Abstract
Objective: To investigate the expression of death decoy receptor 3 (DcR3) and survivin in colorectal carcinoma. Methods: Tumor and normal tissues were taken from a total of 100 colorectal carcinoma patients during surgery, and the expression of DcR3 and survivin was examined by immunohistochemistry, Western blotting, and reverse transcription-polymerase chain reaction (RT-PCR) analyses. Results: RT-PCR showed that the expression levels of DcR3 mRNA (0.846±0.242, P<0.01) and survivin mRNA (0.7835±0.2392, P<0.01) in colorectal cancer tissues were significantly higher than those in adjacent normal tissues. Western blotting showed that the expression levels of DcR3 protein (0.795±0.261, P<0.01) and survivin protein (0.6765±0.1351, P<0.01) in tumor tissues were significantly higher than those in non-cancer tissues. The immunohistochemical streptavidin-peroxidase (SP) method showed that the positive expression rates of DcR3 and survivin were 67.0% and 58.0% in colorectal cancer tissues, and 18.0% and 3.0% in non-cancerous colorectal tissues (P<0.05), respectively. The positive correlations of DcR3 (P<0.01) and survivin (P<0.01) to the differentiation of colorectal carcinoma cells, lymph node metastasis, and pathological stage were observed. The expression of DcR3 and survivin was found to be positively correlated to clinicopathologic parameters of colorectal carcinoma. Conclusion: The overexpressed DcR3 and survivin in colorectal cancer may contribute to the development of the cancer. The monitoring of these two proteins may be useful for the diagnosis, differentiation, metastasis, and determination of stages of colorectal carcinoma.
Keywords: Death decoy receptor 3 (DcR3), Survivin, Colorectal carcinoma
INTRODUCTION
Death decoy receptor 3 (DcR3), also know as trapping receptor 3, is a new member of tumor necrosis factor receptor (TNFR) superfamily. DcR3 can bind ligands such as LIGHT, Fas ligand (FasL), and TL1A, inhibiting various immunoregulatory functions that are induced by the corresponding ligands. On the other hand, DcR3 can bind the death ligand FasL and inhibit the association of death receptor Fas with FasL, resulting in blocking apoptosis that is induced by the Fas, and enabling malignant tumor cell to escape venenosus aggression of FasL expression immunocytes (Connor and Felder, 2008; Fayad et al., 2006; Lee et al., 2008; Chen and Yang, 2008). DcR3 can also closely connect with LIGHT, a member of tumor necrosis factor (TNF) superfamily, which inhibits the combination of HVEM/TR2 and lymphotoxin β receptor/TNFRSF3 (LTβR) with LIGHT. Consequently, DcR3 disturbs apoptosis induced by LIGHT and makes malignant tumor cell escape immune surveillance (Inoue et al., 2008; Chang et al., 2008; Gill et al., 2007; Kim et al., 2005; Hayashi et al., 2007). Thus, DcR3 has the counteracting or antagonist activities in immune response and regulation, and a high expression of DcR3 in many human tissues is closely associated with genesis, development, and prognosis of the tumor (Chen et al., 2008; Roth et al., 2001; Elnemr et al., 2001; Takahama et al., 2002; Macher-Goeppinger et al., 2008). At genetic and protein levels, DcR3 is overexpressed in many human malignant tumors. In addition, the expression of DcR3 modulates the chemotherapeutical sensitivity of 5-fluorouracil/mitomycin (Fu/MMC), and a high expression of DcR3 reduces the chemotherapeutical sensitivity.
A host mechanism to maintain homeostasis, apoptosis is an important physiological function in organisms. The severe disequilibrium of apoptosis would generate disastrous consequence to the host. The relation between tumor genesis and development and the severe disequilibrium of apoptosis has been intensively studied. The long survival of the malignant cells is in part attributed to the low activity of apoptosis gene products and the activation of the counteracting apoptosis genes. Survivin, which is cloned in recent years, is an inhibitor of apoptotic protein (IAP) family (Pitti et al., 1998). It shares a homologous sequence and similar characteristic biological features with other IAPs. Survivin binds caspase-3 and caspase-7 to inhibit apoptosis (Tamm et al., 1998). In addition, survivin can combine with caspases indirectly through P21 and block apoptosis at last. In most cases, survivin is not expressed in normal tissues but highly in many malignant tumors. The expression of survivin can reduce the therapeutic sensitivity of tumor cells. Therefore, it may play an important role in the pathogenesis of malignant tumors.
DcR3 and survivin expression in colorectal cancer tumor tissues has not been adequately studied yet. In the present study, we examined the expression of DcR3 and survivin in colorectal carcinoma and normal tissues by reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemical methods, and Western blotting, and explored the relationship between their expression levels and clinical and pathological features of colorectal carcinoma. The relationship between DcR3 and survivin was also analyzed. The results help to understand the genesis, development, prognosis, and gene therapy of colorectal cancer.
MATERIALS AND METHODS
Materials
Colorectal cancer specimens were obtained from a total of 100 patients who underwent surgical removal from March 2006 to March 2008 in the Department of Surgery, the Affiliated Hospital of Guangdong Medical College, Guangzhou, China. There were 62 males and 38 females, and the age range was 28 to 77 years with a median age of 59 years. The average ages for colon carcinoma and rectal cancer patients were 57 and 43 years, respectively. All the patients were pathologically diagnosed and received no chemotherapy or radiotherapy before surgical therapy. The cancers were classified according to “The Diagnosis and Treatment Criterion for Common Cancer in China” (Li et al., 2005), including 57 tubular adenoma, 29 papillary adenocarcinoma, and 14 mucinous adenocarcinoma. According to Duke’s staging, 5 cases were in stage A, 38 in stage B, 34 in stage C, and 23 in stage D. The removed specimens were snap-frozen at liquid nitrogen before use or being paraffin-embedded. The control group was the normal tissue specimens that parted about 5 cm from colorectal cancer tissues.
Mouse anti-human DcR3 monoclone antibody was purchased from Jingmei Biotech Co., Ltd, Shenzhen, China. Rabbit anti-human survivin monoclone antibody and streptavidin-peroxidase (SP) kit were purchased from Zhongshan Golden Bridge Biotech Co., Ltd, Beijing, China. Trizol kit was purchased from Invitrogen Biotech Ltd, USA, one step RT-PCR kit from TaKaRa Biotech Ltd, Japan, and Western blot relational kit from Beyotime Biotech Ltd, Haimen, China.
RNA extraction and RT-PCR assay
Total RNA was extracted from colorectal cancer tissues and non-cancerous colorectal tissues using Trizol kit, according to the manufacturer’s instruction. The concentration of total RNA was measured by spectrophotometry and the integrity of RNA was determined by agarose gel electrophoresis. 2 μg total RNA was reversely transcribed with avian myeloblastosis virus (AMV) reverse transcriptase (TaKaRa Biotech Ltd), and the complementary DNA (cDNA) was amplified by PCR. β-actin served as the internal reference. DcR3, survivin, and β-actin primers were synthesized by TaKaRa Biotech Ltd (Dalian, China) (Table 1). The PCR conditions were 30 cycles of 94 °C 2 min, 94 °C 45 s, 55 °C 45 s, and 72 °C 1 min, with a final extension at 72 °C for 5 min. RT-PCR products were analyzed by 1.5% (w/v) agarose gel electrophoresis, and scanned by ultraviolet photometry (UVP), and the grey value of them were determined. The relational expression levels of DcR3 and survivin mRNAs were calculated according to the proportionality of DcR3/survivin and β-actin (grey value).
Table 1.
Sequences of the primer sets used for amplification of DcR3, survivin, and β-actin mRNAs
| Upstream primer sequence | Downstream primer sequence | PCR size (bp) | |
| DcR3 | 5′-GTCGCTGCTGTGCCGGTGT-3′ | 5′-AAGCCCGTGCCTCCTCCTCA-3′ | 287 |
| Survivin | 5′-TTCTCAAGGACCACCGCATCT-3′ | 5′-GAAAGCGCAACCGGACGA-3′ | 290 |
| β-actin | 5′-ATTCTTGGCTATTACGACA-3′ | 5′-GAGACCTTCCATCCCTTC-3′ | 328 |
Immunohistochemistry assay
All specimens were fixed with 10% (w/v) formaldehyde, and sections were embedded by paraffin and observed microscopically after hematoxylin and eosin (H&E) staining. Serial sections of 4~6 μm were prepared from the cut surface of paraffin-embedded blocks. Paraffinum in these sections were separated from water, and immunohistochemical staining was carried out by the standard S-P technique according to the S-P kit specification. The known sections were used as the positive substitution control and phosphate buffered saline (PBS) replaced primary antibody as the negative control. The presence of granules dyed claybank in the cytoplasm of cells viewed using a light microscope, was taken as a positive indicator of the expression of survivin. When the positive cells were less than 10% of the whole visual field under high power lens, the section was considered as negative. The cellular expression of DcR3 was indicated by the presence of claybank or brown dye in the cytoplasm, and the way to determine DcR3 positivity was similar to survivin.
Western blot assay
Total proteins of colorectal cancer tissues and non-cancerous colorectal tissues were extracted with lysis buffer. The protein concentration was detected by Bradford procedure (Bio-Rad) and the lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE). After electroblotted to nitrocellulose membranes for 1 h at 120 V, the membranes were blocked with 5% (w/v) skimmed milk for 1 h by rocking. The membranes were probed with mouse anti-human DcR3 monoclonal antibody (1:400) (Jingmei Biotech Co., Ltd), rabbit anti-human survivin monoclonal antibody (1:400) (Zhongshan Golden Bridge Biotech Co., Ltd) and anti-β-actin antibody (1:3000) (Beyotime Biotech Ltd) for 1 h at room temperature or overnight at 4 °C. The membranes were then washed and conjugated with the horseradish peroxidase (HRP)-linked secondary antibody (1:5000) for 1 h at 4 °C and Western blotting eluant was added for 3 times. The protein was detected by BeyoECL kit (Beyotime Biotech Ltd), according to the manufacturer’s instruction. Protein bands were visualized by using enhanced chemiluminescence (ECL) kit. The protein of interest and inner reference β-actin were determined by using Kodak software package for the analysis of 2-D gel, and optical density value (ODV) was detected. The relational expression levels of DcR3 and survivin proteins were calculated according to the proportionality of DcR3/survivin and β-actin (ODV).
Statistical analysis
All statistical analyses were performed by the SPSS 13.0 software package for Macintosh. Variables of the relationships between the expression of DcR3 and survivin and the clinical pathology of colorectal cancer were analyzed by χ 2 test, Student’s t-test, Student’s t′-test, and spearman correlation analysis. P<0.05 was considered significant, and P<0.01 remarkably significant.
RESULTS
Expression of DcR3 and survivin mRNAs and proteins by RT-PCR and Western blot analyses
The results show that the expression of DcR3 and survivin was both higher in colorectal cancer tissues than that in non-cancerous tissues (P<0.01) by RT-PCR and Western blot analyses (Tables 2 and 3).
Table 2.
Expression of DcR3 and survivin mRNAs
| DcR3 |
Survivin |
|||||
| Positive rate (%) | DcR3/β-actin | P | Positive rate (%) | Survivin/β-actin | P | |
| Colorectal cancer tissues | 71 (71/100) | 0.846±0.242 | <0.01 | 63 (63/100) | 0.7835±0.2392 | <0.01 |
| Non-cancerous tissues | 15 (15/100) | 0.198±0.066 | 6 (6/100) | 0.4722±0.2582 | ||
Table 3.
Expression of DcR3 and survivin proteins
| DcR3 |
Survivin |
|||||
| Positive rate (%) | DcR3/β-actin | P | Positive rate (%) | Survivin/β-actin | P | |
| Colorectal cancer tissues | 68 (68/100) | 0.795±0.261 | <0.01 | 65 (65/100) | 0.6765±0.1351 | <0.01 |
| Non-cancerous tissues | 13 (13/100) | 0.219±0.083 | 5 (5/100) | 0.1869±0.0428 | ||
DcR3 and survivin protein levels by immunohistochemistry analysis
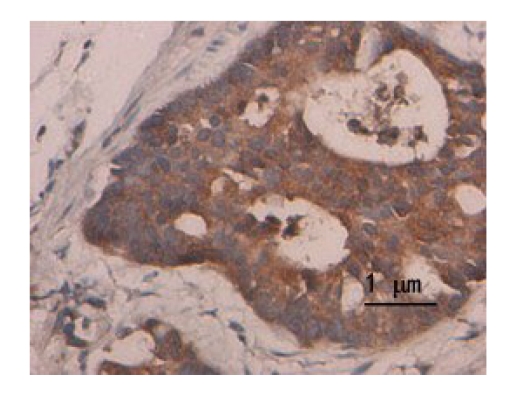
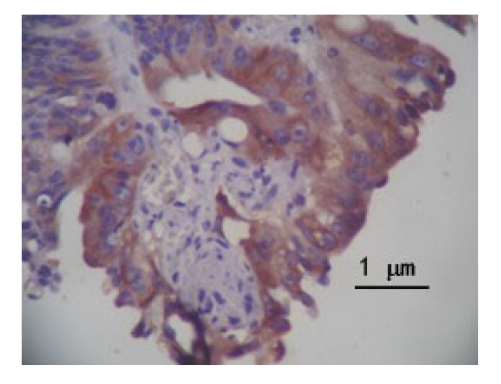
S-P technique results show that the expression of DcR3 protein was dyed claybank or brown in the cytoplasm (Fig.1) and the survivin protein was dyed claybank in the cytoplasm (Fig.2). The positive expression rate of DcR3 protein was observed 67.0% (67/100) in colorectal cancer tissues, and 18.0% (18/100) in non-cancerous tissues (χ 2=49.13, P<0.01). The positive expression rate of survivin protein was 58.0% (58/100) in colorectal cancer tissues, and 3.0% (3/100) in non-cancerous tissues (χ 2=68.78, P<0.01).
Fig.1.
Expression of DcR3 protein in colorectal cancer tissues (SP)
Fig.2.
Expression of survivin protein in colorectal cancer tissues (SP)
Relationships between DcR3 and survivin protein levels and clinicopathological factors
The expression of DcR3 and survivin was both related to the differentiated degree, Duke’s stage and lymph node metastasis (P<0.01). The relationships between the expression of DcR3 in colorectal cancer tissues and clinicopathological factors are shown in Table 4, and the relationships between the expression of survivin in colorectal cancer tissues and clinicopathological factors in Table 5.
Table 4.
Relationships between the expression of DcR3 in colorectal cancer tissues and clinicopathological factors
| nTotal | nNegative | nPositive | Positive rate (%) | χ2 | P | |
| Age | ||||||
| ≤60 | 69 | 23 | 46 | 66.67 | 0.0112 | 0.916 |
| >60 | 31 | 10 | 21 | 67.74 | ||
| Sexuality | ||||||
| Female | 62 | 20 | 42 | 67.74 | 0.0406 | 0.841 |
| Male | 38 | 13 | 25 | 65.79 | ||
| Tumor size (cm) | ||||||
| ≤5 | 44 | 14 | 30 | 68.18 | 0.0496 | 0.824 |
| >5 | 56 | 19 | 37 | 66.07 | ||
| Tissue type | ||||||
| Tubular gland | 57 | 19 | 38 | 66.67 | 0.0302 | 0.985 |
| Papillary adenocarcinoma | 29 | 10 | 19 | 65.52 | ||
| Mucous gland | 14 | 4 | 10 | 71.43 | ||
| Differentiated degree | ||||||
| Well-differentiated | 33 | 19 | 14 | 42.42 | 16.4540 | <0.01 |
| Moderately differentiated | 42 | 12 | 30 | 71.43 | ||
| Poorly differentiated | 25 | 2 | 23 | 92.0 | ||
| Lymph node metastasis | ||||||
| Yes | 54 | 11 | 43 | 79.63 | 8.4690 | <0.01 |
| No | 46 | 22 | 24 | 52.17 | ||
| Duke’s stage | ||||||
| A+B | 43 | 23 | 20 | 46.51 | 14.3230 | <0.01 |
| C+D | 57 | 10 | 47 | 82.46 | ||
Table 5.
Relationships between the expression of survivin in colorectal cancer tissues and clinicopathological factors
| nTotal | nNegative | nPositive | Positive rate (%) | χ2 | P | |
| Age | ||||||
| ≤60 | 69 | 30 | 39 | 56.53 | 0.1997 | 0.655 |
| >60 | 31 | 12 | 19 | 61.23 | ||
| Sexuality | ||||||
| Female | 62 | 25 | 37 | 59.68 | 0.1885 | 0.664 |
| Male | 38 | 17 | 21 | 55.26 | ||
| Tumor size (cm) | ||||||
| ≤5 | 44 | 18 | 26 | 59.09 | 0.0384 | 0.845 |
| >5 | 56 | 24 | 32 | 57.14 | ||
| Tissue type | ||||||
| Tubular gland | 57 | 25 | 32 | 56.14 | 0.0810 | 0.960 |
| Papillary adenocarcinoma | 29 | 12 | 17 | 58.62 | ||
| Mucous gland | 14 | 5 | 9 | 64.29 | ||
| Differentiated degree | ||||||
| Well-differentiated | 33 | 24 | 9 | 27.27 | 22.7080 | <0.01 |
| Moderately differentiated | 42 | 15 | 27 | 64.29 | ||
| Poorly differentiate | 25 | 3 | 22 | 88.00 | ||
| Lymph node metastasis | ||||||
| Yes | 54 | 14 | 40 | 74.07 | 12.4510 | <0.01 |
| No | 46 | 28 | 18 | 39.13 | ||
| Duke’s stage | ||||||
| A+B | 43 | 27 | 16 | 37.21 | 13.3860 | <0.01 |
| C+D | 57 | 15 | 42 | 73.68 |
Relationship between DcR3 and survivin protein levels in colorectal cancer
The expression of DcR3 and survivin by immunohistochemical analysis is shown in Table 6. Correlation analysis showed that the expression of DcR3 and survivin in colorectal cancer is positively correlated (Spearman related coefficient (r s)=0.255, P<0.01).
Table 6.
Correlation between DcR3 and survivin in colorectal cancer
| DcR3 | Survivin |
||
| nNegative | nPositive | nTotal | |
| nNegative | 20 | 13 | 33 |
| nPositive | 22 | 45 | 67 |
| nTotal | 42 | 58 | 100 |
DISCUSSION
DcR3 is a new member of TNFR superfamily. It binds LIGHT and FasL. LIGHT is of lymphotoxin analogs and can bind herpesvirus encroachment medium (HVEM) to induce apoptosis. DcR3 binds LIGHT and blocks the bioactivity of HVEM, and therefore inhibits apoptosis. FasL is a death factor, a native ligand for Fas in vivo, and the receptor/ligand interaction leads to recruitment and activation of the initiator caspase, e.g., caspase-8 and caspase-10, and initiates downstream cascade reaction, thus triggering apoptosis. DcR3 can precipitate cells that express Fas protein, and inhibit the binding of FasL and Fas, consequently suppressing tumor apoptosis.
Many researchers found that DcR3 gene is overexpressed in many malignant tumors. Pitti et al.(1998) found that DcR3 mRNA is normally expressed in embryonic lung, brain, liver and in adult spleen, colon and lung tissues and is overexpressed in colon carcinoma cell line SW480. Bai et al.(2000) found that the expression of DcR3 mRNA was 20 times higher in tumor tissues than in non-cancerous tissues. Some observed that DcR3 mRNA is overexpressed in liver and breast carcinomas but not in non-cancerous tissues, and the expression of DcR3 decreased after surgical therapy. Consequently, the detection of DcR3 level in pre- and post-operative patients can be used to judge the prognosis (Wu et al., 2003; Shen et al., 2003). Mild et al.(2002) found that the expression level of DcR3 influenced the sensibility of chemotherapeutics. In the Fu/MMC treatment, the sensitivity of Fu/MMC is lower in the individuals with a higher DcR3.
The current results show that the expression of DcR3 was significantly higher at mRNA and protein levels in colorectal cancer tissues, consistent with others’ reports (Pitti et al., 1998). We consider that the expression of DcR3 may play a facilitative role for the genesis and development of colorectal cancer, and the mechanism of action was related to its anti-apoptosis function. Compared with the relationship between the expression of DcR3 in colorectal cancer tissues and clinicopathological parameters, it was found that DcR3 is positively correlated with differentiation, lymph node metastasis, and pathological stage of colorectal carcinoma, regardless of sex, age, pathological type, tumor localization, and the vertical extent of infiltration of patients. The results show that the expression rate of DcR3 rises when the grade of tumor malignancy increases, and the mechanism of action may be that the high expression of DcR3 protein can competitively bind with LIGHT and FasL, inhibiting apoptosis. This inhibition could make tumor cell escape from immune surveillance and clearance, and the high expression of DcR3 protein could reduce demic immune function, resulting in a massive tumor cell proliferation and an enhancement of their invasiveness and metastasis. Therefore, the expression level of DcR3 protein can be used to evaluate the malignancy of colorectal cancer and direct colorectal cancer treatment.
Survivin can bind to caspase-3 and caspase-7, inhibiting apoptosis, of which the mechanism is that survivin can specifically bind to the microtubule of karyokinesis spindles and regulate the mitotic cycle, causing the inactivation of caspase-3 and caspase-7 to inhibit apoptosis at last. Deferent levels of survivin had been detected in various malignant tumor tissues, 48.2% in gastric cancer (Zhu et al., 2003), 70.7% breast cancer (Tanaka et al., 2000), 88% in pancreatic carcinoma (Sarela et al., 2000), 63.5% in colorectal cancer (Sarela et al., 2002), and 100% in celiothelioma (Xia et al., 2002). These studies suggest that the expression of survivin plays an important role in the genesis and development of malignant tumors.
In the present study, survivin at both mRNA and protein levels was found significantly higher in colorectal caner tissues than that in control tissues, indicating that survivin gene was activated in the genesis stage of colorectal cancer. Therefore, survivin gene could be as a molecular marker for an early diagnosis of colorectal cancer. The relationships between the expression of survivin in colorectal cancer tissues and clinicopathological factors indicated that survivin was closely associated with the differentiation degree, Duke’s stage, and lymph node metastasis, regardless of sex, age, pathological type, and tumor size. Therefore, it is believed that survivin gene plays an important role in the development and metastasis of colorectal cancer, and the detection of survivin could determine the malignant degree and prognosis of colorectal cancer.
We also found that the expression of DcR3 and survivin co-existed in the carcinogenesis of colorectal cancer. While its mechanism remains unclear, the detection of the co-expression of DcR3 and survivin in colorectal cancer tissues could be also used in clinic.
References
- 1.Bai C, Connolly B, Metzker ML, Hilliard CA, Liu X, Sandig V, Soderman A, Galloway SM, Liu Q, Austin CP, Caskey CT. Overexpression of M68/DcR3 in human gastrointestinal tract tumors independent of gene amplification and its location in a four-gene cluster. Proc. Natl. Acad. Sci. USA. 2000;97(3):1230–1235. doi: 10.1073/pnas.97.3.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang PM, Chen PM, Hsieh SL, Tzeng CH, Liu JH, Chiou TJ, Wang WS, Yen CC, Gau JP, Yang MH. Expression of a soluble decoy receptor 3 in patients with diffuse large B-cell lymphoma predicts clinical outcome. Int J Oncol. 2008;33(3):549–554. [PubMed] [Google Scholar]
- 3.Chen C, Zhang C, Zhuang G, Luo H, Su J, Yin P, Wang J. Decoy receptor 3 overexpression and immunologic tolerance in hepatocellular carcinoma (HCC) development. Cancer Invest. 2008;26(10):965–974. doi: 10.1080/07357900801975256. [DOI] [PubMed] [Google Scholar]
- 4.Chen PH, Yang CR. Decoy receptor 3 expression in AsPC-1 human pancreatic adenocarcinoma cells via the phosphatidylinositol 3-kinase-, Akt-, and NF-kappaB-dependent pathway. J Immunol. 2008;181(12):8441–8449. doi: 10.4049/jimmunol.181.12.8441. [DOI] [PubMed] [Google Scholar]
- 5.Connor JP, Felder M. Ascites from epithelial ovarian cancer contain high levels of functional decoy receptor 3 (DcR3) and is associated with platinum resistance. Gynecol Oncol. 2008;111(2):330–335. doi: 10.1016/j.ygyno.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, Ninomiya I, Fushida S, Nishimura GI, Shimizu K, Miwa K. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001;18(2):311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- 7.Fayad R, Brand MI, Stone D, Keshavarzian A, Qian L. Apoptosis resistance in ulcerative colitis: high expression of decoy receptors by lamina propria T cells. Eur J Immunol. 2006;36(8):2215–2222. doi: 10.1002/eji.200535477. [DOI] [PubMed] [Google Scholar]
- 8.Gill RM, Coleman NM, Hunt JS. Differential cellular expression of LIGHT and its receptors in early gestation human placentas. J Reprod Immunol. 2007;74(1-2):1–6. doi: 10.1016/j.jri.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi S, Miura Y, Nishiyama T, Mitani M, Tateishi K, Sakai Y, Hashiramoto A, Kurosaka M, Shiozawa S, Doita M. Decoy receptor 3 expressed in rheumatoid synovial fibroblasts protects the cells against Fas-induced apoptosis. Arthritis Rheum. 2007;56(4):1067–1075. doi: 10.1002/art.22494. [DOI] [PubMed] [Google Scholar]
- 10.Inoue Y, Morinaga A, Takizawa F, Saito T, Endo M, Haruta C, Nakai T, Moritomo T, Nakanishi T. Molecular cloning and preliminary expression analysis of banded dogfish (Triakis scyllia) TNF decoy receptor 3 (TNFRSF6B) Fish Shellfish Immunol. 2008;24(3):360–365. doi: 10.1016/j.fsi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Fotiadu A, Kotoula V. Increased expression of soluble decoy receptor 3 in acutely inflamed intestinal epithelia. Clin Immunol. 2005;115(3):286–294. doi: 10.1016/j.clim.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Lee CS, Hu CY, Tsai HF, Wu CS, Hsieh SL, Liu LC, Hsu PN. Elevated serum decoy receptor 3 with enhanced T cell activation in systemic lupus erythematosus. Clin Exp Immunol. 2008;151(3):383–390. doi: 10.1111/j.1365-2249.2007.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li DL, Yu QZ, Feng XG, Li M, Lu W. The measures and experiences on actualizing “The Diagnosis and Treatment Criterion for Common Cancer in China” in Shanghai. Bull Chin Cancer. 2005;14(1):3–7. (in Chinese) [Google Scholar]
- 14.Macher-Goeppinger S, Aulmann S, Wagener N, Funke B, Tagscherer KE, Haferkamp A, Hohenfellner M, Kim S, Autschbach F, Schirmacher P, Roth W. Decoy receptor 3 is a prognostic factor in renal cell cancer. Neoplasia. 2008;10(10):1049–1056. doi: 10.1593/neo.08626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mild G, Bachmann F, Boulay JL, Glatz K, Laffer U, Lowy A, Metzger U, Reuter J, Terracciano L, Herrmann R, Rochlitz C. DcR3 locus is a predictive marker for 5-fluorouracil-based adjuvant chemotherapy in colorectal cancer. Int J Cancer. 2002;102(3):254–257. doi: 10.1002/ijc.10711. [DOI] [PubMed] [Google Scholar]
- 16.Pitti RM, Marsters SA, Lawrence DA, Roy M, Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396(17):699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 17.Roth W, Isenmann S, Nakamura M, Platten M, Wick W, Kleihues P, Bahr M, Ohgaki H, Ashkenazi A, Weller M. Soluble decoy receptor 3 is expressed by malignant gliomas and suppresses CD95 ligand-induced apoptosis and chemotaxis. Cancer Res. 2001;61(6):2759–2765. [PubMed] [Google Scholar]
- 18.Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46(5):645–650. doi: 10.1136/gut.46.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86(6):886–892. doi: 10.1038/sj.bjc.6600133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen HW, Wu YL, Peng SY. Overexpression and genomic amplification of decoy receptor 3 in hepatocellular carcinoma and significance thereof. Natl Med J China. 2003;83(9):744–747. (in Chinese) [PubMed] [Google Scholar]
- 21.Takahama Y, Yamada Y, Emoto K, Fujimoto H, Takayama T, Ueno M, Uchida H, Hirao S, Mizuno T, Nakajima Y. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5(2):61–68. doi: 10.1007/s101200200011. [DOI] [PubMed] [Google Scholar]
- 22.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58(23):5315–5320. [PubMed] [Google Scholar]
- 23.Tanaka K, Iwamoto S, Gon G, Nohara T, Iwamoto M, Tanigawa N. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6(1):127–134. [PubMed] [Google Scholar]
- 24.Wu Y, Han B, Sheng H, Lin M, Moore PA, Zhang J, Wu J. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105(5):724–732. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- 25.Xia C, Xu Z, Yuan X, Uematsu K, You L, Li K, Li L, McCormick F, Jablons DM. Induction of apoptosis in mesothelioma cells by antisurvivin oligonucleotides. Mol Cancer Ther. 2002;1(9):687–694. [PubMed] [Google Scholar]
- 26.Zhu XD, Lin GJ, Qian LP, Chen ZQ. Expression of survivin in human gastric carcinoma and gastric carcinoma model of rats. World J Gastroenterol. 2003;9(7):1435–1438. doi: 10.3748/wjg.v9.i7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]