Abstract
Momentary reductions of attention can have extremely adverse outcomes, but it remains unclear whether increased distraction from irrelevant stimuli contributes to such outcomes. To investigate this hypothesis, we examined trial-by-trial relationships between brain activity and response time in twenty healthy adults while they performed a cross-modal selective attention task. In each trial, participants identified a relevant visual letter while ignoring an irrelevant auditory letter, which was mapped either to the same response as the visual letter (congruent trials) or to a different response (incongruent trials). As predicted, reductions of attention (i.e., increases of response time) were associated not only with decreased activity in sensory regions that processed the relevant visual stimuli, suggesting a failure to enhance the processing of those stimuli, but also with increased activity in sensory regions that processed the irrelevant auditory stimuli, suggesting a failure to suppress the processing of those stimuli. Reductions of attention were also linked to larger increases of activity in incongruent than in congruent trials in anterior cingulate regions that detect response conflict, suggesting that failing to suppress the sensory processing of the irrelevant auditory stimuli during attentional reductions allowed those stimuli to more readily activate conflicting responses in incongruent trials. These findings indicate that heightened levels of distraction during momentary reductions of attention likely stem, at least in part, from increased processing of irrelevant stimuli.
Keywords: attention, auditory, visual, response conflict, fMRI, cognitive
Introduction
Although often innocuous, momentary reductions of attention can have dire outcomes. For example, when experiencing a reduction of attention drivers take longer to step on the brake when an unexpected event occurs (Beede and Kass, 2006). Reductions of attention also profoundly disrupt behavior in numerous clinical syndromes, such as attention-deficit and hyperactivity disorder (ADHD) (Castellanos et al., 2005; Reimer et al., 2005), Alzheimer’s disease (Berardi et al., 2005), and drug addiction (Hendricks et al., 2006). Understanding and minimizing reductions of attention therefore has tremendous theoretical and clinical importance.
Current models provide important clues as to which processes might be adversely affected by momentary reductions of attention. Specifically, they posit that attention facilitates performance not only by enhancing the processing of relevant stimuli, but also by limiting the processing of irrelevant stimuli (Desimone, 1998; Handy et al., 2001; Hasher and Zacks, 1988; Lavie et al., 2004). Thus, attentional reductions should both impair the processing of relevant stimuli and permit greater processing of irrelevant stimuli.
Using functional magnetic resonance imaging (fMRI), we recently investigated the effects of momentary reductions of attention on the processing of relevant stimuli in an intramodal visual selective attention task (Weissman et al., 2006). Natural variations in response time can serve as good dynamic markers of variations in attention (Castellanos et al., 2005). When a person experiences a reduction of attention, their responses to external stimuli become slower, and the degree of slowing depends on the severity of the reduction. Therefore, in our prior study we investigated the neural bases of momentary reductions of attention by correlating brain activity with response time (RT) on a trial-by-trial basis. Our findings provided strong evidence that reductions of attention (i.e., increases of RT) impair the processing of relevant stimuli. For example, reductions of attention were associated with reduced activity in sensory regions that processed behaviorally-relevant stimuli, suggesting that attention had failed to enhance the perceptual processing of those stimuli (Weissman et al., 2006).
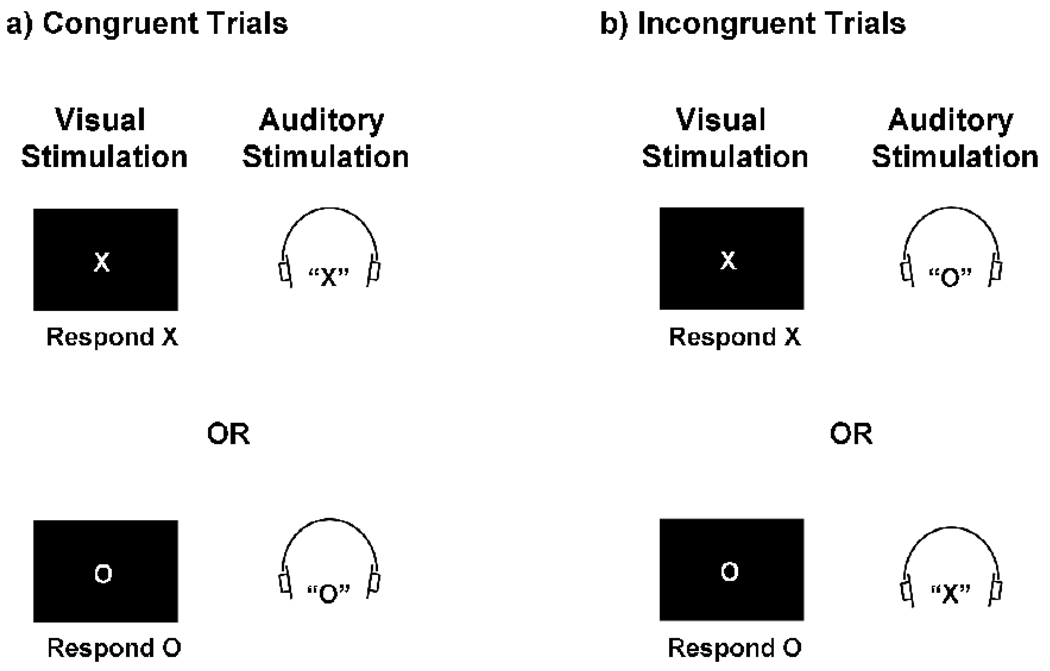
In the present study, we used fMRI to investigate whether reductions of attention permit greater processing of irrelevant stimuli in a multisensory audiovisual selective attention task (Fig 1). In each trial, participants identified a visual letter (X or O) while ignoring a simultaneously-presented auditory letter (X or O). The irrelevant auditory letter was equally likely to be congruent with the visual letter (i.e., both letters were Xs or Os; Fig 1a), in which case the relevant and the irrelevant letter were mapped to the same response, or incongruent (i.e., one letter was an X, the other was an O; Fig 1b), in which case the irrelevant letter was mapped to a different response than the relevant letter. Participants responded to the identity of the visual letter via a button press with either the left or the right thumb. Because low-level sensory aspects of visual and auditory stimuli are processed in mostly nonoverlapping regions of the cerebral cortex (Kandel et al., 2000), we were able to distinguish sensory activity for the irrelevant auditory letter from sensory activity for the relevant visual letter.
Figure 1.
Experimental stimuli. In each trial, participants identified a visual letter (X: 1.66° × 1.81° or O: 1.75° × 1.84°) while ignoring an irrelevant, simultaneously-presented auditory letter (X or O) that was mapped to either (A) the same response as the visual letter (congruent trials) or (B) a different response (incongruent trials).
We made two predictions. First, we predicted that reductions of attention (i.e., increases of RT to correctly identify the relevant visual stimulus) would be associated not only with reduced activity in sensory regions that processed the relevant visual stimuli (Weissman et al., 2006), but also with increased activity in sensory regions that processed the irrelevant auditory stimuli. Second, we predicted that increased sensory processing of the irrelevant auditory stimuli during reductions of attention would allow those stimuli to more strongly activate the responses to which they were associated, thereby leading to greater conflict-related activity (i.e., activity that is greater in incongruent than in congruent trials) in anterior cingulate regions that detect response conflict (MacDonald et al., 2000; Weissman et al., 2004).
Materials and Methods
Participants
Twenty-two healthy participants (12 male, age range: 19–29, all right-handed) took part in the study. All had normal or corrected-to-normal vision with no history of serious neurological trauma or disorders. Furthermore, none reported any problems with their hearing. Two participants were excluded due to excessive head motion leaving twenty participants in the final analyses (10 male, age range: 19–29 years, all right-handed). Participants gave informed consent prior to the experiment in accordance with the Duke University Medical Center human subjects institutional review board. Before the MR session, each participant briefly practiced the task. Participants were paid $20 per hour for their participation, which lasted approximately 2 hours.
Task
As discussed earlier, participants performed a cross-modal audiovisual selective attention task (Fig 1). In each 2.5-second trial, participants identified a visual letter (X or O) while ignoring a simultaneously-presented auditory letter (X or O), which was equally likely to be congruent with the visual letter (i.e., both letters were Xs or Os; Fig 1a) or incongruent (i.e., one letter was an X, the other was an O; Fig 1b). Participants identified the visual letter via a button press with either the left or the right thumb.
Within each of six runs, there were a total of 96 trials (48 congruent and 48 incongruent; stimulus duration, 350 ms). Congruent and incongruent trials were presented in a first-order counterbalanced order such that each trial type was preceded equally often by every trial type in the design. Moreover, the inter-trial-interval (ITI) ranged from 0 to 4 TRs following a roughly exponential distribution that favored short ITIs. Such jittering of the ITI increases the efficiency with which response estimates for distinct trial types are made in a multiple regression framework (Miezin et al., 2000).
Data Acquisition
We used a PC running Presentation software (Neurobehavioral Systems, Inc.) to present the animations to participants through an MR-compatible goggle system. Structural images for each participant were collected using a T1-weighted spin echo sequence on a 4-Tesla GE whole-body scanner (TR=500 ms, TE=14 ms, flip angle=90°, 24 contiguous 5-mm-thick slices – in-plane resolution =0.94 mm X 0.94 mm). Functional images, which measured the blood oxygenation level-dependent (BOLD) signal, were collected using a spiral imaging sequence (TR=1.25 s, TE=40 ms, flip angle=90°, 24 contiguous 5-mm-thick slices – in-plane resolution, 3.75 mm X 3.75 mm). Each participant completed six runs of the experimental task. During each run, 297 brain volumes were collected. The first six functional images of each run contained no trials and were discarded.
Data Analysis
SPM2 (Friston et al., 1995) was used to correct for asynchronous acquisition of functional images, to correct the functional images for head motion, to normalize the functional images to standard Montreal Neurological Institute (MNI) space, and to spatially smooth the functional images with a three-dimensional Gaussian filter (FWHM = 8 mm). The time series for each functional run was analyzed using customized software, which implements a version of the general linear model that makes no assumptions about the shape of the blood oxygen level dependent (BOLD) response. This linear regression approach, sometimes called the finite impulse response (FIR) model, estimates the average stimulus-locked fMRI response associated with each trial type and has been validated in numerous prior studies (Miezin et al., 2000; Shulman et al., 1999; Weissman et al., 2004). The linear model for each run included 18 regressors to model the average response for each trial type, beginning 7.5 seconds (6TRs) prior to stimulus presentation and ending 15 seconds (12 TRs) following stimulus presentation.
RT Regressors
We also included 18 additional regressors for each trial type to determine whether and how the magnitude of the fMRI response in each trial varied with RT. These parametric regressors, which we term RT regressors, modeled trial-to-trial variance in the average fMRI signal for a trial type that scaled linearly with trial-to-trial variance in correct RTs for that trial type. Relative RT for each trial was measured as the mean-subtracted RT score: RT for that trial (in seconds) minus the mean RT for correct trials of that trial type in that functional run. In the linear model, every time a trial type was presented and a correct response was made, we included not only 18 regressors of unit value (i.e., 1), which corresponded to each time point of the average fMRI response, but also 18 RT regressors with a value of the mean-subtracted RT for that trial. Each of the 18 RT regressors for a particular trial type modeled the effect of RT on fMRI activity at one time point of the relevant trial type’s average fMRI response.
Since the average of any distribution from which the mean is subtracted is zero, the regressors that coded the mean-subtracted RT for each trial type were orthogonal, on a time point–by–time point basis, to the regressors that coded for the average fMRI response for that trial type (i.e., the dot product of two corresponding columns in the linear model was zero). Thus, each set of 18 RT regressors modeled the degree to which an individual trial’s fMRI response scaled linearly with response time without changing the estimate of that trial type’s average fMRI response. Error trials (i.e., trials with an omitted or incorrect response, and trials in which a correct response time was more than three standard deviations from the mean response time for that trial type in that run) comprised 4.0% of the trials on average and were coded with separate regressors (without RT regressors). Therefore, only response times in correct trials contributed to the parameter estimates for the RT regressors. Finally, the linear model for each run included six regressors for head motion (i.e., SPM2 motion estimates) and two separate regressors for the linear trend and y-intercept terms.
An assumption of our parametric approach is that BOLD activity varies with RT in a linear fashion across the entire RT distribution. Of importance, we have verified this assumption in a recent study using a global/local selective attention task (Chee et al., 2008). In particular, we found that linear relationships between BOLD signal and RT contributed much more to trial-by-trial variations in BOLD signal than did more complex relationships (e.g., quadratic, cubic, etc.), which contributed very little or not at all to such variations. We also found that our parametric analyses produced results that matched up well with those that were yielded by comparing the slowest 10% of correct trials with the fastest 10% of correct trials. Thus, there is strong evidence to justify our assumption that the BOLD signal varies in a predominantly linear fashion with RT.
Units of Estimated FMRI Responses
The units of measurement for the parameter estimates corresponding to the RT regressors were initially MR units per second of increased RT. This is because the parametric values that comprised the RT regressors were the response times for individual trials (in units of seconds). The parameter estimates for each run were then converted to units of percent change from the session-specific fixation baseline (i.e., the y-intercept term for that run). Following this conversion, the parameter estimates for the RT regressors were in units of percent change in fMRI signal per second of increased RT. Finally, the converted parameter estimates for each regressor were averaged across runs within each participant.
Region of Interest (ROI) Analyses
ROIs in the visual cortex, the auditory cortex, and the anterior cingulate cortex were defined by performing a voxel-wise, repeated-measures one-way analysis of variance (ANOVA) across participants on the average stimulus-evoked response from 0–15 seconds post-stimulus onset, which we thresholded at F(11, 209) = 10.0, p < 2 × 10 −14 (5 contiguous voxels). For the auditory cortex, our analyses focused on two superior temporal regions of interest: the most highly activated region in the left hemisphere and the most highly activated region in the right hemisphere. For the visual cortex, our analyses focused on two middle occipital regions of interest, one in the left hemisphere and one in the right hemisphere, whose posterior locations likely included the retinotopic representations of our centrally-presented visual stimuli. For the anterior cingulate cortex, which is a midline structure, we selected the single most activated anterior cingulate region in the brain.
Each ROI was a 27-voxel cube centered on a local maximum in the overall trial-related activation map. Parameter estimates for individual trial types were averaged across all 27 voxels in each ROI. As noted in the Results section, in some analyses the parameter estimates for congruent and incongruent trials were averaged together in order to reveal how overall activity varied with RT. In others, however, the parameter estimates for congruent and incongruent trials were averaged separately, so that they could be directly compared. Peak activity was defined separately for each ROI to allow for regional differences in the timing and/or duration of the peak response. As in our prior work on momentary reductions of attention (Weissman et al., 2006), peak activity occurred between 3.75 and 5 seconds of stimulus onset, consistent with the known temporal characteristics of the hemodynamic response to a brief event (Buckner et al., 1996). All statistical tests were conducted using random effects analyses so that our conclusions would generalize to the population. P-values less than 0.05 were considered to be significant (all t-tests were one-tailed unless otherwise noted in the Results section).
Results
Overall behavior
As expected, performance was significantly faster in congruent (482 ms), than in incongruent trials (500 ms), F(1,19) = 20.52, p < 0.001. Mean error rates in congruent (3.8%) and incongruent (4.2%) trials did not significantly differ, F(1,19) < 1.
FMRI
We posit that attention varies linearly and continuously as a function of response time, with the very fastest RT likely correlating with the most focused attention and the very slowest RT likely correlating with the least focused attention. From this perspective, even the second-fastest RT in the distribution could be a small attentional reduction relative to the fastest RT. More generally, the longer an individual trial’s RT, the larger the reduction of attention it could reflect relative to the trial with the fastest RT. Thus, the neural correlates of momentary reductions of attention may be revealed by identifying changes in brain activation that are associated with parametric increases of RT.
To investigate the neural correlates of momentary reductions of attention, we used a parametric regression approach (see Materials and Methods). This approach estimates the slope of the best-fitting line that relates trial-by-trial variations in fMRI signal to trial-by-trial variations in RT. For example, a positive slope indicates that an increase of RT is usually associated with an increase of fMRI signal or, equivalently, that a decrease of RT is typically associated with a decrease of fMRI signal. One may describe a positive slope by stating that variations of attention (as reflected by variations of response time) are positively related to variations in fMRI signal. Alternatively, one may describe a positive slope by stating that reductions of attention (i.e., increases of RT) are associated with an increase of fMRI signal. Both types of description are accurate, but in the present study we favor the latter type as it emphasizes the changes in brain activation that are associated with increases of RT (i.e., reductions of attention).
Reductions of attention are linked to increased activity in sensory regions that process irrelevant stimuli, but to decreased activity in sensory regions that process relevant stimuli
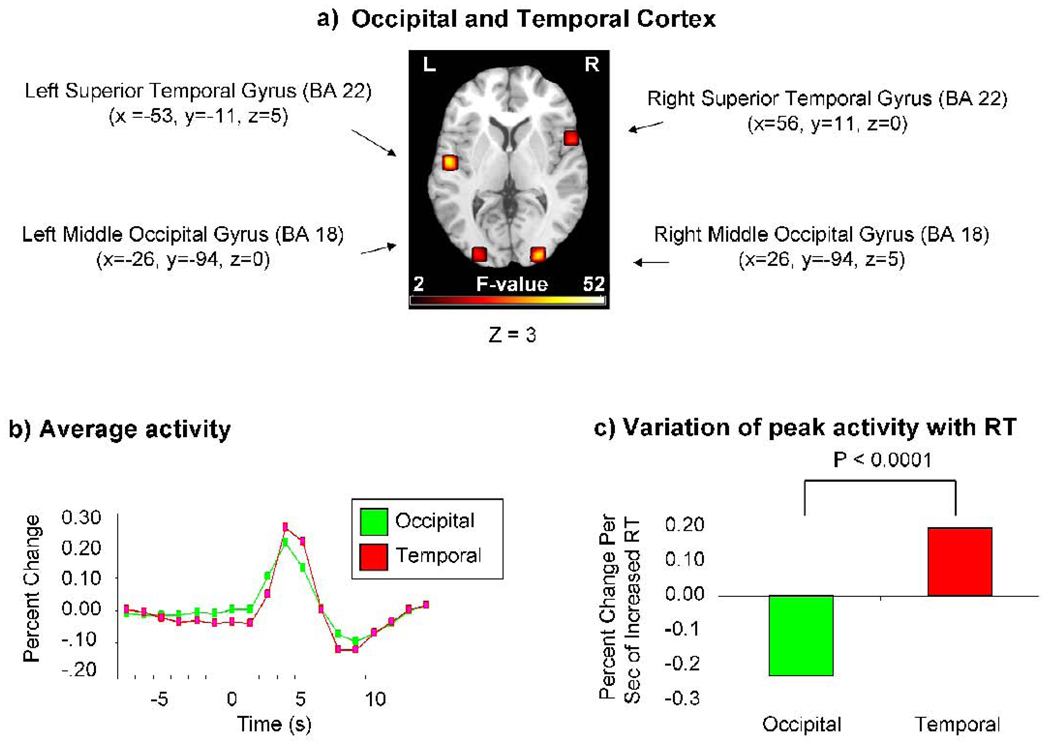
Our first prediction was that reductions of attention (i.e., increases of RT) would be associated with increased activity in sensory regions that processed the irrelevant auditory stimuli, but with reduced activity in sensory regions that processed the relevant visual stimuli. To test this hypothesis, we performed region of interest (ROI) analyses in the visual and auditory cortex (Fig 2a; see Materials and Methods). These analyses revealed robust responses to our multisensory audiovisual stimuli in bilateral superior temporal (auditory) and in bilateral middle occipital (visual) sensory cortex (Fig 2b).
Figure 2.
RT-related reductions of attention (i.e., increases of RT) were associated with increased activity in sensory regions that process irrelevant, but not relevant, stimuli. (A) Regions of interest in superior temporal (auditory) and middle occipital (visual) cortex in each cerebral hemisphere, which were identified in a contrast of all stimuli versus baseline. These regions are overlaid on a normalized anatomical brain, which is in Montreal Neurological Institute (MNI) space. (B) On average, the multisensory audiovisual stimuli evoked robust responses in both superior temporal (auditory) and occipital (visual) regions. (C) RT-related reductions of attention were associated with increased peak activity in superior temporal regions that processed the irrelevant auditory stimuli, but with reduced peak activity in occipital regions that processed the relevant visual stimuli. X, Y, and Z coordinates refer to MNI space. L, left; R, right. BA, Brodmann Area.
Most important, these ROI analyses (averaged across the left and right hemispheres) also revealed that increases of RT were associated with significantly larger increases of overall peak activity (i.e., activity averaged across congruent and incongruent trials at either 3.75 or 5 seconds after stimulus onset) in the auditory cortex (the irrelevant modality sensory cortex) than in the visual cortex (the relevant modality sensory cortex), t(19) = 5.29, p < 0.0001 (Fig 2c). The size of this effect did not differ significantly for congruent and incongruent trials, F(1,19) < 1. Further tests revealed that, consistent with predictions, increases of RT were associated with a significant increase of overall peak activity in the auditory cortex, t(19) = 2.41, p < 0.01, but with a significant reduction of overall peak activity in the visual cortex, t(19) = −3.29, p < 0.002. Neither of these effects differed significantly for congruent and incongruent trials (auditory cortex, t(19) = 1.33, p > 0.09; visual cortex, t(19) = 1.38, p > 0.09). Thus, as predicted, reductions of attention (i.e., increases of RT) were associated with increased activity in superior temporal regions that processed the irrelevant auditory stimuli, but with decreased activity in occipital regions that processed the relevant visual stimuli.
Reductions of attention are associated with heightened conflict-related activity in the anterior cingulate cortex
Our second prediction was that greater sensory processing of the irrelevant auditory stimuli during reductions of attention would lead to greater activity in regions of the anterior cingulate cortex that detect response conflict, and that this effect would be larger when an irrelevant auditory stimulus was mapped to a different response than the relevant visual stimulus (i.e., in incongruent trials) than when it was mapped to the same response (i.e., in congruent trials). As expected, ROI analyses in the anterior cingulate cortex (Fig 3a) revealed that peak activity (i.e., average activity at the fourth and fifth post-stimulus TRs, 3.75 – 5 seconds after stimulus onset) was significantly greater in incongruent than in congruent trials, t(19) = 1.97, p < 0.04 (Fig 3b). Increases of RT were not associated with significantly larger increases of peak activity in incongruent than in congruent trials (p > 0.15) when both peak time points were considered (i.e., averaging across the fourth and fifth post-stimulus TRs, 3.75 – 5 seconds after stimulus onset). In line with predictions, however, increases of RT were associated with significantly larger increases of peak activity in incongruent than in congruent trials at the later peak time point (i.e., the fifth post-stimulus TR, 5 seconds after stimulus onset), t(19) = 2.17, p < 0.025 (Fig. 3c). This result is consistent with our prior finding that increases of RT increase conflict-related activity (i.e., activity that is greater in incongruent than in congruent trials) maximally one time point after the peak response in the ACC (see Fig 4d, page 974, Weissman et al., 2006), and may indicate that hemodynamic signals are sometimes slightly delayed in trials with relatively long response times. In sum, consistent with our second prediction momentary reductions of attention were associated with increased conflict-related activity in the anterior cingulate cortex.
Figure 3.
RT-related reductions of attention (i.e., increases of RT) were associated with increased conflict-related activity in anterior cingulate regions that detect response conflict. (A) The most highly activated region of anterior cingulate cortex in a contrast of all stimuli versus baseline. This region is overlaid on a normalized anatomical brain in MNI space. (B) On average, incongruent stimuli evoked significantly greater peak activity than congruent stimuli (i.e., conflict-related activity) in the anterior cingulate cortex. (C) RT-related reductions of attention were associated with significantly larger increases of peak activity for incongruent stimuli than for congruent stimuli in the anterior cingulate cortex (i.e., increased conflict-related activity). X, Y, and Z coordinates refer to MNI space. L, left; R, right; BA, Brodmann Area.
Discussion
Current models posit that attention serves not only to enhance the processing of relevant stimuli, but also to limit the processing of irrelevant stimuli (Desimone, 1998; Handy et al., 2001; Hasher and Zacks, 1988; Lavie et al., 2004). In the present study, we used an audiovisual selective attention task to conduct a novel investigation of this view, which involved determining whether momentary reductions of attention (i.e., increases of RT) when correctly identifying relevant stimuli) are associated with failures to not only (1) enhance the processing of relevant stimuli (Weissman et al., 2006), but also (2) limit the processing of irrelevant stimuli. Our findings robustly confirmed each of these predictions.
Reductions of attention are linked to opposing changes of activity in sensory regions that process relevant and irrelevant stimuli
First, reductions of attention were associated not only with decreased activity in occipital regions that processed sensory aspects of the relevant visual stimuli (Weissman et al., 2006), but also with increased activity in superior temporal regions that processed sensory aspects of the irrelevant auditory stimuli. This double dissociation is highly consistent with models in which attention not only enhances the sensory processing of relevant stimuli (Handy et al., 2001; Hopfinger et al., 2000; Weissman et al., 2004), but also limits the sensory processing of irrelevant stimuli (Desimone, 1998; Hasher and Zacks, 1988; Lavie et al., 2004). Moreover, it suggests that failures to limit the sensory processing of irrelevant stimuli may indeed contribute to some of the adverse outcomes that are associated with reductions of attention in everyday life, such as work-related injuries (Czeisler et al., 2005) and traffic accidents (Beede and Kass, 2006).
Reductions of attention are associated with heightened conflict-related activity in the anterior cingulate cortex
Second, reductions of attention were associated with larger increases of anterior cingulate cortex activity when the irrelevant auditory stimulus was mapped to a different response than the relevant visual stimulus (i.e., in incongruent trials) than when it was mapped to the same response (i.e., in congruent trials). Anterior cingulate regions are thought to detect response conflict in incongruent trials (Kerns et al., 2004; MacDonald et al., 2000; Miller and Cohen, 2001). Thus, similar to our findings in a prior study (Weissman et al., 2006), the present results appear to indicate that reductions of attention are associated with heightened levels of response conflict in incongruent trials. Unlike our prior findings, however, the present results have shown that heightened conflict-related activity in the anterior cingulate cortex co-occurs with increased sensory processing of irrelevant stimuli. We speculate that failures to suppress the sensory processing of the irrelevant auditory stimuli during reductions of attention allowed those stimuli to more readily activate conflicting responses in incongruent trials. For example, reductions of attention may have allowed higher-quality perceptual representations of the irrelevant auditory stimuli to gain access to response selection mechanisms which, in incongruent trials, led to heightened levels of response conflict.
Possible sources of momentary reductions in attention
Our findings in the sensory cortices raise interesting questions about the sources of dynamic, trial-by-trial fluctuations of attention. One possibility is that such fluctuations stem from competitive interactions between a task-positive fronto-parietal network that supports externally-oriented attention and a so-called ‘default-mode’ network that supports internally-oriented attention (Raichle et al., 2001) including that which occurs during mind-wandering (Mason et al., 2007). Consistent with the existence of such competitive interactions, spontaneous activity in these two networks is negatively correlated (Fox et al., 2005). Moreover, participants who show stronger negative correlations between these networks exhibit less response time variability across trials than subjects who show weaker negative correlations (Kelly et al., 2008). Such findings suggest that momentary reductions of attention (i.e., increases in RT) may stem, at least in some cases, from transient failures of the task-positive network to inhibit mind-wandering that is supported by the default-mode network, Consistent with this possibility, mind wandering is associated with increases of RT, our operational definition of momentary reductions of attention, when study participants are aware that they are engaging in off-task processing (Smallwood et al., 2007). Future studies investigating how functional connectivity varies with response time on a trial-by-trial basis may provide a complementary method for determining whether dynamic fluctuations of attention stem from competitive interactions between distinct brain networks.
Broader relationships between the present findings and various models of attention and cognitive control
The present findings in the sensory cortices may also inform studies of the perceptual load hypothesis: the view that perceptual attentional resources that go unused by relevant stimuli are automatically allocated to irrelevant stimuli (Lavie and Tsal, 1994). Prior findings from neuroimaging suggest that the perceptual load hypothesis may not hold when relevant and irrelevant stimuli are presented in different sensory modalities (Rees et al., 2001), consistent with models in which each sensory modality has access to a somewhat distinct pool of attentional resources (Navon and Gopher, 1979; Talsma et al., 2006). However, we found that, during attentional reductions, paying less attention to the relevant visual letter was associated with increasing attention to the irrelevant auditory letter, due possibly to a failure of prefrontal regions to suppress the processing of the irrelevant distracter stimulus (Gazzeley et al., 2007). Although we did not explicitly manipulate perceptual load, this result suggests that perceptual processing in different sensory modalities may rely, at least to some extent, on a common pool of attentional resources. Given that the present findings appear to conflict on this issue with some prior results (Rees et al., 2001), future studies should be aimed at better understanding the conditions under which the perceptual load hypothesis applies in cross-modal paradigms.
Our findings in the anterior cingulate cortex also have important implications for current models of attention and cognitive control. In particular, they are some of the first to reveal a role for anterior cingulate regions in detecting response conflict that cannot be accounted for by differences in task difficulty that usually differentiate congruent and incongruent trials. In most studies of response conflict, incongruent trials are associated with longer response times than congruent trials (Banich et al., 2000; Botvinick et al., 1999; Carter et al., 2000; MacDonald et al., 2000). Therefore, it is not easy to determine whether greater anterior cingulate activity in incongruent than in congruent trials stems from increased response conflict or, alternatively, from increased task difficulty. In the present study, however, our analyses revealed that a unit increase of task difficulty, as reflected by a unit increase of response slowing (say, for example, 100 ms), was associated with a larger increase of anterior cingulate activity in incongruent than in congruent trials. Thus, the present findings, along with other recent data (Weissman et al., 2006), indicate a role for the anterior cingulate cortex in detecting response conflict that cannot be accounted for by differences in overall task difficulty that typically distinguish congruent and incongruent trials.
Limitations of the present work
Our interpretations of the present data are well-motivated by current models of selective attention, but it is important to consider whether the effects that we have observed truly reflect momentary reductions, or variations, in attention. For example, we did not explicitly manipulate the sensory modality (visual, auditory) that was relevant in the present study. Thus, it is possible that reductions of attention are somehow linked to decreased activity in the visual cortex and increased activity in the auditory cortex regardless of which sensory modality is attended. While this possibility seems highly unlikely, additional research will be necessary to definitively rule it out. As another example, response time variability across trials, our index of attentional reductions, may reflect other processes such as repetition priming (Buckner et al., 1998) or strategic changes in response strategy (e.g., emphasizing speed versus accuracy) (Kerns et al., 2004). Although we cannot rule out the possibility that such factors influenced response time in the present study, they cannot easily explain the brain-behavior relationships that we observed. First, repetition priming is usually associated with positive relationships between activity and RT (i.e., less activity being associated with faster response time) in sensory regions that process relevant stimuli (Buckner et al., 1998), rather than with the negative relationships between activity and RT (i.e., less activity being associated with slower response time) that we observed. Second, changes in response strategy (e.g., emphasizing speed versus accuracy) do not appear to modulate activity in the sensory cortices (Van Veen et al., in press). Thus, although many variables influence response time, the brain-behavior relationships that we have observed appear to be most consistent with reductions of attention.
Summary
In conclusion, our findings provide a system-wide perspective on how momentary reductions of attention affect the processing of irrelevant stimuli. More broadly, they illustrate that novel brain-cognition relationships can be revealed by investigating how brain activity varies with response time on a trial-by-trial basis. New studies that make use of similar approaches may therefore deepen our understanding of how the functioning of the brain gives rise to cognitive processes and, in turn, behavior.
Acknowledgements
This research was supported by NIH grants to D.H.W (1RO3DA021345-01) and to M.G.W. (R01-NS051048 and NSF-BCS-05-24031). We thank William Gehring, Patricia Reuter-Lorenz, Jerome Prado, and Joshua Carp for useful discussions and Kristina Visscher for useful discussions and helpful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, Magin R. FMRI studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. Journal of Cognitive Neuroscience. 2000;12:988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- Beede KE, Kass SJ. Engrossed in conversation: The impact of cell phones on simulated driving performance. Accident Analysis & Prevention. 2006;38:415–421. doi: 10.1016/j.aap.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Berardi AM, Parasuraman R, Haxby JV. Sustained attention in mild Alzheimer's disease. Developmental Neuropsychology. 2005;28:507–537. doi: 10.1207/s15326942dn2801_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Bandettini PA, O'Craven KM, Savoy RL, Petersen SE, Raichle ME, Rosen BR. Detection of cortical activation during averaged single trials of a cognitive task using functional magnetic resonance imaging. Proceedings of the National Academy of Sciences. 1996;93:14878–14883. doi: 10.1073/pnas.93.25.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino AD, Hyde C, Walters JR. Varieties of Attention-Deficit/Hyperactivity Disorder-Related Intra-Individual Variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Tan JC, Zheng H, Parimal S, Weissman DH, Zagorodnov V, Dinges DF. Lapsing during sleep deprivation is associated with distributed changes in brain activation. The Journal of Neuroscience. 2008;28:5519–5528. doi: 10.1523/JNEUROSCI.0733-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Walsh JK, Roth T, Hughes RJ, Wright KP, Kingsbury L, Arora S, Schwartz JRL, Niebler GE, Dinges DF, Black JE, Bogan RK, Bonnet MH, Carskadon MA, Cook JS, Corser BC, Erman MK, Feldman NT, Ferguson JM, Furman Y, Hardy SC, Harsh JR, Hirshkowitz M, Hull SG, Mahajan VK, Pegram GV, Pinto J, Richardson GS, Rosenberg R, Rosenthal MH, Schmidt MH, Schweitzer PK, Seiden D, Wagner DR, Wells CC, Wyatt JK, Zammit GK. Modafinil for excessive sleepiness associated with shift-work sleep disorder. New England Journal of Medicine. 2005;353:476–486. doi: 10.1056/NEJMoa041292. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gazzeley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, D'Esposito M. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cerebral Cortex. 2007;17:125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy TC, Soltani M, Mangun GR. Perceptual load and visuocortical processing: ERP evidence of sensory-level selection. Psychological Science. 2001;12 doi: 10.1111/1467-9280.00338. 213-128. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawl effects. Psychopharmacology (Berl) 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nature Neuroscience. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of Neural Science. Fourth ed. McGraw-Hill; 2000. [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functiional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AWr, RY C, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, deFockert JW, Viding E. Load Theory of Selective Attention and Cognitive Control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Perception & Psychophysics. 1994;56:183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;19:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Navon D, Gopher D. On the economy of the human processing system. Psychological Review. 1979;86:214–255. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G, Frith C, Lavie N. Processing of irrelevant visual motion during performance of an auditory attention task. Neuropsychologia. 2001;39:937–949. doi: 10.1016/s0028-3932(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Reimer B, D'Ambrosia LA, Gilbert J, Coughlin JF, Biederman J, Surman C, Fried R, Aleardi M. Behavior differences in drivers with attention deficit hyperactivity disorder: The driving behavior questionnaire. Accident Analysis & Prevention. 2005;37:996–1004. doi: 10.1016/j.aap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, Corbetta M. Areas involved in encoding and applying directional expectations to moving objects. The Journal of Neuroscience. 1999;19:9480–9496. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, McSpadden M, Schooler JW. The lights are on, but no one's home: Meta-awareness and the decoupling of attention when the mind wanders. Psychonomic Bulletin & Review. 2007;14:527–533. doi: 10.3758/bf03194102. [DOI] [PubMed] [Google Scholar]
- Talsma D, Doty TJ, Strowd R, Woldorff MG. Attentional capacity for processing concurrent stimuli is larger across sensory modalities than within a modality. Psychophysiology. 2006;43:541–549. doi: 10.1111/j.1469-8986.2006.00452.x. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. The Journal of Neuroscience. 2004;24:10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]