; Vashlishan et al, 2008). Furthermore, our co-purification of NRA-2/NRA-4 with L-AChRs argues for direct interactions (Gottschalk et al, 2005).
Are Nicalin and NOMO involved in nAChR assembly in vertebrates? This is not unlikely, given the conservation of the proteins, and the fact that human Nicalin, expressed in C. elegans muscle, partially rescued nra-2 phenotypes. However, as vertebrate muscle does not express such a large set of nAChR subunits as C. elegans muscle, it may be worthwhile to study the function of Nicalin/NOMO in nAChR subunit choice or assembly in neurons.
Materials and methods
C. elegans strains
Nematodes were grown under standard conditions (Brenner, 1974). Mutant strains were backcrossed four to six times. Transgenic strains were generated following standard procedures (Fire, 1986). nra-4(hd127) was isolated from an EMS mutagenized library by a poison primer approach (Edgley et al, 2002), using primers 5′-GATTACGGTTCCCGGTCTTAAC-3′, 5′-CATCAACAAATGGATTCATGCT-3′ and 5′-TCGACTATTCCCAGTTGAAGGT-3′.
Strains used or generated: N2 (wild type), lin-15(n765ts), ZZ37: unc-63(x37), ZZ20: unc-38(x20), RB1195: acr-8(ok1240), ZZ15: lev-8(x15), RB918: acr-16(ok789), RM509: ric-3(md1181), NW987: unc-129(ev554), ZX383: nra-2(tm1453), RB1480: nra-2(ok1731), ZX441: nra-4(hd127), ZX544: nra-4(tm2656), ZX453: nra-2(ok1731); nra-4(hd127), ZX455: nra-2(tm1453); nra-4(hd127), ZX543: nra-2(ok1731); nra-4(tm2656), ZX395: nra-2(tm1453); unc-38(x20), ZX502: nra-2(ok1731); unc-38(x20), ZX500: nra-2(ok1731); acr-8(ok1240), ZX621: nra-2(ok1731); lev-8(x15), ZX445: nra-2(tm1453); acr-16(ok789), ZX575: nra-4(hd127); acr-16(ok789), ZX501: nra-2(tm1453); ric-3(md1181), LT186: sma-6(wk7), DR960: daf-1(m402), LT121: dbl-1(wk70), CB1372: daf-7(e1372).
Transgenic strains: ZX15: ljEx42[punc-38::unc-38::MYC::6xHIS::2xMYC; rol-6d], ZX56: zxEx51[punc-38::unc-38::MYC::6xHIS-2xMYC; podr-2::odr-2::HA; rol-6d], ZX275: zxIs1[plev-1::lev-1::HA-6xHIS-3xHA; rol-6] (ZX15, 56, 275 were as described; (Gottschalk and Schafer, 2006), ZX387: nra-2(tm1453); zxIs1, ZX386: nra-2(ok1731); zxIs1, ZX568: nra-4(hd127); zxIs1, ZX569: nra-2(ok1731); nra-4(hd127); zxIs1, ZX525: nra-2(tm1453); ljEx42, ZX524: nra-2(ok1731); ljEx42, ZX523: nra-4(hd127); ljEx42, ZX522: nra-2(ok1731); nra-4(hd127); ljEx42, ZX574: zxEx52[pnra-4::GFP; rol-6d], ZX556: nra-2(tm1453); zxEx53[pmyo3::nra-2(cDNA)::GFP; rol-6d], ZX578: nra-2(ok1731); ljEx42; zxEx54[pmyo-3::nra-2(cDNA)::GFP; lin15+], ZX640: nra-2(ok1731); lin-15(n765ts); zxEx54, ZX579: zxEx55[pmyo-3::nra-2(cDNA)::YFP; rol-6d], ZX576: zxEx56[pmyo-3::nra-2(cDNA)::mCherry; punc-29::unc-29::GFP; rol-6d], ZX577: zxEx57[pmyo-3::nra-2(cDNA)::mCherry; psec23::sec23::GFP; rol-6d], ZX628: nra-2(ok1731); zxEx58[pmyo-3::Nicalin(human cDNA)::GFP; rol-6d], ZX629: nra-4(hd127); zxEx59[pnra-4::nra-4(cDNA)::GFP; rol-6d], ZX636: lin-15(n765ts); zxEx60[pnra-4::nra-4(cDNA)::VC155; lin-15+]; zxEx61[pmyo-3::nra-2(cDNA)::VN173; rol-6d], ZX639: lin-15(n765ts); zxEx60; zxEx62[pmyo-3::unc-1::VN173; rol-6d], ZX627: nra-2(ok1731); zxEx63[pmyo-3::3xHA::nra-2(cDNA)::GFP; rol-6d], ZX699: N2; zxEx64[plev-8::lev-8::3xHA; punc-38::unc-38-MYC::6xHIS-2xMYC; rol-6d], ZX700: nra-2(ok1731); zxEx64, ZX701: N2; zxEx65[pacr-8::acr-8::6xHIS-3xHA::6xHIS-3xHA; punc-38::unc-38-MYC::6xHIS-2xMYC; rol-6d], ZX702: nra-2(ok1731); zxEx65, ZX703: N2; zxEx66[pnra-2::GFP; rol-6d], ZX460; N2; zxIs6[punc-17::ChR2(H134R)::YFP; lin-15+]IV ((Liewald et al, 2008)), ZX499: N2; zxIs5[punc-17::ChR2(H134R)::YFP; lin-15+]X, ZX704: nra-2(ok1731); zxIs6, ZX705: acr-16(ok789); zxIs5, ZX706: unc-38(x20); zxIs5.
Bimolecular fluorescence complementation
BiFC experiments were essentially as described (Chen et al, 2007; Shyu et al, 2008). pnra-4∷nra-4(cDNA)∷VC155 was first injected (10 ng/μl) into lin-15(n765ts) animals. Stable lines were obtained, and into one of those, either pmyo-3∷nra-2(cDNA)∷VN173 (7 ng/μl), or, as a negative control, pmyo-3∷UNC-1∷VN173 (wp646; 15 ng/μl) were injected with rol-6d(pRF4) as a marker. Stable lines were analysed for reconstituted Venus fluorescence.
Behavioural assays
Paralysis assays, as well as swimming assays, were as described (Gottschalk et al, 2005).
Electrophysiology
Recordings of agonist- or photo-induced PSCs from dissected C. elegans body muscle cells were as described (Liewald et al, 2008; Biala et al, 2009). Single-channel recordings from embryonic muscle cells were as described earlier (Christensen et al, 2002; Rayes et al, 2007).
More detailed and additional Materials and methods are presented in Supplementary data.
Supplementary Material
Supplementary Information
Review Process File
Acknowledgments
We thank M Treinin, W Schafer and J-L Bessereau for comments. We are indebted to K Zehl for expert technical assistance, B Chen, Z-W Wang and C-D Hu for plasmids and advice and M Brauner for injections. We are grateful to the C. elegans knockout consortium, S Mitani and the CGC for genomic deletions and for providing strains. We thank Y Kohara, B Roberts, I Johnstone, J Culotti and J Rand for cDNA clones, plasmids, mutants and antibodies. This work was funded by grants from the Deutsche Forschungsgemeinschaft (SFB628-P17, GO1011/2-1 and the Cluster of Excellence Frankfurt), BMBF and HMWK to AG, by a grant from the Canadian Institutes of Health Research to HH and by grants from CONICET, ANPCyT, Florencio Fiorini and Loreal UNESCO to CB.
Author contributions: RBA, JFL, GH, DR, CS, TS, JP and AG performed the experiments, RBA, JFL, CB, CS and AG analysed the data, RBA, JFL, CB and AG prepared the figures, RBA, JFL, CB, HH and AG wrote the paper.
References
- Biala Y, Liewald JF, Cohen Ben-Ami H, Gottschalk A, Treinin M (2009) The conserved RIC-3 coiled-coil domain mediates receptor-specific interactions with nicotinic acetylcholine receptors. Mol Biol Cell 20: 1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount P, Merlie JP (1991) BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J Cell Biol 113: 1125–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL (2008) Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci USA 105: 18590–18595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J, Edelstein S (2005) Nicotinic Acetylcholine Receptors. New York: Odile Jacob Publishing Corporation [Google Scholar]
- Chen B, Liu Q, Ge Q, Xie J, Wang ZW (2007) UNC-1 regulates gap junctions important to locomotion in C. elegans. Curr Biol 17: 1334–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara DC, Cohen JB (1997) Identification of amino acids contributing to high and low affinity d-tubocurarine sites in the Torpedo nicotinic acetylcholine receptor. J Biol Chem 272: 32940–32950 [DOI] [PubMed] [Google Scholar]
- Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM III, Strange K (2002) A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron 33: 503–514 [DOI] [PubMed] [Google Scholar]
- Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG (1998) Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science 281: 706–709 [DOI] [PubMed] [Google Scholar]
- Culetto E, Baylis HA, Richmond JE, Jones AK, Fleming JT, Squire MD, Lewis JA, Sattelle DB (2004) The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J Biol Chem 279: 42476–42483 [DOI] [PubMed] [Google Scholar]
- Edgley M, D'Souza A, Moulder G, McKay S, Shen B, Gilchrist E, Moerman D, Barstead R (2002) Improved detection of small deletions in complex pools of DNA. Nucleic Acids Res 30: e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer S, Gottschalk A, Hengartner M, Horvitz HR, Richmond J, Schafer WR, Bessereau JL (2007) Regulation of nicotinic receptor trafficking by the transmembrane Golgi protein UNC-50. EMBO J 26: 4313–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A (1986) Integrative transformation of Caenorhabditis elegans. EMBO J 5: 2673–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JT, Squire MD, Barnes TM, Tornoe C, Matsuda K, Ahnn J, Fire A, Sulston JE, Barnard EA, Sattelle DB, Lewis JA (1997) Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci 17: 5843–5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RM, Watson JD, Von Stetina SE, McDermott J, Brodigan TM, Fukushige T, Krause M, Miller DM III (2007) The embryonic muscle transcriptome of Caenorhabditis elegans. Genome Biol 8: R188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Evans SP, Jensen M, Madsen DM, Mancuso J, Norman KR, Maricq AV (2005) The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron 46: 581–594 [DOI] [PubMed] [Google Scholar]
- Francis MM, Mellem JE, Maricq AV (2003) Bridging the gap between genes and behavior: recent advances in the electrophysiological analysis of neural function in Caenorhabditis elegans. Trends Neurosci 26: 90–99 [DOI] [PubMed] [Google Scholar]
- Gally C, Eimer S, Richmond JE, Bessereau JL (2004) A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature 431: 578–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M (2007) Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74: 1102–1111 [DOI] [PubMed] [Google Scholar]
- Gottschalk A, Almedom RB, Schedletzky T, Anderson SD, Yates JR, Schafer WR (2005) Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J 24: 2566–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk A, Schafer WR (2006) Visualization of integral and peripheral cell surface proteins in live Caenorhabditis elegans. J Neurosci Methods 154: 68–79 [DOI] [PubMed] [Google Scholar]
- Gray PC, Harrison CA, Vale W (2003) Cripto forms a complex with activin and type II activin receptors and can block activin signaling. Proc Natl Acad Sci USA 100: 5193–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Camacho P, Gardner P, Hall ZW (1991) Identification of two amino acid residues in the epsilon subunit that promote mammalian muscle acetylcholine receptor assembly in COS cells. Neuron 6: 879–887 [DOI] [PubMed] [Google Scholar]
- Haffner C, Dettmer U, Weiler T, Haass C (2007) The Nicastrin-like protein Nicalin regulates assembly and stability of the Nicalin-nodal modulator (NOMO) membrane protein complex. J Biol Chem 282: 10632–10638 [DOI] [PubMed] [Google Scholar]
- Haffner C, Frauli M, Topp S, Irmler M, Hofmann K, Regula J, Bally-Cuif L, Haass C (2004) Nicalin and its binding partner Nomo are novel Nodal signaling antagonists. EMBO J 23: 3041–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevi S, McKay J, Palfreyman M, Yassin L, Eshel M, Jorgensen E, Treinin M (2002) The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J 21: 1012–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R (2001) The chaperone protein 14-3-3eta interacts with the nicotinic acetylcholine receptor alpha 4 subunit. Evidence for a dynamic role in subunit stabilization. J Biol Chem 276: 28281–28290 [DOI] [PubMed] [Google Scholar]
- Jones AK, Davis P, Hodgkin J, Sattelle DB (2007) The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: an update on nomenclature. Invert Neurosci 7: 129–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3: 102–114 [DOI] [PubMed] [Google Scholar]
- Keller SH, Lindstrom J, Taylor P (1996) Involvement of the chaperone protein calnexin and the acetylcholine receptor beta-subunit in the assembly and cell surface expression of the receptor. J Biol Chem 271: 22871–22877 [DOI] [PubMed] [Google Scholar]
- Keller SH, Lindstrom J, Taylor P (1998) Inhibition of glucose trimming with castanospermine reduces calnexin association and promotes proteasome degradation of the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem 273: 17064–17072 [DOI] [PubMed] [Google Scholar]
- Keller SH, Taylor P (1999) Determinants responsible for assembly of the nicotinic acetylcholine receptor. J Gen Physiol 113: 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienkamp HJ, Maeda RK, Sine SM, Taylor P (1995) Intersubunit contacts governing assembly of the mammalian nicotinic acetylcholine receptor. Neuron 14: 635–644 [DOI] [PubMed] [Google Scholar]
- Lewis JA, Fleming JT, McLafferty S, Murphy H, Wu C (1987) The levamisole receptor, a cholinergic receptor of the nematode Caenorhabditis elegans. Mol Pharmacol 31: 185–193 [PubMed] [Google Scholar]
- Liewald JF, Brauner M, Stephens GJ, Bouhours M, Schultheis C, Zhen M, Gottschalk A (2008) Optogenetic analysis of synaptic function. Nat Methods 5: 895–902 [DOI] [PubMed] [Google Scholar]
- Mishina M, Takai T, Imoto K, Noda M, Takahashi T, Numa S, Methfessel C, Sakmann B (1986) Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 321: 406–411 [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB (2003) Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J Neurobiol 55: 134–150 [DOI] [PubMed] [Google Scholar]
- Rayes D, De Rosa MJ, Sine SM, Bouzat C (2009) Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J Neurosci 29: 6022–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayes D, Flamini M, Hernando G, Bouzat C (2007) Activation of single nicotinic receptor channels from Caenorhabditis elegans muscle. Mol Pharmacol 71: 1407–1415 [DOI] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL (1996) Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 274: 1389–1391 [DOI] [PubMed] [Google Scholar]
- Richmond JE (2006) Electrophysiological recordings from the neuromuscular junction of C.elegans. In The C.elegans Research Community, WormBook (ed). doi:10.1895/wormbook.1.112.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM (1999) One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci 2: 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B, Clucas C, Johnstone IL (2003) Loss of SEC-23 in Caenorhabditis elegans causes defects in oogenesis, morphogenesis, and extracellular matrix secretion. Mol Biol Cell 14: 4414–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage-Dunn C (2005) TGF-β signaling. In The C.elegans Research Community, WormBook (ed). doi:10.1895/wormbook.1.22.1, http://www.wormbook.org [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE III, Sudhof T, Yu G (2005) Nicastrin functions as a gamma-secretase-substrate receptor. Cell 122: 435–447 [DOI] [PubMed] [Google Scholar]
- Shyu YJ, Hiatt SM, Duren HM, Ellis RE, Kerppola TK, Hu CD (2008) Visualization of protein interactions in living Caenorhabditis elegans using bimolecular fluorescence complementation analysis. Nat Protoc 3: 588–596 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yandell MD, Roy PJ, Krishna S, Savage-Dunn C, Ross RM, Padgett RW, Wood WB (1999) A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development 126: 241–250 [DOI] [PubMed] [Google Scholar]
- Touroutine D, Fox RM, Von Stetina SE, Burdina A, Miller DM III, Richmond JE (2005) acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem 280: 27013–27021 [DOI] [PubMed] [Google Scholar]
- Towers PR, Edwards B, Richmond JE, Sattelle DB (2005) The Caenorhabditis elegans lev-8 gene encodes a novel type of nicotinic acetylcholine receptor alpha subunit. J Neurochem 93: 1–9 [DOI] [PubMed] [Google Scholar]
- Treinin M, Gillo B, Liebman L, Chalfie M (1998) Two functionally dependent acetylcholine subunits are encoded in a single Caenorhabditis elegans operon. Proc Natl Acad Sci USA 95: 15492–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashlishan AB, Madison JM, Dybbs M, Bai J, Sieburth D, Ch'ng Q, Tavazoie M, Kaplan JM (2008) An RNAi screen identifies genes that regulate GABA synapses. Neuron 58: 346–361 [DOI] [PubMed] [Google Scholar]
- Wanamaker CP, Christianson JC, Green WN (2003) Regulation of nicotinic acetylcholine receptor assembly. Ann N Y Acad Sci 998: 66–80 [DOI] [PubMed] [Google Scholar]
- Wang JM, Zhang L, Yao Y, Viroonchatapan N, Rothe E, Wang ZZ (2002) A transmembrane motif governs the surface trafficking of nicotinic acetylcholine receptors. Nat Neurosci 5: 963–970 [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Hardy SF, Hall ZW (1996) Assembly of the nicotinic acetylcholine receptor. The first transmembrane domains of truncated alpha and delta subunits are required for heterodimer formation in vivo. J Biol Chem 271: 27575–27584 [DOI] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407: 48–54 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Review Process File