Patients with diffuse large B-cell lymphoma with an intermediate/high or high-risk according to the age-adjusted International Prognostic Index have a dismal prognosis. This clinical trial suggests that the addition of rituximab to high-dose chemotherapy is effective and safe in diffuse large B-cell lymphoma with a poor prognosis. See related perspective article on page 1194.
Keywords: diffuse large B-cell lymphoma, autologous stem cell transplantation, dose-dense chemotherapy, rituximab, poor prognosis, high-dose chemotherapy
Abstract
Background
We investigated the addition of rituximab to dose-dense and high-dose chemotherapy with autologous stem cell transplantation in patients with untreated poor-prognosis diffuse large B-cell lymphoma.
Design and Methods
Ninety-four young patients (age, 18–60) with stage III–IV diffuse large B-cell lymphoma at intermediate/high or high risk according to the age-adjusted International Prognostic Index were enrolled into a phase II trial. The treatment was as follows: four courses of bi-weekly rituximab-cyclophosphamide-epirubicin-vincristine-prednisone (R-MegaCEOP14), two courses of rituximab-mitoxantrone-cytarabine-dexamethasone (R-MAD) and carmustine-etoposide-cytarabine-melphalan (BEAM) with autologous stem cell transplantation.
Results
The complete response and toxic death rates were 82% and 5%, respectively. Failure-free survival and overall survival rates at 4 years were 73% and 80%, respectively. The outcomes of these patients were retrospectively compared to those of 41 patients with similar characteristics enrolled into a previous phase II trial of high-dose chemotherapy without rituximab. This historical group was treated with eight weekly infusions of methotrexate-doxorubicin-cyclophosphamide-vincristine-prednisone-bleomycin (MACOP-B), two courses of MAD and BEAM with autologous stem cell transplantation. The 4-year failure-free survival rates for the rituximab and historical groups were 73% versus 44%, respectively (p=0.001); the 4-year overall survival rates were 80% and 54%, respectively (p=0.002). A Cox’s multivariable model was applied to adjust the effect of treatment for unbalanced or important prognostic factors: failure and death risks were significantly reduced in the rituximab group compared to the historical group, with an adjusted hazard ratio of 0.44 (p=0.01) for failure-free survival and 0.46 (p=0.02) for overall survival.
Conclusions
These results suggest that the addition of rituximab to high-dose chemotherapy is effective and safe in diffuse large B-cell lymphoma with a poor-prognosis and such regimens need to be compared to dose-dense chemoimmunotherapy without autologous stem cell transplantation in randomized trials.
Introduction
Patients with diffuse large B-cell lymphoma (DLBCL) with an intermediate/high or high-risk according to the age-adjusted International Prognostic Index (aa-IPI) have a dismal prognosis with 5-year survival rates of 46% and 32%, respectively.1–3 High-dose chemotherapy (HDC) with autologous stem cell transplantation (ASCT) was shown to be an effective salvage treatment for chemo-sensitive relapsed patients.4 These results prompted many investigators to apply this approach as part of the initial therapy for patients with DLBCL, especially for those with a poor prognosis. So far, no clear benefits have been shown in such patients, and conflicting results were generated in randomized studies, with similar survival rates in patients receiving either first-line HDC and ASCT, or standard chemotherapy without rituximab.5–11
In elderly or in young low-risk patients with DLBCL, the addition of rituximab to CHOP21 or dose-dense CHOP14 significantly improves overall and event-free survival rates compared to those achieved with CHOP alone.12–15 However, fewer data are available concerning young DLBCL patients with a poor prognosis.16,17 On this background, we explored the combination of rituximab with dose-dense chemotherapy and HDC with ASCT in untreated DLBCL patients with a poor prognosis. Here, we report the results of a prospective phase II trial, and compare these results with those from a historical cohort of patients treated in the pre-rituximab era in a previously published phase II study.18
Design and Methods
Rituximab-high-dose chemotherapy phase II study
The rituximab-HDC study was a phase II multicenter trial of the treatment of young patients with DLBCL with a poor prognosis conducted by the GIMURELL. From June 2002 to December 2005, 97 consecutive patients were enrolled (R-HDC study group). The study, registered at http://www.clinicaltrials.gov, under study NCT00556127, was performed in accordance with the Helsinki declaration and approved by the ethics review committees of all participating centers. All patients gave written informed consent.
Patients
The inclusion criteria were: previously untreated aggressive B-cell lymphoma (DLBCL, primary mediastinal lymphoma, follicular lymphoma grade IIIb);19 age 18–60 years; III–IV Ann Arbor stage; 0–2 Eastern Cooperative Oncology Group performance status (PS); intermediate/high and high risk score according to the aa-IPI.1 The exclusion criteria were: major organ dysfunction; seropositivy for human immunodeficiency, hepatitis B or hepatitis C virus; central nervous system (CNS) involvement at diagnosis. Histological diagnoses of all patients were reviewed at the Pathology Department of the University of Turin by DN. The mandatory baseline assessment included: physical examination; chest and abdominal computed tomography scans; bone marrow (BM) biopsy; full laboratory work-up and MUGA scan or echocardiography. Bulky disease was defined as a mass >10 cm in one diameter or more than one-third of the chest diameter in the mediastinum. Patients were retrospectively classified according to the revised International Prognostic Index (R-IPI).20
Treatment plan
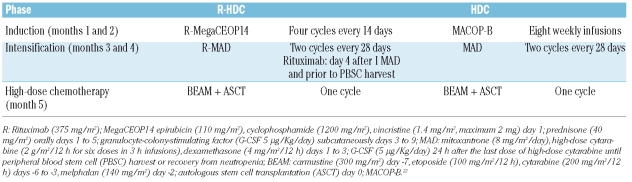
The trial design is shown in Table 1. The treatment consisted of three phases: (i) an induction phase lasting 2 months during which four courses of a dose-dense chemotherapy regimen, rituximab, cyclophosphamide, epirubicin, vincristine and prednisone (rituximab-MegaCEOP14) were given at 2-week intervals with granulocyte colony-stimulating factor (G-CSF) support;9 (ii) an intensification phase with two cycles of high-dose chemoimmunotherapy, rituximab, mitoxantrone, cytarabine and dexamethasone (rituximab-MAD) every 28 days with G-CSF;18 two doses of rituximab 375 mg/m2 were administered on day 4, as well as prior to peripheral blood stem cell harvest during the first MAD course as an in vivo purging; (iii) a consolidation phase consisting of myeloablative chemotherapy according to the carmustine, etoposide, cytarabine and melphalan (BEAM) regimen,21 followed by ASCT with at least 3×106 peripheral blood CD34+ cells/Kg body weight.
Table 1.
Treatment regimens.
At the end of the treatment, involved field radiotherapy 25–30 Gy was planned to be administered to areas of previous bulky disease. Patients with bone marrow, hard palate, orbital or paranasal sinus involvement received CNS prophylaxis with four doses of 12 mg intrathecal methotrexate during the induction phase. Supportive care during the intensification and the consolidation phases was given according to local guidelines.
Historical comparison: high-dose chemotherapy group
The R-HDC study group was retrospectively compared to 41 consecutive DLBCL patients enrolled by the same co-operative Group between August 1991 and August 1995 into a phase II trial of HDC and ASCT without rituximab, (HDC historical control group). This study was previously reported elsewhere.18 Patients from the control group with a T-cell phenotype or a histological subtype other than DLBCL or primary mediastinal lymphoma were excluded. The inclusion/exclusion criteria and the staging of the HDC historic control group study were the same as those of the R-HDC study group.
Treatment in the HDC historical control group also consisted of three phases (Table 1): (i) an induction phase with 8 weeks of methotrexate, doxorubicin, cyclophosphamide, vincristine, prednisone and bleomycin (MACOP-B) chemotherapy;22 (ii) an intensification phase composed of two courses of the MAD regimen which was identical to that used in the study group, except for the absence of rituximab; (iii) a consolidation phase with BEAM followed by ASCT with peripheral blood stem cells.
The criteria for CNS prophylaxis, radiotherapy and stem cell harvesting were the same as those for the R-HDC study group.
Assessment of response
In both studies, response was assessed 1 month after the end of the program by the treating physician according to the criteria described by Cheson et al.23 No response was defined as any response less than a partial response (PR), stable disease, progressive disease or any death during treatment period.
Study design and statistical methods
According to the evidence available when the R-HDC study was planned, the sample size was calculated using a Fleming’s single-stage design with failure-free survival (FFS) as the principal end-point. Given a FFS rate of 50% at 3 years with the HDC regimen, the sample size was calculated in order to show at least a FFS rate of 65% with the new R-HDC treatment, with an α error of 0.025 (one-sided) and a β error of 0.20; the required sample size was 85 patients, although 97 patients were enrolled to take into account 10% losses to follow-up. All the enrolled patients were considered assessable and results were analyzed on an intention-to-treat basis.
Treatment failure was defined as progression of disease at any time during treatment, less than a complete/complete undefined response (CR/CRu) at the end of treatment, relapse or death from any cause. The FFS and overall survival (OS) rates were calculated from the date of diagnosis to the date of treatment failure or death or the last follow-up without any event and reported with 95% confidence intervals (95% CI).
Comparisons between study and historic control group characteristics were performed using the χ2 test or Fisher’s exact test; means of continuous variables were compared using two-sided t-tests. To improve the comparison with the historical control group, all OS and FFS times were censored at the 60th month of follow-up or at the date of last contact. All curves were plotted according to the Kaplan and Meier method,24 and evaluated by the log-rank test. A Cox proportional hazard model was used to evaluate the effect of R-HDC treatment. The hazard ratios (HR) and corresponding 95% CI were adjusted for unbalanced or important prognostic factors (age, aa-IPI, bone marrow involvement, bulky disease, number of extranodal sites involved and B symptoms).25 A subgroup analysis was performed for FFS and OS using a statistical test for the interaction between the aa-IPI score and treatment. All calculations were carried out using the SAS (v. 8.2) package.
Results
Rituximab-high-dose chemotherapy phase II study
Patients’ characteristics
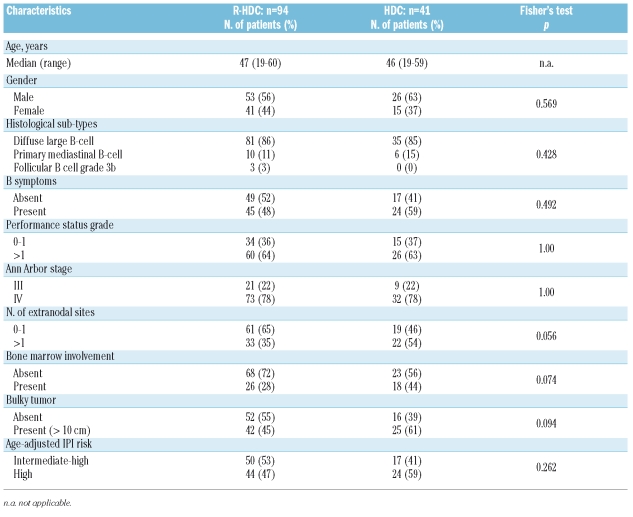
Ninety-seven consecutive patients were enrolled. Three patients were excluded because of a change of diagnosis following the central pathology review (two had follicular lymphoma grade 3a and one had a mantle cell blastoid variant). Therefore, 94 patients fulfilled the inclusion criteria and were included in the R-HDC study group. The median age was 47 years (range, 19–60). The clinical characteristics of these patients are listed in Table 2.
Table 2.
Clinical characteristics of the patients, divided by study group.
According to the aa-IPI, 50 patients (53%) had an intermediate/high and 44 (47%) a high risk score; according to the R-IPI, 34 patients (36%) had a score of 2 and 60 (64%) had a score of 3 or 4.
Feasibility of the treatment
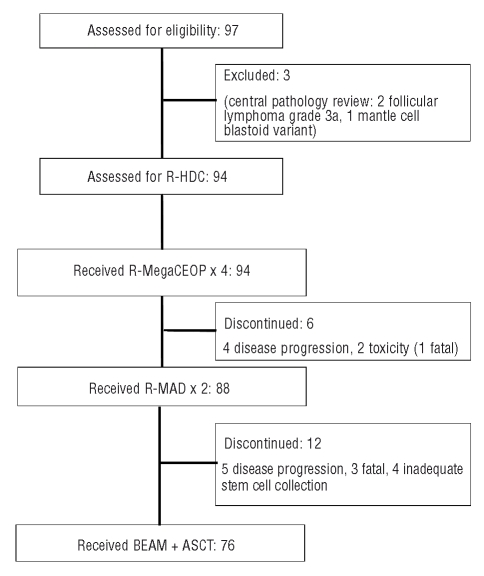
Seventy-six (81%) of the 94 patients completed treatment and underwent ASCT. Reasons for not completing the planned treatment in the remaining 18 patients included disease progression in nine patients, toxic death in four, pancreatic hemorrhage in one, and inadequate stem cell collection in four patients (Figure 1). Thirty-two (34%) patients were delivered involved field radiotherapy to an area of previous bulky disease after completion of the chemotherapy.
Figure 1.
Flow through the study of the 97 patients enrolled into the R-HDC study.
Response to treatment and outcome
Seventy-seven patients (82%, 95% CI: 73–88%) achieved a CR/CRu and one experienced PR. Progressive disease was documented in 11 patients (12%); four of these patients, none of whom had received intrathecal prophylaxis, had CNS progression before ASCT. Five patients (5%) died of toxicity.
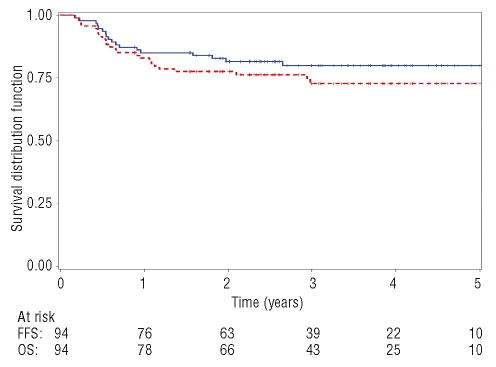
The median follow-up for censored patients was 49 months. Over the first 4 years, 24 R-HDC patients (26%) were defined as having treatment failure and 18 patients (19%) died. The 4-year FFS rate was 73% (95% CI: 63.5–82.5%) and the 4-year OS rate was 80% (95% CI: 71.6–88.4%) (Figure 2). Of the 24 patients in whom treatment failed, 11 had progressive disease during treatment and died of lymphoma; five patients died of acute toxicity. One patient in PR, who progressed early after treatment, is currently alive after second-line chemotherapy. Seven patients relapsed: five of them are alive after different salvage treatments and two died of lymphoma.
Figure 2.
The 4-year OS (blue solid line) and 4-year FFS (red dashed line) for the R-HDC study group. OS at 4 years: 80%; (95% CI: 71.6%–88.4%). FFS at 4 years: 73%; (95% CI: 63.5%–82.5%).
Subgroup analyses of OS according to the aa-IPI and R-IPI showed that the 4-year OS for patients with an aa-IPI score of 2 was 87%, whereas that for patients with an aa-IPI score of 3 was 73%; the 4-year OS for patients with an R-IPI score of 2 and 3–4 were 87% and 76%, respectively.
Hematologic engraftment and safety
All 76 patients who underwent ASCT achieved complete hematologic engraftment. The median times to recovery of an absolute neutrophil count greater than 0.5×109/L and to self-sustaining platelet recovery (>50×109/L) were 9 days (3–27 days) and 13 days (1–72 days), respectively.
Hematologic toxicity was mild during the R-MegaCEOP induction phase: according to the World Health Organization (WHO) toxicity criteria grading system, a grade 3 or higher hematologic toxicity for neutrophils was recorded in 30% of the total number of R-MegaCEOP courses delivered and for platelets and hemoglobin occurred in less than 10% of the courses. The following transfusion support was required during R-MAD and BEAM with ASCT: a median of 2.2 and 5 platelet concentrates, respectively and a median of 1.4 and 3 packed red cell transfusions, respectively.
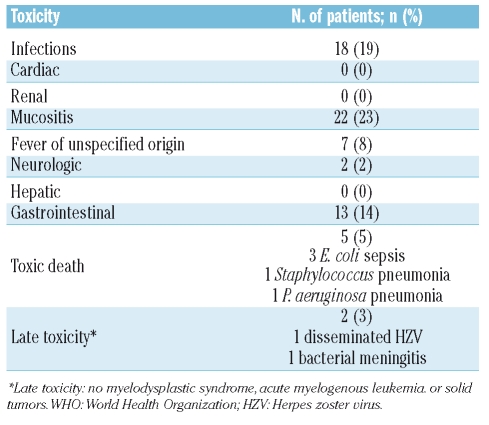
Severe non-hematologic toxicities of WHO grade 3 or more were reported in 50 patients (53.2%; 95%CI: 43.2–63.0) (Table 3). As expected, mucositis and gastrointestinal toxicity were frequently observed during the myeloablative phase. No cardiac, renal or hepatic events were recorded. Overall, 18 (19%) episodes of acute severe infection were reported in the 94 R-HDC patients. Five patients died of toxicity during treatment: three patients died of Escherichia coli sepsis; one of Staphylococcus pneumonia and one died of Pseudomonas aeruginosa pneumonia (Figure 1).
Table 3.
Non-hematologic toxicity of R-HDC (WHO grade ≥3).
Two late infections occurred at 11 and 13 months after treatment: one was a severe disseminated herpes zoster virus infection and one was bacterial meningitis. Both patients recovered completely. So far, no cases of secondary acute myelogenous leukemia, myelodysplastic syndrome or solid tumor have occurred.
Historical comparison
The outcomes in the R-HDC study group were compared to those of the HDC historical group. As shown in Table 2, the baseline characteristics of the patients treated with R-HDC were comparable to those of the patients treated with HDC, except that a lower percentage of R-HDC patients had involvement of more than one extranodal site (35% vs. 54%; p=0.056). However, the distribution of patients into aa-IPI subgroups (intermediate/high and high risk) did not differ between the R-HDC and HDC groups.
In the HDC historical control group, 31 (76%) of the 41 patients completed treatment and underwent ASCT. The reasons for not completing the planned treatment in the remaining ten patients were: disease progression in seven patients, toxic death in two patients, and inadequate stem cell harvest in one. Ten (24%) patients were given involved field radiotherapy after completion of the chemotherapy, this not being different from the R-HDC group (χ2= 0.265).
The details of the feasibility, toxicity and response to HDC in the historical control group have already been reported.18
The median follow-up for censored patients was 72 months for the HDC group and 49 months for the R-HDC group. Due to differences in the duration of follow-up between the two studies, the outcome comparisons were made at 4 years to ensure comparable follow-up times.
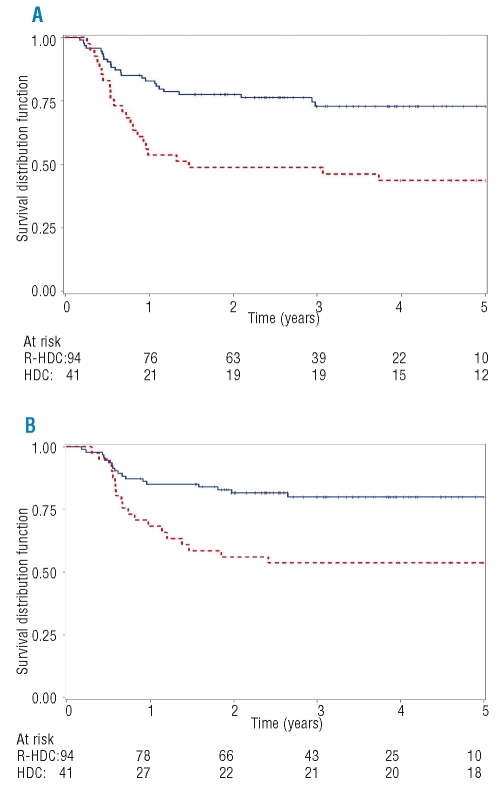
The 4-year FFS rate was 73% for the R-HDC group and 44% for the HDC control group, with a crude HR of 0.39 (95% CI: 0.22–0.69, p=0.001) (Figure 3a). The actuarial OS rate at 4 years was 80% for the R-HDC group and 54% for the HDC group, with a crude HR of 0.37 (95% CI: 0.19–0.71, p=0.002) (Figure 3B).
Figure 3.
FFS and OS, by study group. The 4-year FFS (A) and 4-year OS (B) for the R-HDC study group (blue solid line) and the HDC historical control group (red dashed line). (A) FFS at 4 years: R-HDC study group 73%; HDC historical control group 44%. Hazard ratio= 0.39 (95% CI: 0.22–0.69, p=0.001) (B) OS at 4 years: R-HDC study group 80%; HDC historical control group: 54%. Hazard ratio= 0.37 (95% CI: 0.19–0.71, p=0.002).
A Cox model was performed to adjust the comparison of treatments for potential confounders such as age, aa-IPI, bone marrow involvement, bulky disease, number of extranodal sites involved and B symptoms. This analysis confirmed that the risk of treatment failure or death was significantly reduced in the R-HDC group; the adjusted HR for FFS (R-HDC vs. HDC) was 0.44 (95% CI: 0.24–0.81, p=0.009), and the adjusted HR for OS (R-HDC vs. HDC) was 0.46 (95% CI: 0.22–0.90, p=0.023).
Subgroup analyses according to the aa-IPI score confirmed a better outcome for both intermediate/high and high risk patients treated with R-HDC (4-year FFS aa-IPI score 2: R-HDC 80%, HDC 53%; 4-year FFS aa-IPI score 3: R-HDC 64%, HDC 37%; 4-year OS aa-IPI score 2: R-HDC 87%, HDC 59%; 4-year OS aa-IPI score 3: R-HDC 73%, HDC 50%). The statistical tests for interaction did not indicate any meaningful effects upon the advantage of R-HDC versus HDC treatment by aa-IPI subgroups for either FFS (p for interaction term=0.565) or OS (p for interaction term=0.402).
Discussion
The aim of this multicenter, prospective phase II trial was to assess the potential benefit of adding rituximab to a dose-dense chemotherapy regimen followed by HDC and ASCT in untreated DLBCL patients with a poor prognosis (i.e. at intermediate/high or high risk according to aa-IPI score). The results demonstrate that R-HDC is effective as a first-line treatment in a large cohort of patients with a poor prognosis with a prolonged and adequate follow-up. The CR rate was high (82%) and the long-term outcome was also very favorable with 4-year FFS and OS rates of 73% and 80%, respectively.
There are reports of some trials in which rituximab was administered to relapsed patients with aggressive and follicular lymphoma before and after ASCT. The results indicate that this approach is safe and possibly effective.26–29 However, so far, few data have been reported on the use of HDC and ASCT supplemented with rituximab as first-line treatment in high-risk DLBCL and the information available is usually in abstract form.28,30–32 The feasibility of this approach is a major issue when setting up intensified regimens with autografting, and HDC with ASCT programs yielded better results in studies in which the drop-out rate of patients was less than 25%.10 Our R-HDC program was feasible in a multicenter setting with a drop-out rate limited to 19%. This low rate may have further contributed to the positive outcome of this study.
The impact of rituximab on hematologic and non-hematologic toxicities in lymphoma patients undergoing ASCT has been controversial. Rituximab was reported to affect hematologic engraftment after ASCT in some studies, but not in more recent ones.26,27,33,34 We did not observe a delay in either platelet or neutrophil engraftment in our R-HDC study.
With respect to non-hematologic toxicities, concerns have been raised regarding increased infection rates in rituximab-treated patients, while others did not confirm these data.34,35 In our study, the incidence of acute toxicities was as expected. The rate of fatal infections was not negligible; indeed, five patients died of bacterial infections. This rate is, however, similar to that reported in a recent meta-analysis including 15 randomized trials involving 2728 patients with aggressive non-Hodgkin’s lymphoma treated with HDC and ASCT or conventional chemotherapy in the pre-rituximab era, in which the treatment mortality rate was 5.7% in patients receiving HDC.11 In our R-HDC study, two patients developed late infections 1 year after ASCT. Overall, these data indicate that patients treated with dose-dense chemotherapy and/or HDC supplemented with rituximab require adequate anti-microbial prophylaxis and prolonged close clinical surveillance to avoid or optimize management of infections. Notably, no secondary malignancies have been recorded thus far in our R-HDC group.
The results of our study compare favorably with those of with two recent trials performed in patients with aggressive lymphomas with a poor prognosis treated with chemotherapy schedules characterized by early dose intensification and autografting, but without rituximab. The 5-year FFS rates in these studies ranged from 56% to 62%.7,36
To further validate our observations, we compared the results of the R-HDC study with those in a historical group of patients treated with HDC and ASCT without rituximab. With the limits of a retrospective, non-randomized comparison, our results suggest that the R-HDC scheme may indeed improve the outcome of DLBCL patients with a poor prognosis compared to that achieved with traditional HDC without rituximab. Some limits of this historical comparison should be highlighted, including minor differences in the populations of patients and in the first part of chemotherapy as well as different follow-up times between the two studies. In order to minimize these differences, the comparison was limited to the 4-year time point and adjusted for several potential confounders. The benefit of the R-HDC regimen was shown in a multivariate analysis after adjustment for age, aa-IPI, bone marrow involvement, bulky disease, number of extranodal sites involved and B symptoms. The risk of both treatment failure and death was confirmed to be significantly reduced in the R-HDC group by more than 50%. Moreover, the improvement for patients treated with R-HDC occurred in both those at intermediate/high and high risk according to the aa-IPI. Nevertheless, the benefit observed with the new scheme may not be only attributable to the addition of rituximab but could be due to the whole new scheme.
The efficacy of R-MegaCEOP + R-MAD + BEAM and ASCT may be explained by the rapid tumor reduction during the first part of dose-dense chemoimmunotherapy, and by the addition of non-cross-resistant high-dose cytarabine chemotherapy supplemented with rituximab (R-MAD), which further increases the response rate and avoids the onset of resistant clones. Indeed, in the R-HDC group, only 12% of the patients were refractory to treatment and progressed during therapy. It is noteworthy that this improvement occurred in patients with aa-IPI scores of 2 and 3. A relevant proportion of such patients are refractory to either conventional treatment or HDC and ASCT without rituximab.1,9,37 More intensive induction therapy before ASCT, as applied in our study, may play a favorable role in improving the outcome of patients with poor-prognosis aggressive lymphomas, even without rituximab, as suggested by the encouraging results of a prospective trial with CHOP followed by a dose-intensive cyclophosphamide, etoposide, cisplatin cycle and high-dose BEAM chemotherapy with ASCT.38
The current standard therapy for advanced stage DLBCL is R-CHOP chemotherapy, based on the results of randomized trials conducted either in elderly or in young low-risk patients and by a historical comparison with a population-based study.12–15,39 The appropriate therapy for young patients with intermediate/high and high risk DLBCL is still a subject of debate. Several phase II non-randomized studies incorporating rituximab into dose-dense or dose-intense schemes, namely R-CHOP14, but without ASCT showed that such approaches are feasible and likely effective in high-risk young DLBCL patients.16,17,32 However from these studies it is difficult to gain an estimate of the outcome of young patients with poor prognosis who were analyzed as a subgroup. Overall, the reported 2 and 5-year progression-free survival rates for patients with an aaIPI intermediate/high or high risk score ranged from 45% to 61% suggesting that 40–50% of these patients are unlikely to be cured by standard R-CHOP.16,17,32
Recently, the R-IPI was retrospectively applied to patients with DLBCL treated with R-CHOP distinguishing three separate prognostic groups with different 4-year OS rates: very good risk 94%, good risk 79% and poor risk 55%.20 We retrospectively classified our patients according to the R-IPI and found that 36% were at good risk and 64% at poor risk with 4-year OS rates of 87% and 76%, respectively. Although our data are not strictly comparable because we included only patients under 60 years old, the results reported here are encouraging and support further studies to evaluate the efficacy of R-HDC with ASCT compared with R-CHOP-like regimens in randomized trials in the group with poor prognosis.
Intensified chemoimmunotherapy with HDC and ASCT is one possible strategy to treat DLBCL patients with a poor prognosis. Alternatively, the evaluation at diagnosis of biological markers or models such as gene expression profiling, microvascular density and others may allow better identification of patients who are likely to fail to benefit from R-CHOP alone.40–42 The interim response evaluated with early positron emission tomography and computed tomography imaging has shown promise as a prognostic factor in retrospective series of patients with DLBCL, but requires further investigation because, unlike in Hodgkin’s disease, contradictory results have been reported in DLBCL.43–45 Both issues are worthwhile areas of future research in prospective trials.
In conclusion, the encouraging results reported here suggest that the R-HDC and ASCT approach may be effective in young DLBCL patients with a poor prognosis. However, the issue of whether R-HDC may be more effective than rituximab-dose-dense chemotherapy in these patients will only be resolved by randomized phase III trials that are currently being conducted by the major cooperative groups such as Groupe d’Etude des Lymphomes de l’Adulte, the North American Intergroup Study and others. The results of the present study have provided the rationale for an ongoing, prospective, phase III randomized trial, conducted by the Italian Lymphoma Intergroup (registered at http://www.clinicaltrials.gov:NCT00499018), which is testing the potential benefit of adding rituximab to HDC compared with dose-dense chemoimmunotherapy without ASCT to better define the proper therapy for young DLBCL patients with a poor prognosis.
Acknowledgments
the authors are indebted to Mrs. Lina De Masi for collecting data and wish to thank the staff at the various hematology units for their expert care of the patients.
Footnotes
Authorship and Disclosures
UV conceived and designed the trial, performed research as the principal investigator and wrote the paper. All authors contributed to writing the paper and checked the final version. UV, EA, GR, AML, MGC, VP and AL planned the study and wrote the study protocol; DN performed the pathology review; AC and BB collected and checked the accuracy of the data; GC performed the statistical evaluations; GG, LF, RF, LO, EP, DRS, FS, ATo and ATu were co-investigators in performing the research, treating and documenting patients and editing the manuscript.
The authors reported no potential conflicts of interest.
Funding: this study was supported by a grant from the Special Project “Oncology”, Compagnia SanPaolo/FIRMS, Torino, Italy.
References
- 1.Shipp MA, Harrington DP. A predictive model for aggressive NHL: the international non-Hodgkin’s lymphoma prognostic factors project. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 2.Coltman AC, Jr, Dahlberg S, Jones ES. CHOP is curative in thirty per cent of patients with large cell lymphoma: a twelve year Southwest Oncology Group follow-up. In: Skarin AT, editor. Advances in Cancer Chemotherapy: Update on Treatment for Diffuse Large Cell Lymphoma. New York, NY: Park Row; 1986. pp. 71–7. [Google Scholar]
- 3.Fisher RI, Gaynor ER, Dahlberg R, Oken MM, Grogan TM, Mize EM, et al. Comparison of standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med. 1993;328:1002–6. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 4.Philip T, Guglielmi C, Hagenbeek A, Somers R, van Der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 5.Gianni AM, Bregni M, Siena S, Brambilla C, Di Nicola M, Lombardi F, et al. High dose chemotherapy and autologous bone marrow transplantation compared with MACOP-B in aggressive B-cell lymphoma. N Engl J Med. 1997;336:1290–7. doi: 10.1056/NEJM199705013361804. [DOI] [PubMed] [Google Scholar]
- 6.Haioun C, Lepage E, Gisselbrecht C, Salles G, Coiffier B, Brice P, et al. Survival benefit of high dose therapy in poor risk non Hodgkin’s lymphoma: final analysis of the prospective LNH87-2 protocol, a groupe d’Etude des Lymphomes de l’Adulte study. J Clin Oncol. 2000;18:3025–30. doi: 10.1200/JCO.2000.18.16.3025. [DOI] [PubMed] [Google Scholar]
- 7.Milpied N, Deconinck E, Gaillard F, Delwail V, Foussard C, Berthou C, et al. Initial treatment of aggressive lymphoma with high-dose chemotherapy and autologous stem-cell support. N Engl J Med. 2004;350:1287–95. doi: 10.1056/NEJMoa031770. [DOI] [PubMed] [Google Scholar]
- 8.Martelli M, Gherlinzoni F, De Renzo A, Zinzani PL, De Vivo A, Cantonetti M, et al. Early autologous stem-cell transplantation versus conventional chemotherapy as front-line therapy in high-risk, aggressive non-Hodgkin’s lymphoma: an Italian multicenter randomised trial. J Clin Oncol. 2003;21:1255–62. doi: 10.1200/JCO.2003.01.117. [DOI] [PubMed] [Google Scholar]
- 9.Vitolo U, Liberati AM, Cabras MG, Federico M, Angelucci E, Baldini L, et al. High dose sequential chemotherapy with autologous transplantation versus dose-dense chemotherapy MegaCEOP as first line treatment in poor-prognosis diffuse large cell lymphoma: an “Intergruppo Italiano Linfomi” randomized trial. Haematologica. 2005;90:793–801. [PubMed] [Google Scholar]
- 10.Strehl J, Mey U, Glasmacher A, Djulbegovic B, Mayr C, Gorschluter M, et al. High-dose chemotherapy followed by autologous stem cell transplantation as first-line therapy in aggressive non-Hodgkin’s lymphoma: a meta-analysis. Haematologica. 2003;88:1304–15. [PubMed] [Google Scholar]
- 11.Greb A, Bohlius J, Trelle S, Schiefer D, De Souza CA, Gisselbrecht C, et al. High-dose chemotherapy with autologous stem cell support in first-line treatment of aggressive non-Hodgkin lymphoma - results of a comprehensive meta-analysis. Cancer Treat Rev. 2007;33:338–46. doi: 10.1016/j.ctrv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Vose JM, Link BJ, Grossbard M, Czuczman M, Grillo-Lopez A, Gilman P, et al. Phase II study of rituximab in combination with CHOP chemotherapy in patients with previously untreated, aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2001;19:389–97. doi: 10.1200/JCO.2001.19.2.389. [DOI] [PubMed] [Google Scholar]
- 13.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 14.Pfreundschuh M, Shubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–16. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 15.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 16.Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: a phase II study of feasibility and toxicity. Haematologica. 2006;91:496–502. [PubMed] [Google Scholar]
- 17.Halaas JL, Moskowitz CH, Horwitz S, Portlock C, Noy A, Straus D, et al. R-CHOP-14 in patients with diffuse large B-cell lymphoma: feasibility and preliminary efficacy. Leuk Lymphoma. 2005;46:541–7. doi: 10.1080/10428190400029932. [DOI] [PubMed] [Google Scholar]
- 18.Vitolo U, Cortelazzo S, Liberati AM, Freilone R, Falda M, Bertini M, et al. Intensified and high dose chemotherapy with granulocyte colony-stimulating factor and autologous stem-cell transplantation support as first-line therapy in high-risk diffuse large-cell lymphoma. J Clin Oncol. 1997;15:491–8. doi: 10.1200/JCO.1997.15.2.491. [DOI] [PubMed] [Google Scholar]
- 19.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361–92. [PubMed] [Google Scholar]
- 20.Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109:1857–61. doi: 10.1182/blood-2006-08-038257. [DOI] [PubMed] [Google Scholar]
- 21.Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol. 1995;13:588–95. doi: 10.1200/JCO.1995.13.3.588. [DOI] [PubMed] [Google Scholar]
- 22.Klimo P, Connors JM. MACOP-B chemotherapy for the treatment of advanced diffuse large cell lymphoma. Ann Intern Med. 1985;102:596–602. doi: 10.7326/0003-4819-102-5-596. [DOI] [PubMed] [Google Scholar]
- 23.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an International Workshop to standardize response criteria for non Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete information. J Am Stat Assoct. 1958;53:547–81. [Google Scholar]
- 25.Cox DR. Regression model and life tables (with discussion) JR Stat Soc. 1972;34:187–22. [Google Scholar]
- 26.Hoerr AL, Gao F, Hidalgo J, Tiwari D, Blum KA, Mathews V, et al. Effects of pretransplantation treatment with rituximab on outcomes of autologous stem-cell transplantation for non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:4561–6. doi: 10.1200/JCO.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 27.Khouri IF, Saliba RM, Hosing C, Okoroji GJ, Acholonu S, Anderlini P, et al. Concurrent administration of high-dose rituximab before and after autologous stem-cell transplantation for relapsed aggressive B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23:2240–7. doi: 10.1200/JCO.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Tarella C, Zanni M, Magni M, Benedetti F, Patti C, Barbui T, et al. Rituximab improves the efficacy of high-dose chemotherapy with autograft for high-risk follicular and diffuse large B-cell lymphoma: a multi-center Gruppo Italiano Terapie Innnovative nei Linfomi survey. J Clin Oncol. 2008;26:3166–75. doi: 10.1200/JCO.2007.14.4204. [DOI] [PubMed] [Google Scholar]
- 29.Vellenga E, van Putten WLJ, van’t Veer MB, Zijlstra JM, Fibbe WE, van Oers MHJ, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+ NHL: a prospective randomized HOVON trial. Blood. 2008;111:537–43. doi: 10.1182/blood-2007-08-108415. [DOI] [PubMed] [Google Scholar]
- 30.Tarella C, Zanni M, Di Nicola M, Patti C, Calvi R, Pescarollo A, et al. Prolonged survival in poor-risk diffuse large B-cell lymphoma following front-line treatment with rituximab-supplemented, early intensified chemotherapy with multiple autologous hematopoietic stem cell support: a multicenter study by GITIL (Gruppo Italiano Terapie Innovative nei Linfomi) Leukemia. 2007;21:1802–11. doi: 10.1038/sj.leu.2404781. [DOI] [PubMed] [Google Scholar]
- 31.Gisselbrecht C, Fitoussi O, Belhadj K, Mounier N, Feugier P, Coiffier B, et al. Survival impact of rituximab combined to ACVBP (R-ACVBP) in 209 poor risk diffuse large B-cell lymphoma (DLBCL) patients treated with upfront high–dose consolidative autotransplantation (HDC): a GELA phase II study. Blood. 2008;112:771. [Google Scholar]
- 32.Glass B, Kloess M, Reisen M, Metzner B, Trumper L, Loffler M, et al. Repetitive high-dose therapy followed by autologous stem cell transplantation (MegaCHOEP) for primary treatment of aggressive NHL: the impact of rituximab on outcome and toxicity Bone Marrow Transplant 20063723916327812 [Google Scholar]
- 33.Kamezaki K, Kikushige Y, Numata A, Miyamoto T, Takase K, Henzan H, et al. Rituximab does not compromise the mobilization and engraftment of autologous peripheral blood stem cells in diffuse-large B-cell lymphoma. Bone Marrow Transplant. 2007;39:523–7. doi: 10.1038/sj.bmt.1705649. [DOI] [PubMed] [Google Scholar]
- 34.Benekli M, Hahn T, Shafi F, Qureshi A, Alam AR, Czuczman MS, et al. Effect of rituximab on peripheral blood stem cell mobilization and engraftment kinetics in non-Hodgkin’s lymphoma patients. Bone Marrow Transplant. 2003;32:139–43. doi: 10.1038/sj.bmt.1704106. [DOI] [PubMed] [Google Scholar]
- 35.Horwitz SM, Negrin RS, Blume KG, Breslin S, Stuart MJ, Stockerl-Goldstein KE, et al. Rituximab as adjuvant to high-dose therapy and autologous hematopoietic cell transplantation for aggressive non-Hodgkin lymphoma. Blood. 2004;103:777–83. doi: 10.1182/blood-2003-04-1257. [DOI] [PubMed] [Google Scholar]
- 36.Glass B, Kloess M, Bentz M, Schlimok G, Berdel WE, Feller A, et al. Dose-escalated CHOP plus etoposide (MegaCHOEP) followed by repeated stem cell transplantation for primary treatment of aggressive high-risk non-Hodgkin lymphoma. Blood. 2006;107:3058–64. doi: 10.1182/blood-2005-04-1570. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser U, Uebelacker I, Abel U, Birkmann J, Trumper L, Schmalenberg H, et al. Randomized study to evaluate the use of high-dose therapy as part of primary treatment for “aggressive” lymphoma. J Clin Oncol. 2002;20:4413–9. doi: 10.1200/JCO.2002.07.075. [DOI] [PubMed] [Google Scholar]
- 38.Stewart DA, Bahlis N, Valentine K, Balogh A, Savoie L, Morris DG, et al. Upfront high-dose chemotherapy with DICEP followed by BEAM and autologous stem cell transplantation for poor-prognosis aggressive non-Hodgkin’s lymphoma. Blood. 2006;107:4623–7. doi: 10.1182/blood-2005-12-4898. [DOI] [PubMed] [Google Scholar]
- 39.Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–33. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 40.Malumbres R, Chen J, Tibshirani R, Johnson NA, Sehn LH, Natkunam Y, et al. Paraffin-based 6-gene model predicts outcome in diffuse large B-cell lymphoma patients treated with R-CHOP. Blood. 2008;111:5509–14. doi: 10.1182/blood-2008-02-136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rimsza LM, Leblanc ML, Unger JM, Miller TP, Grogan TM, Persky DO, et al. Gene expression predicts overall survival in paraffin-embedded tissues of diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2008;112:3425–33. doi: 10.1182/blood-2008-02-137372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signature in large B-cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupuis J, Gaulard P, Hemery F, Itti E, Gisselbrecht C, Rahmouni A, et al. Respective prognostic values of germinal center phenotype and early (18)fluorodeoxyglucose-positron emission tomography scanning in previously untreated patients with diffuse large B-cell lymphoma. Haematologica. 2007;92:778–83. doi: 10.3324/haematol.10895. [DOI] [PubMed] [Google Scholar]
- 44.Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25:3746–52. doi: 10.1200/JCO.2007.11.6525. [DOI] [PubMed] [Google Scholar]
- 45.Cashen A, Dehdashti F, Luo J, Bartlett NL. Poor predictive value of FDG-PET/CT performed after 2 cycles of R-CHOP in patients with diffuse large B-cell lymphoma (DLCL) Blood. 2008;112:371. [Google Scholar]