Abstract
AIM: To explore the effects of fentanyl on insulin release from freshly isolated rat pancreatic islets in static culture.
METHODS: Islets were isolated from the pancreas of mature Sprague Dawley rats by common bile duct intraductal collagenase V digestion and were purified by discontinuous Ficoll density gradient centrifugation. The islets were divided into four groups according to the fentanyl concentration: control group (0 ng/mL), group I (0.3 ng/mL), group II (3.0 ng/mL), and group III (30 ng/mL). In each group, the islets were co-cultured for 48 h with drugs under static conditions with fentanyl alone, fentanyl + 0.1 μg/mL naloxone or fentanyl + 1.0 μg/mL naloxone. Cell viability was assessed by the MTT assay. Insulin release in response to low and high concentrations (2.8 mmol/L and 16.7 mmol/L, respectively) of glucose was investigated and electron microscopy morphological assessment was performed.
RESULTS: Low- and high-glucose-stimulated insulin release in the control group was significantly higher than in groups II and III (62.33 ± 9.67 μIU vs 47.75 ± 8.47 μIU, 39.67 ± 6.18 μIU and 125.5 ± 22.04 μIU vs 96.17 ± 14.17 μIU, 75.17 ± 13.57 μIU, respectively, P < 0.01) and was lowest in group III (P < 0.01). After adding 1 μg/mL naloxone, insulin release in groups II and III was not different from the control group. Electron microscopy studies showed that the islets were damaged by 30 ng/mL fentanyl.
CONCLUSION: Fentanyl inhibited glucose-stimulated insulin release from rat islets, which could be prevented by naloxone. Higher concentrations of fentanyl significantly damaged β-cells of rat islets.
Keywords: Fentanyl, Inhibition, Insulin release, Islets
INTRODUCTION
In recent years, anesthetists and surgeons have had to manage an increasing number of diabetic patients. A concern for the anesthetist is how to control blood glucose and protect islet function in the clinical setting. Because the risk of surgery and anesthesia is higher for these patients, it is particularly important to maintain whole-body glucose homeostasis during the perioperative period. It is well known that many drugs used during surgery for anesthesia or pain relief have effects on pancreatic islets, for example, one study has demonstrated that β-endorphin, an endogenous opioid peptide, can inhibit insulin secretion[1]. Therefore, when administering drugs to diabetic patients, the surgeons and anesthetists must consider the protection of islet function in these patients. Fentanyl was first synthesized 60 years ago and is currently the most popular opioid used in the preoperative period because of its safety and efficacy. In addition, fentanyl transdermal patches have been developed to manage chronic pain associated with diabetic neuropathy. These patches have been designed to provide continuous, rate-controlled systemic delivery of the fentanyl base for up to 72 h[2,3].
The minimum effective plasma concentration of fentanyl is 0.63 ng/mL after intravenous administration and the therapeutic plasma concentration is 1-2 ng/mL. However, the plasma concentration often exceeds 3 ng/mL because many practitioners prefer to administer high doses of fentanyl[4,5]. In most cancer cases secondary to disease progression, the initial median transdermal fentanyl dose is generally 60-70 μg/h (release rate), which increases to about 170 μg/h over time[6]. Furthermore, a small proportion of patients require doses of between 400 and 1000 μg/h in the latter stages of therapy and the mean treatment period is often longer than 50 d. These large doses are associated with high plasma concentrations (up to 14.5 ng/mL) which are linearly related to the dose[7,8]. Furthermore, fentanyl has been commonly used for patients undergoing cardiac operations because high doses of fentanyl can stabilize the cardiovascular circulatory system during operations. After administration of 50-100 μg/kg fentanyl, the plasma concentration of fentanyl in these patients is commonly above 20-30 ng/mL[9]. However, high plasma concentrations of fentanyl are associated with clinical side effects such as nausea and vomiting, constipation, skin itching and respiratory depression. So far, the potential effects of fentanyl on pancreatic islet β-cells remain unknown.
Because some opioid receptor agonists affect insulin release[10,11], it would be expected that high-doses of fentanyl would have an effect on islet insulin secretion. Studies have suggested that some opiates inhibit insulin secretion[12]. In mouse pancreatic islets incubated under static conditions, glucose-stimulated insulin release is inhibited by β-endorphin and endomorphin-1, endogenous opioid receptor agonists. This inhibition could be prevented by naloxone, an opioid receptor antagonist[13]. Therefore, we hypothesized that fentanyl would affect insulin release. Therefore, we investigated the effect of fentanyl on rat islets in a static culture model and believe the results have important implications on the use of fentanyl in clinical situations, particularly in people with diabetes.
MATERIALS AND METHODS
Animals
Male SD rats weighing 250-300 g were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences and housed under constant conditions of temperature (20-22°C) and artificial lighting (12-h light-dark cycle) before taking part in the study. The study was carried out in accordance with the Guidelines for Animal Experimentation, Tongji University of Shanghai, China, and all the tests were approved by the Animal Experimentation Committee of Tongji University of Shanghai.
Islet preparation
After male adult rats were anesthetized with 50-75 mg/kg sodium pentobarbital, the abdominal wall was cut open and 10 mL of Hank’s buffered saline solution (HBSS) containing collagenase V 1.0-1.2 mg/mL (Sigma Chemical Co., St Louis, MO, USA) was injected into the common bile duct of the rat. The pancreas, which was swollen with the digestion solution, was quickly excised and immersed into a plastic culture bottle containing HBSS and incubated with shaking for 13-15 min at 37°C. The digested suspension was obtained by filtering the suspension through a 0.5-mm metal mesh and washed with HBSS containing 2% bovine serum albumin (BSA). A total of 300-400 islets were obtained from each rat by discontinuous Ficoll density gradient centrifugation (density: 1.100, 1.077) (Ficoll, Sigma Chemical Co., St Louis, MO, USA). After being washed with HBSS containing 2% BSA, the islets were cultured for 24 h with 5% CO2 and collected for further tests[14]. The islets were identified by dithizone (Sigma, USA) staining. Cells stained red under light microscopy were considered to be islets[15]. The combination dyes acridine orange (AO) and propidium iodide (PI) (AO: 0.67 μmol/L; PI: 75 μmol/L) were applied to differentiate between viable and non-viable whole islets; the dyes stained living cells green and dead cells red using minimal background fluorescence[16].
Groups
The islets were divided into four groups according to the fentanyl concentration used: control group (0 ng/mL), group I (0.3 ng/mL), group II (3.0 ng/mL), and group III (30 ng/mL). In each group, the islets were co-cultured for 48 h with drugs as follows: fentanyl alone, fentanyl + 0.1 μg/mL naloxone and fentanyl + 1.0 μg/mL naloxone. There were 12 wells in each group with 30 IEQ (islet equivalent, the diameter of an islet of 150-200 μm equates to 1.7 IEQ) islets/well. An additional two groups were co-cultured with naloxone alone at concentrations of 0.1 or 1.0 μg/mL.
Islet viability
The effect of fentanyl on cell viability was measured by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, USA) assay to display a direct correlation with cell metabolism. After co-culturing with fentanyl for 48 h, 100 μL MTT solution was added to each well to a final concentration of 0.5 mg/mL per well and the plates were cultured for 4 h at 37°C. The supernatant was then removed by the addition of DMSO to each well to dissolve the deposition, and the optical density (OD) disparity was read at 490 nm using a spectrophotometer microplate reader (Labsystems, Finland). The inhibition of islet cells caused by the different concentrations of fentanyl was calculated using the formula: inhibition rate (%) = (1 - ODdrug exposure/ODcontrol) × 100[17,18].
Glucose-stimulated insulin release assay in static culture
After incubating with the drugs for 48 h, the islets were washed with serum-free and glucose-free medium twice. The insulin release stimulation test was performed by first incubating the islets in low (2.8 mmol/L) and then high (16.7 mmol/L) concentrations of glucose in static culture medium for 1 h each. Supernatant from each well was collected and the insulin level was determined using a rat insulin radioimmunoassay kit (Linco Research, Inc, St Charles, MO, USA). The stimulation index (SI) was calculated by dividing the value of high glucose-stimulated secretion by the value of low glucose-stimulated secretion[19,20].
Electron microscopy studies
After co-culturing with fentanyl for 48 h, the islets in the control group and those in group III were cut into thin sections and mounted on slides. Samples were stained with 2% uranyl acetate and lead citrate. The sections were viewed and photographed on a JEOL JEM-1230 transmission electron microscope (Jeol Ltd., Japan) at 80 kV.
Statistical analysis
Statistical and graphic analyses were performed with SPSS 13.0 software. Differences between groups were evaluated with one-way ANOVA or Kruskal-Wallis H tests, as appropriate, and the differences between two groups were analyzed with the LSD test or Mann-Whitney U test. P < 0.05 was considered statistically significant.
RESULTS
Islet viability assessment
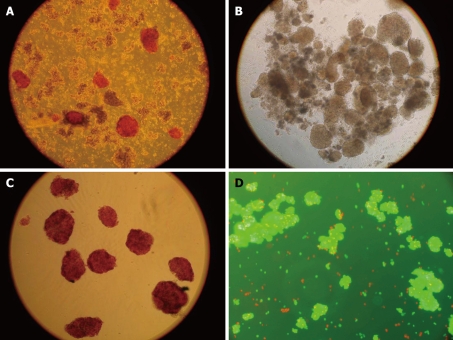
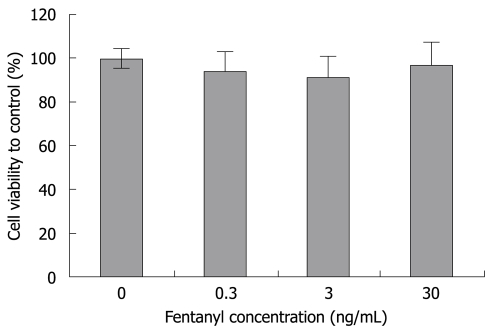
In this study, the rat islets were isolated from the rat pancreas with good quality. The viability was about 90% and the purification rate was about 95% (Figure 1). Fentanyl had no effect on cell viability (Figure 2).
Figure 1.
The procedure of islets preparation. A: The islets were stained red by DTZ in digested pancreatic tissue; B: Pure islets isolated from digested pancreatic tissue; C: Pure islets stained by DTZ; D: The dye AO-PI stained living cells green and dead cells red in minimal background fluorescence (× 100).
Figure 2.
The cell viability after co-culture with fentanyl. The viability measured by MTT was 100% in the control group. The viability of islets exposed to 0.3, 3 and 30 ng/mL fentanyl was 94.3%, 91.3% and 96.9%, respectively. There was no difference between groups. The data represent means ± SE.
Insulin release test
Fentanyl significantly inhibited the low and high concentration glucose-stimulated insulin release in a concentration-dependent manner (P < 0.01), and the insulin release was lowest at the concentration of 30 ng/mL (P < 0.01). After adding 0.1 μg/mL naloxone, fentanyl still significantly inhibited glucose-stimulated insulin release (P < 0.01). However, after adding 1 μg/mL naloxone, the insulin release showed no difference from that of controls. Naloxone had no effect on insulin release (Table 1). There was no difference in SI between any of the groups (Table 2).
Table 1.
The glucose-stimulated insulin release (μIU) in response to fentanyl with different concentrations of naloxone (mean ± SD, n = 12 )
|
Fentanyl alone |
Fentanyl + 0.1 μg/mL naloxone |
Fentanyl + 1 μg/mL naloxone |
||||
| Low glucose | High glucose | Low glucose | High glucose | Low glucose | High glucose | |
| Control (0 ng/mL) | 62.33 ± 9.67 | 125.5 ± 22.04 | 62.33 ± 9.67 | 125.5 ± 22.04 | 62.33 ± 9.67 | 125.5 ± 22.04 |
| I (0.3 ng/mL) | 54.75 ± 5.93a | 118.17 ± 16.81 | 54.33 ± 8.99 | 110.4 ± 15.69 | 61.5 ± 8.13 | 126.75 ± 16.48 |
| II (3 ng/mL) | 47.75 ± 8.47b | 96.17 ± 14.17b | 45.92 ± 7.63b | 88.25 ± 11.22d | 59.75 ± 8.42 | 118.33 ± 21.09 |
| III (30 ng/mL) | 39.67 ± 6.18b | 75.17 ± 13.57b | 36.33 ± 5.79b | 68.67 ± 11.99d | 61.08 ± 8.07 | 126.0 ± 15.54 |
| Naloxone | 61.67 ± 9.16 | 120.3 ± 18.04 | 60.67 ± 9.15 | 123.0 ± 20.89 | ||
P < 0.05,
P < 0.01 vs control group (Mann-Whitney U test);
P < 0.01 vs control group (LSD test). Low- and high-glucose-stimulated insulin release in the 3 and 30 ng/mL fentanyl groups was significantly lower than in the control group (P < 0.01), and was lowest with 30 ng/mL fentanyl (P < 0.01). After adding 0.1 μg/mL naloxone, insulin secretion in the 3 and 30 ng/mL fentanyl groups remained significantly lower than the control group (P < 0.01) and was lowest with 30 ng/mL fentanyl (P < 0.01).
Table 2.
Stimulation index at different concentrations of fentanyl and naloxone (mean ± SD, n = 12)
| Fentanyl | Fentanyl + 0.1 μg/mL naloxone | Fentanyl + 1 μg/mL naloxone | |
| Control (0 ng/mL) | 2.01 ± 0.21 | 2.02 ± 0.21 | 2.02 ± 0.21 |
| I (0.3 ng/mL) | 2.17 ± 0.36 | 2.07 ± 0.35 | 2.08 ± 0.26 |
| II (3 ng/mL) | 2.04 ± 0.26 | 1.95 ± 0.23 | 1.99 ± 0.34 |
| III (30 ng/mL) | 1.89 ± 0.16 | 1.89 ± 0.19 | 2.08 ± 0.27 |
| Naloxone | 1.97 ± 0.29 | 2.04 ± 0.28 |
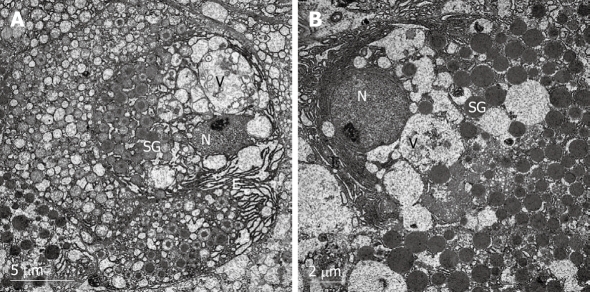
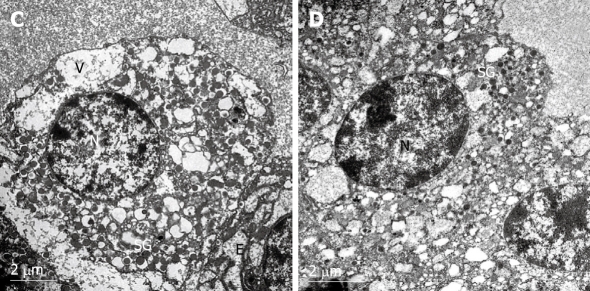
Evaluation of electron microscopy studies
Electron micrography of β-cells in rat islets from the control group showed no pathological changes (Figure 3). After exposure to 30 ng/mL fentanyl for 48 h, the β-cells were in a poor morphological condition, and exhibited chromatin margination and severe cytoplasmic vacuolization and degeneration (Figure 4).
Figure 3.
Electron micrographs of β-cells in rat pancreatic islets of control group. N: Nucleus; SG: Secretory granule; V: Vesicle; E: Endoplasmic reticulum. The typical β-cells had an abundance of cytoplasmic granules and endoplasmic reticulum. The electron density of the granules increased when the granules were maturating in the vesicles. The mature secretory granules displayed a highly electron-dense core surrounded by a wide electron-lucent halo. The granules had a space between the core and the membrane. The vesicles showed a normal round outline (A, × 6000). There were many nascent granules in the β-cells; subsequent maturation involved further condensation of the matrix constituents and a reduction in granule diameter (B, × 8000).
Figure 4.
Electron micrographs of rat pancreatic islet β-cells after exposure to 30 ng/mL fentanyl for 48 h. N: Nucleus; SG: Secretory granules; V: Vesicle; E: Endoplasmic reticulum. Chromatin margination and severe cytoplasmic vacuolization and degeneration were observed in the islets. Large and small vacuoles are present in the cytoplasm. The secretory granules and endoplasmic reticulum were much smaller than in the control group (A, × 10 000). In addition, endoplasmic reticulum and normal vesicles were almost never observed (B, × 12 000).
DISCUSSION
Many studies have focused on islet isolation, application and function in the diabetes field. Many factors affect the isolation of rat pancreatic islets; therefore, the yields and function of the isolated islets vary considerably, despite the introduction of novel or improved methods. Large variations in yields and function have been found, even if the same sources of collagenase are used[21]. In our study, the islets were isolated from the rat pancreas with good quality (viability rate was about 90% and purification rate was about 95%). Generally, we obtained 300-400 islets from each rat. This indicates that our method is efficient and provides high yields of islets. The high yields and favorable function of the isolated islets were attributed to the pancreatic tissue being infused and fully digested. Low yields of islets typically result from insufficient digestion; therefore, we suggest that the digestion time and collagenase concentration should be optimized precisely for islet isolation.
Over the last two decades, despite the development of more potent, safer, faster onset, and shorter- and longer-lasting opioids, fentanyl has remained the mainstay of anesthesiologists and Certified Registered Nurse Anesthetists in the perioperative period, and for physicians involved in pain management. Because diabetic patients undergo higher risk procedures during the period of operation and anesthesia, anesthetists and surgeons should protect islet function and try to minimize any harmful medical effects on islets during the procedure. Although some studies have suggested that some opiates inhibit insulin secretion, it was unknown whether fentanyl has a similar effect[12].
Before our current study, a number of studies have demonstrated direct effects of endogenous and selective opioid receptor agonists on insulin release[10,11]. Some opioid receptor agonists, such as methadone, were found to improve multiple-low-dose streptozotocin-induced type 1 diabetes in mice, which suggests that the opioid receptor agonists improve insulin release in vivo[22]. However, other studies have suggested that some opiates inhibit insulin secretion[12]. β-endorphin seems to inhibit glucose-induced insulin secretion but, conversely, an excitatory effect has been reported in many studies[11,23]. These observations have yielded conflicting results with marked variations between species. Therefore, it could not be hypothesized whether fentanyl inhibits insulin release[13].
Our results clearly demonstrate that fentanyl inhibited glucose-stimulated insulin release from islets in vitro. Electron microscopy studies showed that the cells in the control group exhibited good viability but, after the cells were exposed to 30 ng/mL fentanyl for 48 h, chromatin margination and severe cytoplasmic vacuolization were observed, indicating that the cells were in a poor condition. Thus, our test suggests that the islets were injured by exposure to fentanyl at the concentrations tested.
We know that glucose uptake by tissues is as likely to play a decisive role as does the release of insulin. In the presence of insulin, opioid receptors in the pancreas have been reported to regulate plasma glucose and the activation of mu-opioid receptors, which could increase the utilization of glucose in peripheral tissue to lower the plasma glucose[24,25]. In vivo, a potent opiate was shown to lower glucose levels by enhancing peripheral glucose utilization without improving insulin release[26]. Also, serum glucose levels are responsible for the altered potency of mu-opioid agonists only during the early stages of diabetes[27]. Thus, the plasma glucose-lowering response induced by mu-opioid receptor activation in vivo is not attributed to improved insulin release.
Endogenous opioid receptors are expressed in endocrine pancreas and studies have indicated that endogenous opioid peptides and selective opiate receptor agonists suppress insulin release[28]. Thus, the effect of fentanyl on insulin release might be related to opiate receptor activation. Activation of the opioid receptor initiates a cascade of events that result in an array of biological effects, which inhibit insulin release[29,30]. It seems that fentanyl-induced mu-opioid receptor activation leads to the inhibition of glucose-stimulated insulin release. This inhibitory effect could be reversed by high doses of naloxone, an opioid receptor antagonist. Therefore, the activation of opioid receptors induced by fentanyl appears to be a route by which insulin release is inhibited.
There are two processes that take place during insulin secretion: hormone biosynthesis and release. Many drugs affect insulin secretion, although the exact mechanism varies. Many drugs have different effects on either of the two processes. For example, long-chain fatty acids powerfully increase insulin release and inhibit glucose-stimulated pro-insulin biosynthesis[31,32]. In the present study, it is unknown which of these processes are affected by fentanyl. Because fentanyl may be involved in both insulin release and biosynthesis[33,34], the role of opioid receptor activation on insulin secretion requires further investigation.
There are certain limitations in our experiment. The pharmacology of the mu receptor signaling pathway involves G-protein coupling which, when activated, closes Ca2+ channels and opens K+ channels, resulting in a decline in intracellular Ca2+ levels and hyperpolarization; all of which are short-term effects. The magnitude of the insulin response to glucose is related not only to the absolute level of glucose but also to the rate of change in the glucose level. There are two phases of insulin release. An acute increase in glucose level elicits a rapid and transient secretion of insulin, called the first or acute-phase response, which subsides within 10 min. The second phase response begins when glucose levels increase slowly and progressively for up to 4 h[35]. As in many other studies, we measured the insulin release over 60 min in static culture after incubating in low (2.8 mmol/L) and then high (16.7 mmol/L) glucose concentrations[19,20,36]. Therefore, the effect of fentanyl on glucose-stimulated insulin release in rat islets in dynamic culture also needs to be investigated. In addition, perfusion of islets with glucose provides a dynamic profile of the characteristics of glucose-stimulated insulin release, which could be used to fully determine the effect of fentanyl on the inhibition of insulin release, and the ability of the cells to down-regulate insulin secretion after a fentanyl challenge are a focus of our future studies.
In conclusion, fentanyl inhibited glucose-stimulated insulin release from islets, and the inhibition could be reversed by naloxone. Because islet function varies between animal species, translation of our results to the clinical setting will necessitate further studies. However, our results indicate the potential for fentanyl to inhibit insulin release; thus, it is necessary to test the plasma insulin level and the islet function regularly in patients treated with fentanyl, particularly patients treated with fentanyl for a long term and/or with high doses of fentanyl. Further studies are needed to explore the exact mechanism by which fentanyl affects islet insulin release.
COMMENTS
Background
Fentanyl citrate is a potent synthetic narcotic analgesic extensively used for anesthesia and analgesia in the operating room and intensive care unit. In recent years, anesthetists and surgeons have had to manage an increasing number of diabetic patients. However, the effects of fentanyl on the glucose-stimulated insulin secretion capacity of rat β-cells in vitro has remained unclear. Although some studies have suggested that some opiates inhibit insulin secretion, it could not be concluded whether fentanyl has a similar effect, because the earlier studies yielded conflicting results, with marked species variation. Therefore, this study investigated the effects of fentanyl on rat islets in static culture.
Research frontiers
This study is a novel field in diabetes investigation that has been largely overlooked. The research team used freshly isolated islets to study the effects of the opiate fentanyl on insulin release, which is an important subject for anesthetists and surgeons. In this study, the results demonstrated that fentanyl inhibited glucose-stimulated insulin release in rat islets and higher concentrations of fentanyl significantly damaged rat islets.
Innovations and breakthroughs
The study of effects of anesthetic and analgesic drugs on pancreatic islets is a novel field in diabetes management. This is the first study to report that fentanyl inhibits the release of insulin from rat islets. These findings would be helpful for clinicians who administer fentanyl.
Applications
The results may stimulate further investigation of diabetes management in the anesthesia field. These findings suggest that it is important to regularly test the plasma insulin level and islet function in patients treated with fentanyl for the long term and/or at high doses, and could help to develop novel approaches to help people with diabetes to maintain whole-body glucose homeostasis during the perioperative period.
Peer review
The author has used rat islets as a model to study the effect of the mu-opioid agonist fentanyl on glucose-stimulated insulin release. Generally, this is a topic that has been overlooked in the past, thus this study is important and unique. Also, the islet preparation and EM technology were good.
Footnotes
Peer reviewer: Ming Li, Associate Professor, Tulane University Health Sciences Center, 1430 Tulane Ave Sl-83, New Orleans 70112, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
References
- 1.Giugliano D, Cozzolino D, Salvatore T, Torella R, D'Onofrio F. Beta-endorphin-induced inhibition and stimulation of insulin secretion in normal humans is glucose dependent. Diabetes. 1988;37:1265–1270. doi: 10.2337/diab.37.9.1265. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann KA, Zech D. Transdermal fentanyl: clinical pharmacology. J Pain Symptom Manage. 1992;7:S8–S16. doi: 10.1016/0885-3924(92)90048-m. [DOI] [PubMed] [Google Scholar]
- 3.Grond S, Radbruch L, Lehmann KA. Clinical pharmacokinetics of transdermal opioids: focus on transdermal fentanyl. Clin Pharmacokinet. 2000;38:59–89. doi: 10.2165/00003088-200038010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kussman BD, Zurakowski D, Sullivan L, McGowan FX, Davis PJ, Laussen PC. Evaluation of plasma fentanyl concentrations in infants during cardiopulmonary bypass with low-volume circuits. J Cardiothorac Vasc Anesth. 2005;19:316–321. doi: 10.1053/j.jvca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Gilson AM, Ryan KM, Joranson DE, Dahl JL. A reassessment of trends in the medical use and abuse of opioid analgesics and implications for diversion control: 1997-2002. J Pain Symptom Manage. 2004;28:176–188. doi: 10.1016/j.jpainsymman.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Sloan PA, Moulin DE, Hays H. A clinical evaluation of transdermal therapeutic system fentanyl for the treatment of cancer pain. J Pain Symptom Manage. 1998;16:102–111. doi: 10.1016/s0885-3924(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 7.Sittl R, Nuijten M, Nautrup BP. Patterns of dosage changes with transdermal buprenorphine and transdermal fentanyl for the treatment of noncancer and cancer pain: a retrospective data analysis in Germany. Clin Ther. 2006;28:1144–1154. doi: 10.1016/j.clinthera.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Hair PI, Keating GM, McKeage K. Transdermal matrix fentanyl membrane patch (matrifen): in severe cancer-related chronic pain. Drugs. 2008;68:2001–2009. doi: 10.2165/00003495-200868140-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bedirli N, Boyaci A, Akin A, Esmaoglu A. Comparison of the effects of fentanyl and remifentanil on splanchnic tissue perfusion during cardiac surgery. J Anesth. 2007;21:94–98. doi: 10.1007/s00540-006-0457-y. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano D, Torella R, Lefèbvre PJ, D'Onofrio F. Opioid peptides and metabolic regulation. Diabetologia. 1988;31:3–15. doi: 10.1007/BF00279126. [DOI] [PubMed] [Google Scholar]
- 11.Ahrén B. Effects of beta-endorphin, met-enkephalin, and dynorphin A on basal and stimulated insulin secretion in the mouse. Int J Pancreatol. 1989;5:165–178. doi: 10.1007/BF02924417. [DOI] [PubMed] [Google Scholar]
- 12.Schleicher RL. Beta-endorphin inhibits insulin secretion from isolated pancreatic islets. Endocrinology. 1989;124:1254–1258. doi: 10.1210/endo-124-3-1254. [DOI] [PubMed] [Google Scholar]
- 13.García-Barrado MJ, Iglesias-Osma MC, Rodríguez R, Martín M, Moratinos J. Role of mu-opioid receptors in insulin release in the presence of inhibitory and excitatory secretagogues. Eur J Pharmacol. 2002;448:95–104. doi: 10.1016/s0014-2999(02)01897-6. [DOI] [PubMed] [Google Scholar]
- 14.Hyder A. Effect of the pancreatic digestion with liberase versus collagenase on the yield, function and viability of neonatal rat pancreatic islets. Cell Biol Int. 2005;29:831–834. doi: 10.1016/j.cellbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827–830. [PubMed] [Google Scholar]
- 16.Bank HL. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cell Dev Biol. 1988;24:266–273. doi: 10.1007/BF02628826. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Ge J, Wang K, Qian J, Zou Y. Evaluation of MTT assay for measurement of emodin-induced cytotoxicity. Assay Drug Dev Technol. 2006;4:203–207. doi: 10.1089/adt.2006.4.203. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Zhu JS, Zhang Y, Shen WM, Zhang Q. Predictive value of MTT assay as an in vitro chemosensitivity testing for gastric cancer: one institution's experience. World J Gastroenterol. 2008;14:3064–3068. doi: 10.3748/wjg.14.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novelli M, Piaggi S, De Tata V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced impairment of glucose-stimulated insulin secretion in isolated rat pancreatic islets. Toxicol Lett. 2005;156:307–314. doi: 10.1016/j.toxlet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Vosough-Ghanbari S, Sayyar P, Pournourmohammadi S, Aliahmadi A, Ostad SN, Abdollahi M. Stimulation of insulin and glucagon synthesis in rat Langerhans islets by malathion in vitro: Evidence for mitochondrial interaction and involvement of subcellular non-cholinergic mechanisms. Pestic Biochem Phys. 2007;89:130–136. [Google Scholar]
- 21.de Haan BJ, Faas MM, Spijker H, van Willigen JW, de Haan A, de Vos P. Factors influencing isolation of functional pancreatic rat islets. Pancreas. 2004;29:e15–e22. doi: 10.1097/00006676-200407000-00063. [DOI] [PubMed] [Google Scholar]
- 22.Amirshahrokhi K, Dehpour AR, Hadjati J, Sotoudeh M, Ghazi-Khansari M. Methadone ameliorates multiple-low-dose streptozotocin-induced type 1 diabetes in mice. Toxicol Appl Pharmacol. 2008;232:119–124. doi: 10.1016/j.taap.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Curry DL, Bennett LL, Li CH. Stimulation of insulin secretion by beta-endorphins (1-27 & 1-31) Life Sci. 1987;40:2053–2058. doi: 10.1016/0024-3205(87)90097-x. [DOI] [PubMed] [Google Scholar]
- 24.Locatelli A, Spotti D, Caviezel F. The regulation of insulin and glucagon secretion by opiates: a study with naloxone in healthy humans. Acta Diabetol Lat. 1985;22:25–31. doi: 10.1007/BF02591089. [DOI] [PubMed] [Google Scholar]
- 25.Liu IM, Chi TC, Chen YC, Lu FH, Cheng JT. Activation of opioid mu-receptor by loperamide to lower plasma glucose in streptozotocin-induced diabetic rats. Neurosci Lett. 1999;265:183–186. doi: 10.1016/s0304-3940(99)00226-8. [DOI] [PubMed] [Google Scholar]
- 26.Werther GA, Joffe S, Artal R, Sperling MA. Opiate modulation of glucose turnover in dogs. Metabolism. 1985;34:136–140. doi: 10.1016/0026-0495(85)90122-2. [DOI] [PubMed] [Google Scholar]
- 27.Kamei J, Kawashima N, Kasuya Y. Serum glucose level-dependent and independent modulation of mu-opioid agonist-mediated analgesia in diabetic mice. Life Sci. 1993;52:53–60. doi: 10.1016/0024-3205(93)90288-e. [DOI] [PubMed] [Google Scholar]
- 28.Khawaja XZ, Green IC, Thorpe JR, Titheradge MA. The occurrence and receptor specificity of endogenous opioid peptides within the pancreas and liver of the rat. Comparison with brain. Biochem J. 1990;267:233–240. doi: 10.1042/bj2670233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cetin Y. Immunohistochemistry of opioid peptides in the guinea pig endocrine pancreas. Cell Tissue Res. 1990;259:313–319. doi: 10.1007/BF00318454. [DOI] [PubMed] [Google Scholar]
- 30.Ko WC, Liu TP, Cheng JT, Tzeng TF, Liu IM. Effect of opioid mu-receptors activation on insulin signals damaged by tumor necrosis factor alpha in myoblast C2C12 cells. Neurosci Lett. 2006;397:274–278. doi: 10.1016/j.neulet.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 31.Stein DT, Stevenson BE, Chester MW, Basit M, Daniels MB, Turley SD, McGarry JD. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest. 1997;100:398–403. doi: 10.1172/JCI119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skelly RH, Bollheimer LC, Wicksteed BL, Corkey BE, Rhodes CJ. A distinct difference in the metabolic stimulus-response coupling pathways for regulating proinsulin biosynthesis and insulin secretion that lies at the level of a requirement for fatty acyl moieties. Biochem J. 1998;331(Pt 2):553–561. doi: 10.1042/bj3310553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knoch KP, Meisterfeld R, Kersting S, Bergert H, Altkrüger A, Wegbrod C, Jäger M, Saeger HD, Solimena M. cAMP-dependent phosphorylation of PTB1 promotes the expression of insulin secretory granule proteins in beta cells. Cell Metab. 2006;3:123–134. doi: 10.1016/j.cmet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Hutton JC. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia. 1994;37 Suppl 2:S48–S56. doi: 10.1007/BF00400826. [DOI] [PubMed] [Google Scholar]
- 35.Torres N, Noriega L, Tovar AR. Nutrient modulation of insulin secretion. Vitam Horm. 2009;80:217–244. doi: 10.1016/S0083-6729(08)00609-2. [DOI] [PubMed] [Google Scholar]
- 36.de Barros Reis MA, Arantes VC, Cunha DA, Latorraca MQ, Toyama MH, Carneiro EM, Boschero AC. Increased L-CPT-1 activity and altered gene expression in pancreatic islets of malnourished adult rats: a possible relationship between elevated free fatty acid levels and impaired insulin secretion. J Nutr Biochem. 2008;19:85–90. doi: 10.1016/j.jnutbio.2007.01.005. [DOI] [PubMed] [Google Scholar]