Abstract
The artificial endocrine pancreas is a feedback control instrument that regulates insulin delivery on a minute-by-minute basis according to measured blood glucose levels. Only one type of bedside-type artificial endocrine pancreas is now available in Japan: STG-22 (Nikkiso Co. Ltd., Japan). In the insulin infusion algorithm, insulin is infused on the basis of its proportional and derivative actions, to blood glucose concentrations with a constant time delay. The bedside-type artificial endocrine pancreas has been proven to be useful not only as a therapeutic tool for diabetes mellitus, but also as an elegant research tool for investigating the pathophysiology of the disease, by using the euglycemic hyperinsulinemic glucose clamp technique. The wearable type of closed-loop system has been developed recently. The breakthrough is the establishment of a needle-type glucose sensor. The development of closed-loop glycemic control systems that enable long-term physiological regulation has focused on implantable devices. Much effort has been expended to realize these devices.
Keywords: Diabetes mellitus, Artificial pancreas, Blood glucose, Insulin, Infusion
INTRODUCTION
The strategy used in the therapy of diabetes mellitus can be divided into three periods. First, doctors attempted to prevent acute metabolic derangements and prolong the lifespan of patients through use of insulin (discovered in 1921 by Banting and Best) and through the introduction of oral hypoglycemic agents. Second, in association with prolongation of lifespan, an increase in chronic complications, especially microangiopathy, was observed. To counter these, the techniques of hemodialysis or kidney transplantation were established. The application of photocoagulation for preventing the blindness caused by diabetic retinopathy became popular. We are now in the third stage, in which we are attempting to prevent the onset of these chronic complications. Many retrospective studies and recently performed prospective studies have revealed that strict glycemic regulation is essential to prevent the onset of microangiopathy[1-3].
HISTORY OF ARTIFICIAL ENDOCRINE PANCREAS
As early as 1959, Professor E Perry McCullagh, an endocrinologist at The Cleveland Clinic, demonstrated the concept of an implantable artificial endocrine pancreas. The closed-loop regulatory system, which consisted of a glucose monitoring device, transmitter, and insulin syringe, was looked upon as the future treatment device for diabetes mellitus.
The development of an artificial endocrine pancreas to substitute for the diseased pancreatic β cell function has been attempted widely. Albisser et al[4] in Toronto in 1974 and Shichiri et al[5] in Osaka in 1975 succeeded with the clinical use of an artificial endocrine pancreas that consisted of an autoanalyzer for blood glucose determination, a minicomputer system, and a pump driving system. Next, the size of the whole system was reduced, which created a bedside-type artificial endocrine pancreas; Biostator (Miles Laboratory Inc., Elkhart, IN, USA) was developed (no longer available)[6] and another type was developed by the Osaka University group[7]. These devices have been used clinically on a short-term basis, and have a good reputation as elegant research tools to study the pathophysiology of diabetes mellitus.
BEDSIDE-TYPE ARTIFICIAL ENDOCRINE PANCREAS
Principle of the system
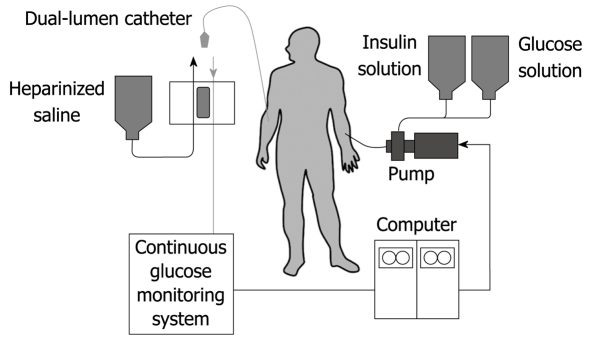
The artificial endocrine pancreas is a device that is composed of a sensor, computer and set of pumps. These components are connected in such a way as to form a closed loop for the subjects. The system is shown in Figure 1. By means of an indwelling dual-lumen catheter, venous blood is drawn into an analyzer that is modified for continuous blood glucose measurement. The computer receives the electrical signals generated by the glucose analyzer and interprets these in accordance with its internal algorithms that are programmed with specific parameters. In turn, the computer instructs one pump to delivery insulin, the amount varying according to the level of the blood glucose and its rate of change. Similarly, glucose or glucagon may be administered by another pump in a counter-regulatory manner when hypoglycemia tends to occur.
Figure 1.
Schematic diagram of the bedside-type artificial endocrine pancreas.
Glucose sensor
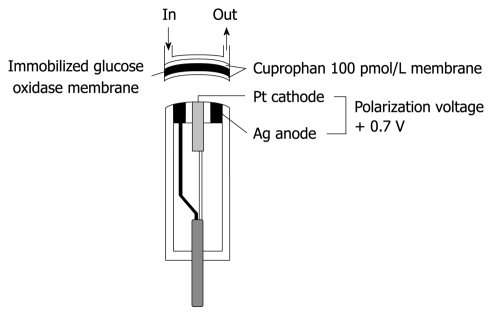
In our first artificial endocrine pancreas system, continuous glucose measurement was conducted with a Technicon AutoAnalyzer II using a modification of the glucose oxidase method[5-7]. However, to minimize the blood sampling volume and to make the whole system smaller, a glucose sensor for continuous glucose monitoring of the whole blood was developed by combining glucose oxidase membrane with an electrode that measures hydrogen peroxide, one of the reaction products, polarographically. A key component of a low-noise blood glucose sensor with long-term stability is its membrane, therefore, hydrophilic Cuprophan 100 pmol/L with a pore size of 3 nm, was applied to cover the immobilized glucose oxidase (Figure 2).
Figure 2.
Structure of a glucose sensor of the bedside-type artificial endocrine pancreas. The immobilized glucose oxidase was covered with hydrophilic Cuprophan 100 pmol/L.
Intravenous insulin infusion algorithm
To develop an insulin infusion algorithm, mathematical models and a computer algorithm are required. By applying the mechanical control theory, Albisser et al[4] have proposed a set of relationships to translate information about blood glucose levels into rates of delivery of insulin and glucagon (or glucose). In this algorithm, the rate of insulin infusion is regulated by the proportional (static) and derivative (dynamic) control mechanisms, which provides for single-phase insulin release, as well as a biphasic response to measured blood glucose concentrations. However, glucose infusion is necessary to prevent severe hypoglycemia caused by excessive insulin infusion in response to a hyperglycemic state.
It is well known that, in rat islets perfused with glucose, a biphasic response of insulin secretion is observed. With the aid of a control theory, we assume that insulin secretion responds not only to the glucose concentration itself, but also to the rate of change in glucose concentration. In other words, against the stepwise input of glucose concentration, an initial rapid insulin secretion is achieved by the derivative action and a second milder increase in secretion is achieved by the proportional action as an output. This relationship has been simulated successfully by using the transfer function with the first-order delay in both proportional and derivative action as shown in Figure 3[8]. By applying this principle, an insulin infusion algorithm has been developed.
Figure 3.
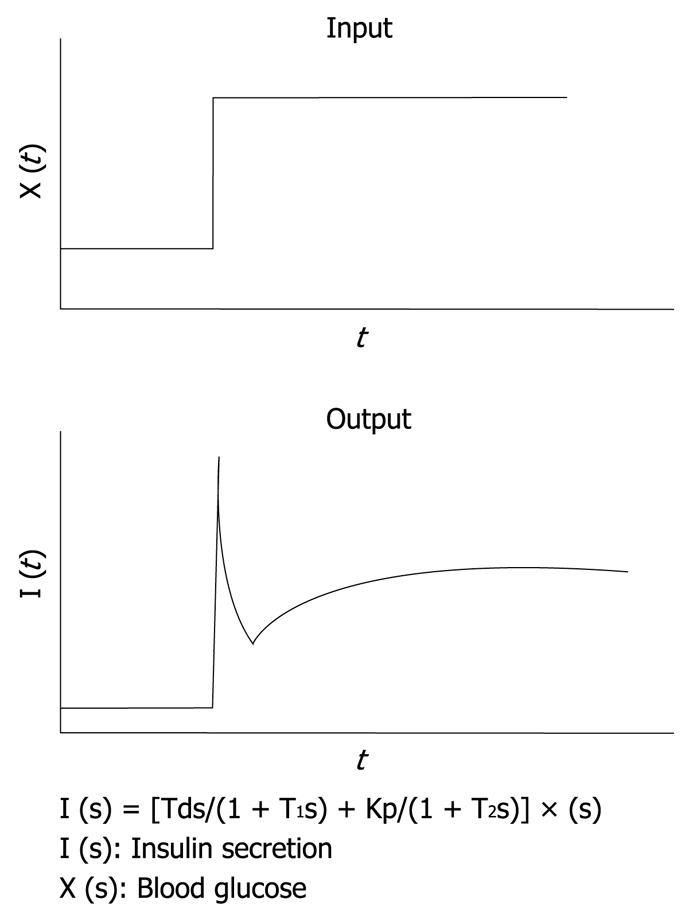
Mathematical model for insulin infusion algorithm. The stepwise input of glucose concentration [X (t)] and biphasic response of insulin secretion [I (t)] are depicted. The relationship between input and output is expressed in the transfer function. I (s), X (s) and dX (s) are the Laplace transformed form of I (t), X (t) and dX (t)/dt, respectively. T1 and T2 are the first order delay in response.
The characteristics of our insulin infusion algorithm are as follows: (1) the amount of insulin infusion is small enough to keep or mimic the physiological plasma insulin concentration; and (2) glucagon infusion is not necessarily needed because the negative derivative action of blood glucose concentration reduces the insulin infusion rate when blood glucose is falling. The details of the computer algorithm have been described previously[6,8-10].
Self-adaptive control algorithm for compensating insulin sensitivity changes
Even though the insulin secretory dynamics of healthy subjects are accomplished in patients with diabetes whose insulin sensitivity is low or super-normal, it is necessary to change manually the parameters for deciding insulin infusion rates for the adaptive control of blood glucose. Therefore, a computer algorithm for self-adaptive control has been established. Firstly, under the blood glucose regulation with artificial endocrine pancreas, the real rate of change in blood glucose concentration in each subject is calculated, and the difference between this and the projected rate of change in blood glucose concentration is assumed to be the index of insulin sensitivity. Secondly, according to the calculated insulin sensitivity, the computer automatically changes the parameters that regulate the insulin infusion rate[11].
Glucose infusion algorithm
A counter-regulatory system might be useful and safe for the prevention of hypoglycemia caused by increased endogenous insulin secretion, and the change in insulin sensitivity that is observed frequently during insulin treatment with the artificial endocrine pancreas in patients with diabetes. In the glucose infusion algorithm, glucose is infused on the basis of proportional and derivative actions of blood glucose concentration with a time delay constant between blood withdrawal and initiation of glucose infusion[10,12].
Clinical application of bedside-type artificial endocrine pancreas
Only one type of bedside-type artificial endocrine pancreas is now available in Japan: STG-22 (Nikkiso Co. Ltd., Japan; Figure 4). Using this system, perfect blood glucose regulation with physiological plasma insulin profiles can be obtained in patients with diabetes. At present, clinical applications of the bedside-type artificial endocrine pancreas on a short-term basis include: blood glucose control in diabetic coma or diabetic ketoacidosis[13], during surgery[14-16], delivery[17], during hemodialysis in diabetic nephropathy[18], the prediction of the insulin requirement[19], and blood glucose control in a hypoglycemic state such as in the case of insulinoma[20].
Figure 4.
Bedside-type artificial endocrine pancreas (STG-22, Nikkiso Co. Ltd. Japan).
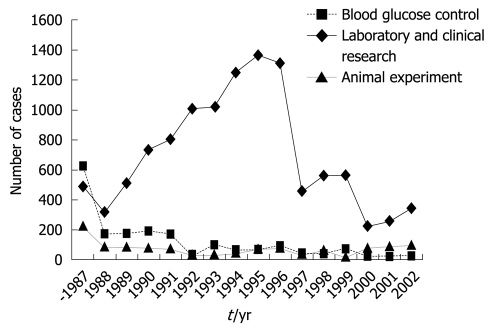
In addition, by using this system, the euglycemic hyperinsulinemic glucose clamp study for the determination of insulin sensitivity in patients with diabetes is now applied widely[21,22]. The euglycemic hyperinsulinemic clamp study was performed using an artificial pancreas according to the method of DeFronzo et al[23]. In brief, insulin is infused in a continuous fashion at a rate of 1.12-1.50 mU/kg per minute, after the priming insulin infusion during the first 10 min of the clamp at the same doses. Blood glucose levels were determined every 5 min during the 2-h clamp study, and euglycemia (5.0 mmol/L) was maintained by infusion of variable amounts of 10%-20% glucose solution. The total-body glucose disposal rate was evaluated as the mean of the glucose infusion rate during the last 30 min of the clamp. The insulin resistance index by the clamp was calculated by dividing the mean glucose infusion rate by the steady-state plasma insulin levels during the last 30 min of the clamp. The Artificial Organs Registry in Japan shows that the clinical applications of this bedside-type artificial endocrine pancreas have been increasing over time. The cumulative number of cases, including clinical and experimental applications, reached 14 418 from 1983 to 2002. The number of applications was 465 in 2002 (29 for blood glucose control, 341 for laboratory and clinical research, and 95 for animal experiments) (Figure 5).
Figure 5.
Number of cases to whom the bedside-type artificial endocrine pancreas had been applied in Japan. Artificial Organs Registry Report in Japan, 2002.
TREND IN THE DEVELOPMENT OF ARTIFICIAL ENDOCRINE PANCREAS
Wearable artificial endocrine pancreas
The ultimate goal of the development of the artificial endocrine pancreas is to achieve long-term strict glycemic regulation. In 1982, we succeeded in miniaturizing a glucose monitoring system to a needle-type, which consisted of a platinum anode and a silver cathode (0.4 mm in diameter and 2 cm in length). The electrode loaded with 0.6 V polarographic voltage measures hydrogen peroxide produced. It has been demonstrated that this sensor possesses excellent sensor characteristics suitable for application in vivo. We then developed a wearable type artificial endocrine pancreas, which consisted of a needle-type glucose sensor, microcomputer system, insulin and glucagon infusion pump systems, and a battery. The total system was packed into a small unit (12 cm × 15 cm × 6 cm in size and 400 g in weight)[24-26]. However, the major obstacle in extending the term of glycemic control is the lack of a stable and reliable glucose sensor.
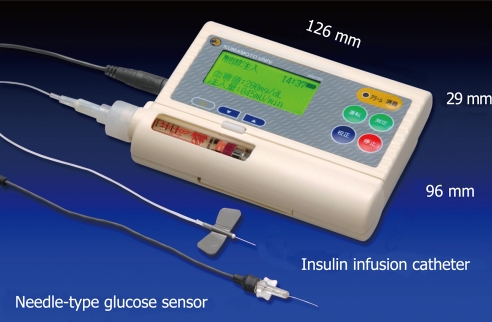
To obtain stable and reliable measurement of subcutaneous tissue glucose concentrations for at least 4 d, we have developed two types of glucose sensors: a miniaturized extracorporeal glucose monitoring system based on a microdialysis sampling method[27]; and a ferrocene-mediated needle-type glucose sensor covered with biocompatible membrane[28]. We have also reported that subcutaneous tissue glucose concentration was monitored continuously using these systems for 4 d. Furthermore, we have developed a newly designed wearable type artificial endocrine pancreas (12.6 cm × 2.9 cm × 9.6 cm in size and 250 g in weight) (KAP-003; Nikkiso, Tokyo, Japan), which consists of a glucose sensing system, microcomputer system, syringe pump for insulin infusion, and a battery[29] (Figure 6).
Figure 6.
Newly designed wearable artificial endocrine pancreas. The total system is packed into a small unit (12.6 cm ¡Á 2.9 cm ¡Á 9.6 cm) weighing 250 g (KAP-003, Nikkiso). A glucose sensor is placed in the subcutaneous tissue and measures glucose concentration continuously. The blood glucose concentration (BG, in mg/dL) and the insulin infusion rate (IIR, in mU/min) appear every minute on a large LCD display.
Implantable artificial endocrine pancreas
The trend in the development of an artificial endocrine pancreas is now from a wearable to an implantable one. However, many problems remain to be solved for each part of the devices. Technology derived from the development of various implantable artificial organs will be beneficial for the accomplishment of the long-term clinical application of the devices.
Our study has suggested that although closed-loop portal and peripheral venous insulin delivery systems are equally effective in terms of blood glucose control and insulin requirements, portal insulin delivery is superior to peripheral delivery in maintaining more appropriate hepatic glucose handling and physiological insulin profiles. These results indicate that the portal vein is the most suitable insulin delivery route for the implantable artificial endocrine pancreas[30,31].
With regard to the metabolic efficacy and insulin requirement, intraportal insulin therapy is expected to be more effective than intraperitoneal insulin therapy. However, because the technique of placing an insulin catheter into the portal vein in humans is associated with severe invasion and high risks, such as infection and catheter thrombosis, there have been few reports of applying intraportal insulin therapy to patients with diabetes. Recently, there have been several reports of new methods that overcome these problems[32,33]. Thus, with technical improvement, it should be possible to safely infuse insulin intraportally.
The application of a one-chip microcomputer could make the processor system smaller. The technology derived in the process of developing the clinical applications of implantable insulin infusion pumps has contributed to the completion of the effector implantation. Implantation of the entire system requires a small and powerful long-life battery. A transcutaneous energy transmission system is one of the candidates for this. As a stable and reliable long-life implantable glucose sensor is not yet available, it is not practical to implant all these parts of these apparatus intracorporeally. It would be adequate to implant the computer and infusion pumps and retain the glucose sensor extracorporeally to regulate insulin infusion rates by telemetric means. In the near future, an implantable artificial endocrine pancreas with a telecommunications system will be available for the treatment of diabetes mellitus.
CONCLUSION
Successful glycemic control in patients with diabetes using the artificial pancreas emphasizes the importance of continuous glycemic monitoring for strict glycemic control. However, the major obstacle for extending the term of glycemic control in patients with diabetes is the development of an implantable, high-precision glucose sensor for tissue glucose determination. A needle-type glucose sensor, which is a miniature hydrogen peroxide electrode covered by membrane with biological activity, can be implanted easily and is exchangeable. The sensor has the in vitro and in vivo characteristics suitable for continuous tissue glucose monitoring. A wearable artificial endocrine pancreas, which incorporates a needle-type glucose sensor, has been devised and has regulated glycemia physiologically in patients with diabetes for > 6 d. Further improvements in sensor design, especially in membranes with biocompatibility, might reduce the host reactions to the sensor implanted in tissue and thus extend its biological life.
Footnotes
Peer reviewer: Christa Buechler, PhD, Regensburg University Medical Center, Internal Medicine I, Franz Josef Strauss Allee 11, 93042 Regensburg, Germany
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 3.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 4.Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W, Schipper H, Gander R. Clinical control of diabetes by the artificial pancreas. Diabetes. 1974;23:397–404. doi: 10.2337/diab.23.5.397. [DOI] [PubMed] [Google Scholar]
- 5.Shichiri M, Kawamori R, Yamasaki Y, Inoue M, Shigeta Y, Abe H. Computer algorithm for the artificial pancreatic beta cell. Artif Organs. 1978;2 suupl:247–250. [Google Scholar]
- 6.Goriya Y, Kawamori R, Shichiri M, Abe H. The development of an artificial beta cell system and its validation in depancreatized dogs: the physiological restoration of blood glucose homeostasis. Med Prog Technol. 1979;6:99–108. [PubMed] [Google Scholar]
- 7.Pfeiffer EF, Thum C, Clemens AH. The artificial beta cell--a continuous control of blood sugar by external regulation of insulin infusion (glucose controlled insulin infusion system) Horm Metab Res. 1974;6:339–342. doi: 10.1055/s-0028-1093841. [DOI] [PubMed] [Google Scholar]
- 8.Nomura M, Shichiri M, Kawamori R, Yamasaki Y, Iwama N, Abe H. A mathematical insulin-secretion model and its validation in isolated rat pancreatic islets perifusion. Comput Biomed Res. 1984;17:570–579. doi: 10.1016/0010-4809(84)90021-1. [DOI] [PubMed] [Google Scholar]
- 9.Kawamori R, Shichiri M, Goriya Y, Yamasaki Y, Shigeta Y, Abe H. Importance of insulin secretion based on the rate of change in blood glucose concentration in glucose tolerance, assessed by the artificial beta cell. Acta Endocrinol (Copenh) 1978;87:339–351. doi: 10.1530/acta.0.0870339. [DOI] [PubMed] [Google Scholar]
- 10.Shichiri M, Kawamori R. Optimized algorithms for closed-loop glycemia control systems. In: Beyer J, Albisser AM, J Schrezenmeir, Lehmann L, editors. Computer systems for insulin adjustment in diabetes mellitus. Hedingen: Pancsienta-Verlag; 1985. pp. 171–183. [Google Scholar]
- 11.Kawamori R, Shichiri M, Kikuchi M, Yamasaki Y, Abe H. Perfect normalization of excessive glucagon responses to intravenous arginine in human diabetes mellitus with the artificial beta-cell. Diabetes. 1980;29:762–765. doi: 10.2337/diab.29.9.762. [DOI] [PubMed] [Google Scholar]
- 12.Yamasaki Y, Shichiri M, Kawamori R, Goriya Y, Sasai T, Morishima T, Nomura M, Tohdo R, Abe H. Counterregulatory system in an artificial endocrine pancreas. Glucose infusion algorithm. Artif Organs. 1979;3:265–270. doi: 10.1111/j.1525-1594.1979.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 13.Berg G, Sailer D. Treatment of diabetic ketoacidosis with a computer-controlled bedside monitoring and infusion system. Horm Metab Res Suppl. 1979;3:146–150. [PubMed] [Google Scholar]
- 14.Pfeiffer EF, Beishcher W, Kerner W. The artificial endocrine pancreas in clinical research. Horm Metab Res. 1977;Suppl 7:95–114. [PubMed] [Google Scholar]
- 15.Okabayashi T, Hnazaki K, Nishimori I, Sugimoto T, Maeda H, Yatabe T, Dabanaka K, Kobayashi M, Yamashita K. Continuous post-operative blood glucose monitoring and control using a closed-loop system in patients undergoing hepatic resection. Dig Dis Sci. 2008;53:1405–1410. doi: 10.1007/s10620-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita K, Okabayashi T, Yokoyama T, Yatabe T, Maeda H, Manabe M, Hanazaki K. Accuracy and reliability of continuous blood glucose monitor in post-surgical patients. Acta Anaesthesiol Scand. 2009;53:66–71. doi: 10.1111/j.1399-6576.2008.01799.x. [DOI] [PubMed] [Google Scholar]
- 17.Verpooten G, De Leeuw I, Abs R. The artificial pancreas and the management of the pregnant diabetic. Eur J Obstet Gynecol Reprod Biol. 1980;10:375–379. doi: 10.1016/0028-2243(80)90022-2. [DOI] [PubMed] [Google Scholar]
- 18.Slama G, Klein JC, Delage A, Rottembourg J, Marouani A, Jacobs C. The use of the artificial pancreas in uremic diabetic patients. Horm Metab Res Suppl. 1979;10:178–183. [PubMed] [Google Scholar]
- 19.Magyar I, Tamás G Jr, Bányai Z. Experiences in the insulin requirement of diabetics. Endokrinologie. 1981;77:79–86. [PubMed] [Google Scholar]
- 20.Gin H, Erny P, Perissat J, Doutre LP, Aubertin J. Artificial pancreas in the diagnosis and treatment of insulinoma: a report of five cases. Br J Surg. 1988;75:584–585. doi: 10.1002/bjs.1800750630. [DOI] [PubMed] [Google Scholar]
- 21.Hazama Y, Matsuhisa M, Ohtoshi K, Gorogawa S, Kato K, Kawamori D, Yoshiuchi K, Nakamura Y, Shiraiwa T, Kaneto H, et al. Beneficial effects of nateglinide on insulin resistance in type 2 diabetes. Diabetes Res Clin Pract. 2006;71:251–255. doi: 10.1016/j.diabres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama H, Mori K, Emoto M, Araki T, Teramura M, Mochizuki K, Tashiro T, Motozuka K, Inoue Y, Nishizawa Y. Non-oxidative glucose disposal is reduced in type 2 diabetes, but can be restored by aerobic exercise. Diabetes Obes Metab. 2008;10:400–407. doi: 10.1111/j.1463-1326.2007.00716.x. [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 24.Shichiri M, Kawamori R, Yamasaki Y, Hakui N, Abe H. Wearable artificial endocrine pancrease with needle-type glucose sensor. Lancet. 1982;2:1129–1131. doi: 10.1016/s0140-6736(82)92788-x. [DOI] [PubMed] [Google Scholar]
- 25.Shichiri M, Kawamori R, Goriya Y, Yamasaki Y, Nomura M, Hakui N, Abe H. Glycaemic control in pancreatectomized dogs with a wearable artificial endocrine pancreas. Diabetologia. 1983;24:179–184. doi: 10.1007/BF00250158. [DOI] [PubMed] [Google Scholar]
- 26.Shichiri M, Kawamori R, Hakui N, Yamasaki Y, Abe H. Closed-loop glycemic control with a wearable artificial endocrine pancreas. Variations in daily insulin requirements to glycemic response. Diabetes. 1984;33:1200–1202. doi: 10.2337/diab.33.12.1200. [DOI] [PubMed] [Google Scholar]
- 27.Hashiguchi Y, Sakakida M, Nishida K, Uemura T, Kajiwara K, Shichiri M. Development of a miniaturized glucose monitoring system by combining a needle-type glucose sensor with microdialysis sampling method. Long-term subcutaneous tissue glucose monitoring in ambulatory diabetic patients. Diabetes Care. 1994;17:387–396. doi: 10.2337/diacare.17.5.387. [DOI] [PubMed] [Google Scholar]
- 28.Nishida K, Sakakida M, Ichinose K, Uemura T, Uehara M, Kajiwara K, Miyata T, Shichiri M, Ishihara K, Nakabayashi N. Development of a ferrocene-mediated needle-type glucose sensor covered with newly designed biocompatible membrane, 2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate. Med Prog Technol. 1995;21:91–103. [PubMed] [Google Scholar]
- 29.Ichimori S, Nishida K, Shimoda S, Sekigami T, Matsuo Y, Ichinose K, Shichiri M, Sakakida M, Araki E. Development of a highly responsive needle-type glucose sensor using polyimide for a wearable artificial endocrine pancreas. J Artif Organs. 2006;9:105–113. doi: 10.1007/s10047-005-0326-8. [DOI] [PubMed] [Google Scholar]
- 30.Sekigami T, Shimoda S, Nishida K, Matsuo Y, Ichimori S, Ichinose K, Shichiri M, Sakakida M, Araki E. Comparison between closed-loop portal and peripheral venous insulin delivery systems for an artificial endocrine pancreas. J Artif Organs. 2004;7:91–100. doi: 10.1007/s10047-004-0251-2. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo Y, Shimoda S, Sakakida M, Nishida K, Sekigami T, Ichimori S, Ichinose K, Shichiri M, Araki E. Strict glycemic control in diabetic dogs with closed-loop intraperitoneal insulin infusion algorithm designed for an artificial endocrine pancreas. J Artif Organs. 2003;6:55–63. doi: 10.1007/s100470300009. [DOI] [PubMed] [Google Scholar]
- 32.Liang HL, Yang CF, Pan HB, Chen CK, Chang JM. Percutaneous transsplenic catheterization of the portal venous system. Acta Radiol. 1997;38:292–295. doi: 10.1080/02841859709172066. [DOI] [PubMed] [Google Scholar]
- 33.Shishko PI, Kovalev PA, Goncharov VG, Zajarny IU. Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes. 1992;41:1042–1049. doi: 10.2337/diab.41.9.1042. [DOI] [PubMed] [Google Scholar]