Upfront or second line allogeneic hematopoietic stem cell transplantation (HSCT) offers a good disease-free survival option for patients suffering from severe idiopathic aplastic anemia. This report shows improved outcome of reduced-intensity versus conventional conditioning in HLA-identical sibling HSCT for older aplastic anemia patients.
Keywords: aplastic anemia, stem cell transplantation, conditioning regimen, fludarabine
Abstract
Older age is a limitation for HLA-identical sibling hematopoietic stem cell transplantation (HSCT) as first-line therapy for severe acquired idiopathic aplastic anemia (SAA). Fludarabine (Flu)-based conditioning might improve outcome in older patients. We analyzed retrospectively 30 patients older than 30 years receiving such reduced-intensity conditioning HSCT according to recommendations of the European Group for Blood and Marrow Transplantation (EBMT) and compared their outcome to a control group receiving the standard regimen (cyclophosphamide+/−antithymocyte globulin) over the same study period (1998–2007). Patients conditioned with Flu had a higher probability of overall survival than the control group (p=0.04) when adjusting for recipient’s age. This might be related to a trend towards a reduced incidence of graft failure in patients receiving Flu (0% vs. 11%, p=0.09), while no difference was observed regarding graft-versus-host disease incidence. Flu-based conditioning regimen may reduce the negative impact of age in older patients with SAA receiving an HLA-identical sibling HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) from an HLA-identical sibling donor is the first-line treatment of choice for newly diagnosed patients with severe acquired idiopathic aplastic anemia (SAA) if they are younger than 30–40 years.1,2 However, for older patients aged 30–40 years or over, the decision whether to treat with immunosuppressive therapy (IST), namely antithymocyte globulin (ATG) and cyclosporine, or to transplant upfront with an HLA-identical sibling donor remains a key question. Two large studies from the Seattle group3 and the European Group for Blood and Marrow Transplantation (EBMT) SAA Working Party4 that examined survival by age group did not show an advantage for either HSCT or IST in older patients. This was notably related to a lower long-term survival after HSCT for older patients, i.e. over 40 years in the Seattle cohort3 and over 20 years in the European study.4 The negative impact of older age at transplant has been further confirmed in several other studies.5–7 In one of those published more recently,7 recipient’s older age was identified as one factor associated with a lower survival in multivariate analysis, with 54% of patients older than 30 years surviving long term.
The standard conditioning regimen for HLA identical sibling HSCT relies on cyclophosphamide (Cy) combined or not with ATG. In order to improve survival in patients older than 30–40 years, the use of less cytotoxic but more immunosuppressive regimens including low-dose cyclophosphamide (below the standard dose of 200 mg/kg) in combination with ATG, while adding fludarabine (Flu), might be an option to explore with the aim of reducing transplant-related mortality.8,9 Such Flu-based regimens have been explored in various non-malignant diseases without conferring a significant survival advantage when compared to conventional myeloablative regimens.10 In patients with SAA specifically, several previous case reports11,12 or non-comparative studies13–16 have reported encouraging results in the setting of HLA-matched related HSCT. The EBMT SAA working party has recently reported a favorable experience using Flu-based HSCT in SAA patients transplanted with unrelated (n=33) or family mismatched (n=5) donors.17 In combination with ATG and very low-dose Cy (1200 mg/m2, i.e. approximately 20 mg/kg), such a preparative regimen provided a 2-year actuarial survival of 73%. In view of these encouraging results, and given the need to improve outcome for older patients after HLA-identical sibling HSCT, the SAA Working Party of the EBMT proposed to apply such a conditioning regimen in patients older than 30 years receiving a matched related HSCT. Here we analyze 30 patients older than 30 years treated according to this protocol and compare their outcome to a control group of similar patients registered in the EBMT database receiving the standard Cy ± ATG regimen over the same study period (1998–2007).
Design and Methods
In the Flu group, all but 4 patients received the conditioning regimen according to the EBMT SAA-WP recommendation associating Flu (30 mg/m2/day × 4 days), low-dose Cy (300 mg/m2/day × 4 days,) and ATG (Thymoglobuline™ 3.75 mg/kg/day × 4 days), in 6 different centers. The 4 other patients, all from an additional center, received Flu combined with Cy but without ATG. The control group comprised patients older than 30 years receiving a first transplant using Cy alone (n=121) or Cy + ATG (n=118) in 109 European centers. Update of data was performed as of August 2008.
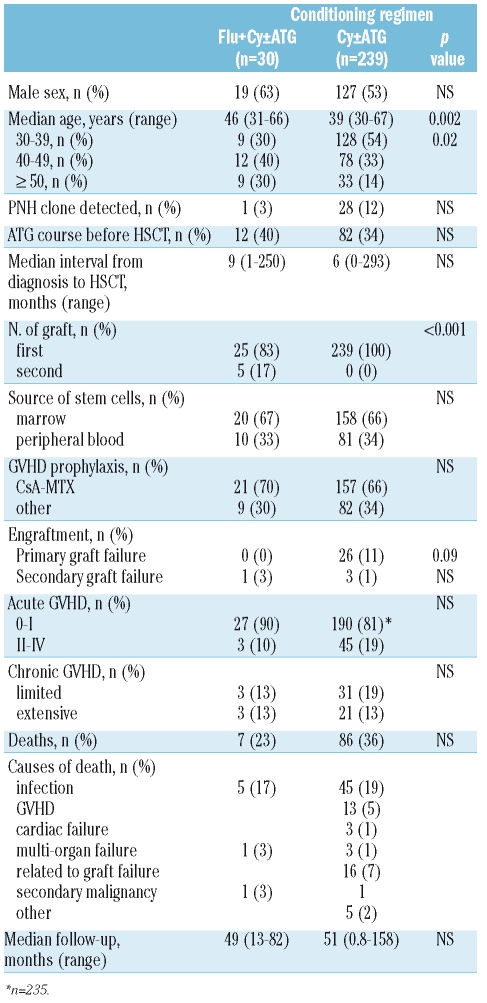
Patients’ and transplant characteristics of the 2 groups are compared in Table 1. No significant difference was observed regarding the detection of a PNH clone, the median interval between diagnosis and HSCT, or the proportion of patients who received a course of immunosuppressive therapy prior to HSCT, the source of stem cells and graft-versus-host disease (GVHD) prophylaxis. However, patients in the Flu group were significantly older at the time of HSCT (46 vs. 39 years, p<0.01). Additionally, 5 patients in the Flu group were included after failure of a first standard allogeneic HSCT from the same donor. These received conditioning with Cy (200 mg/kg) combined with ATG (n=4) or TBI (n=1, 8 Gray in 3 fractions) for their first transplant. Differences in categorical variables were evaluated by χ2 analysis. The Fisher’s exact test was also used to confirm the χ2 test, when at least one factor modality related to less than 5 individuals. Comparisons of continuous variable medians were performed using a Mann-Whitney test. Probabilities of overall survival were estimated from the time of transplantation according to the Kaplan-Meier product-limit method and compared using the log rank statistic. Probabilities of overall survival adjusted for recipient age were estimated according to a Cox regression model. All tests were two-sided.
Table 1.
Patients’ characteristics and outcome according to conditioning regimen.
Results and Discussion
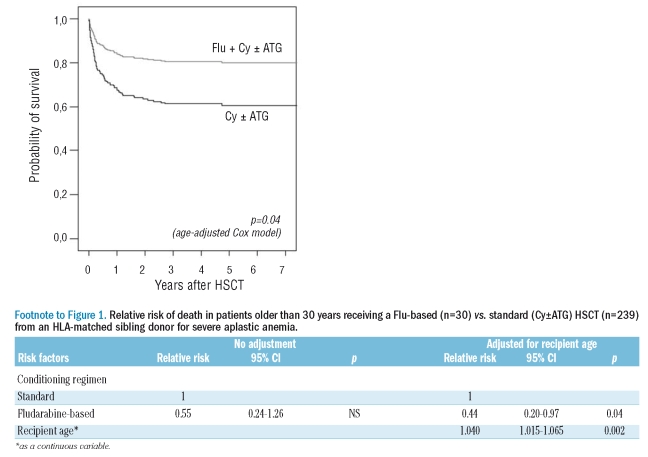
With comparable median follow-up of surviving patients of 49 and 51 months, respectively, patients receiving a Flu-based regimen had a non-significant higher 5-year probability of survival than the control group (77±8% vs. 60±3%, p=0.14 by the log-rank test). As the 2 groups differed significantly by age, outcome is presented in a Cox regression model adjusting for recipient’s age and graphically as age-adjusted survival curves (Figure 1 and Footnote). Adjusted for age, the Flu group had a significant lower relative risk (RR) of death compared to the control group (RR=0.44 [95% CI, 0.20–0.97], p=0.04). Of note, age entered as a continuous variable was highly significantly associated with survival, even in this selected group of patients over the age of 30 (p=0.002).
Figure 1.
Age-adjusted comparison of overall survival in SAA patients older than 30 years receiving either a Flu-based (n=30) or conventional (Cy±ATG) HSCT (n=239) from an HLA-matched sibling donor. Probabilities of overall survival adjusted for recipient age were estimated from the time of HSCT according to a Cox regression model.
Possible reasons for better survival in the Flu group were found in a trend towards a lower incidence of primary graft failure in comparison with the control group (0% vs. 11% of cases, p=0.09, Table 1). This was noted despite the fact that 5 out of the 30 patients receiving a Flu-based regimen had previously experienced graft failure following a standard conditioning HSCT from the same donor. All of these patients primarily fully engrafted (one experienced secondary graft failure with autologous reconstitution), suggesting that the addition of Flu had reduced the host-versus-graft reaction, as previously reported in such patients,16 even when heavily immunized after repeated transfusions.13 This effect of Flu should also be further explored in light of a recent study which failed to demonstrate a significant contribution of ATG in reducing graft failure.18 All surviving patients in the Flu group were transfusion independent or independent from other SAA specific treatment at last follow-up.
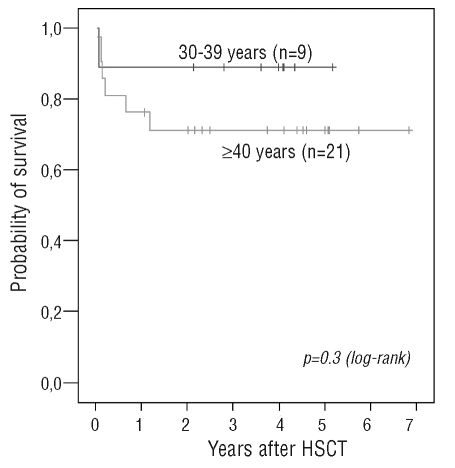
Conversely, no difference was observed regarding the incidence of acute and chronic GVHD between the 2 groups of patients (Table 1). Of note, all but 2 patients among those who received Flu and engrafted long term (29 out of 30) had full donor chimerism as determined by molecular or karyotypic analyses. These last 2 had persistent mixed chimerism, indicating that in this setting, as also reported after standard HSCT,19 persistent mixed chimerism may be accompanied by normal blood cell counts. Seven out of 30 patients died in the Flu group, 6 of those being older than 40 years. Figure 2 shows survival in the Flu group by age (< and > 40 years). The main cause of death was infection (5 out of 7). Specific attention should be given to potential Epstein-Barr virus (EBV) reactivation in these patients since one of these died of EBV-associated lymphoproliferative disease, as also previously reported in a 62-year old patient receiving the same conditioning regimen.12
Figure 2.
Overall survival in SAA patients receiving a Flu-based HSCT from an HLA-matched sibling donor according to recipient age at HSCT.
In conclusion, we show in this comparative study that a Flu-based regimen might be a way to improve outcome of older SAA patients after HLA-matched related HSCT, as also suggested for unrelated HSCT.17 These results reinforce encouraging survival estimates observed in previously reported studies without a comparative group, using such Flu-based regimens independently of patient age.13–16 In older patients, the comparison of Flu-based to standard HSCT will certainly need to be further evaluated in a still larger number of patients to allow a definitive conclusion to be drawn.
Acknowledgments
Romaine Viollier, Sandra Acholonu and Grace-Julia Okoroji for data management.
Footnotes
Authorship and Disclosures
SM, AB, PA, MA, JM, GS and JRP designed the study; RO provided data management; SM and JRP performed the statistical analysis and prepared the manuscript; all authors participated in interpretation of data and approved the final manuscript.
The authors reported no potential conflict of interest.
References
- 1.Marsh JC, Ball SE, Darbyshire P, Gordon-Smith EC, Keidan AJ, Martin A, et al. Guidelines for the diagnosis and management of acquired aplastic anaemia. Br J Haematol. 2003;123:782–801. doi: 10.1046/j.1365-2141.2003.04721.x. [DOI] [PubMed] [Google Scholar]
- 2.George ER, Storb R. Allogeneic Hematopoietic Cell Transplantation for Aplastic Anemia. In: Blume KG, Forman SJ, Thomas ED, editors. Thomas’ Hematopoietic Cell Transplantation. Boston: Blackwell; 2004. pp. 981–1001. [Google Scholar]
- 3.Doney K, Leisenring W, Storb R, Appelbaum FR. Primary treatment of acquired aplastic anemia: outcomes with bone marrow transplantation and immunosuppressive therapy. Seattle Bone Marrow Transplant Team. Ann Intern Med. 1997;126:107–15. doi: 10.7326/0003-4819-126-2-199701150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Bacigalupo A, Hows J, Gluckman E, Nissen C, Marsh J, Van Lint MT, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177–82. doi: 10.1111/j.1365-2141.1988.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 5.Passweg JR, Socie G, Hinterberger W, Bacigalupo A, Biggs JC, Camitta BM, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858–64. [PubMed] [Google Scholar]
- 6.Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J, et al. Treatment of acquired severe aplastic anemia: bone marrow transplantation compared with immunosuppressive therapy--The European Group for Blood and Marrow Transplantation experience. Semin Hematol. 2000;37:69–80. doi: 10.1016/s0037-1963(00)90031-3. [DOI] [PubMed] [Google Scholar]
- 7.Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H, Ribaud P, Devergie A, Traineau R, Gluckman E, Socie G. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103:2490–7. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 8.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–19. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh JC. Treatment of acquired aplastic anemia. Haematologica. 2007;92:2–5. doi: 10.3324/haematol.11107. [DOI] [PubMed] [Google Scholar]
- 10.Ringden O, Remberger M, Svenberg P, Svahn BM, Dahllof G, Gustafsson B, et al. Fludarabine-based disease-specific conditioning or conventional myeloablative conditioning in hematopoietic stem cell transplantation for treatment of non-malignant diseases. Bone Marrow Transplant. 2007;39:383–8. doi: 10.1038/sj.bmt.1705602. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Prem S, Mahapatra M, Seth T, Chowdhary DR, Mishra P, et al. Fludarabine, cyclophosphamide and horse antithymocyte globulin conditioning regimen for allogeneic peripheral blood stem cell transplantation performed in non-HEPA filter rooms for multiply transfused patients with severe aplastic anemia. Bone Marrow Transplant. 2006;37:745–9. doi: 10.1038/sj.bmt.1705321. [DOI] [PubMed] [Google Scholar]
- 12.Itala M, Aho H, Remes K. Reduced-intensity conditioning and blood stem cell transplantation from an HLA-identical sibling for severe aplastic anaemia: two patients with successful engraftment but a fatal post-transplant lymphoproliferative disorder in the other. Hematol J. 2004;5:440–3. doi: 10.1038/sj.thj.6200381. [DOI] [PubMed] [Google Scholar]
- 13.Srinivasan R, Takahashi Y, McCoy JP, Espinoza-Delgado I, Dorrance C, Igarashi T, et al. Overcoming graft rejection in heavily transfused and allo-immunised patients with bone marrow failure syndromes using fludarabine-based haematopoietic cell transplantation. Br J Haematol. 2006;133:305–14. doi: 10.1111/j.1365-2141.2006.06019.x. [DOI] [PubMed] [Google Scholar]
- 14.Resnick IB, Aker M, Shapira MY, Tsirigotis PD, Bitan M, Abdul-Hai A, et al. Allogeneic stem cell transplantation for severe acquired aplastic anaemia using a fludarabine-based preparative regimen. Br J Haematol. 2006;133:649–54. doi: 10.1111/j.1365-2141.2006.06084.x. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Almaguer D, Vela-Ojeda J, Jaime-Perez JC, Gutierrez-Aguirre CH, Cantu-Rodriguez OG, Sobrevilla-Calvo P, et al. Allografting in patients with severe, refractory aplastic anemia using peripheral blood stem cells and a fludarabine-based conditioning regimen: the Mexican experience. Am J Hematol. 2006;81:157–61. doi: 10.1002/ajh.20467. [DOI] [PubMed] [Google Scholar]
- 16.George B, Mathews V, Viswabandya A, Kavitha ML, Srivastava A, Chandy M. Fludarabine and cyclophosphamide based reduced intensity conditioning (RIC) regimens reduce rejection and improve outcome in Indian patients undergoing allogeneic stem cell transplantation for severe aplastic anemia. Bone Marrow Transplant. 2007;40:13–8. doi: 10.1038/sj.bmt.1705669. [DOI] [PubMed] [Google Scholar]
- 17.Bacigalupo A, Locatelli F, Lanino E, Marsh J, Socie G, Maury S, et al. Fludarabine, cyclophosphamide and anti-thymocyte globulin for alternative donor transplants in acquired severe aplastic anemia: a report from the EBMT-SAA Working Party. Bone Marrow Transplant. 2005;36:947–50. doi: 10.1038/sj.bmt.1705165. [DOI] [PubMed] [Google Scholar]
- 18.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–5. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casado LF, Steegmann JL, Pico M, Requena MJ, Ramirez M, Madero L, et al. Study of chimerism in long-term survivors after bone marrow transplantation for severe acquired aplastic anemia. Bone Marrow Transplant. 1996;18:405–9. [PubMed] [Google Scholar]