Abstract
Adenoid cystic carcinoma rarely occurs within the subglottic larynx. In this study, a case of subglottic adenoid cystic carcinoma was reported. A 54 year-old Chinese woman developed a sudden onset of chest distress and cough worsening after physical exertion, and was diagnosed with, and treated as, bronchial asthma. Regular anti-asthmatic therapy did not improve the symptoms. Until a sudden dyspnea, a cervicothoracic computerized tomography (CT) revealed that her upper airway was obstructed by a laryngeal tumor. The patient was diagnosed with a subglottic adenoid cystic carcinoma and treated with complete surgical excision and adjuvant radiation therapy. Follow-up endoscopy and laryngeal magnetic resonance imaging (MRI) at six months showed no recurrence of the tumor. The diagnosis of subglottic adenoid cystic carcinoma should be considered in patients who are characterized by dyspnea, cough, and stridor, but do not respond to regular anti-asthmatic therapy.
Keywords: Subglottic adenoid cystic carcinoma, Larynx, Asthma
INTRODUCTION
Laryngeal malignant tumors are mainly squamous carcinomas. Adenoid cystic carcinomas originated from the salivary gland epithelium are rare. They account for less than 1% of all malignant tumors in the larynx (Damborenea Tajada et al., 1999; Aydin et al., 2008). Adenoid cystic carcinomas are very slow growing tumors. The symptoms in patients with early stage subglottic tumor were not significant and characterized by dyspnea, cough, and stridor, which were often wrongly diagnosed as asthma. The disease is often diagnosed at a late stage, leading to delay in treatment (Stack and Steckler, 1990; Javadi et al., 2002; Horiuchi et al., 1987; Sur et al., 1997).
Tracheal tumors usually affect the pulmonary function. The pulmonary function test may reveal disorder of the pulmonary ventilatory function, and the diagnosis of the tracheal tumor can be clarified by fiberoptic bronchoscopy (Kokturk et al., 2005). However, the subglottic tumor may exert less influence on the ventilatory function, so the pulmonary ventilatory function may appear normal. In addition, because the subglottic region is anatomically more occult, the fiberoptic bronchoscopy does not necessarily reveal the tumor in this area. The iconographic examination, particularly cervical computerized tomography (CT) or magnetic resonance imaging (MRI), is of great importance in the diagnosis of subglottic tumor.
A case with subglottic adenoid cystic carcinoma that had been previously diagnosed as asthma is reported in this paper.
CASE PRESENTATION
A 54 year-old Chinese woman had a sudden onset of chest distress and cough 5 months ago, which was worsening after physical exertion, and was diagnosed with bronchial asthma in a local county-level hospital. She received anti-infection and anti-asthma treatments for three times. The symptoms had some improvement, but were still uncontrolled. Half month ago, the symptoms became worse, and the patient could not lie flat at night. She came to our out-patient clinic, and was suspected of bronchial asthma again. The patient had no history of cigarette smoking.
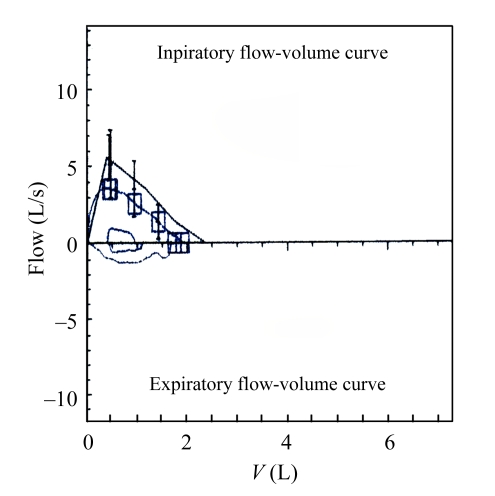
On admission to the Respiratory Department of our hospital, physical examination showed that her temperature was at 36.9 °C, pulse 90/min, blood pressure 120/80 mmHg, and leukocyte count 10.4×109 L−1. The patient was treated as bronchial asthma with cefminox 2 g twice a day, levofloxacin 500 mg once a day, montelukast sodium 10 mg once a day, ketoprofen 1 mg twice a day, methylprednisolone sodium succinate 40 mg for micro-pump administration twice a day, doxofylline 0.2 g for micro-pump twice a day, and budesonide 1 ml+salbutamol sulphate 1 ml+ipratropium bromide 1 ml for aerosol inhalation 4 times a day. In order to exclude the possibility of upper airway obstruction, thyroid B-ultrasonography, chest CT and fiberoptic bronchoscopy were performed. Thyroid B-ultrasonography showed that the bilateral cervical lymph nodes were detectable, and right thyroid nodule was complicated by calcification. Pulmonary function tests revealed normal spirometric values except low diffusion capacity, forced vital capacity (FVC) was 80.6% of the predicted, forced expiratory volume in one second (FEV1) was 88.3% of the predicted, and FEV1/FVC ratio was 91.9% (Fig.1). Chest CT revealed a nodule in the left lower lobe, which unobstructed the airway lumen. Fiberoptic bronchoscopy showed that no evident abnormality was found in the bilateral bronchi, and a smear for cytological examination found no tumor cells. These findings suggested that the asthmatic symptoms were not obviously improved.
Fig.1.
Flow-volume loop displays suggestive normal spirometric values
FVC: 1.9 L (80.6%); FEV1: 1.74 L (88.3%); FEV1/FVC:91.9%
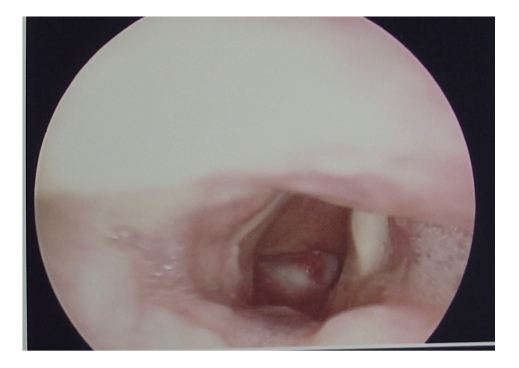
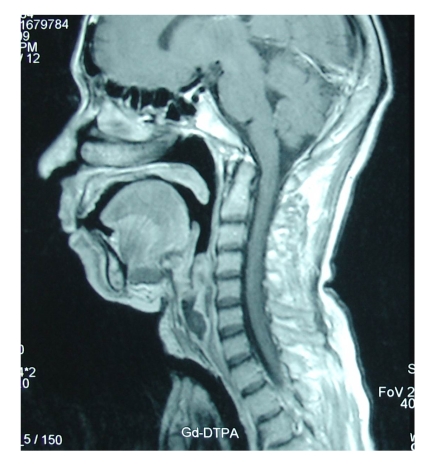
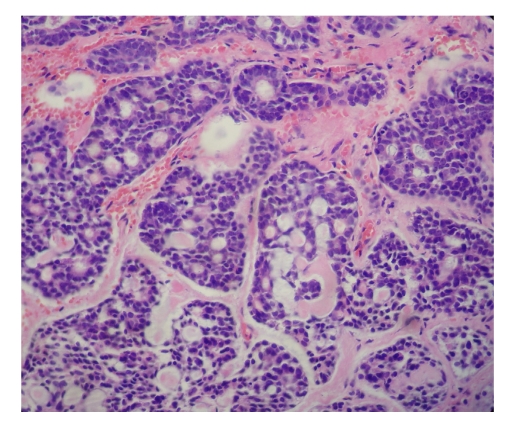
Ten days later, the patient had a sudden dyspnea, and underwent an urgent endotracheal intubation in the Intensive Care Unit. Decannulation was performed two days later, but the dyspnea relapsed immediately with stridor, and then the endotracheal intubation was performed again. Considering the presence of an upper airway obstruction, we performed a cervicothoracic CT on this patient and found that her upper airway was obstructed by a laryngeal tumor. Then tracheotomy was performed and direct laryngoscopy revealed a hemispherical projection at the posterior wall of the subglottic region, blocking up approximately 90% of the airway lumen. The mucous membrane surface of the tumor was smooth (Fig.2). Esophagoscopy was performed and showed that the esophagus and the trachea were no invaded by the tumor. Laryngeal MRI showed obvious thickening of the laryngeal soft tissue, and T1W1 appeared with low signal and T2W1 with high signal, showing remarkable heterogeneous potentialization of the lesion after enhancement and evident narrowing of the airway passage (Fig.3). The patient was then treated with laryngofissure subglottic resection of the tumor, and the postoperative pathology reported an adenoid cystic carcinoma (Fig.4). After the operation, the patient underwent adjuvant radiation therapy. Follow-up examination with endoscopy and laryngeal MRI six months later showed no relapse of the tumor.
Fig.2.
Dynamic Laryngoscopic finding of subglottic tumor
Fig.3.
Imaging of sagittal MRI with subglottic tumor
Fig.4.
Imaging of microscope with adenoid cystic carcinoma (hematoxylin and eosin (H&E), ×400)
DISCUSSION
Subglottic adenoid cystic carcinoma is rare and often disregarded until it reaches an advanced stage (Damborenea Tajada et al., 1999). The symptoms with subglottic or tracheal tumor include prolonged cough and wheezing. It can be misdiagnosed as asthma (Garas and McGuirt, 2006; Dahm et al., 1998; Kokturk et al., 2005; Goyal et al., 2005).
Precise diagnosis of tracheal tumor is extremely challenging (Kokturk et al., 2004). It usually becomes symptomatic when it starts occluding more than 75% of the lumen (Goyal et al., 2005).
Subglottic tumor may exert little influence on the pulmonary ventilatory function. The patient in this report with subglottic tumor occupying 90% of the airway showed basically normal in the pulmonary function tests. The possibility of a central obstructing lesion should be considered if the severity of asthma increased under adequate anti-asthmatic therapies (Kokturk et al., 2005). CT scan and/or MRI would yield valuable data of the tracheal lesion (Allen, 1993).
If a patient with an atypical asthma was unresponsive to regular anti-asthmatic therapy, it should be considered that a tumor, such as adenoid cystic carcinoma, presented in the larynx or trachea. The diagnostic procedures such as fiberoptic laryngoscope, fiberoptic bronchoscope, and laryngotracheal CT/MRI can be decisive (Shi, 1987). The treatment of adenoid cystic carcinoma is mainly surgery and postoperative radiotherapy (Sur et al., 1997). Partial surgery is possible in the selected cases, but total laryngectomy is usually recommended because of the high propensity for submucosal spread and perineural and lymphovascular invasion (Ganly et al., 2006). However, the prognosis of adenoid cystic carcinoma is much worse, with 15- to 20-year survival rates being less than 20% (Morais et al., 1999). In adenoid cystic carcinoma, regional recurrence is rare, but distant recurrence is common and may occur up to 10 years after the index therapy (Ganly et al., 2006).
In the present case, the upper airway mass was not considered at first, because the symptoms were similar to asthma, and anti-asthma treatments could relieve the patient’s condition sometimes. Moreover, pulmonary function test was normal, because the tumor was fixed at the posterior wall of the subglottic region, and was not moveable along with patient’s respiration thus not affecting the airflow. Bronchoscopy did not find the subglottic mass because the tumor was located at a hidden position of the subglottic region, and a subglottic mass was not expected. Chest CT showed no evident abnormality, because it did not scan the subglottic region. First anesthesia intubation failed to detect the subglottic tumor, because it was performed urgently to save the life of the patient. However, when the patient presented with dyspnea after the removal of the intubation, and the respiratory obstruction disappeared again after the intubation was made, the upper airway obstruction became obvious. Cervicothoracic CT was performed and suggested the existence of a subglottic tumor, which was further demonstrated by tracheotomy and direct laryngoscopy. Other possible diagnoses such as tracheal compression from mediastinum, tracheal stenosis, airway foreign body, thyroid tumor, and esophageal tumor were ruled out in this case. Laryngeal MRI showed that the airway passage became evidently narrow. Laryngofissure subglottic resection of the tumor was performed and afterward adjuvant radiation therapy was given. No relapse of the tumor was noted in the half-year examination.
CONCLUSION
Adenoid cystic carcinomas originated from the subglottic region are rarely seen. It is difficult to discover the tumors at early stages, and it is likely to misdiagnose such a patient to have asthma. The diagnosis of a subglottic adenoid cystic carcinoma should be considered in patients who are characterized by dyspnea, cough, and stridor, but are not responsive to regular anti-asthmatic therapies, even if pulmonary function test, chest CT, and fiberoptic bronchoscopy show no evident abnormality.
References
- 1.Allen MS. Malignant tracheal tumours. Mayo Clin. Proc. 1993;68(7):680–684. doi: 10.1016/s0025-6196(12)60604-1. [DOI] [PubMed] [Google Scholar]
- 2.Aydin O, Ustundag E, Iseri M, Ercin C. Laryngeal adenoid cystic carcinoma in an adolescent. Kulak Burun Bogaz Ihtis Derg. 2008;18(5):319–322. [PubMed] [Google Scholar]
- 3.Dahm JD, Sessions DG, Paniello RC, Harvev J. Primary subglottic cancer. Laryngoscope. 1998;108(5):741–746. doi: 10.1097/00005537-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Damborenea Tajada J, Campos del Alamo MA, Marín García J, Leache Pueyo J, Liorente Arenas E, Fernández Liesa R. Adenoid cystic carcinoma of the larynx: case report and literature review. An Otorrinolaringol Ibero Am. 1999;26(3):257–263. [PubMed] [Google Scholar]
- 5.Ganly I, Patel SG, Coleman M, Ghossein R, Carlson D, Shah JP. Malignant minor salivary gland tumors of the larynx. Arch Otolaryngol Head Neck Surg. 2006;132(7):767–770. doi: 10.1001/archotol.132.7.767. [DOI] [PubMed] [Google Scholar]
- 6.Garas J, McGuirt WF. Squamous cell carcinoma of the subglottis. Am J Otolaryngol. 2006;27(1):1–4. doi: 10.1016/j.amjoto.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Goyal A, Tyagi I, Tewari P, Agarwal SK, Syal R. Management of difficult airway in intratracheal tumor surgery. BMC Ear Nose Throat Disorders. 2005;5(1):4. doi: 10.1186/1472-6815-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horiuchi J, Shibuya H, Suzuki S, Takeda M, Takagi M. The role of radiotherapy in the management of adenoid cystic carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1987;13(8):1135–1141. doi: 10.1016/0360-3016(87)90185-4. [DOI] [PubMed] [Google Scholar]
- 9.Javadi M, Bafrouee FM, Monseni M. Laryngeal adenoid cystic carcinoma in a child: a case report. Ear Nose Thorat J. 2002;81(1):34–35. [PubMed] [Google Scholar]
- 10.Kokturk N, Demir N, Kervan F, Ninc E, Koybasioqlu A, Turktas H. A subglottic mass mimicking near-fatal asthma: a challenge of diasnosis. J Emerg Med. 2004;29(1):104–105. doi: 10.1016/j.jemermed.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kokturk N, Demircan S, Kurul C, Turktas H. Tracheal adenoid cystic carcinoma masquerading asthma: a case report. BMC Pulm Med. 2005;19(4):10. doi: 10.1186/1471-2466-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morais PD, Cortejoso HA, Borau CM. Adenoid cystic carcinoma of the larynx. Acta Otorrinolaringol Esp. 1999;50(8):660–663. [PubMed] [Google Scholar]
- 13.Shi ML. X-ray diagnosis of trachea and large tracheal primary non-squamous epithelial carcinoma: analysis of 23 cases. Chin J Tumour. 1987;9:208–210. (in Chinese) [Google Scholar]
- 14.Stack PS, Steckler RM. Tracheal neurilemmoma:case report and review of the literature. Head Neck. 1990;12(5):436–439. doi: 10.1002/hed.2880120512. [DOI] [PubMed] [Google Scholar]
- 15.Sur RK, Donde B, Levin V, Pacella J, Kotzen J, Cooper K, Hale M. Adenoid cystic carcinoma of the salivary glands: a review of 10 years. Laryngoscope. 1997;107(9):1276–1280. doi: 10.1097/00005537-199709000-00022. [DOI] [PubMed] [Google Scholar]