Translocation of chromosomes 8 and 21, t(8;21), resulting in the AML1-ETO fusion gene, is associated with acute myeloid leukemia (AML). The findings of this study indicate that genomic alterations and KIT-D816 mutation confer a poor prognosis in t(8;21) AML patients.
Keywords: t(8;21), AML1-ETO, CNN-LOH, SNP-chip, KIT, PIM1
Abstract
Translocation of chromosomes 8 and 21, t(8;21), resulting in the AML1-ETO fusion gene, is associated with acute myeloid leukemia. We searched for additional genomic abnormalities in this acute myeloid leukemia subtype by performing single nucleotide polymorphism genomic arrays (SNP-chip) analysis on 48 newly diagnosed cases. Thirty-two patients (67%) had a normal genome by SNP-chip analysis (Group A), and 16 patients (33%) had one or more genomic abnormalities including copy number changes or copy number neutral loss of heterozygosity (Group B). Two samples had copy number neutral loss of heterozygosity on chromosome 6p including the PIM1 gene; and one of these cases had E135K mutation of Pim1. Interestingly, 38% of Group B and only 13% of Group A samples had a KIT-D816 mutation, suggesting that genomic alterations are often associated with a KIT-D816 mutation. Importantly, prognostic analysis revealed that overall survival and event-free survival of individuals in Group B were significantly worse than those in Group A.
Introduction
The t(8;21)(q22;q22) translocation occurs in 40% of patients with acute myeloid leukemia (AML) of the FAB-M2 subtype, and constitutes 12% of all newly diagnosed cases of AML. This translocation leads to a fusion product of AML1 (also called RUNX1 or CBFβ) and ETO (also called MTG8). Data have suggested that the translocation is an early event in leukemogenesis.1 Furthermore, the t(8;21) translocation can be found in neonatal Guthrie blood spots of infants that later developed AML1-ETO leukemia, suggesting that the translocation can precede development of AML by up to ten years.2,3
Several murine models have demonstrated that AML1-ETO alone is not sufficient to induce leukemia. Murine bone marrow cells expressing tetracycline-inducible AML1-ETO transgene did not develop leukemia:4 but developed myeloproliferative disorders.5 In contrast, 30–55% of AML1-ETO-expressing mice treated with the DNA-alkylating mutagen N-ethyl-N-nitrosourea (ENU) developed AML.6,7 These findings strongly suggest that a secondary hit is necessary for the development of t(8;21) AML.
The protooncogene KIT is a receptor tyrosine kinase. Activating mutations of KIT including those in either the extracellular (exon 8) region or the protein kinase domains (D816 mutation) are found in 2% and 11% of t(8;21) AML samples, respectively.8,9 FLT3 is also a receptor tyrosine kinase. Two frequent activating mutations of FLT3, FLT3-internal tandem duplication (ITD) and FLT3-tyrosine kinase domain (TKD) mutation, are detected in a range of 2–8% and 2–4% of samples of t(8;21) AML, respectively.10,11 Mutation of NRAS at either codon 12, 13 or 61 is found in 9% of t(8;21) AML samples.10,11
High-density single nucleotide polymorphism genomic arrays (SNP-chip) allow the detection of copy number changes, as well as copy number neutral loss of heterozygosity (CNN-LOH) in leukemia samples.12–18 In order to screen for secondary alteration(s) that potentially could cause AML1-ETO transformed cells to develop acute myeloid leukemia, we performed SNP-chip analysis of 48 t(8;21) AML samples. The use of CNAG (copy number analysis for Affymetrix GeneChips) program12 and an algorithm AsCNAR (allele-specific copy number analysis using anonymous references)13 allows identification of hidden abnormalities and novel disease-related genomic regions in the leukemia samples. Here, we found that genomic changes detected by SNP-chip analysis are associated with a poor overall and event-free survival in t(8;21) AML.
Design and Methods
Patient samples, determination of mutant genes and statistical analysis
Genomic DNA of 48 anonymized samples of t(8;21) AML cells were obtained from Chang-Gung Memorial Hospital, Chang-Gung University in Taiwan after obtaining informed consent. These samples had been frozen over a span of 14 years (July 1990 to July 2004). Sample information is shown in the Online Supplementary Table S1. The study has been approved by Cedars-Sinai Medical Center (IRB number 4485).
To detect an AML1-ETO fusion transcript, RT-PCR was performed using specific primers as described previously.19 Mutation analysis of the KIT gene for the t(8;21) AML samples was reported previously.20 Statistical analysis is described in the Online Supplementary Design and Methods section.
SNP-chip analysis
Genomic DNA isolated from t(8;21) AML cells was subjected to GeneChip Human mapping microarray (SNP-chip, Affymetrix, Santa Clara, CA, USA) as described previously;12,13 ten samples (cases #47, #51, #52, #54, #56, #57, #59, #60, #61 and #62) were examined with the 250 K array, and the other 38 samples were analyzed with the 50 K array. The allele-specific copy numbers (AsCNs) were estimated using normal genomic DNA from peripheral blood of normal volunteers as controls.13 The array does not contain Y-chromosome probes; therefore, we summarize the SNP-chip data without sex chromosomes. Size, position and location of genes were identified with UCSC Genome Browser (http://genome.ucsc.edu/). Copy number changes previously described as copy number variants (http://projects.tcag.ca/variation/) were excluded.
Fluorescence in situ hybridization (FISH) analysis
Interphase hybridizations were performed following the manufacturer’s instructions and standard protocols. Probes for the SNRPN gene, 15q telomere, 7p telomere and 7q telomere were obtained from Cytocell (Cambridge, United Kingdom); and probes for the MYC gene as well as the centromere of chromosome 8 were purchased from Abbott Molecular (Abbott Park, IL, USA). Fifty interphase cells were scored for each sample, with 20 cells scored in controls (bone marrow controls with normal karyotypes). Signal patterns were normal for all controls with all probe sets.
Analysis of the PIM1 gene
Six coding exons of the PIM1 gene were amplified using specific primers from genomic DNA of cases #39 and #41. After purification of the PCR products from agarose gel, nucleotide sequences were determined. Primer sequences will be provided upon request. These 6 exons of other t(8;21) AML samples were examined by single strand conformation polymorphism (SSCP) as described in the Online Supplementary Design and Methods section.
To determine the frequency of missense mutations of the PIM1 gene within exon 4, this region of 34 t(8;21) AML samples and 40 normal blood DNA samples were amplified by PCR using specific primer (5′-TCC TGG AGA GGC CCG AGC-3′ and 5′-TTG AGG TCG ATA AGG ATG-3′). The PCR product (178 bp) was treated with a restriction enzyme Hpy188III for 1h. PCR products from wild-type allele are not digested but mutated allele are digested by the restriction enzyme.
Results and Discussion
SNP-chip analysis of 48 t(8;21) acute myeloid leukemia samples
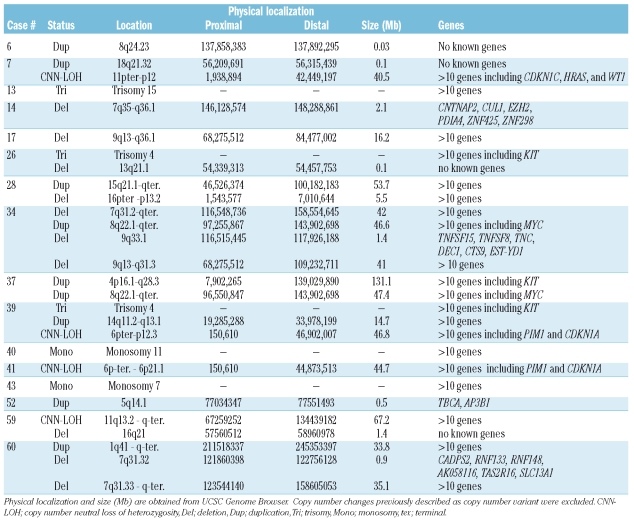
SNP-chip analysis of 48 t(8;21) acute myeloid leukemia (AML) samples revealed several genomic copy number changes, as well as copy number neutral loss of heterozygosity (CNN-LOH). As shown in Table 1 and Online Supplementary Figure S1, 32 patients (67%) had a normal genome by SNP-chip analysis (Group A, mean age is 23, range 2–74). In contrast, 16 patients (33%) had one or more genomic abnormalities (Group B, mean age is 31, range 4–61). Thus, these copy number changes probably harbor dysregulated leukemia-associated genes in t(8;21) AML. Cytogenetics showed that case #33 had trisomy 4 in 2 out of 15 cells (13%), and case #34 had monosomy 18 in 6 out of 24 cells (25%) (Online Supplementary Table S1). These minor clones were not detected by SNP-chip analysis. Case #40 had tetraploidy in 23 out of 25 cells; 2-fold gene-dosage in all chromosomes was masked and detected as normal gene-dosage.
Table 1.
Chromosomal regions with copy number changes and copy number neutral loss of heterozygosity in t(8; 21) acute myeloid leukemia samples.
Next, we compared SNP-chip results and gene mutations. Ten out of 48 samples (18%) had a KIT-D816 mutation. Interestingly, 6 (case #7, #14, #26, #37, #40, and #52) of the 10 samples were found in Group B, demonstrating that KIT-D816 mutation is significantly associated with Group B (p<0.05, χ2 test). This result suggests that copy number changes are often involved in cases with a KIT-D816 mutation in t(8;21) AML.
Recurrent copy number changes in t(8;21) acute myeloid leukemia samples
Two cases (#34 and #37) had a duplication on chromosome 8 from 8q22.1 to q-terminal including the MYC gene; and 2 cases (#13 and #28) had a trisomy/duplication on chromosome 15 with common duplicated region at 15q21.1-15q-terminal (53.7 Mb). Four cases (#14, #34, #43 and #60) had a deletion/monosomy on chromosome 7 with a common deleted region at 7q35 - 7q36.1 (2.1 Mb) including the CUL1 and EZH2 genes; and 2 cases (#17 and #34) had a deletion on chromosome 9 with the common deleted region at 9q13 -9q36.1 (16.2 Mb). Interestingly, a frequent large duplication was found on chromosome 4. Two cases had trisomy 4, and one case had a large region of duplication on chromosome 4 from 4p16.1 to q28.3 (131.1 Mb). All of these amplifications covered the region of the KIT gene; and 3 of these cases (#26, #37 and #39) had a KIT mutation of either D816Y, D816V or D820G. Amplification of chromosome 4 linked to KIT mutations has previously been described in systemic masto-cytosis.21 Thus, the probable increased expression of the mutated form of KIT by trisomy 4 or duplication in the region of the gene should give the clone a proliferative advantage.
Validation of copy number change by fluorescence in situ hybridization (FISH)
To validate some of these copy number changes, we used an interphase FISH approach. Case #34 had a duplication of 8q22.1-8q-terminal (46.6 Mb) including the MYC gene and a deletion of 7q31.2-7q-terminal (42.0 Mb). The 8q duplication was confirmed using FISH probes for MYC and the centromere of chromosome 8 (Online Supplementary Figure S2A). In the same case, deletion of chromosome 7q (q31.2 to q-terminal) was confirmed using FISH probes for 7p telomere and 7q telomere (Online Supplementary Figure S2B). Case #28 had a large duplication of chromosome 15 (53.7 Mb). The duplication was confirmed using FISH probes for SNRPN and 15q telomere (Online Supplementary Figure S2C). These results suggest that abnormalities detected by SNP-chip analysis reflected real alterations in AML cells.
Chromosomal regions and candidate genes in genomic areas with copy number neutral loss of heterozygosity (CNN-LOH)
Four cases (8%) had CNN-LOH (Table 1 and Online Supplementary Figure S1). Case #7 has CNN-LOH at 11p-terminal-11p12 (40.5 Mb) which included the CDKN1C, HRAS, WT1 and LMO2 genes. Case #39 and #41 have CNN-LOH at 6p-terminal - 6p12.3 (46.8 Mb) and at 6p-terminal - 6p21.1 (44.7 Mb), respectively; and the region contained the PIM1 and CDKN1A genes (Table 1 and Online Supplementary Figure S3). Case #59 had CNN-LOH at 11q13.2-q-terminal (67.2 Mb). Raghavan et al.15 showed that approximately 20% AML samples had CNN-LOH, and Gondek et al.16 found that 20% of MDS, 23% of MDS-derived AML, and 35% of MDS/MPD patients had CNN-LOH. In additional studies, we found that 32% of normal karyotype AML samples and 15% of t(15;17) APL samples had CNN-LOH.17,18 CNN-LOH in t(8;21) AML is less frequent than many other types of leukemia.
Acquired mutation of the PIM1 gene
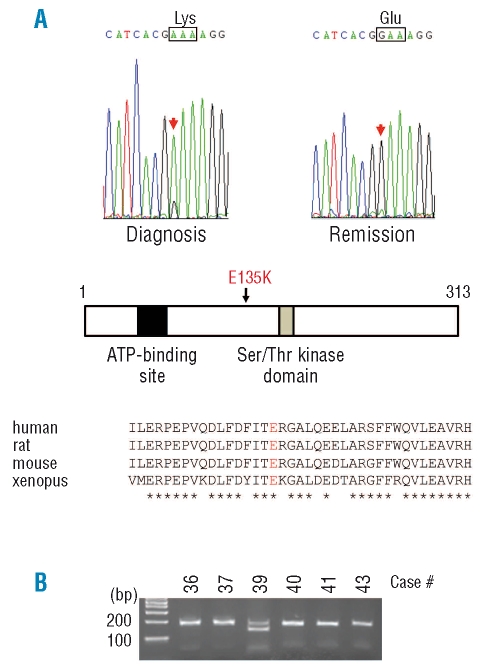
The protooncogene PIM1, which encodes the serine-threonine protein kinase, is located on chromosome 6p, and 2 cases had CNN-LOH in the region. All exons of the PIM1 gene for these 2 cases were examined for mutations. As shown in Figure 1A, case #39 had a nucleic acid change of G to A at exon 4 of the PIM1 gene leading to an amino acid change of glutamic acid (E) to lysine (K) at codon 135 (E135K). The amino acid change occurred between the ATP-binding site and serine-threonine kinase domain. The wild-type amino acid is conserved between human, rat, mouse and xenopus. Importantly, the complete remission sample of the same individual showed the wild-type sequence, demonstrating that the nucleic acid change was a disease-specific acquired alteration.
Figure 1.
Acquired mutation of the PIM1 gene in case #39. (A) Exon 4 of the PIM1 gene in case #39 had a missense mutation in the sample at diagnosis but not at remission (top panel). The mutation leads to the amino acid change of glutamic acid (E) to lysine (K) at amino acid 135 (E135K) of PIM1 protein. This mutated amino acid is located between the ATP binding domain and serine-threonine kinase domain of the protein (middle panel). The wild-type amino acid (E) is highly conserved among human, rat, mouse and xenopus (bottom). Note, *identical amino acid. (B) The mutated DNA sequence produced a Hpy188III restriction enzyme recognition sequence. The region was amplified by PCR, digested with Hpy188III, and subjected to agarose gel electrophoresis. The PCR product from only case #39 was digested.
The missense mutation in the PIM1 gene change produces the recognition site of a restriction enzyme, Hpy188III. A total of 34 t(8;21) AML samples and 40 normal blood DNA samples were examined for this mutation by Hpy188III digestion. The PCR product (178 bp) encompassing the mutation was only digested in case #39 (Figure 1B), but not the DNA from the other AML samples or normal blood DNA (data not shown), suggesting it is infrequent in the AML subtype. We also examined all exons of the PIM1 gene by SSCP using 34 t(8;21) AML samples, but no shifted bands were detected other than exon 4 of case #39. The PIM1 E135K mutant was also detected in B-cell diffuse large-cell lymphoma;22 and another mutant (E135Q) was discovered in primary diffuse large B-cell lymphomas.23 It remains to be clarified whether the E135K mutant is activated constitutively.
Prognostic significance of genomic change
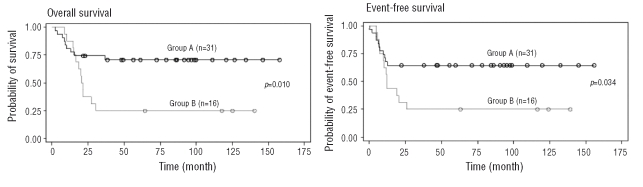
Overall survival of t(8;21) AML patients of Group A (no genomic abnormality observed by SNP-chip) was significantly better than individuals in Group B (genomic abnormality observed by SNP-chip) (hazard ratio=2.992 [95% confidence interval, 1.247–7.179], p=0.01) (Figure 2). The event-free survival of individuals of Group A was also significantly better than those in Group B (hazard ratio=2.360 [95% confidence interval, 1.037–5.372], p=0.0347). We also compared the prognosis of individuals with the KIT-D816 mutation (6 cases) to those without the alteration (10 cases) in Group B, but found no significant difference (data not shown). These results strongly suggest that genomic changes in t(8;21) AML are associated with a poor overall and event-free survival.
Figure 2.
Comparison of overall survival and event-free survival of t(8;21) acute myeloid leukemia patients either with or without genomic changes. Overall survival (left) and event-free survival (right) were compared between Groups A and B. Black and grey lines indicate Group A (no genomic abnormality by SNP-chip) and Group B (genomic abnormality by SNP-chip), respectively.
A recent study showed that a KIT-D816V mutation is associated with a poor prognosis in t(8;21) AML patients.9 Also, secondary cytogenetic abnormalities including trisomy of chromosome 8 and 4, deletion/duplication of chromosome 7, as well as deletion of chromosome X and Y in t(8;21) AML have previously been reported to be associated with a poor prognosis.24 Taken together, these findings indicate that genomic alterations and KIT-D816 mutation confer a poor prognosis in t(8;21) AML patients. Further studies in a larger cohort of patients will begin to stratify prognostically the patients in relation to the genomic changes; and new therapeutic targets should be discovered.
Acknowledgments
we thank members of our laboratories for helpful discussions. Funding: this work was supported by NIH grants, Parker Hughes Fund (HPK); and the grant support of NHRI-EX96-9434SI (LS) and MMH-E-96009 (DL). HPK is the holder of the Mark Goodson endowed Chair in Oncology Research and is a member of the Jonsson Cancer Center and the Molecular Biology Institute, UCLA. MD is supported by the European Leukemia Network (funded by the 6th Framework Program of the European Community). This article is dedicated to the memory of David Golde, a mentor and friend.
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
TA performed research, analyzed the data and wrote the paper; LS and DL determined mutation of genes; SO, MS, and YN performed SNP-chip analysis and developed CNAG; NK, SD, and JS assisted data analysis; JG and MM performed statistical analysis; VZ and AN performed the methylation analysis; SRM and RS performed FISH analysis; and SL and HPK directed the overall study. TA, LS and SO contributed equally in this work; and SL and HPK are co-last authors.
The authors reported no potential conflicts of interest.
References
- 1.Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing non-leukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA. 2000;97:7521–6. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, Donaldson C, et al. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci USA. 2002;99:8242–7. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiemels JL, Xiao Z, Buffler PA, Maia AT, Ma X, Dicks BM, et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood. 2002;99:3801–5. doi: 10.1182/blood.v99.10.3801. [DOI] [PubMed] [Google Scholar]
- 4.Rhoades KL, Hetherington CJ, Harakawa N, Yergeau DA, Zhou L, Liu LQ, et al. Analysis of the role of AML1-ETO in leukemogenesis, using an inducible transgenic mouse model. Blood. 2000;96:2108–15. [PubMed] [Google Scholar]
- 5.Fenske TS, Pengue G, Mathews V, Hanson PT, Hamm SE, Riaz N, et al. Stem cell expression of the AML1/ETO fusion protein induces a myeloproliferative disorder in mice. Proc Natl Acad Sci USA. 2004;101:15184–9. doi: 10.1073/pnas.0400751101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan Y, Zhou L, Miyamoto T, Iwasaki H, Harakawa N, Hetherington CJ, et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci USA. 2001;98:10398–403. doi: 10.1073/pnas.171321298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higuchi M, O’Brien D, Kumaravelu P, Lenny N, Yeoh EJ, Downing JR. Expression of a conditional AML1-ETO oncogene bypasses embryonic lethality and establishes a murine model of human t(8;21) acute myeloid leukemia. Cancer Cell. 2002;1:63–74. doi: 10.1016/s1535-6108(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Care RS, Valk PJ, Goodeve AC, Abu-Duhier FM, Geertsma-Kleinekoort WM, Wilson GA, et al. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121:775–7. doi: 10.1046/j.1365-2141.2003.04362.x. [DOI] [PubMed] [Google Scholar]
- 9.Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spiekermann K, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107:1791–9. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 10.Schessl C, Rawat VP, Cusan M, Deshpande A, Kohl TM, Rosten PM, et al. The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice. J Clin Invest. 2005;115:2159–68. doi: 10.1172/JCI24225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchenbauer F, Schnittger S, Look T, Gilliland G, Tenen D, Haferlach T, et al. Identification of additional cytogenetic and molecular genetic abnormalities in acute myeloid leukaemia with t(8;21)/AML1-ETO. Br J Haematol. 2006;134:616–9. doi: 10.1111/j.1365-2141.2006.06229.x. [DOI] [PubMed] [Google Scholar]
- 12.Nannya Y, Sanada M, Nakazaki K, Hosoya N, Wang L, Hangaishi A, et al. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res. 2005;65:6071–9. doi: 10.1158/0008-5472.CAN-05-0465. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto G, Nannya Y, Kato M, Sanada M, Levine RL, Kawamata N, et al. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotide-polymorphism genotyping microarrays. Am J Hum Genet. 2007;81:114–26. doi: 10.1086/518809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamata N, Ogawa S, Zimmermann M, Kato M, Sanada M, Hemminki K, et al. Molecular allelokaryotyping of pediatric acute lymphoblastic leukemias by high-resolution single nucleotide polymorphism oligonucleotide genomic microarray. Blood. 2008;111:776–84. doi: 10.1182/blood-2007-05-088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raghavan M, Lillington DM, Skoulakis S, Debernardi S, Chaplin T, Foot NJ, et al. Genome-wide single nucleotide polymorphism analysis reveals frequent partial uniparental disomy due to somatic recombination in acute myeloid leukemias. Cancer Res. 2005;65:375–8. [PubMed] [Google Scholar]
- 16.Gondek LP, Tiu R, O’Keefe CL, Sekeres MA, Theil KS, Maciejewski JP. Chromosomal lesions and uniparental disomy detected by SNP arrays in MDS, MDS/MPD, and MDS-derived AML. Blood. 2008;111:1534–42. doi: 10.1182/blood-2007-05-092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akagi T, Ogawa S, Dugas M, Kawamata N, Yamamoto G, Nannya Y, et al. Frequent genomic abnormalities in acute myeloid leukemia/myelodysplastic syndrome with normal karyotype. Haematologica. 2009;94:213–23. doi: 10.3324/haematol.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akagi T, Shih LY, Kato M, Kawamata N, Yamamoto G, Sanada M, et al. Hidden abnormalities and novel classification of t(15;17) acute promyelocytic leukemia (APL) based on genomic alterations. Blood. 2009;113:1741–8. doi: 10.1182/blood-2007-12-130260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downing JR, Head DR, Curcio-Brint AM, Hulshof MG, Motroni TA, Raimondi SC, et al. An AML1/ETO fusion transcript is consistently detected by RNA-based polymerase chain reaction in acute myelogenous leukemia containing the (8;21) (q22;q22) translocation. Blood. 1993;81:2860–5. [PubMed] [Google Scholar]
- 20.Shih LY, Liang DC, Huang CF, Chang YT, Lai CL, Lin TH, et al. Cooperating mutations of receptor tyrosine kinases and Ras genes in childhood core-binding factor acute myeloid leukemia and a comparative analysis on paired diagnosis and relapse samples. Leukemia. 2008;22:303–7. doi: 10.1038/sj.leu.2404995. [DOI] [PubMed] [Google Scholar]
- 21.Beghini A, Ripamonti CB, Castorina P, Pezzetti L, Doneda L, Cairoli R, et al. Trisomy 4 leading to duplication of a mutated KIT allele in acute myeloid leukemia with mast cell involvement. Cancer Genet Cytogenet. 2000;119:26–31. doi: 10.1016/s0165-4608(99)00221-6. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Küppers R, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–6. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 23.Montesinos-Rongen M, Van Roost D, Schaller C, Wiestler OD, Deckert M. Primary diffuse large B-cell lymphomas of the central nervous system are targeted by aberrant somatic hypermutation. Blood. 2004;103:1869–75. doi: 10.1182/blood-2003-05-1465. [DOI] [PubMed] [Google Scholar]
- 24.Marcucci G, Mrózek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:5705–17. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]