Abstract
At the core of iron homeostasis is hepcidin, a small acute phase antimicrobial peptide that now also appears to synchronously orchestrate the response of iron transporter and regulatory genes. In this perspective article, Drs Bayele and Srai discuss cis and trans acting factors that may influence hepcidin variation in humans and their potential role in iron metabolism control. See related papers on page 1293 and 1297.
Iron homeostasis, like other physiological processes, relies on precise and timely interactions between key proteins involved in either its uptake or release. At the core of this is hepcidin, a small acute phase antimicrobial peptide that now also appears to synchronously orchestrate the response of iron transporter and regulatory genes to ensure proper balance between how much dietary iron is absorbed by the small intestine or released into the circulation by macrophages.1 Several studies suggest that there are strong genetic components that underlie hepcidin regulation beyond the usual suspects (i.e. infection, inflammation, erythropoiesis, hypoxia and iron), in a manner that could impinge on phenotypic differences in susceptibility to iron-overload or anemia. Based on variation in hepcidin expression phenotypes, new emerging data suggest that there are heritable regulatory polymorphisms within the promoter that are linked to diseases of iron metabolism. Here we provide a perspective of what factors could determine such variability, giving some insight into how gene-gene, gene-environment, gene-nutrient interactions and even circadian rhythms may contribute to hepcidin expression variation and diseases associated with such variation.
Role of human genetics in hepcidin expression variation
Susceptibility to diseases of iron metabolism is often due to inappropriate levels of hepcidin expression or ferroportin resistance to its effects.2 Evidence suggests that these diseases cannot be fully explained by mutations in susceptibility genes alone i.e. those intimately linked to iron metabolism since most of these genes may have no mutations at all. This is particularly true for hepcidin because only a few mutations have been identified in the human hepcidin gene yet there are large variations in iron and hepcidin levels between individuals.3–5 In other words, there are heritable differences in hepcidin expression that may determine phenotypic variation in iron metabolism between individuals. This is because like most other genes, hepcidin does not express at the same levels or in the same temporal order in every individual, a phenomenon known as the genomics of gene expression or expression level polymorphisms.6
Hepcidin regulation: the story so far
For a whole host of reasons, gene expression is invariably stochastic. Thus, a random population-sampling would reveal wide variations in gene expression profiles and in hepcidin levels. Variation in hepcidin expression may be sexually dimorphic or it may depend on age, iron levels, and infection/inflammation or simply on time of day. For example, estradiol has been shown to repress hepcidin transcription in fish7 suggesting that differences in the complement of sex hormones could induce some variation in hepcidin expression within and between the sexes; this may underlie variation in hepcidin expression and liver iron loading between males and females.3–5,7–9
Regulatory variation in hepcidin expression may be determined by polymorphic cis-acting, non-coding regions of the gene. Thus these regions are just as crucial to quantitative differences in its expression as point mutations within its open-reading frame (ORF) because some of these regions contain transcription factor-binding sites. Trans-acting factors also determine hepcidin expression variation; these include transcription factors and iron regulatory or modifier proteins.2 Structural variation in the hepcidin gene i.e. gene dosage or copy number polymorphism, inversions and insertions,10 may also determine variability in its expression. We conjecture that where certain individuals inherit different copy numbers or structural variants of the hepcidin gene, there may be consequential variation in hepcidin expression and iron absorption. Although conceptually possible, this type of variation has not yet been identified.
Cis-acting regulatory polymorphisms in hepcidin expression level variation
A CCAAT-enhancer-binding protein (C/EBP) recognition site within the hepcidin promoter provided the first evidence for cis-acting regulation of its expression by C/EBPα.11 Subsequently, we showed that hepcidin expression was also regulated by Upstream Stimulatory Factor (USF) and c-Myc/Max through several E-boxes with the consensus sequence CAnnTG (n is any other nucleotide); these are binding sites for the basic helix-loophelix leucine zipper family of transcription factors.12 Genes that are regulated through E-boxes including the Clock genes period, timeless and clock tend to be under circadian rhythmic transcriptional control,13 suggesting that hepcidin may also be transcribed in pulses. This may account for the wide diurnal variations in hepcidin expression5 which may cause cyclical changes in iron levels. We also showed that single nucleotide polymorphisms (SNPs) within the cognate promoters of the genes in different mouse strains could contribute to variability in mouse hepcidin gene expression as some of these SNPs constituted USF binding sites.14 Similarly hepcidin expression by STAT3 (Signal Transducer and Activator of Transcription 3) is thought to be mediated by the STAT response element (also referred to as interferon-γ activation sequence, GAS), TTCTTGGAA.15 In support of the contribution of regulatory SNPs in hepcidin expression variation and iron metabolism, Island et al. found a C>T polymorphism (underlined) in one of two bone morphogenetic protein response elements, BMP-RE, (GGCGCC→GGTGCC) in the promoter that impaired transcription of the gene, its IL-6-responsiveness and binding by Smads.16 Similarly, Marco et al. found association between a −582A>G polymorphism in the hepcidin promoter and iron overload in thalassemia major.17 Porto et al. previously reported a SNP (a G to A substitution) in the 5′UTR of the human hepcidin gene which correlated with severe hemochromatosis. This SNP generated a short ORF upstream of the gene, causing a marked reduction in hepcidin expression.18 Such short ORFs abound in the human genome; they are highly polymorphic and some have been linked to a variety of human diseases because they cause significant reductions in the expression of proximal downstream genes.19
Trans-acting regulatory variation in hepcidin expression
Transcriptional regulators that may cause variation in hepcidin expression levels between individuals are primarily involved in the inflammation arm of hepcidin regulation i.e. the JAK/STAT (IL-6/STAT3), and BMP/Smad signaling pathways.20 However, the signals that culminate in iron-dependent hepcidin transcription or the cognate proteins that mediate this pathway remain obscure. Although we showed that USF regulates hepcidin expression,12 the underlying signal transduction pathway remains unclear. The increasing number of transcription factors for hepcidin expression (STAT3, Smads, USF1/2, c-Myc/Max, C/EBPα, and HIF-1) has major implications because quantitative or qualitative differences in their expression (i.e. due to regulatory polymorphisms or structural variation, respectively), could determine phenotypic variation in hepcidin expression between individuals. For example, Huang et al.21 found an association between biliary atresia and a polymorphism in USF2 that decreased hepcidin expression by this transcription factor.
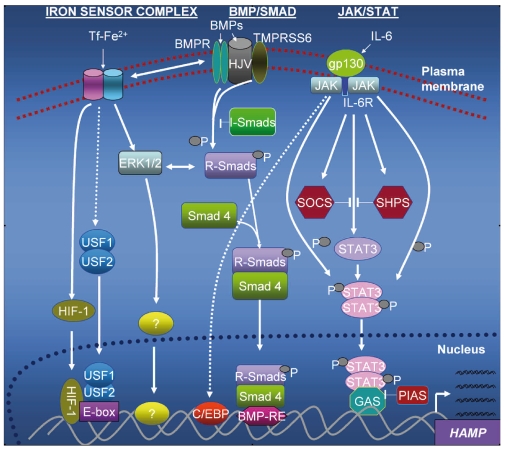
Mouse genetics suggests that there may be epistatic interactions between the hepcidin gene and TMPRSS6, HFE, TfR2 and HJV. TMPRSS6 mutations that increase systemic hepcidin levels in humans have been found to cause iron-deficiency anemia2,22,23 while mutations or deletions of TfR2, HJV and HFE invariably reduce hepcidin levels and cause iron-overload.2 It is therefore probable that polymorphisms or mutations at any of these loci or their upstream regulators could cause significant variability in hepcidin expression and differential iron-loading. Thus the potential for hepcidin expression variation increases exponentially with every identifiable regulator along the pathways depicted in Figure 1.
Figure 1.
Regulatory pathways in hepcidin expression. In the BMP/Smad pathway, the binding of BMPs to the BMP receptor induces receptor regulated Smads (R-Smads); following phosphorylation, R-Smads heterodimerize with Smad 4 (common Smad) and co-migrate to the nucleus where they bind to the BMP response elements (BMP-RE) in the hepcidin promoter. R-Smads can be inhibited by inhibitory Smads (I-Smads). An iron sensor complex which may include HFE, TfR2, HJV and TMPRSS6, is regulated by transferrin-bound iron (Tf-Fe). This (hypothetical) complex transmits iron signals via ERK1/2 for activation of a putative iron-responsive transcription factor which binds to the hepcidin promoter or modulates Smad phosphorylation and influences levels of hepcidin expression. Homo- and/or heterodimers of USF1/USF2 compete with HIF-1 for binding to the E-boxes; the signals for this may be generated by the iron sensor complex. The inflammatory (JAK-STAT) pathway engages IL-6 and its receptor, causing phosphorylation of the Janus kinase; this phosphorylates STAT3 which subsequently forms homodimers and translocates to the nucleus where they bind to the interferon γ-activation sequence (GAS) on the hepcidin promoter to drive transcription. The C/EBPs may also be regulated by this pathway (shown with a stippled arrow). The JAK-STAT pathway can be inhibited by the suppressors of cytokine signaling (SOCS), phosphotyrosine phosphatases (SHPS) and PIAS (Protein Inhibitor of Activated STAT). Both the SOCS and SHPs are induced by IL-6 but inhibit JAK-STAT signaling in a negative feed-back loop.
Epigenetic regulation of hepcidin expression
The most important epigenetic modifiers of hepcidin expression are the environment and diet because of their potential to influence chromatin structure e.g. through DNA methylation.24 For example, individuals that are exposed to infectious diseases may express more hepcidin than those in relatively sterile environments. Similarly, diets that are rich in iron may increase hepcidin synthesis. On the other hand, individuals living in chronically hypoxic environments (e.g. high altitude) may express reduced levels of hepcidin compared with those at sea-level. Unfortunately these assumptions are based on our working knowledge of hepcidin expression dynamics as no epidemiological data are available to support them. Nevertheless, it is highly likely that gene-environment and gene-nutrient interactions may critically modify hepcidin expression levels between individuals or populations, and their predisposition to iron-overload or anemia.
Concluding remarks
The exquisite sensitivity of hepcidin to fluctuations in systemic iron levels would make it a good reporter of iron metabolism but this is confounded by its equal sensitivity to inflammatory mediators and environmental vagaries. In this perspective, we have described how hepcidin regulation is multi-pronged and that hepcidin-dependent susceptibility to disorders of iron metabolism may be highly complex. A number of suggestions could be made to untangle this complexity.
For hepcidin to be a veritable diagnostic biomarker and a faithful reporter of disease, we must be able to distinguish between spurious or normal inconsequential biological processes that result in transient changes in its expression, from those that inform us of incipient disease.
Reference values are also urgently required to enable early disease diagnosis and staging, patient stratification and response to treatment.
Re-sequencing efforts should be made to identify polymorphisms in hepcidin genes particularly in population clusters with idiopathic iron-overload or deficiency.
The increasing complexity of iron metabolism requires a bottom-up, systems biology approach that begins with a computational assemblage of all the information available on hepcidin regulation. This would provide a unified, plug-and-play even if imperfect executable model to test new hypotheses and to validate existing pathways for its regulation. Such an approach will enable our understanding of how hepcidin integrates and/or controls iron metabolism in health and disease.
Footnotes
Dr. Henry K Bayele is a Senior Research Fellow in the Research Department of Structural and Molecular Biology, Division of Biosciences, University College London, UK.
Dr. Surjit Kaila Singh Srai is a Professor of Biochemistry and Molecular Biology, post-graduate tutor, Intercalated B.Sc. tutor in Molecular Medicine in the Research Department of Structural and Molecular Biology, Division of Biosciences, University College London, UK. This work was funded by a grant from the Biotechnology and Biological Sciences Research Council.
References
- 1.Chung B, Chaston T, Marks J, Srai SK, Sharp PA. Hepcidin decreases iron transporter expression in vivo in mouse duodenum and spleen and in vitro in THP-1 macrophages and intestinal Caco-2 cells. J Nutr . 2009 Jun 23; doi: 10.3945/jn.108.102905. [Epub ahead of print] PubMed PMID: 19549758. [DOI] [PubMed] [Google Scholar]
- 2.Lee PL, Beutler E. Regulation of hepcidin and iron-overload disease. Annu Rev Pathol. 2009;4:489–515. doi: 10.1146/annurev.pathol.4.110807.092205. [DOI] [PubMed] [Google Scholar]
- 3.Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ. Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr. 2009;89:1088–91. doi: 10.3945/ajcn.2008.27297. [DOI] [PubMed] [Google Scholar]
- 4.Van Deuren M, Kroot JJC, Swinkels DW. Time-course analysis of serum hepcidin, iron and cytokines in a C282Y homozygous patient with Schnitzler’s syndrome treated with IL-1 receptor antagonist. Haematologica. 2009;94:1297–300. doi: 10.3324/haematol.2009.005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroot JJ, Hendriks JC, Laarakkers CM, Klaver SM, Kemna EH, Tjalsma H, et al. (Pre)analytical imprecision, between-subject variability, and daily variations in serum and urine hepcidin: implications for clinical studies. Anal Biochem. 2009;389:124–9. doi: 10.1016/j.ab.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Stamatoyannopoulos JA. The genomics of gene expression. Genomics. 2004;84:449–57. doi: 10.1016/j.ygeno.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Robertson LS, Iwanowicz LR, Marranca JM. Identification of centrarchid hepcidins and evidence that 17beta-estradiol disrupts constitutive expression of hepcidin-1 and inducible expression of hepcidin-2 in largemouth bass (Micropterus salmoides) Fish Shellfish Immunol. 2009;26:898–907. doi: 10.1016/j.fsi.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Courselaud B, Troadec MB, Fruchon S, Ilyin G, Borot N, Leroyer P, et al. Strain and gender modulate hepatic hepcidin 1 and 2 mRNA expression in mice. Blood Cells Mol Dis. 2004;32:283–9. doi: 10.1016/j.bcmd.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Young MF, Glahn RP, Ariza-Nieto M, Inglis J, Olbina G, Westerman M, et al. Serum hepcidin is significantly associated with iron absorption from food and supplemental sources in healthy young women. Am J Clin Nutr. 2009;89:533–8. doi: 10.3945/ajcn.2008.26589. [DOI] [PubMed] [Google Scholar]
- 10.Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85–97. doi: 10.1038/nrg1767. [DOI] [PubMed] [Google Scholar]
- 11.Courselaud B, Pigeon C, Inoue Y, Inoue J, Gonzalez FJ, Leroyer P, et al. C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem. 2002;277:41163–70. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 12.Bayele HK, McArdle H, Srai SKS. Cis and trans regulation of hepcidin expression by Upstream Stimulatory Factor. Blood. 2006;108:4237–45. doi: 10.1182/blood-2005-07-027037. [DOI] [PubMed] [Google Scholar]
- 13.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 14.Bayele HK, Srai SK. Regulatory variation in hepcidin expression as a heritable quantitative trait. Biochem Biophys Res Commun. 2009;384:22–7. doi: 10.1016/j.bbrc.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–9. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Island ML, Jouanolle AM, Mosser A, Deugnier Y, David V, Brissot P, et al. A new mutation in the hepcidin promoter impairs its BMP response and contributes to a severe phenotype in HFE related hemochromatosis. Haematologica. 2009;94:720–4. doi: 10.3324/haematol.2008.001784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreani M, Radio FR, Testi M, De Bernardo C, Troiano M, Majore S, et al. Association of hepcidin promoter C.−582 α>γ variant and iron overload in thalassemia major. Haematologica. 2009;94:1293–6. doi: 10.3324/haematol.2009.006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porto G, Roetto A, Daraio F, Pinto JP, Almeida S, Bacelar C, et al. A Portuguese patient homozygous for the −25G>A mutation of the HAMP promoter shows evidence of steady-state transcription but fails to up-regulate hepcidin levels by iron. Blood. 2005;106:2922–3. doi: 10.1182/blood-2005-04-1630. [DOI] [PubMed] [Google Scholar]
- 19.Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci USA. 2009;106:7507–12. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–9. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 21.Huang YH, Huang CC, Chuang JH, Hsieh CS, Lee SY, Chen CL. Upstream stimulatory factor 2 is implicated in the progression of biliary atresia by regulation of hepcidin expression. J Pediatr Surg. 2008;43:2016–23. doi: 10.1016/j.jpedsurg.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Melis MA, Cau M, Congiu R, Sole G, Barella S, Cao A, et al. A mutation in the TMPRSS6 gene, encoding a transmembrane serine protease that suppresses hepcidin production, in familial iron deficiency anemia refractory to oral iron. Haematologica. 2008;93:1473–9. doi: 10.3324/haematol.13342. [DOI] [PubMed] [Google Scholar]
- 23.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–11. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JK, Kim Y-K. Epigenetic regulation and the variability of gene expression. Nat Genet. 2008;40:141–7. doi: 10.1038/ng.2007.58. [DOI] [PubMed] [Google Scholar]