Abstract
AIM: To evaluate the clinical role of 18F-fluorodeo-xyglucose positron emission and computed tomography (18F-FDG PET/CT) in detection of gastric cancer recurrence after initial surgical resection.
METHODS: In the period from January 2007 to May 2008, 23 patients who had previous surgical resection of histopathologically diagnosed gastric cancer underwent a total of 25 18F-FDG PET/CT scans as follow-up visits in our center. The standard of reference for tumor recurrence consisted of histopathologic confirmation or clinical follow-up information for at least 5 mo after PET/CT examinations.
RESULTS: PET/CT was positive in 14 patients (61%) and negative in 9 (39%). When correlated with final diagnosis, which was confirmed by histopathologic evidence of tumor recurrence in 8 of the 23 patients (35%) and by clinical follow-up in 15 (65%), PET/CT was true positive in 12 patients, false positive in 2, true negative in 8 and false negative in 2. Overall, the accuracy of PET/CT was 82.6%, the negative predictive value (NPV) was 77.7%, and the positive predictive value (PPV) was 85.7%. The 2 false positive PET/CT findings were actually chronic inflammatory tissue lesions. For the two patients with false negative PET/CT, the final diagnosis was recurrence of mucinous adenocarcinoma in the anastomosis in one patient and abdominal wall metastasis in the other. Importantly, PET/CT revealed true-positive findings in 11 (47.8%) patients who had negative or no definite findings by CT. PET/CT revealed extra-abdominal metastases in 7 patients and additional esophageal carcinoma in one patient. Clinical treatment decisions were changed in 7 (30.4%) patients after introducing PET/CT into their conventional post-operative follow-up program.
CONCLUSION: Whole body 18F-FDG PET/CT was highly effective in discriminating true recurrence in post-operative patients with gastric cancer and had important impacts on clinical decisions in a considerable portion of patients.
Keywords: 18F-fluorodeoxyglucose, Positron emission tomography/computed tomography, Gastric cancer, Follow-up, Recurrence
INTRODUCTION
Gastric cancer is the second most common cause of cancer death worldwide[1]. Within the Asian region, high incidence areas include Japan, China and Korea. It is a major health burden in the Asia-Pacific region[2]. Complete surgical resection of gastric cancer is considered potentially curative, but its long-term survival is frequently reported as poor. In fact, despite successful surgery, the five-year survival rate is approximately 35%; and even with adjuvant chemoradiotherapy in selected patients, the survival rate is 40%[3]. After curative surgery, about 80% of the patients die within a short period of time from locoregional recurrence (87%) and/or distant metastasis (30%)[4].
Positron emission tomography (PET) and, particularly, positron emission tomography/computed tomography (PET/CT) are widely accepted imaging methods in the management of a variety of cancers[5]. Variable uptake of 18F-fluorodeoxyglucose (18F-FDG) has been noticed in PET studies of gastric carcinoma patients, with low uptake occurring especially in some particular histological subtypes and early carcinomas. Larger or more advanced tumors with nodal involvement have a higher detection rate by PET. In the initial staging of gastric cancer, preoperative PET was useful for the detection of nodal involvement and distant metastasis[6,7]. The role of PET/CT in patients with gastric cancer after operation however, is not clear. In this study, we aimed to analyze the value of 18F-FDG PET/CT scans in the follow up of post-operative patients with gastric cancer.
MATERIALS AND METHODS
Patients
A retrospective review of our electronic database of 23 post-operative patients with gastric cancer (15 males and 8 female; age range: 27-84 years; mean age: 55.4 years) imaged by 18F-FDG PET/CT between January 2007 and May 2008 was performed to select and analyze PET/CT scan findings for patients with or without clinically and/or radiologically suspicious findings for tumor recurrence. Only the patients with correlative histopathological data were included. The standard of reference for tumor recurrence consisted of histopathologic confirmation (n = 8) or clinical follow-up information (n = 15) for at least 5 mo after PET/CT.
18FDG PET/CT technique
The patients were asked to fast for at least 4 h before undergoing 18F-FDG PET/CT. Their blood glucose level should be within the normal range (70-120 mg/dL) prior to intravenous injection of 18F-FDG. The patients received an intravenous injection of 370-666 MBq (10-18 mCi) of 18F-FDG. Data acquisition by an integrated PET/CT system (Discovery STE; GE Medical Systems, Milwaukee, WI, USA) was performed within 60 min after injection. The data acquisition procedure was as follows: CT scanning was first performed, from the head to the pelvic floor, with 110 kV, 110 mA, a tube rotation time of 0.5 s, and a 3.3-mm section thickness which was matched to the PET section thickness. Immediately after CT scanning, a PET emission scan that covered the identical transverse field of view was obtained. Acquisition time was 3 min per table position. PET image data sets were reconstructed iteratively by applying the CT data for attenuation correction, and coregistered images were displayed on a workstation.
Definitive diagnoses of positive and negative findings
Reviewer 1 and Reviewer 2, who were aware of other clinical or imaging information, read the 18F-FDG PET/CT images on a high-resolution computer screen. The reviewers reached a consensus in cases of discrepancy. Reviewer 1 had 20 years of experience in both nuclear medicine and radiology, and Reviewer 2 had 5 years of experience in both nuclear medicine and radiology. 18F-FDG PET/CT scan was considered positive or suspicious when abnormal non-physiologic metabolic activity was identified. Focal hypermetabolic activity within the liver which was greater than adjacent normal liver tissue was considered abnormal. Diffuse mild activity in the intestinal tract was considered normal physiologic uptake. Quantification of tumor metabolic activity was obtained using the Standardized Uptake Value (SUV) normalized to body weight. Mean ± SD of maximum-pixel SUV (SUVmax) of the lesions were calculated.
RESULTS
A total of 25 18F-FDG PET/CT studies in 23 patients after gastric cancer were reviewed. 18F-FDG PET/CT was ordered in 12 patients due to suspected disease recurrence on conventional image examinations, on history and physical exam. The remaining 11 patients underwent 18F-FDG PET/CT as part of routine post-operative follow-up. The characteristics of the patients are summarized in Table 1. At the time of recurrent gastric cancer being suspected, the mean patient age was 57 years with a tendency to male gender distribution (65%). All the patients had undergone surgical resection, with chemotherapy prior to or following the resection. Surgical exploration was undertaken within 1 mo after 18F-FDG PET/CT scan in 5 patients.
Table 1.
Patient characteristics
| Clinical characteristics | Data |
| Mean age (yr) | 55.4 |
| Gender | |
| Male | 15 |
| Female | 8 |
| Mean time after operation to PET/CT exam | 2 mo-10 yr; mean 25 mo |
| Mean follow up time after PET/CT exam | 5-18 mo; mean 9 mo |
18F-FDG PET/CT scan was considered negative in 9 patients (39%) and positive in 14 (61%, Table 2). Final diagnoses were confirmed by histopathologic evidence of tumor recurrence in 8 of the 23 patients (Figure 1) and by clinical follow-up in 15 patients (Figure 2). Overall, the accuracy of 18F-FDG PET/CT diagnosis was 82.6%, negative predictive value (NPV) was 77.7%, and positive predictive value (PPV) was 85.7%. Of the 14 positive 18F-FDG PET/CT scans, 12 were true positive and two were false positive. For the two patients with false positive 18F-FDG PET/CT scans, the final diagnosis was anastomosis inflammation with high metabolism. Seven patients were found to have true negative 18F-FDG PET/CT scans while two were false negative. For the patients with false negative 18F-FDG PET/CT scans, the final diagnosis was recurrence of mucinous adenocarcinoma in the anastomosis in one patient and metastasis in the abdominal wall of another patient (Figure 3).
Table 2.
PET/CT findings in 14 patients with positive PET/CT scans
| No. | PET/CT findings | SUV max | Pathology of resected GC | Interval time |
| 1 | Retroperitoneal lymph nodes | 6.0 | MPD tubular adeno ca and mucinous adeno Ca | 12 mo |
| 2 | Retroperitoneal lymph nodes | 5.2 | PD adeno ca and signet-ring cell Ca | 3 mo |
| Supraclavicular lymph nodes | 6.6 | |||
| Osseous metastasis | 3.8 | |||
| 3 | Esophageal carcinoma | 14.2 | MPD adeno Ca | 10 yr |
| 4 | Anastomosis recurrence with pancreas involvement | 5.6 | PD adeno Ca | 5 mo |
| 5 | Osseous metastasis | 2.5 | MPD adeno Ca | 25 mo |
| 6 | Liver metastasis | 7.2 | MPD signet-ring cell Ca | 15 mo |
| 7 | Greater omentum lymph nodes | 1.3 | PD signet-ring cell Ca | 8 mo |
| 8 | Abdominal wall metastasis | 2.5 | PD signet-ring cell Ca | 24 mo |
| Ovarian metastasis | ||||
| 9 | Liver metastasis | 5.3 | PD adenosquamous Ca | 3 mo |
| Intraperitoneal lymph nodes | 17.6 | |||
| 10 | Abdominal wall metastasis | 3.2 | MPD adeno Ca | 24 mo |
| 11 | Intraperitoneal lymph nodes | 7.7 | MPD adeno Ca | 36 mo |
| 12 | Brain metastasis | 8.1 | PD adeno Ca | 6 mo |
| 13 | Osseous metastasis | 12.6 | MPD adeno Ca | 20 mo |
| Retroperitoneal lymph nodes | 7.0 | |||
| 14 | Anastomosis recurrence | 4.3 | MPD adeno Ca | 5 mo |
MPD: Moderately to poorly differentiated; PD: Poorly differentiated; GC: Gastric cancer; Interval time: Time period from operation to PET/CT scan; Ca: Carcinoma.
Figure 1.
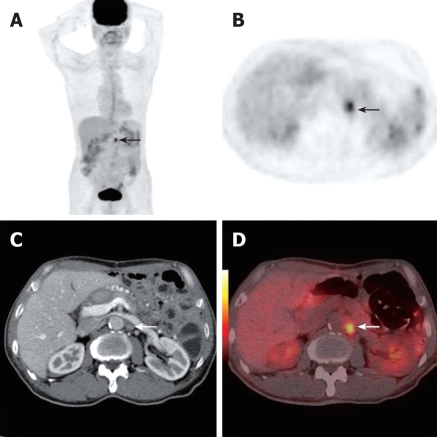
A 56-year-men who had had gastric cancer resection 3 years previously underwent PET/CT because of suspected disease recurrence upon barium swallow examination (white arrows, A), Axial contrast CT demonstrated local thickened stomach wall at anastomosis (white arrows, C). Axial PET (black arrows, B) and PET/CT fusion images (white arrow, D) showed focal hypermetabolic activity in the remnant stomach, which was later verified as malignant by histopathology.
Figure 2.
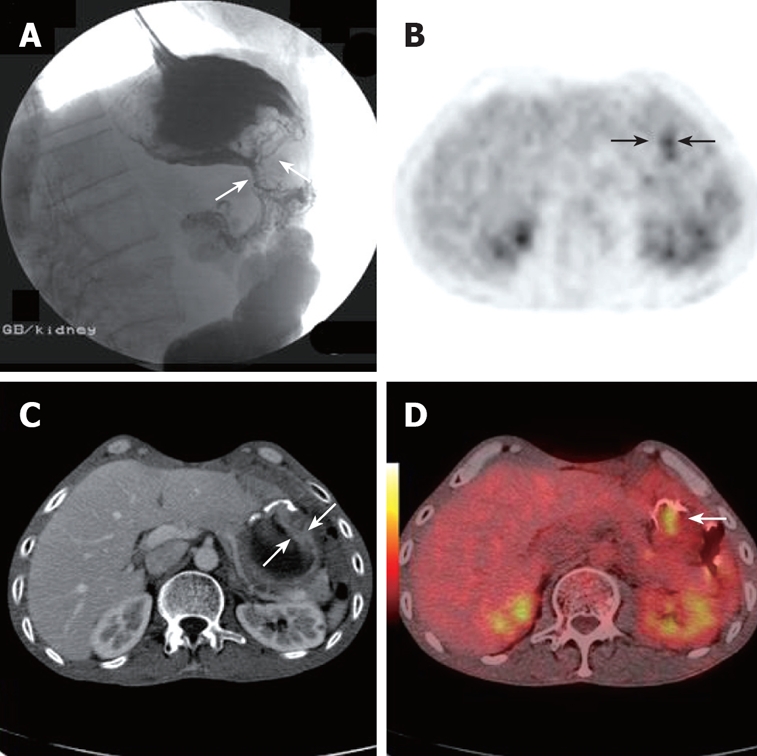
A 75-year-old asymptomatic man who had gastric cancer resection 1 year previously underwent PET/CT as part of routine post-operative surveillance. Whole body PET projection image and axial PET image showed focal hypermetabolic activity in the abdomen (black arrow, A and B). Axial contrast CT detected a small lymph node at the same position (white arrow, C). PET/CT fusion images showed a focus of highly metabolic metastasis in retroperitoneal lymph node (white arrow, D).This was later verified by follow up. The case illustrated the value of early discovery by PET/CT in asymptomatic patients after surgery.
Figure 3.
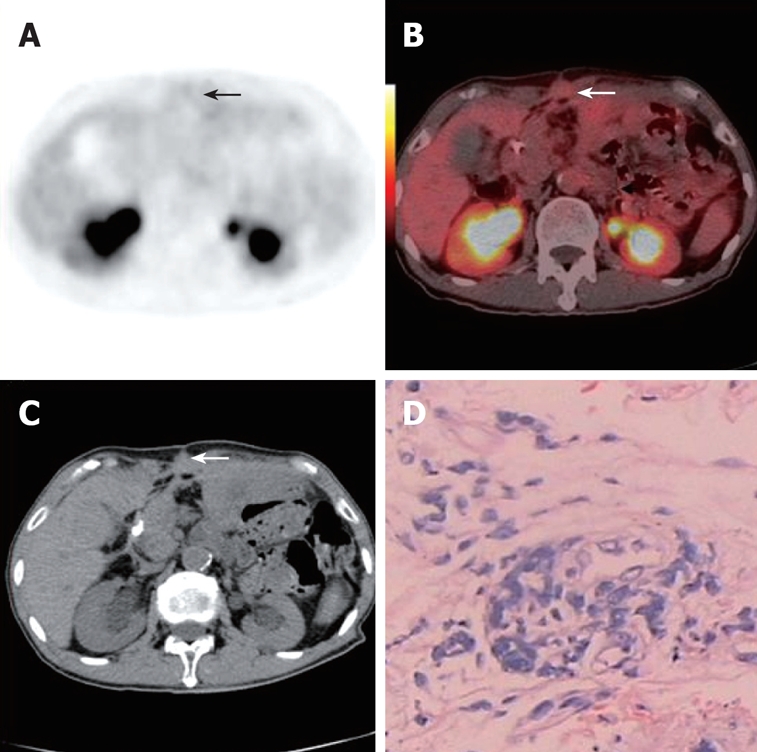
A 71-year-old asymptomatic man underwent PET/CT as part of routine post-operative surveillance after gastric cancer resection was performed 2.5 years previously. Axial PET and PET/CT fusion images (arrows, A and B) showed no focal hypermetabolic activity in the abdominal wall. Axial contrast CT (white arrow, C) demonstrated local thickness in the abdominal wall. This was later verified as malignant by histopathological assessment of a CT guided core tissue biopsy (D).
Importantly, tumor recurrence was revealed in 27.2% (3/11) of the patients who underwent PET/CT as part of routine post-operative surveillance; these patients were asymptomatic, with no evidence of disease. 18F-FDG PET/CT revealed true-positive findings in 11 patients who had either negative or no definite CT findings. 18F-FDG PET/CT demonstrated extra-abdominal metastases in 7 patients (Figure 4) and new esophageal carcinoma in one patient. Clinical treatment decisions were changed in 7 (30.4%) patients after introducing 18F-FDG PET/CT into their conventional post-operative follow up program.
Figure 4.
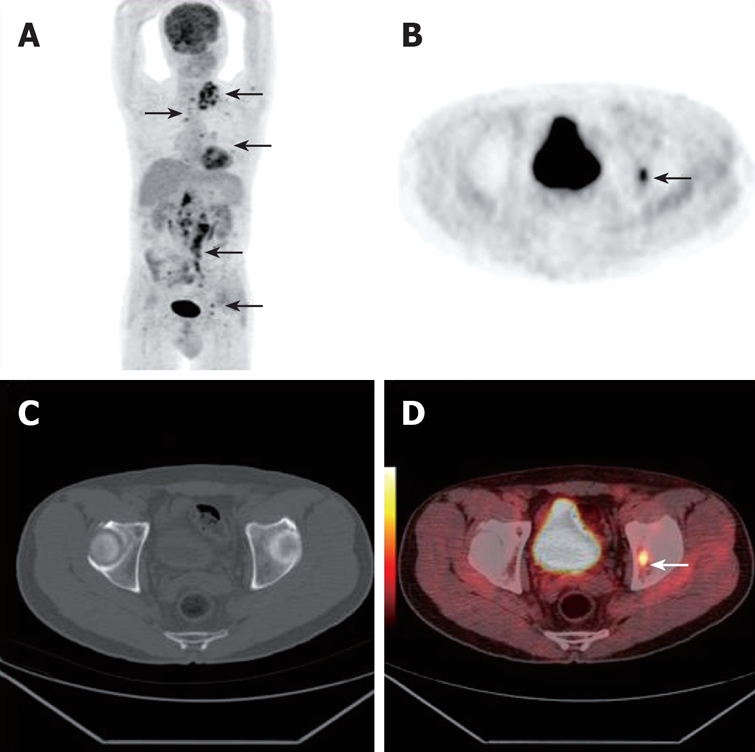
A 67-year-old man underwent PET/CT due to clinically suspected disease recurrence in supraclavicular lymph nodes after his gastric cancer resection which was performed 2 mo previously. Whole body PET projection image showed retroperitoneal and supraclavicular metastatic lymph nodes and bone metastasis. (arrows, A, B and D). Axial CT failed to reveal early bone metastasis (C).
In our study, 11 (84.5%) cases of recurrence after curative resection occurred within 25 mo and 2 (15.5%) occurred 3 years after the resection. A high percentage of first failures presented as regional nodal metastases and distant metastases. Only 4 (17.3%) patients with recurrence had increased serum tumor markers.
DISCUSSION
At the time of diagnosis, gastric cancer is found to be localized and surgically resectable in approximately 50% of patients; however, regional nodal metastases or direct invasion into surrounding organs or structures are frequently encountered and preclude cure by surgery alone in many patients[8,9]. Analyses of patterns of relapse after complete surgical resection demonstrate that subsequent relapse of cancer is common in the tumor bed and nodal regions as well as all over the body[10,11]. The optimal method for assessing early recurrence in patients with gastric cancer is unknown[12]. Conventional imaging (ultrasonography, CT and magnetic resonance imaging) has represented the standard for staging and restaging of gastric cancer[13,14]. Conventional imaging is noninvasive and is the least costly of the available methods, although it has limited value in differentiating post-surgical changes from local tumor recurrence. Therefore, these techniques have limitations in terms of accurate assessment of re-staging results[15,16].
Modern cancer care is critically dependent on imaging technologies, which are used to detect early tumors and guide their therapy or surgery[17,18]. Molecular imaging technologies provide information about the functional or metabolic characteristics of malignancies, tumor stage and therapeutical response, and tumor recurrence; whereas conventional imaging technologies predominantly assess anatomical or morphologic features of the tumor including its size, density, shape, etc[19,20]. There are now various indications for 18F-FDG PET and PET/CT imaging in gastrointestinal malignancies [21]. Detection and staging of recurrent colorectal cancer with 18F-FDG PET has largely been documented[22,23]. However, there is a paucity of data concerning its role in patients with suspected gastric cancer recurrence. In our study, 18F-FDG PET/CT could be applied reliably in such patients to allow for earlier detection of nodal involvement and distant metastasis in 3 asymptomatic patients. Because most metastatic gastric cancers are inoperable, they are usually treated with combination chemotherapy. Early detection and treatment of tumor recurrence may be the only hope to improve long-term survival and made the early management of recurrent gastric cancer possible in three patients in our study.
18F-FDG PET/CT is highly effective in discri-minating true recurrence in patients with suspected recurrence, highly sensitive for detecting recurrence in asymptomatic patients, and has important impacts on clinical decisions in a considerable portion of patients[24]. 18FDG-PET is also of benefit in assessing response to neoadjuvant treatment[25]. 18F-FDG PET/CT has been demonstrated as a useful molecular imaging modality to evaluate the biological response of advanced hepatic metastasis and peritoneal carcinomatosis to Cetuximab plus Endostar in patients after remnant gastric cancer resection[26]. In our study, there was a 55-year-old male who developed advanced hepatic metastasis and peritoneal carcinomatosis after resection of remnant gastric cancer resection 3 mo previously. The patient received a treatment only by epidermal growth factor (EGF) receptor antibody (Cetuximab) plus recombinant human endostatin (Endostar). Anti-tumor activity was assessed by 18F-FDG PET/CT at baseline and every 4 wk thereafter.
18F-FDG PET can reveal recurrent sites in the intraperitoneum, liver, lungs, bones and retroperitoneal lymph nodes[27,28]. However, the minimum tumor size detectable by PET depended on the sites of recurrence. In the two false-negative cases of intraperitoneal tumors by suggested by CT images, 18F-FDG PET/CT was able to detect a solitary small intraperitoneal mass, which was very difficult to reveal with CT alone[29]. 18F-FDG PET/CT imaging was also able to detect normally sized metastatic lymph nodes in 4 patients with retroperitoneal metastasis. Our results demonstrated that the application of PET/CT imaging was useful for early detection of recurrent sites, especially for decision making in determining treatment strategy for patients with recurrent gastric cancer. Positive PET/CT findings did not affect the prognosis in 7 of the 14 recurrent patients; however, the remaining 7 patients consequently underwent combination therapy consisting of surgery and chemotherapy and survived for more than 10 mo after the positive 18F-FDG PETCT results.
Diagnostic contrast-enhanced multiphase CT as part of the combined 18F-FDG PET/CT protocol has the potential to provide considerable augmented value in specific clinical conditions with resultant change of management in a substantial proportion of patients[30]. The greatest benefit of its diagnostic CT is in category localization of pathological FDG uptake and precise tumor delineation, making changes in 18F-FDG PET/CT interpretation for some patients[31]. The reported increase in sensitivity of PET/CT over CT has been attributed to the ability of 18F-FDG PET to detect metabolic abnormalities that precede the morphologic changes seen by CT. The global (from skull base to proximal thighs) nature of the 18F-FDG PET/CT study also contributes to the increased sensitivity through detection of distant metastatic lesions. In our study, 18F-FDG PET/CT demonstrated extra-abdominal metastases (metastases in lymph nodes, the abdominal wall, bones, and the brain) in 7 patients and in one gastric cancer patient who had the comorbidity of a new esophageal carcinoma, which was confirmed by post-operative pathology.
Our study had several limitations. The first was our small sample size which may have limited the robustness of our study in terms of statistics. The second was the retrospective nature of our study. Because of this nature, we were unable to obtain baseline clinical and laboratory data in some of the patients, except for one patient who accepted three PET/CT scans during his treatment. Additionally, there are some disadvantages associated with PET/CT imaging. For example, small, early-stage tumors may go undetected because partial-volume effects result in a falsely low measurement of true 18F-FDG activity, as was indicated in our previous report[32]. Another drawback of PET/CT is that 18F-FDG frequently accumulates in areas of inflammation[33,34]. In our study, two patients had false positive PET/CT scan results, and the final diagnosis was anastomosis inflammation with high metabolism. Low 18F-FDG uptake occurred especially in some particular histological subtypes or at special locations. For the two patients with false negative PET/CT findings, the final diagnosis was recurrence of mucinous adenocarcinoma in the anastomosis in one patient and abdominal wall metastasis in the other.
In conclusion, whole body 18F-FDG PET/CT was highly effective in discriminating true recurrence in gastric cancer patients with suspected recurrence and highly sensitive in detecting recurrence in asymptomatic patients. This had important impacts on clinical decisions in a considerable portion of these patients. Any positive finding in post-operative patients with gastric cancer that could lead to a clinically significant change in patient management should be confirmed by subsequent histopathologic examination because of the risk of false-positive results.
COMMENTS
Background
After curative surgery, about 80% of patients die within a short period of time from locoregional recurrence and/or distant metastasis. The optimal method for assessing early recurrence in patients with gastric cancer is not clear. This study aimed to analyze the value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in the follow up of post-operative patients with gastric cancer.
Research frontiers
18F-FDG PET and, particularly, 18F-FDG PET/CT are widely accepted imaging methods in the management of a wide variety of cancers. However, the role of PET/CT in patients with gastric cancer after surgery is not clear.
Innovations and breakthroughs
Whole body 18F-FDG PET/CT was highly effective in discriminating true recurrence in gastric cancer patients with suspected recurrence and highly sensitive in detecting recurrence in asymptomatic patients. This had important impacts on clinical decisions in a considerable portion of these patients.
Applications
The findings could be helpful for early discrimination of true recurrence in gastric cancer patients with suspected recurrence and highly sensitive at detecting recurrence in asymptomatic patients, especially when recurrence can not be accurately diagnosed by conventional imaging.
Peer review
In this study, the authors reported the clinical significance of 18F-FDG PET/CT for early discrimination of true recurrence in gastric cancer patients. This manuscript arouses interest for readers and provides an important clue to assess whole body, early recurrence in gastric cancer patients. The paper is scientific and innovative and is for rapid communication part of journal.
Peer reviewer: Dusan M Jovanovic, Professor, Institute of Oncology, Institutski Put 4, Sremska Kamenica 21204, Serbia
S- Editor Li DL L- Editor Lalor PF E- Editor Ma WH
References
- 1.Khalighinejad N, Hariri H, Behnamfar O, Yousefi A, Momeni A. Adenoviral gene therapy in gastric cancer: a review. World J Gastroenterol. 2008;14:180–184. doi: 10.3748/wjg.14.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–365. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 5.Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, Bockisch A, Debatin JF, Freudenberg LS. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357–365. [PubMed] [Google Scholar]
- 6.Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597–604. doi: 10.1007/BF02984657. [DOI] [PubMed] [Google Scholar]
- 7.Mukai K, Ishida Y, Okajima K, Isozaki H, Morimoto T, Nishiyama S. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–196. doi: 10.1007/s10120-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 8.Roukos DH. Current advances and changes in treatment strategy may improve survival and quality of life in patients with potentially curable gastric cancer. Ann Surg Oncol. 1999;6:46–56. doi: 10.1007/s10434-999-0046-z. [DOI] [PubMed] [Google Scholar]
- 9.Sasako M, Saka M, Fukagawa T, Katai H, Sano T. Surgical treatment of advanced gastric cancer: Japanese perspective. Dig Surg. 2007;24:101–107. doi: 10.1159/000101896. [DOI] [PubMed] [Google Scholar]
- 10.Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Prognostic benefit of extended radical lymphadenectomy for patients with gastric cancer. Anticancer Res. 2000;20:1285–1289. [PubMed] [Google Scholar]
- 11.Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, Joypaul V, Sydes M, Fayers P. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen EH, Tuttle TM. Preoperative staging and postoperative surveillance for gastric cancer. Surg Oncol Clin N Am. 2007;16:329–342. doi: 10.1016/j.soc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Chen CY, Hsu JS, Wu DC, Kang WY, Hsieh JS, Jaw TS, Wu MT, Liu GC. Gastric cancer: preoperative local staging with 3D multi-detector row CT--correlation with surgical and histopathologic results. Radiology. 2007;242:472–482. doi: 10.1148/radiol.2422051557. [DOI] [PubMed] [Google Scholar]
- 14.Kang BC, Kim JH, Kim KW, Lee DY, Baek SY, Lee SW, Jung WH. Value of the dynamic and delayed MR sequence with Gd-DTPA in the T-staging of stomach cancer: correlation with the histopathology. Abdom Imaging. 2000;25:14–24. doi: 10.1007/s002619910003. [DOI] [PubMed] [Google Scholar]
- 15.Shinya S, Sasaki T, Nakagawa Y, Guiquing Z, Yamamoto F, Yamashita Y. The usefulness of diffusion-weighted imaging (DWI) for the detection of gastric cancer. Hepatogastroenterology. 2007;54:1378–1381. [PubMed] [Google Scholar]
- 16.Jensen EH, Tuttle TM. Preoperative staging and postoperative surveillance for gastric cancer. Surg Oncol Clin N Am. 2007;16:329–342. doi: 10.1016/j.soc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Weissleder R. Molecular imaging in cancer. Science. 2006;312:1168–1171. doi: 10.1126/science.1125949. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman JM, Gambhir SS. Molecular imaging: the vision and opportunity for radiology in the future. Radiology. 2007;244:39–47. doi: 10.1148/radiol.2441060773. [DOI] [PubMed] [Google Scholar]
- 19.Czernin J, Weber WA, Herschman HR. Molecular imaging in the development of cancer therapeutics. Annu Rev Med. 2006;57:99–118. doi: 10.1146/annurev.med.57.080904.190431. [DOI] [PubMed] [Google Scholar]
- 20.Jager PL, de Korte MA, Lub-de Hooge MN, van Waarde A, Koopmans KP, Perik PJ, de Vries EG. Molecular imaging: what can be used today. Cancer Imaging. 2005;5 Spec No A:S27–S32. doi: 10.1102/1470-7330.2005.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abhyankar SA, Nair N. Highlighting the role of FDG PET scan in early response assessment of gastrointestinal stromal tumor treated with imatinib mesylate. Clin Nucl Med. 2008;33:213–214. doi: 10.1097/RLU.0b013e318162db8d. [DOI] [PubMed] [Google Scholar]
- 22.Tan MC, Castaldo ET, Gao F, Chari RS, Linehan DC, Wright JK, Hawkins WG, Siegel BA, Delbeke D, Pinson CW, et al. A prognostic system applicable to patients with resectable liver metastasis from colorectal carcinoma staged by positron emission tomography with [18F]fluoro-2-deoxy-D-glucose: role of primary tumor variables. J Am Coll Surg. 2008;206:857–868; discussion 868-869. doi: 10.1016/j.jamcollsurg.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Sobhani I, Tiret E, Lebtahi R, Aparicio T, Itti E, Montravers F, Vaylet C, Rougier P, Andre T, Gornet JM, et al. Early detection of recurrence by 18FDG-PET in the follow-up of patients with colorectal cancer. Br J Cancer. 2008;98:875–880. doi: 10.1038/sj.bjc.6604263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langenhoff BS, Oyen WJ, Jager GJ, Strijk SP, Wobbes T, Corstens FH, Ruers TJ. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453–4458. doi: 10.1200/JCO.2002.12.134. [DOI] [PubMed] [Google Scholar]
- 25.Smithers BM, Couper GC, Thomas JM, Wong D, Gotley DC, Martin I, Harvey JA, Thomson DB, Walpole ET, Watts N, et al. Positron emission tomography and pathological evidence of response to neoadjuvant therapy in adenocarcinoma of the esophagus. Dis Esophagus. 2008;21:151–158. doi: 10.1111/j.1442-2050.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun L, Ye HY, Zhang YH, Guan YS, Wu H. Epidermal growth factor receptor antibody plus recombinant human endostatin in treatment of hepatic metastases after remnant gastric cancer resection. World J Gastroenterol. 2007;13:6115–6118. doi: 10.3748/wjg.v13.45.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruf J, Lopez Hanninen E, Oettle H, Plotkin M, Pelzer U, Stroszczynski C, Felix R, Amthauer H. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5:266–272. doi: 10.1159/000085281. [DOI] [PubMed] [Google Scholar]
- 28.Langenhoff BS, Oyen WJ, Jager GJ, Strijk SP, Wobbes T, Corstens FH, Ruers TJ. Efficacy of fluorine-18-deoxyglucose positron emission tomography in detecting tumor recurrence after local ablative therapy for liver metastases: a prospective study. J Clin Oncol. 2002;20:4453–4458. doi: 10.1200/JCO.2002.12.134. [DOI] [PubMed] [Google Scholar]
- 29.De Gaetano AM, Calcagni ML, Rufini V, Valenza V, Giordano A, Bonomo L. Imaging of peritoneal carcinomatosis with FDG PET-CT: diagnostic patterns, case examples and pitfalls. Abdom Imaging. 2009;34:391–402. doi: 10.1007/s00261-008-9405-7. [DOI] [PubMed] [Google Scholar]
- 30.Pfannenberg AC, Aschoff P, Brechtel K, Muller M, Klein M, Bares R, Claussen CD, Eschmann SM. Value of contrast-enhanced multiphase CT in combined PET/CT protocols for oncological imaging. Br J Radiol. 2007;80:437–445. doi: 10.1259/bjr/34082277. [DOI] [PubMed] [Google Scholar]
- 31.Brechtel K, Klein M, Vogel M, Mueller M, Aschoff P, Beyer T, Eschmann SM, Bares R, Claussen CD, Pfannenberg AC. Optimized contrast-enhanced CT protocols for diagnostic whole-body 18F-FDG PET/CT: technical aspects of single-phase versus multiphase CT imaging. J Nucl Med. 2006;47:470–476. [PubMed] [Google Scholar]
- 32.Fang YH, Muzic RF Jr. Spillover and partial-volume correction for image-derived input functions for small-animal 18F-FDG PET studies. J Nucl Med. 2008;49:606–614. doi: 10.2967/jnumed.107.047613. [DOI] [PubMed] [Google Scholar]
- 33.Meave A, Soto ME, Reyes PA, Cruz P, Talayero JA, Sierra C, Alexanderson E. Pre-pulseless Takayasu's arteritis evaluated with 18F-FDG positron emission tomography and gadolinium-enhanced magnetic resonance angiography. Tex Heart Inst J. 2007;34:466–469. [PMC free article] [PubMed] [Google Scholar]
- 34.Kao PF, Chou YH, Lai CW. Diffuse FDG uptake in acute prostatitis. Clin Nucl Med. 2008;33:308–310. doi: 10.1097/RLU.0b013e3181662f8b. [DOI] [PubMed] [Google Scholar]