EMBO J 28, 2625–2635 (2009); published online 23 July 2009
Using cohesion to keep a broken chromatid in close proximity to its intact sister chromatid, thereby supporting efficient repair, is a straightforward concept. The responsible protein complex, cohesin, has at least one additional role in the DNA damage response. As Watrin and Peters show in this issue, cohesin, but not cohesion, is required for G1, intra-S and G2–M DNA damage checkpoints. As our knowledge of the distinct functions and properties of cohesin and related complexes expands, it becomes clear that cohesin proteins have evolved to serve in almost every fundamental reaction that concerns chromosomes. Not surprisingly, the questions do not become fewer.
How many more functions of cohesin will still be described and how do they relate to each other? After the initial description of cohesin as a protein complex, which holds sister chromatids in cohesion, additional roles of cohesin and related complexes were discovered, including DNA repair and recombination, regulation of transcription, perhaps a function at the mitotic spindle, and specific roles in meiosis (for reviews, see Strom and Sjogren, 2007; Onn et al, 2008; Peters et al, 2008; Yanagida, 2009). An intra-S DNA damage checkpoint function was suggested, as two cohesin polypeptides, SMC1 and SMC3, become phosphorylated by ATM in response to DNA damage, and non-phosphorylatable mutants are impaired at this checkpoint (Kim et al, 2002; Yazdi et al, 2002). Additional complexes, which, similar to cohesin, contain the SMC1–SMC3 heterodimer, were suggested to be involved in DNA repair and/or in intra-S checkpoint function, such as the BRCA1 supercomplex (BASC) (Yazdi et al, 2002) and the recombination complex RC-1(Jessberger et al, 1996).
In this issue of The EMBO Journal, Watrin and Peters report on a role of cohesin, independent of cohesion, not only in the intra-S but also in the G2–M checkpoint (Watrin and Peters, 2009). They approached this problem by depleting synchronized HeLa cells for the cohesin subunits, SCC1 and SMC3. The response to DNA damage with respect to cell cycle arrest, chromosome stability, formation of irradiation-induced foci, and checkpoint activation was tested. For the intra-S and G2–M checkpoints, the authors compared cohesin-depleted cells with cells depleted for sororin, which, as they showed before, is necessary only to establish and maintain sister chromatid cohesion, and not for chromatin association of cohesin. Sororin depletion did not impair G2–M or intra-S checkpoints, whereas cohesin depletion did. Thus, the function of cohesin in these two checkpoints is cohesion-independent. On cohesin depletion, checkpoint kinase 2 (CHK2) activation is impaired not only in these checkpoints but also in G1. As there are no sister chromatids in G1, this checkpoint function of cohesin is also cohesion independent.
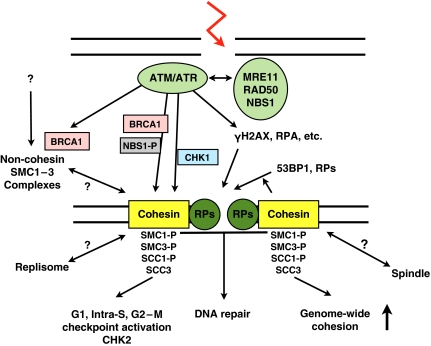
How to understand the cohesion-independent function of cohesin in DNA damage checkpoints (Figure 1)? Cohesin may act as a platform for the recruitment and activation of checkpoint and DNA repair proteins. Cohesin helps to translate checkpoint signals into DNA repair and takes part in both processes. Cohesin's accumulation close to DNA breaks, its contribution to the structure of irradiation-induced foci, the increase in activated forms of ATM, CHK1, and H2AX (γH2AX) in cohesin-depleted cells suffering increased spontaneous DNA damage, and further data fit this model. Watrin and Peters show that recruitment of 53BP1 to DNA breaks is reduced in cohesin-depleted cells. The functional consequence(s) remain to be explored, particularly as 53BP1 behaved normally in cells expressing a non-phosphorylatable mutant of SMC1 (Kitagawa et al, 2004). An open question also concerns the relationship between DNA damage response, CHK2, and cohesin, as both, cohesin-dependent (Watrin and Peters, 2009) and ATM/NBS1/SMC1-independent modes of CHK2 activation (Yazdi et al, 2002), were reported. Cohesion also increases genome wide in G2–M in response to DNA damage. This CHK1-dependent process may serve as a global genome safeguard (Heidinger-Pauli et al, 2008).
Figure 1.
Model for the functions of cohesin in the DNA damage response. DNA damage sensors (ATM, ATR, and the MRE11–RAD50–NBS1 complex) are activated in G1, S, and/or G2–M phase. In S, phosphorylated NBS1 allows ATM to phosphorylate SMC1, possibly SMC3. In G2–M, ATR activates CHK1, which phosphorylates SCC1, causing an increase in genome-wide cohesion. Polo-like kinase phosphorylation of SCC3 may have to be prevented. The model excludes neither overlapping actions of proteins in different checkpoints nor multiple effects of cohesin phosphorylation. The distinction between cohesin and repair proteins (RPs) is for illustrative purpose only, as cohesin takes part in the repair reaction itself. Recruitment of some RPs depends on cohesin. The SMC5–SMC6 complex, included in ‘RPs', loads at DNA breaks. Cohesin-loading factors are not represented. How non-cohesin SMC1–SMC3-based complexes relate to cohesin, and when and where such complexes exist is unknown. It is also unclear whether DNA damage-activated cohesin affects the replisome, for example, by slowing replication, or even spindle formation. How cohesin controls checkpoint function also remains to be described. Assemblies at both ends of the broken DNA are supposed to be identical and are drawn differently only for the purpose of illustration.
There are many more questions to be solved in the future. Is the mode of DNA binding of cohesin different between cohesion-dependent and -independent functions?
Watrin and Peters (2009) suggest a ‘non-cohesive' binding of cohesin to chromatin in sororin-depleted cells. As this requires SCC1, which closes the cohesin ring for cohesion, the question is whether non-cohesive binding is through a ring structure and topological, as is generally considered to be the case for cohesive cohesin (Ivanov and Nasmyth, 2005). Moreover, do the previously described loosely and tightly chromatin-associated fractions of cohesin reflect a non-cohesive and cohesive binding of cohesin? What is the relationship between the abundant cohesin complex and other, minor SMC1/SMC3-based complexes, such as RC1, BASC, or ATM phosphorylation-dependent complex assemblies? Is there, perhaps, a pool of intact cohesin required, of which a small fraction exchanges subunits in response to a biological need, such as the need to repair DNA damage? This hypothesis suggests that depletion of seemingly cohesin-specific subunits, such as SCC1, may indirectly affect other such complexes, the roles, cell- and condition-dependent formation of which are insufficiently understood. Furthermore, how are cohesin's S-phase checkpoint and DNA repair activities related to the replication machinery?
For efficient DNA repair, the cohesion activity of cohesin is necessary, consistent with the idea that cohesin holds DNA strands and ends in repair-proficient proximity. Whether this is cohesin's only role during DNA repair reaction is unlikely, given the interactions with DNA repair proteins such as those present in BASC and RC-1, given the cohesin-dependent architecture of irradiation-induced foci, given the binding of SMC1 or SMC3 protein domains to unusual DNA structures, which may reflect repair intermediates, and given the RAD52 pathway-dependent effect of SMC1 on the balance between homologous recombination and non-homologous end joining. Furthermore, the regulation of transcription by cohesin is likely to be based on its ability to intrachromosomally hold rather distant DNA regions in close proximity. Thus, cohesin may help in establishing and maintaining chromosome loops to control cis-interactions between regulatory elements. Intrachromosomal looping may also benefit certain DNA repair and recombination reactions, such as V(D)J rearrangements of immunoglobulin genes, or may contribute to an expansion or contraction of repetitive DNA elements, and it would not be too surprising if cohesin is involved.
Together, as the intriguing paper by Watrin and Peters (2009) again highlights, research on individual cohesin proteins and on their complexes certainly holds more surprises in store than is generally appreciated even today, about 18 years after the SMC proteins were initially described.
Footnotes
The author declares that he has no conflict of interest.
References
- Heidinger-Pauli JM, Unal E, Guacci V, Koshland D (2008) The kleisin subunit of cohesin dictates damage-induced cohesion. Mol Cell 31: 47–56 [DOI] [PubMed] [Google Scholar]
- Ivanov D, Nasmyth K (2005) A topological interaction between cohesin rings and acircular minichromosome. Cell 122: 849–860 [DOI] [PubMed] [Google Scholar]
- Jessberger R, Riwar B, Baechtold H, Akhmedov AT (1996) SMC proteins constitute two subunits of the mammalian recombination complex RC-1. EMBO J 15: 4061–4068 [PMC free article] [PubMed] [Google Scholar]
- Kim ST, Xu B, Kastan MB (2002) Involvement of the cohesin protein, Smc1, in Atm-dependent and independent responses to DNA damage. Genes Dev 16: 560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa R, Bakkenist CJ, McKinnon PJ, Kastan MB (2004) Phosphorylation of SMC1is a critical downstream event in the ATM-NBS1-BRCA1 pathway. Genes Dev 18: 1423–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn I, Heidinger-Pauli JM, Guacci V, Unal E, Koshland DE (2008) Sister chromatidcohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 24: 105–129 [DOI] [PubMed] [Google Scholar]
- Peters JM, Tedeschi A, Schmitz J (2008) The cohesin complex and its roles in chromosome biology. Genes Dev 22: 3089–3114 [DOI] [PubMed] [Google Scholar]
- Strom L, Sjogren C (2007) Chromosome segregation and double-strand break repair—a complex connection. Curr Opin Cell Biol 19: 344–349 [DOI] [PubMed] [Google Scholar]
- Watrin E, Peters JM (2009) The cohesin complex is required for the DNA damage induced G2/M checkpoint in mammalian cells. EMBO J 28: 2625–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M (2009) Clearing the way for mitosis: is cohesin a target? Nat Rev Mol Cell Biol 10: 489–496 [DOI] [PubMed] [Google Scholar]
- Yazdi PT, Wang Y, Zhao S, Patel N, Lee EY, Qin J (2002) SMC1 is a downstream effector in the ATM/NBS1 branch of the human S-phase checkpoint. Genes Dev 16: 571–582 [DOI] [PMC free article] [PubMed] [Google Scholar]