SUMMARY
Although often overshadowed by factors influencing seizure initiation, seizure termination is a critical step in the return to the interictal state. Understanding the mechanisms contributing to seizure termination could potentially identify novel targets for anticonvulsant drug development and may also highlight the pathophysiological processes contributing to seizure initiation. In this article, we review known physiological mechanisms contributing to seizure termination and discuss additional mechanisms that are likely to be relevant even though specific data are not yet available. This review is organized according to successively increasing “size scales”—from membranes to synapses to networks to circuits. We first discuss mechanisms of seizure termination acting at the shortest distances and affecting the excitable membranes of neurons in the seizure onset zone. Next we consider the contributions of ensembles of neurons and glia interacting at intermediate distances within the region of the seizure onset zone. Lastly, we consider the contribution of brain nuclei, such as the substantia nigra pars reticulata (SNR), that are capable of modulating seizures and exert their influence over the seizure onset zone (and neighboring areas) from a relatively great—in neuroanatomical terms—distance. It is our hope that the attention to the mechanisms contributing to seizure termination will stimulate novel avenues of epilepsy research and will contribute to improved patient care.
Keywords: Status epilepticus, Seizure, Epilepsy, Postictal, Seizure termination, Potassium, Adenosine, Endocannabinoids, Connexin, Gap junction, Substantia nigra pars reticulata, Estrogen, Neuropeptide Y, Neuromodulators
Most seizures are self-limited, lasting no more than a few minutes. The persistence of a seizure lasting longer than several minutes is usually a cause for alarm as physiological mechanisms terminating the seizure may have failed. Why seizures typically do not continue indefinitely, and how intrinsic anticonvulsant mechanisms in the brain lead to seizure termination, are questions that potentially offer new avenues for developing novel treatments for epilepsy, as well as offering insights into brain autoregulatory mechanisms. The topic of how seizures end has been addressed previously, but has not been the sole focus of a review (Timofeev & Steriade, 2004). The availability of additional data, and our increased understanding of relevant mechanisms, make revisiting this topic timely. The methodical consideration of known and possible mechanisms acting to end seizures may hasten the development of novel anticonvulsant therapies.
In adults, typical seizure duration varies somewhat with seizure type. Jenssen et al. reported that the median durations of complex partial seizures and secondarily generalized seizures are 78 and 130 s, respectively (Jenssen et al., 2006). In that study, no self-limited seizure lasted more than 11 min; longer seizures were considered likely to continue as status epilepticus. In children, on the other hand, seizure duration may be longer. Shinnar et al. used structured interviews of parents of children presenting with a first unprovoked seizure to explore this issue (Shinnar et al., 2001). They determined that 50% of children experienced a seizure lasting 5 min or longer, while 29% of children had a first seizure that lasted 10 min or longer. Eight percent of children required medication to terminate their seizure, which lasted longer than 30 min in most cases. Although parents may overestimate seizure duration, these observations nevertheless confirm the common clinical experience that most seizures stop without intervention. The study, however, also suggests that seizure termination might depend on specific mechanisms which may fail in some individuals. In the same study, the duration of a second seizure was highly correlated with the duration of the first. Indeed, children developing status epilepticus with the first seizure were likely to experience status epilepticus with a second seizure.
The inference from these observations on adults and children is that seizure termination results from the specific mechanisms and that such mechanisms may change with developmental age. Additional factors, such as sex, structural injury, and genetic abnormalities, may also affect seizure duration. If specific mechanisms contribute to seizure termination, one might well ask whether the mechanisms available in otherwise “normal” brain are the same as those available in the chronically epileptic brain or in a brain affected by neurodegenerative disease.
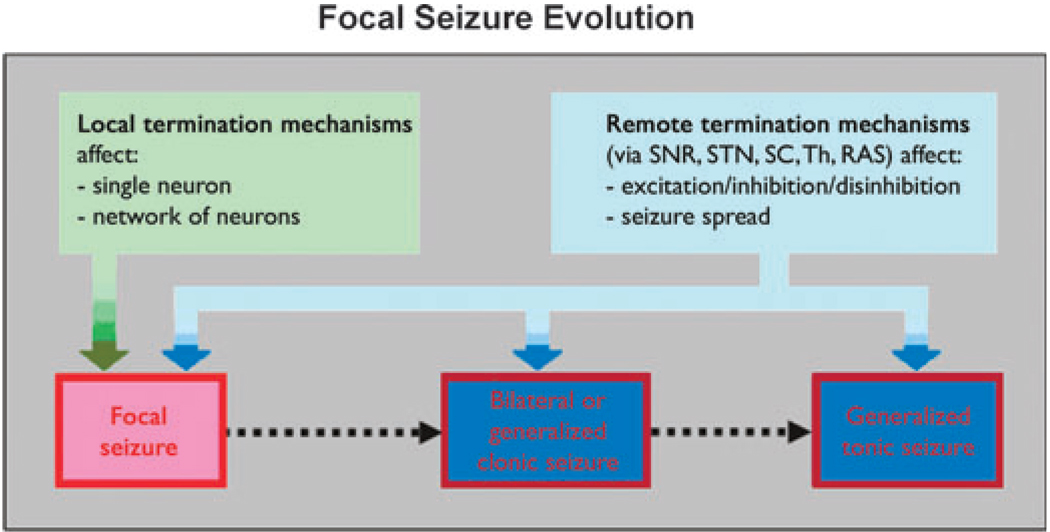
This review will explore known mechanisms of seizure termination, and will offer some hypotheses, based on a synthesis of current insights into the synchronization and desynchronization of neuronal networks, that may be important for seizure termination. We will concentrate on the mechanisms of seizure termination operating on focal seizures arising in neocortex or limbic structures such as the hippocampus. We have organized this review to discuss mechanisms operating progressively from the scale of neuronal membranes and synapses (in the seizure onset zone), to an intermediate scale comprising the networks of neurons and interneurons, to the larger scale characterized by distinct subcortical seizure-modulating circuits that reduce the likelihood of seizure initiation and may attenuate ongoing focal seizure activity by altering the balance between excitation and inhibition (see Fig. 1). Examples of this long-range category are the anticonvulsant actions exerted by the substantia nigra pars reticulata (SNR) and subthalamic nucleus (STN). At each of these scales, increasingly detailed electrophysiological and molecular data focusing on mechanisms of neuronal communication require revision of older notions, and in some cases the replacement of generally accepted theories about seizure evolution and termination.
Figure 1.
Schematic diagram indicating the potential sites of action of seizure terminating mechanisms. SNR, substantia nigra pars reticulate; STN, subthalamic nucleus; SC, superior colliculus; Th, thalamus; RAS, reticular activating system.
Revisiting the question of “How do seizures stop?” is timely, as it coincides with an increased understanding of the mechanisms by which neurons are able to interact. In addition to traditional or classical models of synaptic interactions, it is now clear that neurons may influence each other through the extracellular environment, gap junctions, local neuromodulators released in response to metabolic changes, and neuromodulatory circuits able to affect neuronal excitability globally (Bullock et al., 2005). In the following sections, we review advances in each of these areas and highlight the impact of these mechanisms on seizure termination.
MECHANISMS ACTING AT THE LEVEL OF SINGLE NEURONS
Within a single neuron, prolonged depolarizations with sustained action potential firing may be triggered by a brief depolarizing pulse, as in the paroxysmal depolarizing shift, or may be the result of sustained excitatory synaptic input from neighboring neurons engaged in seizure activity (Ayala, 1983). Intrinsic mechanisms of seizure termination active in a single neuron—discussed below—include: the potassium currents activated by calcium and sodium entry; the loss of ionic gradients—particularly of potassium—leading first to depolarization with increased firing, followed by depolarization blockade of membrane firing and cessation of firing; and possibly the depletion of energy substrates locally, with the decline in adenosine triphosphate (ATP), resulting in cessation of neuronal firing. (ATP), resulting in cessation of neuronal firing.
Intracellular ion-activated potassium currents
The membrane after hyperpolarization that follows bursts of action potential discharge is the result, at least in part, of potassium currents activated by the entry of calcium and sodium. Increased calcium entry during the paroxysmal depolarizing shift, or as a result of the action of glutamate at the postsynaptic membrane, activates a calcium-dependent membrane potassium conductance that allows potassium efflux, membrane hyperpolarization, and cessation of firing (Alger & Nicoll, 1980; Timofeev et al., 2004). Like calcium, sodium entry may also activate a sodium-dependent potassium current that reduces neuronal excitability by hyperpolarizing the membrane and increasing shunt conductance (Schwindt et al., 1989).
Transmembrane ion gradients
The effect of extracellular potassium is multifaceted. Sustained potassium efflux increases extracellular potassium concentration, depolarizing the membrane and moving the intracellular voltage toward the threshold for sodium action potential firing. As extracellular potassium continues to accumulate, there is membrane depolarization and action potential firing increases. With further accumulation, the membrane potential becomes more depolarized than the firing threshold for sodium action potentials, sodium channels inactivate, and neuronal firing ceases. In vitro experiments by Bikson et al. illustrate these effects of extracellular potassium accumulation (Bikson et al., 2003). Electrographic seizure-like activity triggered in hippocampal slices by exposure to low-calcium artificial cerebrospinal fluid (aCSF) manifested as recurrent periods of population firing followed by periods of electrographic silence lasting 12–18 s. The termination of each electrographic discharge by a period of electrographic silence resulted from transient increases in extracellular potassium to plateaus of approximately 12 mM. The depolarized state was maintained by the elevation of extracellular potassium and by the presence of persistent sodium channels that did not inactivate. Depolarization blockade terminating seizure-like discharges has also been observed in neocortical slices in which GABAergic inhibition is partially blocked by picrotoxin (Pinto et al., 2005). Focal or localized increases in potassium may also trigger additional potassium release beyond the initial region of potassium accumulation. Shifts in extracellular potential, and oscillations seen at the end of hippocampal afterdischarges, have been attributed to a rapid rise in extracellular potassium that trigger waves of astrocyte depolarization and a propagating rise in potassium that terminates neuronal firing (Bragin et al., 1997).
In addition to its direct depolarizing effects, increased extracellular potassium may also indirectly result in membrane depolarization through the action of the potassium-chloride cotransporter KCC2. The rise in extracellular potassium can increase intracellular chloride, shifting the chloride reversal potential toward membrane depolarization. In the setting of increased intracellular chloride, the action of GABA to open chloride channels could enhance membrane depolarization. The net effect of shifting chloride equilibrium the excitability would be to increase until neurons depolarized to the point of becoming refractory to further firing of action potentials (Jin et al., 2005; Galanopoulou, 2007).
Extracellular calcium levels also change markedly during paroxysmal neuronal firing and may affect the efficiency of neuron-to-neuron spread of activity. Focal seizure activity results in a decline in extracellular calcium activity of approximately 50% (Heinemann et al., 1977). This decline may inhibit synaptic transmission because synaptic vesicle fusion and neurotransmitter release are dependent on entry of extracellular calcium (King et al., 2001; Cohen & Fields, 2004). Decline in extracellular calcium also potentially affects gap junction function as hemichannel opening increases in low calcium (Thimm et al., 2005). The net effects of increasing gap junction coupling are complex and difficult to predict. As discussed below, there is evidence that the increased coupling between interneuronal populations or between pyramidal cells may increase synchrony and promote seizures. However, enhanced gap junction coupling may also alter glial interactions that either enhance or retard seizures.
Membrane shunting
Neuronal input resistance decreases over the course of a seizure as a consequence of increased opening of synaptic and ion-gated conductances following a period of intense discharge (Matsumoto et al., 1969). Membrane shunting reduces the effect of synaptic currents on the postsynaptic membrane so that one neuron is less likely to trigger firing in a neighboring neuron. Membrane shunting also reduces the effective coupling between neurons linked by gap junctions, acting as a voltage divider to reduce the effects of electrotonic current spread (Llinas et al., 1974). As a result of the activity associated with seizures, the shunting of synaptic and gap junction currents by low membrane resistance can decrease the coupling between neurons, thus disrupting network oscillations that sustain seizure activity.
Energy failure
Sustained neuronal activation also markedly increases energy—namely ATP—utilization to restore ion gradients across the membrane. In some neurons, the presence of an ATP-gated potassium channel (KATP) reduces neuronal activity when ATP levels decline intracellularly (Yamada et al., 2001). When ATP level falls because energy utilization outpaces energy production, potassium channels open and produce membrane hyperpolarization. Indeed, knockout mice lacking functioning KATP channels experience a myoclonic seizure on average 8.9 ± 1.1 s following onset of hypoxia, followed by generalized convulsions and death. A similar hypoxic challenge, however, does not trigger seizures in wild-type mice, indicating that KATP channels in vivo resist membrane depolarization during energy failure.
Reduced levels of energy metabolites, such as glucose, may also affect seizure duration. In vitro recordings show that decreasing extracellular glucose terminates electrographic seizure-like activity in the low magnesium hippocampal slice (Kirchner et al., 2006). The effect of hypoglycemia on seizure-like discharges in vitro was statistically significant, but not immediate. Fifty percent fewer seizure-like discharges occurred in the 24-min period following application of low glucose artificial cerebrospinal fluid (CSF) compared to the frequency of discharges in the 30 min prior to application. Low glucose also reduced the amplitude of the seizure-like discharge by 25%. These effects on the frequency and amplitude of seizure-like discharges were reversed by restoration of normal glucose levels.
While useful in the laboratory investigation of seizures, observations from such in vitro studies must be interpreted cautiously because they cannot be translated directly into an appropriate treatment for seizures in vivo. It is well established that energy deprivation via either hypoxia or hypoglycemia—such as produced by insulin overdose—often results in coma and neuronal death, and is sometimes associated with onset of seizure activity rather than seizure control (Auer, 1986; Patrick & Campbell, 1990).
MECHANISMS ACTING ON A LOCAL NETWORK OF NEURONS
While seizure initiation is driven at least in part by the burst firing properties of the individual neurons, the evolution and spread of the seizures also requires amplification and synchronization among neurons within susceptible networks. Seizure amplification occurs through the action of recurrent excitatory collaterals that form feedback loops, returning excitatory synaptic activity to the neurons within the seizure onset zone (Rutecki et al., 1989; Coulter & DeLorenzo, 1999). Seizure spread depends on the propagation and synchronization of the seizure discharge across synapses that separate neurons in the seizure onset zone from “normal” neurons synaptically connected to the seizure onset zone (MacVicar & Dudek, 1980; Miles & Wong, 1983).
Glutamate depletion
Decrease in synaptic efficacy results in milder postsynaptic excitation, and consequently diminished amplification and spread of the seizure discharge. One mechanism limiting synaptic transmission during a sustained seizure discharge is the depletion of synaptic vesicle containing neurotransmitter. Staley et al. investigated the effects of synaptic depletion in vitro using a model of CA3 electrographic seizure discharges produced by hyperkalemia (Staley et al., 1998). CA3 discharges consist of recurrent neuronal depolarizations with bursts of action potential firing separated by period of electrographic silence. Staley et al. found that the duration of the seizure burst was proportional to the duration of the silent period preceding the burst, consistent with the hypothesis that the seizure burst duration depended on the renewed availability of immediately-releasable glutamate. If glutamate-containing synaptic vesicles are replaced at a steady rate, longer inter-burst periods allow a greater resupply of immediately releasable glutamate, and an increased duration of the subsequent electrographic seizure discharge. Interburst intervals of 2–3 s or longer were necessary to achieve the longest burst durations (up to 420 ms). This dependence of burst duration on the interburst interval persisted even when GABAA and GABAB conductances were blocked. Replacing calcium with strontium, which reduces the rate of synaptic vesicle release, prolonged burst duration in a concentration dependent manner, with the highest concentration of strontium producing a 37-fold increase in burst duration (Jones et al., 2007). Thus, as the seizure discharge develops, it consumes the supply of readily releasable glutamate needed to sustain the seizure, potentially acting as a governor on excitatory drive. As the glutamate reservoir is replenished continuously, however, additional control mechanisms are necessary to prevent reinitiation of seizure activity.
The intra- and extracellular environments
Prolonged neuronal activity during seizure discharges may also have the effect of increasing CO2 or increasing the byproducts of anaerobic metabolism, and produce extracellular acidosis or intracellular acidosis associated with extracellular alkalinosis (Chesler & Kaila, 1992). Glial cells may also contribute to acidification of the extracellular space in response to increases in the extracellular potassium concentration (Chesler & Kraig, 1987). In the hippocampal slice in vitro, acidification of the extracellular space to pH 6.7 terminated seizure-like burst firing facilitated by low-magnesium in the artificial CSF. The attenuation of epileptiform activity began within minutes of lowering pH (Velisek et al., 1994). The mechanisms of action—at least in part—included decreased NMDA receptor function and loss of synaptic long-term potentiation (LTP). A milder reduction of pH to 7.1 also produced milder synaptic impairment with continued loss of LTP (Velisek, 1998). Inhibition of carbonic anhydase, which alters extracellular pH, has some anticonvulsant benefit. In humans, the carbonic anhydrase inhibitor acetozolamide has a mild anticonvulsant effect (Thiry et al., 2007). Knockout mice deficient in carbonic anhydrase are severely acidotic and are resistant to seizures produced by flurothyl gas compared to wild type mice (Velisek et al., 1993).
Intracellular acidification may also contribute to termination of seizure discharges. Spontaneous interictal spiking following focal application of bicuculline in the piriform cortex in an in vitro whole brain preparation was associated with periodic abrupt alkanization of the extracellular space followed by a slow return to baseline pH (de Curtis et al., 1998). These observations were interpreted as evidence of intracellular acidification. Application of ammonium chloride in the perfusing medium to prevent intracellular acidification increased neuronal excitability and resulted in afterdischarges following each spike, and in seizure-like discharges. The investigators hypothesized that the intracellular acidification reduced excitability by reducing gap junction function. Application of octanol, a nonspecific gap junction blocker, abolished spontaneous interictal spiking (de Curtis et al., 1998). Gap junction disruption as a possible mechanism contributing to seizure termination is discussed further below.
Other alterations of the extracellular milieu may disrupt epileptic activity by altering chloride homeostasis. Furosemide applied to an epileptic initiation site, either in vitro or in vivo, terminates the seizure discharge but does not interfere with excitatory synaptic transmission (Hochman et al., 1995). The effect of furosemide appears to be mediated by changes in chloride concentration. Furosemide in the extracellular environment disrupted the synchronization of action potential firing between neurons without affecting synaptic activity (Hochman & Schwartzkroin, 2000).
Altered regulation of intracellular chloride may account for the difficulty in treating and terminating neonatal status epilepticus. The chloride reversal potential is elevated in neonatal neurons compared to mature neurons. It is hypothesized that immaturity of intracellular chloride regulation is the reason that anticonvulsants acting through GABA, such as phenobarbital, are often ineffective for treating status epilepticus. Data from neonatal rats suggest that the blockade of a potassium-chloride cotransporter, NKCC1, with the diuretic butemanide enhances the action of phenobarbital in terminating status epilepticus (Dzhala et al., 2008). Treatment of neonatal status epilepticus using phenobarbital with butemenide is the subject of a prospective clinical trial (K. Staley, personal communication). Lowering intracellular chloride levels could augment the functional effect of GABA in an activity-dependent manner. Increasing activity-dependent inhibition has the potential to minimize side effects such as sedation or confusion seen with GABA agonists by minimizing the increase in inhibition at inactive synapses.
Glial buffering of glutamate
Glial uptake of perisynaptic glutamate is the major mechanism forestalling accumulation of glutamate at the synapse (Benarroch, 2005). Astrocytes have an equally important role in the regulation of extracellular potassium. Astrocytic buffering of potassium maintains extracellular levels below a ceiling of 12 mM (Benarroch, 2005). In some cases, such as the epileptic brain, glia may also release glutamate, thereby prolonging postsynaptic excitation. Tian et al. recently showed that glial release of glutamate contributed to the maintenance of the paroxysmal depolarizing shift that is the hallmark of “epileptic” neurons (Tian et al., 2005). Failure of glia to buffer extracellular glutamate, let alone glutamate release from glia, can be expected to result in prolonged excitory drive and seizure maintenance.
Gap junctions
Synchrony is a hallmark of seizures. The significance and role of synchrony likely depends on the nature and extent of interconnections. For example, the emergence of synchrony across short distances in local small ensembles of networked neurons and interneurons may be important in seizure initiation (Grenier et al., 2003; Traub et al., 2004), and mechanisms disrupting short range synchrony may contribute to seizure termination. Long range synchrony, affecting large areas of cortex and distant subcortical structures, may also play a role in seizure termination (Timofeev & Steriade, 2004; Schindler et al., 2007). The present section considers the effect of increased synchrony in small compact network of neurons and interneurons. The role of long range synchrony is discussed later in the section of mechanisms acting remotely.
Presynaptic volleys arriving simultaneously at the postsynaptic receptor increase the likelihood that the presynaptic activity will result in postsynaptic activation. Perhaps paradoxically, synchronization in the network of inhibitory interneurons has the effect of synchronizing the discharges in excitatory pyramidal cells receiving interneuron input. (Beierlein et al., 2000; Hasenstaub et al., 2005). Inhibitory synapses are located mainly on the proximal dendrites, soma, and initial segment. Excitatory synapses, in constrast, are mostly located on the distal dendrites (DeFelipe & Farinas, 1992). This arrangement enables inhibitory synapses to strongly modulate excitatory synaptic input. Synchronized inhibitory postsynaptic potentials will phasically reduce or block neuronal firing resulting in pyramidal action potential firing that is coupled to and modulated by the synchronized inhibitory input.
Electrical synapses formed by gap junctions between interneurons provide a mechanism for interneuronal synchronization (Mancilla et al., 2007). Gap junctions formed by connexins are found between inhibitory neurons of the same subtype. For example, gap junction connections are common between pairs of fast-spiking (FS) interneurons or between pairs of low-threshold spiking (LTS) interneurons, but are uncommon between FS and LTS neurons (Galarreta & Hestrin, 2001; Hestrin & Galarreta, 2005). Simultaneous recordings from pairs of inhibitory interneurons in the neocortex and hippocampus indicate that interneurons of the same subtype are synchronized with each other (Galarreta & Hestrin, 2001; Hestrin & Galarreta, 2005). It has also been proposed that high frequency synchronization—so called fast ripples—may be mediated by gap junction coupling between the proximal axons of pyramidal neurons (Traub et al., 2002; Hamzei-Sichani et al., 2007).
Connexin coupling to form an open gap junction may be influenced or controlled in several ways. Gap junction opening is dependent on pH (Trexler et al., 1999; Valiunas, 2002); connexins uncouple at acidic pH, leading to decreased gap junction conductance, while alkalotic pH promotes connexin coupling and increases gap junction conductance (Spray et al., 1981). Opening and closing may also be regulated by cyclic nucleotide coupled receptors (DeVries & Schwartz, 1989). In the setting of increased metabolic activity during a seizure, with a drop in pH resulting from increased anaerobic metabolism, gap junction closing may be one mechanism disrupting synchronization of the network of neurons driving the seizure. Administration of gap junction blockers (both broadspectrum blockers such as carbenoxolone and octanol, and the connexin 36-specific blocker quinine) shorted or suppressed seizure discharges produced by focal neocortical injection of 4-aminopyridine in vivo (Szente et al., 2002; Gajda et al., 2005).
A role for gap junctions in seizure maintenance and termination is also supported by in vitro studies. The observations of de Curtis and colleagues (discussed in the section of extra- and intracellular environment) suggest that gap junction decoupling in response to acidosis terminates paroxysmal discharges. Other labs report similar observations that that application of the gap junction blocker carbenoxolone attenuates epileptiform discharges and negates the dependence of burst firing (Schweitzer et al., 2000; Jahromi et al., 2002). Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) second messenger pathways can regulate connexin coupling in neocortex, which could regulate the extent of synchrony induced in a population of neurons.
Additional investigation is needed to clarify the extent to which gap junctional mechanisms contribute to seizure generation and epilepsy, and whether modification of gap junction conductance improves seizure control. Carbenoxolone has low toxicity, crosses the blood–brain barrier, and has already been approved by the FDA for the treatment of gastrointestinal ulcers. Though carbenoxolone has been shown to stop seizures in animal models in vivo and in vitro, the effects on human epilepsy have not been studied.
Increased GABAergic inhibition
A basic mechanism to control focal seizure activity is GABAergic synaptic inhibition mediated by local interneurons. Seizure discharges within the seizure onset zone produce recurrent inhibition within the seizure initiation zone (Kostopoulos et al., 1983; Dorn & Witte, 1995), thus reducing excitatory output. Early investigations of the spike and wave components of “spike-wave” discharges showed that the spike component is associated with a burst of rapid action potential firing, while the wave component is associated with a pause in action potential firing (Dichter & Spencer, 1969; Ayala et al., 1973). The pause in neuronal firing results from synaptic inhibition produced by local inhibitory interneurons activated by the volley of excitatory activity comprising the “spike” component, an example of feedback inhibition.
Feed-forward inhibition is a fundamental feature of cortical processing (Swadlow, 2003). Feed-forward inhibition may also play an important role; an interneuron activated by a principle cell sends inhibitory signals to principal cells outside the focus, inhibiting the propagation of the seizure (Cruikshank et al., 2007; Trevelyan et al., 2007). Recent evidence indicates that a principle cell axon may synapse on the presynaptic terminal of an inhibitory interneuron, bypassing somatic activation of the interneuron altogether by causing transmitter release directly from the inhibitory synaptic terminal (Connors & Cruikshank, 2007).
Synaptic inhibition is mediated by the presynaptic release of the neurotransmitter gamma aminobutyric acid, GABA, which acts on the postsynaptic neuron via receptors located on the soma, dendrites, or presynaptic terminals. GABA receptors are present in two major varieties, GABA-A and GABA-B. GABA-B receptors are metabotropic acting through G-protein second messengers. The pre- and postsynaptic distribution of GABA-B receptors, along with mixed evidence of anti- and proconvulsant effects of GABA-B activation, makes it difficult to determine their role in seizure termination (Vergnes et al., 1997; Zivanovic et al., 2003; Chen et al., 2004).
GABA-A receptors are chloride conducting membrane channels that open rapidly in response to GABA. Desensitization of GABA-A receptors during status epilepticus likely contributes to the failure of seizure termination (Chen et al., 2007). Desensitization of GABA-A receptors is also the basis of the loss of efficacy of benzodiazepine medications used to treat status-epilepticus. Multiple mechanisms appear to contribute to GABA-A receptor desensitization. Increased internalization of GABA-A receptors during status-epilepticus reduces the effect of GABAergic stimulation (Goodkin et al., 2007). Changes in subunit composition may also contribute to GABA-A receptor desensitization, although this process acts over many minutes to hours, and appears to affect long-term neuronal excitability and epileptogenesis rather than seizure termination (Brooks-Kayal, 2005).
Extrasynapatic GABA-A receptors, in contrast, do not desensitize and instead are capable of tonic inhibition which produces long lasting changes in neuronal reactivity. These tonic GABA receptors typically contain particular subunits—delta and possibly gamma—that alter the properties of the receptors (Richerson, 2004). Tonic receptors are activated by micromolar levels of the extrasynaptic GABA, which arrives either by diffusion from a synapse before reuptake, or by release into the extracellular space via nonsynaptic mechanisms. Tonic GABA receptors may play an important role in epilepsy. On the one hand, reduction of tonic GABA currents (as produced experimentally by a mutation in the delta-subunit of tonic GABA receptors) is associated with generalized epilepsy. On the other hand, progesterone-derived neurosteroids enhance tonic GABA currents, and may play a role in preventing seizure genesis, and potentially in terminating ongoing seizures (Stell et al., 2003). It is also clear that the contribution of extrasynaptic GABA-A receptors changes during maturation, and may contribute to changes in seizure susceptibility during development (Chudomel et al., 2006).
Neuromodulators: endocannabinoids, adenosine, and neuropeptide Y
Effects induced by neuromodulators—molecules that modulate the response of a neuron to neurotransmitters—can contribute significantly to seizure termination. Endocannabinoids, adenosine, and neuropeptide Y (NPY) each exert an effect on seizure termination or control. Ingestion or inhalation of tetrahydracannabinoid, the psychoactive component of marijuana, has some anticonvulsant effect (Mechoulam & Lichtman, 2003). It is increasingly recognized that endogenous cannabinoids—“endocannabinoids” synthesized and released from neurons following membrane depolarization—are capable of controlling seizures, possibly by increasing inhibitory interneuron activity within a region of cortex (Marsicano et al., 2003). Extracellular levels of endocannabinoid increase rapidly during seizures (up to threefold), suggesting a role for endocannabinoids in seizure termination. Endocannabinoid effects are mediated through G-protein-coupled cannabinoid receptors (Lutz, 2004). Endocannabinoid receptor CB1 is prevalent throughout the brain. Animal studies show increased CB1 in epileptic hippocampi. Blockade of CB1 increases spontaneous seizure frequency and duration, but does not trigger seizures in normal rats. Like pharmacologic blockade, knocking out the CB1 gene also increases seizure severity (Wallace et al., 2003). Thus, the endocannabinoid system appears to regulate excessive neuronal activity as occurs during seizures and contributes to seizure termination.
Adenosine—a by-product of energy metabolism and ATP utilization—also likely contributes to seizure termination, and the adenosine mechanism may be a feasible target for novel seizure-controlling medications. Adenosine crosses the neuronal membrane by facilitated diffusion between the intra- and extracellular compartments. Extracellular adenosine rises in the setting of increased metabolic activity (McGaraughty et al., 2005). Evidence that seizures increase extracellular adenosine several-fold was initially obtained in animals, and has been confirmed by microdialysis measurements in humans with temporal lobe epilepsy (During & Spencer, 1992). In humans, seizures increased adenosine between 6- and 31-fold, and adenosine remained elevated during the postictal period. It has been known for more than two decades that extracellular adenosine exerts an anticonvulsant effect (Dragunow et al., 1985). Extracellular levels of adenosine in the range of 25–250 nm provide an inhibition of neuronal activity by inhibiting the presynaptic release of neurotransmitter (Dunwiddie & Masino, 2001).
Although four receptor subtypes are known, the major effects of adenosine in seizure control appear to be mediated by the A1 receptor, which is the most abundant subtype present in the hippocampus and neocortex (Boison, 2005). Blockade of the adenosine receptor increases susceptibility to status epilepticus produced by recurrent electrical stimulation in rats. Activation of the A1 receptor, in contrast, terminates ongoing status epilepticus (Young & Dragunow, 1994). Decreased numbers of A1 receptors may contribute to failure of seizure termination. Homozygous A1 receptor knockout mice show a markedly greater susceptibility to seizures and status epilepticus. Following cortical trauma, 50% of A1 knockout mice experienced status epilepticus, while none of the controls exhibited this activity (Kochanek et al., 2006). In human temporal lobes of patients undergoing epilepsy surgery, A1 levels were reduced compared to levels measured in postmortem controls (Glass et al., 1996).
Reduction of extracellular adenosine may also contribute to the failure to terminate seizures or may lower the seizure threshold. Adenosine levels are regulated mainly by adenosine kinase—a cytosolic protein that converts adenosine to adenosine monophosphate (AMP) (Boison, 2005). Increased adenosine kinase activity decreases adenosine levels. Following a focal injection of kainic acid to induce status epilepticus in mice, adenosine kinase labeling in hippocampus increases bilaterally. The cells expressing adenosine kinase also expressed glial fibrillary acidic protein (GFAP), a specific marker of glia. In a mouse model, adenosine kinase levels increased as a result of hippocampal gliosis that followed status epilepticus. Mice receiving an adenosine kinase inhibitor that blocked the removal of adenosine from the extracellular space did not have further seizures or spikes on EEG (Gouder et al., 2004). Mice engineered to constitutively overexpress adenosine kinase had spontaneous seizures without exposure to kainate. In these mice, spontaneous seizures could be suppressed by systemic administration of an adenosine kinase inhibitor (Fedele et al., 2005).
As treatments to control or terminate seizures, adenosine and adenosine receptor agonists have been limited despite the potent effect of these compounds on seizures. Short half-life (adenosine itself persists only seconds in circulation), the multiplicity of potential peripheral and central targets, and the occurrence of adverse effects such as suppression of locomotion and hypothermia in rodents, have all limited the exploration of these drugs. Inhibition of adenosine kinase, however, offers the possibility of augmenting the action of adenosine by suppressing the major metabolic pathway that removes it from the brain. Compared to administration of adenosine or adenosine agonists, suppression of adenosine kinase has additional advantages. An adenosine kinase antagonist augments the adenosine effect in regions where adenosine is physiologically released, but not in areas where adenosine is not released. This selective amplification of adenosines effects offers a means to increase the desired action of adenosine while minimizing adverse undesired effects (McGaraughty et al., 2005).
NPY is another modulator of neuronal activity. Found in GABAergic inhibitory interneurons, it is capable of exerting an endogenous anticonvulsant effect that may contribute to seizure termination. NPY receptors are widely expressed in the CNS and influence diverse functions including regulation of blood pressure, feeding behavior, circadian rhythms, memory, anxiety and cognition (Vezzani et al., 1999). Of relevance to epilepsy, expression of mRNA encoding NPY increases 6–10 h following seizures even though NPY levels decline (Vezzani & Sperk, 2004).NPY expression is high in hippocampal mossy fibers and inhibitory interneurons (Bellmann et al., 1991). The density of NPY receptors is high in the Ammon's horn region of the hippocampus, where NPY released by interneurons and mossy fibers can modulate the activation of hippocampal pyramidal neurons (Colmers&El Bahh, 2003). The action of NPY is mediated by five classes of NPY receptors, Y1–Y5. The most abundant hippocampal receptor, Y2, reduces excitation by reducing presynaptic calcium entry. Activation of Y1 receptors, on the other hand, increase excitability, but this effect is minor compared to that of Y2 receptors. Release of NPY may favor selective control of seizures, as neuropeptide release is increased in neurons firing rapidly at frequencies of 5–40 Hz, as occurs during a seizure. Release of classical neurotransmitters is maximal at lower firing rates of 1–10 Hz(Vezzani et al., 1999).
Animal data supports an anticonvulsant role for NPY and a role in reducing seizure severity. NPY inhibits seizures in epilepsy models in vivo and in vitro. Mice and rats overexpressing NPY had increased resistance to kainic acid induced seizures (Vezzani et al., 2002; Lin et al., 2006). Absence of NPY in genetically modified mice, in contrast, markedly increased death from seizures induced by kainic acid and reduced the threshold for seizures induced by pentylenetetrazole (Baraban et al., 1997). Mice lacking NPY also manifested mild spontaneous seizures (Erickson et al., 1996). Data obtained from NPY receptor knockout mice and from pharmacologic studies in rat indicate that the major anticonvulsant action occurs via Y2 receptors (Lin et al., 2006).
Because replacement of released NPY requires protein synthesis and is relatively slow, the likelihood that NPY will contribute to seizure termination declines with increasing duration of a seizure. Prolonging the action of NPY potentially offers a novel pharmacologic target for seizure control.
MECHANISMS ACTING REMOTELY TO LIMIT EXCITATION AND SEIZURE SPREAD
In addition to mechanisms terminating seizures within the epileptic neuron and within the seizure onset zone, there are also long range interactions between neuronal populations that decrease likelihood of seizure initiation and may contribute to seizure termination. These long range interactions may be observed in the form of increased synchronization between cortical regions during a seizure. Long range effects may also result from actions of subcortical nuclei on cortical seizure threshold, duration, or severity. Some subcortical nuclei form regulatory circuits that modify, and possibly terminate, seizures through mechanisms that are only gradually being elucidated.
Knowledge of the relationship between seizure termination and synchronization between cortical regions or between cortex and subcortical regulatory circuits still remains largely phenomenological rather than mechanistic. Nevertheless, consideration of the effects of regulatory circuits potentially offers two avenues for progress. First, pharmacologic manipulations of regulatory centers to enhance seizure termination or reduce seizure initiation have direct therapeutic potential. Second, hypothesis generation based on available data—though necessarily speculative—may identify novel mechanisms. Synchrony between areas of cortex increases over the course of a seizure, accounting for the progressive increase in amplitude and slowing of the discharge frequency typically seen on EEG. Indeed the increase in intrahemispheric synchrony is greatest immediately before seizure termination. It has been proposed that the rise in intrahemispheric synchrony could result in strong activation of sodium and calcium inflow leading to activation of sodium- and calcium dependent potassium conductances that silence neuronal firing. It may be then, that increased synchrony between brain regions facilitates seizure termination, or at least is a marker for mechanisms mediating seizure termination (Timofeev & Steriade, 2004).
Vagal nerve stimulation (VNS)
There are numerous examples of circuits or brain regions that either reduce seizure likelihood or increase seizure termination or both. For example, electrical stimulation of the vagal nerve, which activates brainstem nuclei such as the nucleus solitarius, reduces seizure frequency in humans. In some patients, activation of VNS at seizure onset may help abort the seizure. The mechanisms of action of VNS are unknown. Theories have focused on the effects of vagal stimulation on cortical activity, and on the diffusely projecting catecholaminergic nuclei of the brainstem such as the locus coeruleus. The locus coeruleus projects extensively to thalamus, amygdala, and hippocampus, as well as diffusely to cortex (Groves & Brown, 2005). Depletion of forebrain norepinephrine facilitates seizures, suggesting norepinephrine release influences seizure termination (Albala et al., 1986). In rats, a lesion in the locus coeruleus abolishes the beneficial effect of VNS (Krahl et al., 1998). In humans, the effects of chronic VNS are not entirely consistent across studies. VNS may increase both catecholamine metabolites and GABA levels in CSF (Carpenter et al., 2004). VNS may also influence synchrony across the cortex, enhancing gamma frequency synchrony over long distances while decreasing theta and delta synchrony (Koo, 2001; Marrosu et al., 2005). Whether this effect of VNS on hemispheric gamma synchrony is mediated by catecholamines is unknown.
Role of subcortical brain regions
Numerous subcortical anatomical regions mediate anticonvulsant effects, such as the deep layers of the superior colliculus, the STN, the mammillothalamic tract and the anterior nucleus of the thalamus, deep cerebellar nuclei, and the SNR. The mechanisms by which these regions influence seizure severity and duration are yet unclear. Anticonvulsant circuits may limit seizures by reducing recruitment and spread of seizure activity from the seizure onset zone to “naive” brain by decreasing excitatory feedback and amplification, by increasing inhibitory tone diffusely, and by regulating the synchrony between cortical regions. The regulation of excitation and inhibition by subcortical nuclei appears to be age-dependent, and may contribute to the age-dependent differences in seizure susceptibility observed in humans.
As one example of a subcortical region capable of modulating seizure threshold, it is instructive to examine the SNR. The SNR is positioned anatomically to receive seizure activity originating in the neocortical hemispheres via the striatum and STN. Cortical discharges may enter the SNR either as glutamatergic activity relayed by the striatum-pallidum-subthalamic “indirect” extrapyramidal pathway, or as GABAergic activity via the “direct” striatal relay. The SNR, in turn, influences hemispheric activity via projections to the thalamus, superior colliculus, and the pedunculopontine nucleus in the rostral brainstem. In animal models, SNR metabolic activity—measured by uptake and utilization of radiolabelled 2-deoxyglucose—increases during seizure activity. The greatest increases are seen with generalized seizures, but partial limbic seizures increase glucose utilization as well (Lothman et al., 1985; Veliskova et al., 2005). Metabolic activation studies, discussed further below, identify the SNR as a relay point, where metabolic activation increases in response to seizure activity in the hemispheres.
Reduction of SNR firing through local application of pharmacological compounds such as the GABA agonist, muscimol, increases seizure threshold (Iadarola & Gale, 1982). Increased SNR firing, as occurs after application of GABA antagonist bicuculline, has proconvulsant effects (Sperber et al., 1989). Together, pharmacologic infusion and lesion studies indicate that decreasing firing in the anterior region of the SNR disinhibits—that is increases the activity of—efferent targets such as the deep layers of the superior colliculus and pedunculopontine tegmental nucleus.
Nigral control of seizure threshold also appears to vary with sex and developmental age. Application of GABA agonists to the substantia nigra can produce anti- or proconvulsant effects that vary by sex and developmental age (Veliskova et al., 1998). Male rats develop two regions in the SNR—anterior and posterior—with anticonvulsant and proconvulsant effects on seizures, respectively (Veliskova & Moshe, 2001). In female rats there is no equivalent proconvulsant posterior region. The differences in functional properties of anterio and posterior SNR are also reflected in differences in GABA receptor composition, neuronal GABA content, and the level of expression of the neuron-specific potassium chloride cotransporter (KCC2) responsible for maintaining intracellular chloride levels and regulating the hyperpolarizing effects of GABA (Galanopoulou & Moshe, 2003).
How might SNR influence of the seizure threshold translate into seizure termination? Metabolic studies measuring regional glucose uptake offers a means of examining the progression of seizure activity and activation of circuits modulating seizure activity. Glucose uptake is largely determined by synaptic metabolism rather than somatic activity (Ackermann et al., 1984). Rats exposed to the convulsant gas flurothyl develop a relatively consistent progression of seizure activity, beginning with brief generalized myoclonic jerks that become increasingly frequent, followed by recurrent clonic seizures involving mainly the forelimbs, followed by tonic seizures affecting all four limbs. One of the first regions to increase glucose uptake during generalized myoclonic seizures is the SNRposterior, which appears to act as a “gateway” for seizure propagation (Veliskova et al., 2005). As seizures worsen in severity, progressing from myoclonic to clonic, glucose uptake increases in the SNRanterior, and decreases in the superior colliculus and striatum, both of which receive GABAergic efferents from the SNR. Silencing the SNR with microinjection of a GABA antagonist such as muscimol decreases the inhibition in the superior colliculus and other targets of the SNR. Direct microinjection of bicuculline, a GABA antagonist, into the deep layers of the superior colliculus or pedunculpontine nucleus produces a similar anticonvulsant effect to inhibiting the SNR (Okada et al., 1989). The deep layers of the superior colliculus overlap with the rostral extent of a brainstem region termed the “DMAZ” that also includes part of the mesencephalic reticular formation. Microinjection of bicuculline in the DMAZ reduces hindlimb extension—a measure of seizure severity—in the maximum electroshock rat model. This anticonvulsant action mediated by the DMAZ is abolished by lesioning the pathways connecting the DMAZ to the ventrolateral pons (Shehab et al., 1995). Although the mechanisms by which the SNR and other brainstem anticonvulsant regions modify focal seizure activity in cortex are unknown, the final common pathway of these anticonvulsant regions may be an increase in neuronal activity in ventral and lateral pontine tegmental nuclei. The nuclei of pontine tegmentum comprise diffusely projecting networks, such as the reticular activating system, capable of strongly influencing thalamic and cortical activity that could counteract the recruitment and synchronization induced by the seizure onset zone.
Nigral influence over seizures varies with development and may be impaired as a result of neuronal injury from prolonged seizures. Seizure incidence is high early in life and greatest in the neonatal period (Moshe et al., 1983; DeLorenzo et al., 1992). Though the immature brain is relatively resistant to neuronal injury following seizures and status epilepticus, early life seizures produce functional changes in hippocampal cortex and SNR (Sperber et al., 1999; Haut et al., 2004). Rat pups experiencing three episodes of status epilepticus before age 6 days have abnormal development of the anterior subregion of the SNR, the area of the SNR that is most involved in seizure control (Veliskova et al., 2005). Rats experiencing early life status epilepticus appear to lose the anticonvulsant activity of the SNR once they mature (Heida et al., 2006). The changes in function of the SNR are paralleled by alterations in the composition of GABA-A receptors, where there is loss of benzodiazepine-sensitive GABA subunits of the GABA-A receptor (Galanopoulou et al., 2006). Thus, current evidence indicates that the SNR is capable of exerting an anticonvulsant effect, but that this mechanism for controlling seizures may be impaired in the epileptic brain because of neuronal death or abnormal development in adult or immature brain, respectively.
In addition to maturation dependent changes in SNR anatomy and function, the SNR may also be affected by prolonged seizures. Status epilepticus induced by a variety of chemoconvulsants in adult rats results acutely in SNR neuronal injury, ranging from mild to total (Nevander et al., 1985; Pineau et al., 1999). SNR metabolism also increases following status epilepticus during the transition to the epileptic state raising the question whether severe seizures or possibly recurrent seizures produce structural or functional changes that affect and possibly diminish the SNR influence over seizures (Dube et al., 2000; Veliskova & Moshe, 2006). It reasonable to suppose that the nigral action to raise seizure threshold interictally may manifestictally as retardation of seizure spread or possibly facilitation of seizure termination. If so, the gradual maturation of SNR influence on seizures during development may account for the increased susceptibility to seizures in young children or the decreased ability to terminate prolonged seizures in children identified in, the study of Shinnar et al. (2001). The impact of acquired injury on SNR function is difficult to assess in humans, particularly because the SNR is likely only one region of many experiencing neuronal injury. Nevertheless, evidence of changes in nigral control of seizure threshold as a consequence of injury suggest changes in seizure termination may also be affected.
The STN, which lies adjacent to the SNR and provides the major excitatory input to the SNR is also capable of modulating seizure activity. The STN receives inhibitory GABAergic input from the pallidum and excitatory glutamatergic input from mainly frontal cortex as well as excitatory inputs from the intralaminar nuclei of the thalamus. Smaller projections arriving from brainstem areas of substantia nigra, pendunculopontine nucleus, and raphe nuclei bring dopaminergic, cholinergic, and serotonergic activity that modulates STN activity (Hamani et al., 2004). Microinfusion of muscimol into STN raises the threshold for seizures in some, but not all animal models (Veliskova et al., 1996; Shehab et al., 2006). Electrical stimulation of the STN in rodents, and humans, raises the seizure threshold and has an anticonvulsant effect (Chabardes et al., 2002; Lado et al., 2003). Anticonvulsant action has also been attributed to the zona incerta, which lies adjacent to the STN.
CONCLUSIONS AND POSSIBLE INTERVENTIONS
How might better understanding of seizure termination translate into improved clinical practice? Importantly, noting a patient's prior seizure history can alert the physician to potential problems before they occur. A child with a history of prolonged febrile seizures or status epilepticus, for example, is more likely to have recurrent prolonged seizures (Shinnar et al., 2001). Considerable work, however, remains to translate the increased understanding of mechanisms terminating seizures into clinically proven treatments. The persistence of seizures among a large subgroup of individuals with epilepsy that is refractory to currently available medications underscores the need for novel targets for drug and device development. Seizure termination mechanisms may present specific targets for preclinical screening of potential anticonvulsants. Agents enhancing, for example, termination of paroxysmal depolarizing shifts might decrease excitatory amplification preferentially in “epileptic” neurons. Modification of the extracellular environment or intracellular ion gradients, either through the use of diuretics or by special electrolytic solutions, may offer another avenue for raising seizure threshold or enhancing termination (Ochoa, 2006). Disruption of the electrotonic coupling between neurons and between interneurons by disrupting gap junction connections has the potential to reduce neuronal synchrony and perhaps facilitate seizure termination. Enhancement of local inhibition within a seizure onset zone, currently the mainstay of acute seizure treatment in the form of benzodiazepine medications, has not become a significant tool for control of chronic epilepsy because of tachyphylaxis and GABA receptor desensitization (Crawford et al., 1987). Drug development targeting the GABA receptor to enhance activity dependent local inhibition without increasing sedation or causing tachyphylaxis would be widely applicable to many seizure types. Modification of the age-dependent depolarizing effects of GABA offer another molecular target that can alter seizure susceptibility. Manipulation of the chloride gradient either through molecular techniques or through the use of drugs that alter chloride transporters NKCC1 and KCC2 are potential treatment for age-dependent seizure syndromes refractory to conventional treatment (Galanopoulou et al., 2003; Dzhala et al., 2005).
Alteration of the hormonal milieu may be another means of enhancing some mechanisms controlling seizures. Novel compounds that activate endogenous seizure-limiting mechanisms without unacceptable side-effects would constitute an entirely new class of anticonvulsants. Agents targeting adenosine kinase are already under development. Similar efforts targeting the endocannibanoid and NPY receptors also hold promise.
Implanted devices are another opportunity to terminate seizure activity before consciousness is impaired or clinical symptoms manifest. Vagal nerve stimulation, for example, initiated by a patient or a caregiver can sometimes halt ongoing seizure activity, raising the hope of creating a device that both detects seizures and produces an action capable of terminating seizure activity (Morris, 2003). This approach was used in a pilot study of four patients with medically refractory epilepsy who received electrical stimulation of the seizure onset zone in response to seizure detection (Kossoff et al., 2004). The study showed promise as a means of terminating seizures, and the approach is now the subject of an ongoing clinical trial (Neuro Pace, 2008). The strategy of seizure detection followed by focal electrical stimulation can also be applied to other seizure modifying circuits such as the anterior thalamus, SNR, STN, or other brainstem sites, may also offer a means of aborting seizures. More research is necessary, however, because the effects of stimulation are not well understood. In one report, for example, stimulation of the anterior thalamus significantly exacerbated seizures in a rat model of chronic limbic epilepsy (Lado, 2006). Neurostimulation, however, is only one approach to focally modifying brain circuits. Alternative technologies may use implantable drug infusion systems to regulate the seizure onset zone or the activity of nuclear targets capable of regulating seizure spread, onset, or termination (Ludvig et al., 2006).
In summary, it can be reasonably hoped that unraveling the mechanisms that result in seizure termination will lead us to novel treatments for patients with epilepsy. It can also be hoped that further investigation of how seizures end will describe fundamental mechanisms at work in the brain and will have implications for other disease states and for our understanding of brain function.
ACKNOWLEDGMENTS
Supported by NINDS grants NS-20253 and NS-43209 to SLM and NS-41340 and NS-48149 to FAL, and the Heffer Family Medical Foundation. SLM is the recipients of the Martin A. and Emily L. Fisher fellowship in Neurology and Pediatrics.
Footnotes
Conflict of interest: The authors have read the Journal's position on issues involved in ethical publication and affirm that this review is consistent with those guidelines. Neither author has any conflicts of interest to disclose.
REFERENCES
- Ackermann RF, Finch DM, Babb TL, Engel J., Jr Increased glucose metabolism during long-duration recurrent inhibition of hippocampal pyramidal cells. J Neurosci. 1984;4:251–264. doi: 10.1523/JNEUROSCI.04-01-00251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albala BJ, Moshe SL, Cubells JF, Sharpless NS, Makman MH. Unilateral peri-substantia nigra catecholaminergic lesion and amygdale kindling. Brain Res. 1986;370:388–392. doi: 10.1016/0006-8993(86)90500-7. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Epileptiform burst after hyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980;210:1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Auer RN. Progress review: hypoglycemic brain damage. Stroke. 1986;17:699–708. doi: 10.1161/01.str.17.4.699. [DOI] [PubMed] [Google Scholar]
- Ayala GF. The paroxysmal depolarizing shift. Prog Clin Biol Res. 1983;124:15–21. [PubMed] [Google Scholar]
- Ayala GF, Dichter M, Gumnit RJ, Matsumoto H, Spencer WA. Genesis of epileptic interictal spikes. New knowledge of cortical feedback systems suggests a neurophysiological explanation of brief paroxysms. Brain Res. 1973;52:1–17. doi: 10.1016/0006-8993(73)90647-1. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Bellmann R, Widmann R, Olenik C, Meyer DK, Maas D, Marksteiner J, Sperk G. Enhanced rate of expression and biosynthesis of neuropeptide Y after kainic acid-induced seizures. J Neurochem. 1991;56:525–530. doi: 10.1111/j.1471-4159.1991.tb08181.x. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bikson M, Hahn PJ, Fox JE, Jeffreys JG. Depolarization block of neurons during maintenance of electrographic seizures. J Neurophysiol. 2003;90:2402–2408. doi: 10.1152/jn.00467.2003. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- Bragin A, Penttonen M, Buzsaki G. Termination of epileptic after-discharge in the hippocampus. J Neurosci. 1997;17:2567–2579. doi: 10.1523/JNEUROSCI.17-07-02567.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Kayal AR. Rearranging receptors. Epilepsia. 2005;46 (Suppl. 7):29–38. doi: 10.1111/j.1528-1167.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Bullock TH, Bennett MV, Johnston D, Josephson R, Marder E, Fields RD. Neuroscience. The neuron doctrine, redux. Science. 2005;310:791–793. doi: 10.1126/science.1114394. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Moreno FA, Kling MA, Anderson GM, Regenold WT, Labiner DM, Price LH. Effect of vagus nerve stimulation on cerebrospinal fluid monoamine metabolites, norepinephrine, and gamma-aminobutyric acid concentrations in depressed patients. Biol Psychiatry. 2004;56:418–426. doi: 10.1016/j.biopsych.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Chabardes S, Kahane P, Minotti L, Koudsie A, Hirsch E, Benabid AL. Deep brain stimulation in epilepsy with particular reference to the subthalamic nucleus. Epileptic Disord. 2002;4 (Suppl. 3):S83–S93. [PubMed] [Google Scholar]
- Chen L, Chan YS, Yung WH. GABA-B receptor activation in the rat globus pallidus potently suppresses pentylenetetrazol-induced tonic seizures. J Biomed Sci. 2004;11:457–464. doi: 10.1007/BF02256094. [DOI] [PubMed] [Google Scholar]
- Chen JW, Naylor DE, Wasterlain CG. Advances in the pathophysiology of status epilepticus. Acta Neurol Scand Suppl. 2007;186:7–15. [PubMed] [Google Scholar]
- Chesler M, Kraig RP. Intracellular pH of astrocytes increases rapidly with cortical stimulation. Am J Physiol. 1987;253:R666–R670. doi: 10.1152/ajpregu.1987.253.4.R666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M, Kaila K. Modulation of pH by neuronal activity. Trends Neurosci. 1992;15:396–402. doi: 10.1016/0166-2236(92)90191-a. [DOI] [PubMed] [Google Scholar]
- Chudomel O, Galanopoulou A, Moshè S. Tonic and phasic GABA currents in GABAergic neurons of the substantia nigra reticulata are dependent on age regardless of sex. American Epilepsy Society Annual Meeting, San Diego. 2006 [Google Scholar]
- Cohen JE, Fields RD. Extracellular calcium depletion in synaptic transmission. Neuroscientist. 2004;10:12–17. doi: 10.1177/1073858403259440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, El Bahh B. Neuropeptide Y and epilepsy. Epilepsy Curr. 2003;3:53–58. doi: 10.1046/j.1535-7597.2003.03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Cruikshank SJ. Bypassing interneurons: inhibition in neocortex. Nat Neurosci. 2007;10:808–810. doi: 10.1038/nn0707-808. [DOI] [PubMed] [Google Scholar]
- Coulter DA, DeLorenzo RJ. Basic mechanisms of status epilepticus. Adv Neurol. 1999;79:725–733. [PubMed] [Google Scholar]
- Crawford TO, Mitchell WG, Snodgrass SR. Lorazepam in childhood status epilepticus and serial seizures: effectiveness and tachyphylaxis. Neurology. 1987;37:190–195. doi: 10.1212/wnl.37.2.190. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Manfridi A, Biella G. Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci. 1998;18:7543–7551. doi: 10.1523/JNEUROSCI.18-18-07543.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Towne AR, Pellock JM, Ko D. Status epilepticus in children, adults, and the elderly. Epilepsia. 1992;33 (Suppl. 4):S15–S25. doi: 10.1111/j.1528-1157.1992.tb06223.x. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter M, Spencer WA. Penicillin-induced interictal discharges from the cat hippocampus. II. Mechanisms underlying origin and restriction. J Neurophysiol. 1969;32:663–687. doi: 10.1152/jn.1969.32.5.663. [DOI] [PubMed] [Google Scholar]
- Dorn T, Witte OW. Refractory periods following interictal spikes in acute experimentally induced epileptic foci. Electroencephalogr Clin Neurophysiol. 1995;94:80–85. doi: 10.1016/0013-4694(94)00214-6. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Goddard GV, Laverty R. Is adenosine an endogenous anticonvulsant? Epilepsia. 1985;26:480–487. doi: 10.1111/j.1528-1157.1985.tb05684.x. [DOI] [PubMed] [Google Scholar]
- Dube C, Boyet S, Marescaux C, Nehlig A. Progressive metabolic changes underlying the chronic reorganization of brain circuits during the silent phase of the lithium-pilocarpine model of epilepsy in the immature and adult Rat. Exp Neurol. 2000;162:146–157. doi: 10.1006/exnr.2000.7324. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances Phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Gouder N, Guttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- Gajda Z, Szupera Z, Blazso G, Szente M. Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia. 2005;46:1581–1591. doi: 10.1111/j.1528-1167.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Moshe SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184:1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshe SL. Sex-specific KCC2 expression and GABA(A) receptor function in rat substantia nigra. Exp Neurol. 2003;183:628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Chudomel O, Heida J, Moshe SL. Neonatal seizures cause long term changes in GABAA receptors in rat substantia nigra. American Epilepsy Society Annual Meeting; American Epilepsy Society; San Diego. 2006. [Google Scholar]
- Galanopoulou AS. Developmental patterns in the regulation of chloride homeostasis and GABA(A) receptor signaling by seizures. Epilepsia. 2007;48 (Suppl. 5):14–18. doi: 10.1111/j.1528-1167.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical synapses between GABA-releasing interneurons. Nat Rev Neurosci. 2001;2:425–433. doi: 10.1038/35077566. [DOI] [PubMed] [Google Scholar]
- Glass M, Faull RL, Bullock JY, Jansen K, Mee EW, Walker EB, Synek BJ, Dragunow M. Loss of A1 adenosine receptors in human temporal lobe epilepsy. Brain Res. 1996;710:56–68. doi: 10.1016/0006-8993(95)01313-x. [DOI] [PubMed] [Google Scholar]
- Goodkin HP, Sun C, Yeh JL, Mangan PS, Kapur J. GABA(A) receptor internalization during seizures. Epilepsia. 2007;48 (Suppl. 5):109–113. doi: 10.1111/j.1528-1167.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy JM, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epilepto-genesis. J Neurosci. 2004;24:692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM. The subthalamic nucleus in the context of movement disorders. Brain. 2004;127:4–20. doi: 10.1093/brain/awh029. [DOI] [PubMed] [Google Scholar]
- Hamzei-Sichani F, Kamasawa N, Janssen WG, Yasumura T, Davidson KG, Hof PR, Wearne SL, Stewart MG, Young SR, Whittington MA, Rash JE, Traub RD. Gap junctions on hippocampal mossy fiber axons demonstrated by thin-section electron microscopy and freeze fracture replica immunogold labeling. Proc Natl Acad Sci USA. 2007;104:12548–12553. doi: 10.1073/pnas.0705281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Haut SR, Veliskova J, Moshe SL. Susceptibility of immature and adult brains to seizure effects. Lancet Neurol. 2004;3:608–617. doi: 10.1016/S1474-4422(04)00881-6. [DOI] [PubMed] [Google Scholar]
- Heida JG, Veliskova J, Velisek L, Chudomel O, Moshe SL, Galanopoulou AS. Early life status epilepticus alters the seizure controlling function of the substantia nigra pars reticulata. American Epilepsy Society Annual Meeting; American Epilepsy Society; San Diego. 2006. [Google Scholar]
- Heinemann U, Lux HD, Gutnick MJ. Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res. 1977;27:237–243. doi: 10.1007/BF00235500. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Hochman DW, Baraban SC, Owens JW, Schwartzkroin PA. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995;270:99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- Hochman DW, Schwartzkroin PA. Chloride-cotransport blockade desynchronizes neuronal discharge in the “epileptic” hippocampal slice. J Neurophysiol. 2000;83:406–417. doi: 10.1152/jn.2000.83.1.406. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–1240. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Jahromi SS, Wentlandt K, Piran S, Carlen PL. Anticonvulsant actions of gap junctional blockers in an in vitro seizure model. J Neurophysiol. 2002;88:1893–1902. doi: 10.1152/jn.2002.88.4.1893. [DOI] [PubMed] [Google Scholar]
- Jenssen S, Gracely EJ, Sperling MR. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia. 2006;47:1499–1503. doi: 10.1111/j.1528-1167.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- Jin X, Huguenard JR, Prince DA. Impaired Cl- extrusion in layer V pyramidal neurons of chronically injured epileptogenic neocortex. J Neurophysiol. 2005;93:2117–2126. doi: 10.1152/jn.00728.2004. [DOI] [PubMed] [Google Scholar]
- Jones J, Stubblefield EA, Benke TA, Staley KJ. Desynchronization of glutamate release prolongs synchronous CA3 network activity. J Neurophysiol. 2007;97:3812–3818. doi: 10.1152/jn.01310.2006. [DOI] [PubMed] [Google Scholar]
- King RD, Wiest MC, Montague PR. Extracellular calcium depletion as a mechanism of short-term synaptic depression. J Neurophysiol. 2001;85:1952–1959. doi: 10.1152/jn.2001.85.5.1952. [DOI] [PubMed] [Google Scholar]
- Kirchner A, Veliskova J, Velisek L. Differential effects of low glucose concentrations on seizures and epileptiform activity in vivo and in vitro. Eur J Neurosci. 2006;23:1512–1522. doi: 10.1111/j.1460-9568.2006.04665.x. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- Koo B. EEG changes with vagus nerve stimulation. J Clin Neurophysiol. 2001;18:434–441. doi: 10.1097/00004691-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, Spencer DD, Bergey GK. Effect of an external responsive neurostimulator on seizures and electrographic discharges during subdural electrode monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G, Avoli M, Gloor P. Participation of cortical recurrent inhibition in the genesis of spike and wave discharges in feline generalized penicillin epilepsy. Brain Res. 1983;267:101–112. doi: 10.1016/0006-8993(83)91043-0. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Lado FA, Velisek L, Moshe SL. The effect of electrical stimulation of the subthalamic nucleus on seizures is frequency dependent. Epilepsia. 2003;44:157–164. doi: 10.1046/j.1528-1157.2003.33802.x. [DOI] [PubMed] [Google Scholar]
- Lado FA. Chronic bilateral stimulation of the anterior thalamus of kainate-treated rats increases seizure frequency. Epilepsia. 2006;47:27–32. doi: 10.1111/j.1528-1167.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- Lin EJ, Young D, Baer K, Herzog H, During MJ. Differential actions of NPY on seizure modulation via Y1 and Y2 receptors: evidence from receptor knockout mice. Epilepsia. 2006;47:773–780. doi: 10.1111/j.1528-1167.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Hatlelid JM, Zorumski CF. Functional mapping of limbic seizures originating in the hippocampus: a combined 2-deoxyglucose and electrophysiologic study. Brain Res. 1985;360:92–100. doi: 10.1016/0006-8993(85)91224-7. [DOI] [PubMed] [Google Scholar]
- Ludvig N, Kuzniecky RI, Baptiste SL, John JE, von Gizycki H, Doyle WK, Devinsky O. Epidural pentobarbital delivery can prevent locally induced neocortical seizures in rats: the prospect of transmeningeal pharmacotherapy for intractable focal epilepsy. Epilepsia. 2006;47:1792–1802. doi: 10.1111/j.1528-1167.2006.00642.x. [DOI] [PubMed] [Google Scholar]
- Lutz B. On-demand activation of the endocannabinoid system in the control of neuronal excitability and epileptiform seizures. Biochem Pharmacol. 2004;68:1691–1698. doi: 10.1016/j.bcp.2004.07.007. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Dudek FE. Local synaptic circuits in rat hippocampus: interactions between pyramidal cells. Brain Res. 1980;184:220–223. doi: 10.1016/0006-8993(80)90602-2. [DOI] [PubMed] [Google Scholar]
- Mancilla JG, Lewis TJ, Pinto DJ, Rinzel J, Connors BW. Synchronization of electrically coupled pairs of inhibitory interneurons inneocortex. J Neurosci. 2007;27:2058–2073. doi: 10.1523/JNEUROSCI.2715-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrosu F, Santoni F, Puligheddu M, Barberini L, Maleci A, Ennas F, Mascia M, Zanetti G, Tuveri A, Biggio G. Increase in 20–50 Hz (gamma frequencies) power spectrum and synchronization after chronic vagal nerve stimulation. Clin Neurophysiol. 2005;116:2026–2036. doi: 10.1016/j.clinph.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Ayala GF, Gumnit RJ. Effects of intracellularly injected currents on the PDS and the hyperpolarizing after-potential in neurons within an epileptic focus. Electroencephalogr Clin Neurophysiol. 1969;26:120. [PubMed] [Google Scholar]
- McGaraughty S, Cowart M, Jarvis MF, Berman RF. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5:43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Lichtman AH. Neuroscience. Stout guards of the central nervous system. Science. 2003;302:65–67. doi: 10.1126/science.1091256. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Single neurones can initiate synchronized population discharge in the hippocampus. Nature. 1983;306:371–373. doi: 10.1038/306371a0. [DOI] [PubMed] [Google Scholar]
- Morris GL., 3rd A retrospective analysis of the effects of magnet-activated stimulation in conjunction with vagus nerve stimulation therapy. Epilepsy Behav. 2003;4:740–745. doi: 10.1016/j.yebeh.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Moshe SL, Albala BJ, Ackermann RF, Engel J., Jr Increased seizure susceptibility of the immature brain. Brain Res. 1983;283:81–85. doi: 10.1016/0165-3806(83)90083-4. [DOI] [PubMed] [Google Scholar]
- NeuroPace. Responsive Neurostimulator (RNS™) System Long-Term Treatment Clinical Investigation. [Accessed April 30, 2008];Bethesda (MD): National Library of Medicine (US) 2000; ClinicalTrials.gov [Internet] 2008 Available from: http://clinicaltrials.gov/ct2/show/NCT00572195 NLM Identifier: NCT00572195.
- Nevander G, Ingvar M, Auer R, Siesjo BK. Status epilepticus in well-oxygenated rats causes neuronal necrosis. Ann Neurol. 1985;18:281–290. doi: 10.1002/ana.410180303. [DOI] [PubMed] [Google Scholar]
- Ochoa JG. Oral electrolyte therapy for refractory epilepsy; American Epilepsy Society Annual Meeting; San Diego. 2006. [Google Scholar]
- Okada R, Negishi N, Nagaya H. The role of the nigrotegmental GABAergic pathway in the propagation of pentylenetetrazol-induced seizures. Brain Res. 1989;480:383–387. doi: 10.1016/0006-8993(89)90212-6. [DOI] [PubMed] [Google Scholar]
- Patrick AW, Campbell IW. Fatal hypoglycaemia in insulin-treated diabetes mellitus: clinical features and neuropathological changes. Diabet Med. 1990;7:349–354. doi: 10.1111/j.1464-5491.1990.tb01403.x. [DOI] [PubMed] [Google Scholar]
- Pineau N, Charriaut-Marlangue C, Motte J, Nehlig A. Pentylene-tetrazol seizures induce cell suffering but not death in the immature rat brain. Brain Res Dev Brain Res. 1999;112:139–144. doi: 10.1016/s0165-3806(98)00158-8. [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Patrick SL, Huang WC, Connors BW. Initiation, propagation, and termination of epileptiform activity in rodent neocortex in vitro involve distinct mechanisms. J Neurosci. 2005;25:8131–8140. doi: 10.1523/JNEUROSCI.2278-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB. Looking for GABA in all the wrong places: the relevance of extrasynaptic GABA(A) receptors to epilepsy. Epilepsy Curr. 2004;4:239–242. doi: 10.1111/j.1535-7597.2004.46008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg. 1989;70:667–675. doi: 10.3171/jns.1989.70.5.0667. [DOI] [PubMed] [Google Scholar]
- Schindler K, Elger CE, Lehnertz K. Increasing synchronization may promote seizure termination: evidence from status epilepticus. Clin Neurophysiol. 2007;118:1955–1968. doi: 10.1016/j.clinph.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Schweitzer JS, Wang H, Xiong ZQ, Stringer JL. pH Sensitivity of non-synaptic field bursts in the dentate gyrus. J Neurophysiol. 2000;84:927–933. doi: 10.1152/jn.2000.84.2.927. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Crill WE. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol. 1989;61:233–244. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- Shehab S, Simkins M, Dean P, Redgrave P. The dorsal midbrain anticonvulsant zone-III. Effects of efferent pathway transections on suppression of electroshock seizures and defence-like reactions produced by local injections of bicuculline. Neuroscience. 1995;65:697–708. doi: 10.1016/0306-4522(94)00517-9. [DOI] [PubMed] [Google Scholar]
- Shehab SA, Ljubisavljevic M, Al-Halhali F, Al-Awadhi A, Madathil M, Abdul-Kareem A, Redgrave P. Experimental manipulations of the subthalamic nucleus fail to suppress tonic seizures in the electroshock model of epilepsy. Exp Brain Res. 2006;173:274–281. doi: 10.1007/s00221-006-0439-1. [DOI] [PubMed] [Google Scholar]
- Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49:659–664. [PubMed] [Google Scholar]
- Sperber EF, Wurpel JN, Moshe SL. Evidence for the involvement of nigral GABAB receptors in seizures of rat pups. Brain Res Dev Brain Res. 1989;47:143–146. doi: 10.1016/0165-3806(89)90117-x. [DOI] [PubMed] [Google Scholar]
- Sperber EF, Veliskova J, Germano IM, Friedman LK, Moshe SL. Age-dependent vulnerability to seizures. Adv Neurol. 1999;79:161–169. [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Longacher M, Bains JS, Yee A. Presynaptic modulation of CA3 network activity. Nat Neurosci. 1998;1:201–209. doi: 10.1038/651. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cereb Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Szente M, Gajda Z, Said Ali K, Hermesz E. Involvement of electrical coupling in the in vivo ictal epileptiform activity induced by 4-aminopyridine in the neocortex. Neuroscience. 2002;115:1067–1078. doi: 10.1016/s0306-4522(02)00533-x. [DOI] [PubMed] [Google Scholar]
- Thimm J, Mechler A, Lin H, Rhee S, Lal R. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem. 2005;280:10646–10654. doi: 10.1074/jbc.M412749200. [DOI] [PubMed] [Google Scholar]
- Thiry A, Dogne JM, Supuran CT, Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem. 2007;7:855–864. doi: 10.2174/156802607780636726. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Grenier F, Steriade M. Contribution of intrinsic neuronal factors in the generation of cortically driven electrographic seizures. J Neurophysiol. 2004;92:1133–1143. doi: 10.1152/jn.00523.2003. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123:299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, Schmitz D. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Michelson-Law H, Bibbig AE, Buhl EH, Whittington MA. Gap junctions, fast oscillations and the initiation of seizures. Adv Exp Med Biol. 2004;548:110–122. doi: 10.1007/978-1-4757-6376-8_9. [DOI] [PubMed] [Google Scholar]
- Trevelyan AJ, Sussillo D, Yuste R. Feedforward inhibition contributes to the control of epileptiform propagation speed. J Neurosci. 2007;27:3383–3387. doi: 10.1523/JNEUROSCI.0145-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velisek L, Moshe SL, Xu SG, Cammer W. Reduced susceptibility to seizures in carbonic anhydrase II deficient mutant mice. Epilepsy Res. 1993;14:115–121. doi: 10.1016/0920-1211(93)90016-z. [DOI] [PubMed] [Google Scholar]
- Velisek L, Dreier JP, Stanton PK, Heinemann U, Moshe SL. Lowering of extracellular pH suppresses low-Mg(2+)-induces seizures in combined entorhinal cortex-hippocampal slices. Exp Brain Res. 1994;101:44–52. doi: 10.1007/BF00243215. [DOI] [PubMed] [Google Scholar]
- Velisek L. Extracellular acidosis and high levels of carbon dioxide suppress synaptic transmission and prevent the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Hippocampus. 1998;8:24–32. doi: 10.1002/(SICI)1098-1063(1998)8:1<24::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velsek L, Moshe SL. Subthalamic nucleus: a new anticonvulsant site in the brain. Neuroreport. 1996;7:1786–1788. [PubMed] [Google Scholar]
- Veliskova J, Kubova H, Friedman LK, Wu R, Sperber EF, Zukin RS, Moshe SL. The expression of GABA(A) receptor subunits in the substantia nigra is developmentally regulated and region-specific. Ital J Neurol Sci. 1998;19:205–210. doi: 10.1007/BF02427602. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann Neurol. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Miller AM, Nunes ML, Brown LL. Regional neural activity within the substantia nigra during peri-ictal flurothyl generalized seizure stages. Neurobiol Dis. 2005;20:752–759. doi: 10.1016/j.nbd.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Update on the role of substantia nigra pars reticulata in the regulation of seizures. Epilepsy Curr. 2006;6:83–87. doi: 10.1111/j.1535-7511.2006.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnes M, Boehrer A, Simler S, Bernasconi R, Marescaux C. Opposite effects of GABAB receptor antagonists on absences and convulsive seizures. Eur J Pharmacol. 1997;332:245–255. doi: 10.1016/s0014-2999(97)01085-6. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF. Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 1999;22:25–30. doi: 10.1016/s0166-2236(98)01284-3. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Michalkiewicz M, Michalkiewicz T, Moneta D, Ravizza T, Richichi C, Aliprandi M, Mule F, Pirona L, Gobbi M, Schwarzer C, Sperk G. Seizure susceptibility and epileptogenesis are decreased in transgenic rats overexpressing neuropeptide Y. Neuroscience. 2002;110:237–243. doi: 10.1016/s0306-4522(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G. Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides. 2004;38:245–252. doi: 10.1016/j.npep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- Young D, Dragunow M. Status epilepticus may be caused by loss of adenosine anticonvulsant mechanisms. Neuroscience. 1994;58:245–261. doi: 10.1016/0306-4522(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Zivanovic D, Bernaskova K, Kaminskij Y, Mares P. Action of GABA-B antagonist on cortical epileptic afterdischarges in rats is similar to that of GABA-A antagonist. Physiol Res. 2003;52:651–655. [PubMed] [Google Scholar]