Abstract
Background and aims
Previous studies suggest that depression increases risk of falls, low bone mineral density, and fractures. Our aim was to evaluate whether depressive symptomatology alone predicts 5-year clinical fracture risk in older adults.
Methods
In this secondary analysis of a communitybased, prospective cohort study including 4175 women and 1652 men in Canada, depressive symptomatology was assessed at baseline by the mental health inventory-5 (MHI-5) and the mental component score (MCS) of the short form 36 questionnaire (SF-36). Fracture events were assessed annually for five years; all reported incident fragility fractures were confirmed radiographically.
Results
Depressive symptomatology did not predict time to first fracture in men (hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.45–1.65) or women (HR 1.09, 95% CI 0.86–1.39). Results were similar after controlling for potential confounders. Depressive symptoms were not significantly associated with baseline bone mineral density at the lumbar spine or femoral neck. Women with depressive symptoms were more likely to report falls in the previous month (odds ratio [OR] 1.52, 95% CI 1.12–2.06, p=0.01). This association did not achieve statistical significance in men (OR 1.71, 95% CI 0.96–3.04, p=0.07).
Conclusion
In this large, community cohort, depressive symptomatology did not predict five-year risk of clinical fracture. Further research is needed to determine if individuals with major depressive disorder (MDD) are at higher fracture risk and whether neuroendocrine or hormonal dysregulation might contribute to such risk in MDD.
Keywords: Bone mineral density, depression, falls, osteoporosis, risk
INTRODUCTION
Depression and osteoporosis are both prevalent, highly morbid geriatric conditions, and it has been suggested that depression may increase the risk of bone fracture (1, 2). However, the etiology of this potential link remains uncertain. Depression is a known risk factor for falls in the elderly (3–5). The use of anti-depressive medications has been associated with increased fall risk and fracture rates (6–8). Several groups have reported that patients with major depressive disorder (MDD) have lower bone mineral density (BMD) than their non-depressed peers (9–14). This finding might reflect altered levels of glucocorticoids or other neuroendocrine modulators of bone metabolism in patients with MDD. Alternatively, lower BMD in depressed patients could be mediated through confounders such as medications, comorbidities, altered mobility patterns, and behaviors. To meet diagnostic criteria for MDD, an individual must exhibit depressive symptoms consecutively for at least two weeks, and the episode must represent a change from baseline functioning and be significant enough to cause distress or impairment in social and occupational functioning (15). To improve our understanding of the apparent link between affective health and bone health, we sought to determine whether depressive symptomatology per se, as opposed to MDD, is associated with higher fracture risk or lower BMD in older adults.
To our knowledge, only two studies have suggested an association between depressive symptoms and fractures (16, 17). One study found that elderly women who scored positively on the Geriatric Depression Scale were more likely to suffer falls, vertebral fractures, and non-vertebral fractures (16). After controlling for the history of falls, the association between depression and non-vertebral fractures retained only borderline statistical significance (OR 1.2, 95% CI 1.0–1.5) (16). Of note, depressive symptoms were not associated with lower BMD in that study (16). A second study, which used data from the National Health and Nutrition Examination Survey (NHANES I), found that high depressive symptomatology detected by the four-item General Well-being Schedule (GWB-D) was predictive of hip fracture during the 22-year follow-up period (17). The investigator concluded that additional large-scale studies were needed to verify the findings, partly because that study relied on International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) codes to detect fractures (17).
We sought to determine whether depressive symptomatology is associated with increased risk of clinical fractures in a large, representative sample of older adults with meticulously collected fracture data. The Canadian Multicentre Osteoporosis Study (CaMos) has collected radiographically-confirmed fracture data on thousands of older adults over a five-year period. In addition, CaMos assessed multiple health, lifestyle, and demographic variables known to affect fracture risk. We hypothesized that individuals with depressive symptomatology at baseline would be more likely to sustain clinical fragility fractures over the next five years. Our second aim was to evaluate whether depressive symptoms at baseline were associated with fall history and lower baseline BMD.
METHODS
Study design and population
The Canadian Multicentre Osteoporosis Study (CaMos) has prospectively followed a randomly selected, population-based cohort of non-institutionalized adults living within 50 km of one of nine urban regional centers. Details of the purpose and methodology of the CaMos cohort have been reported elsewhere (18). Briefly, recruitment for the cohort began in February 1996 and ended in September 1997. At an initial baseline visit, BMD was measured in all available subjects and participants were interviewed by a trained interviewer to assess for osteoporosis and fracture-related risk factors. A fracture questionnaire was mailed to subjects annually and a second intensive interview was conducted five years after enrollment (between July 2000 and January 2003) to reassess fracture risk factors. The present analysis is restricted to those participants aged 50 years or older at baseline who were enrolled at one of seven regional centers that obtained radiographic verification of all reported incident fragility fractures. The study was approved by regional institutional ethics review boards, all participants provided written informed consent, and the research activities were in compliance with the Helsinki Declaration.
Depressive symptoms
Depressive symptomatology was assessed at baseline with the mental health inventory-5 (MHI-5) and the mental component score (MCS) of the short form 36 questionnaire (SF-36) (19). The MCS provides a validated measure of mental health and exhibits a strong association with severity of depressive symptoms (20). The MHI-5 is another validated mental health measure, and the cut-point of <52 has been established to indicate the presence of depressive symptomatology (21, 22). In the Medical Outcomes Study population, a cut-point of <42 on the MCS demonstrated optimal test characteristics for detection of depression (sensitivity 73.7%, specificity 80.6%) and confirmed the cut-point of <52 for the MHI-5 (sensitivity 66.8%, specificity 86.2%) when validated against the National Institute for Mental Health Diagnostic Interview Schedule (19, 23). To maximize sensitivity, depressive symptomatology was defined as a score <52 on the MHI-5 scale or <42 on the MCS.
Clinical fragility fractures
After the baseline interview, participants were mailed annual questionnaires to ascertain if they had experienced a fracture since their last contact. The completion rate was excellent with 98.4% of participants returning at least one follow-up questionnaire. All reported clinical fragility fractures were confirmed radiographically by the study investigators and the date of fracture was recorded. Clinical fragility fractures were defined as fractures that occurred due to minimal trauma (e.g. falling from standing height, bed or chair). This definition was intended to identify fractures likely to be related to osteopenia or osteoporosis, rather than pathologic fractures or fractures sustained through major trauma such as a motor vehicle accident. Follow-up continued for fracture outcomes until the end of the fifth year of the study.
Bone mineral density (BMD)
Six of seven centers measured bone mineral density of the lumbar spine (L1-L4) and hip using dual energy X-ray absorptiometry (DXA) with Hologic QDR 1000, 2000 and 4500 while one center used a Lunar DPX densitometer. All BMD results were converted to a Hologic standard, using the method described by Genant et al. (24). Machine calibration occurred daily. Daily and weekly quality assurance tests were performed as recommended by manufacturers. Cross-calibration was performed by the same medical physicist using the same European Spine Phantom at each site (25).
Falls
Fall history was assessed at baseline by self report. Specifically, participants were asked whether they had fallen in the last month.
Other variables
Additional baseline variables known to affect fracture risk were chosen a priori for inclusion in the analysis models. To create appropriately parsimonious models predicting fracture, three authors [HEW, DTG, KWL] deliberated and agreed to include the following covariates: age, body mass index (BMI), smoking status, use of psychotropic medication, use of steroids, history of previous fracture, comorbidity index, falls, bone mineral density. Participants’ age was calculated from date of birth. BMI was calculated from the subject’s weight in kilograms divided by the square of his or her height in meters. Participants reported their current smoking status and medication use. Dichotomous variables were created for smoking (cigarettes, pipes, or cigars), steroid use (inhaled, oral, or injection steroids in the last month), and use of psychoactive medications (tricyclic antidepressants, selective serotonin reuptake inhibitors, short-acting benzodiazepines, long-acting benzodiazepines, antipsychotics, or barbiturates). Complete fracture history was obtained at baseline and a variable was created for history of any minimal trauma fracture prior to enrollment. A modified Charlson index was based on the following comorbidities: liver disease, heart disease, stroke, diabetes (type 1 or 2), kidney disease, rheumatoid arthritis, chronic obstructive pulmonary disease, breast cancer, uterine cancer, hypertension, dementia, and prostate cancer (26). One point was given for each condition which was diagnosed by a physician; in addition, a point was awarded for dementia in patients who scored <24 on the Mini-Mental Status Exam (MMSE) (27). Race was not included as a covariate because of the racial homogeneity of the cohort with respect to fracture risk (fewer than 1.0% of this cohort was black). Family history of osteoporosis was not included as a covariate because >25% of the cohort was missing data for this variable.
Statistical analysis
Because depressive symptomatology and bone health are both strongly influenced by sex (28–30); any associations between them may differ importantly in men and women. Thus, all analyses were stratified by sex. This decision was made prior to analysis, to minimize issues related to multiple model testing. Descriptive statistics were generated to characterize men and women at baseline, with means, standard deviations, medians and inter-quartile ranges calculated for continuous variables and percentages calculated for dichotomous or ordinal variables.
Cox proportional hazards regression models assessed the association between depressive symptomatology at baseline and incident clinical fragility fractures. This methodology accounts for the impact of drop-out (due to death or other factors) on subject’s time at risk of fracture. For the adjusted models, covariate selection occurred a priori with an intention to include the most important potential confounders while producing a model that was appropriately parsimonious. Proportional hazards assumptions were met. Kaplan-Meier curves were produced to display the probability of remaining fracture free over time, among participants with or without depressive symptoms at baseline. The association between depressive symptomatology and fall history at baseline was assessed using logistic regression methods. To compare the adjusted mean BMDs between subjects with and without depressive symptomatology, an analysis of covariance (AN-COVA) was performed. All analyses were performed using SAS V.9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
The analysis included 1652 older men and 4175 older women who were enrolled at the seven CaMos sites where all reported clinical bone fractures were radiographically confirmed. Depressive symptomatology was detected in 148 (9.0%) of the men and 572 (13.7%) of the women. The majority of depressive symptomatology was detected by a MCS score <42; depressive symptomatology was detected solely by MHI score <52 in only 8 of 148 men (5.4%) and 41 of 572 women (7.2%). The prevalence of depressive symptomatology in this cohort is similar to the previously reported prevalence of depressive symptoms in the general North American population (31). Descriptive statistics characterizing the male and female cohorts are reported in Table 1. Among men, those with depressive symptoms were more likely to live alone and to use medications including steroids, selective serotonin reuptake inhibitors (SSRIs), tri-cyclic antidepressants (TCAs), and benzodiazepines. Among women, those with depressive symptoms were less educated, reported more chronic diseases and falls, and were more likely to smoke and use steroids, SSRIs, TCAs, and benzodiazepines.
Table 1.
Baseline characteristics of male and female participants with and without depressive symptoms.
| Men |
Women |
|||
|---|---|---|---|---|
| Characteristic | With depressive symptoms n=148 (9.0%) | Without depressive symptoms n=1504 (91.0%) | With depressive symptoms n=572 (13.7%) | Without depressive symptoms n=3603 (86.3%) |
| Age in years, mean±SD | 65.5±10.2 | 66.2±9.3 | 65.8±9.3 | 66.8±9.2* |
| Race, % black | 0.7 | 0.8 | 0.2 | 0.4 |
| Live alone, % | 35.1 | 22.3** | 40.4 | 37.2 |
| Education level | ||||
| <High school, % | 41.2 | 38.5 | 52.1 | 43.9** |
| High school or trade | ||||
| Degree, % | 25.0 | 27.2 | 30.6 | 34.1 |
| College attendance, % | 33.8 | 34.3 | 17.3 | 22.0 |
| Current smoking, % | 18.2 | 17.4 | 18.4 | 12.1** |
| Alcoholic drinks per day | ||||
| none, % | 27.7 | 29.1 | 50.1 | 44.8 |
| ≤ one, % | 50.7 | 51.8 | 44.1 | 49.8 |
| > one, % | 21.6 | 19.1 | 5.8 | 5.4 |
| Total mg/day caffeine in last 12 months, median (IQR) | 273 (104, 413) | 272 (131, 460) | 233 (102, 393) | 247 (93, 393) |
| BMI, mean±SD | 27.0±4.1 | 27.1±4.0 | 27.2±5.3 | 27.0±5.1 |
| % with history of: | ||||
| Stroke/TIA | 3.2 | 5.3 | 5.8 | 3.5* |
| Liver disease | 3.9 | 2.7 | 4.0 | 2.6 |
| Heart disease | 11.8 | 10.6 | 4.6 | 4.8 |
| Diabetes | 10.8 | 8.4 | 5.6 | 6.7 |
| Rheumatoid arthritis | 6.9 | 4.9 | 8.5 | 6.5 |
| Kidney disease | 2.7 | 1.5 | 1.2 | 1.8 |
| COPD | 11.6 | 7.6 | 12.3 | 10.3 |
| Hypertension | 32.4 | 27.4 | 33.3 | 32.2 |
| Breast cancer | 0.0 | 0.1 | 5.8 | 4.8 |
| Uterine cancer | NA | NA | 2.8 | 2.5 |
| Prostate cancer | 3.4 | 4.0 | NA | NA |
| Dementia | 5.4 | 3.0 | 3.5 | 1.6* |
| Charlson Score, mean±SD | 0.89±1.01 | 0.74±0.89 | 0.85±1.00 | 0.75±0.91* |
| Fallen in last month, % | 10.1 | 6.2 | 9.8 | 6.7** |
| Family history of osteoporosis, % | 7.3 | 11.0 | 20.8 | 20.6 |
| Medication history | ||||
| Steroid therapy, % | 16.2 | 9.8* | 18.5 | 12.7** |
| Daily SSRI use, % | 8.1 | 0.9** | 9.3 | 2.1** |
| Daily TCA use, % | 6.8 | 1.0** | 9.8 | 3.1** |
| Daily Benzodiazepines, % | 7.4 | 2.9* | 12.9 | 3.7** |
| Daily Antipsychotics, % | 0.7 | 0.0 | 0.9 | 0.3 |
| Daily Phenobarbiturates, % | 0.7 | 0.3 | 0.5 | 0.2 |
| Femoral Neck BMD (g/cm2), mean±SD | 0.78±0.11 | 0.80±0.13 | 0.70±0.12 | 0.70±0.12 |
| L1–4 BMD (g/cm2), mean±SD | 1.04±0.17 | 1.05±0.18 | 0.93±0.17 | 0.92±0.17 |
SD= standard deviation, mg= milligrams, BMI= body mass index, TIA= transient ischemic attack, COPD= chronic obstructive pulmonary disease, SSRI= selective serotonin reuptake inhibitors, TCA= tricyclic antidepressants, BMD= bone mineral density, Ll-4=lumbar vertebra 1–4. Variables for which a statistically significant difference exists between participants with and without depressive symptoms are indicated
p<0.05
p<0.001.
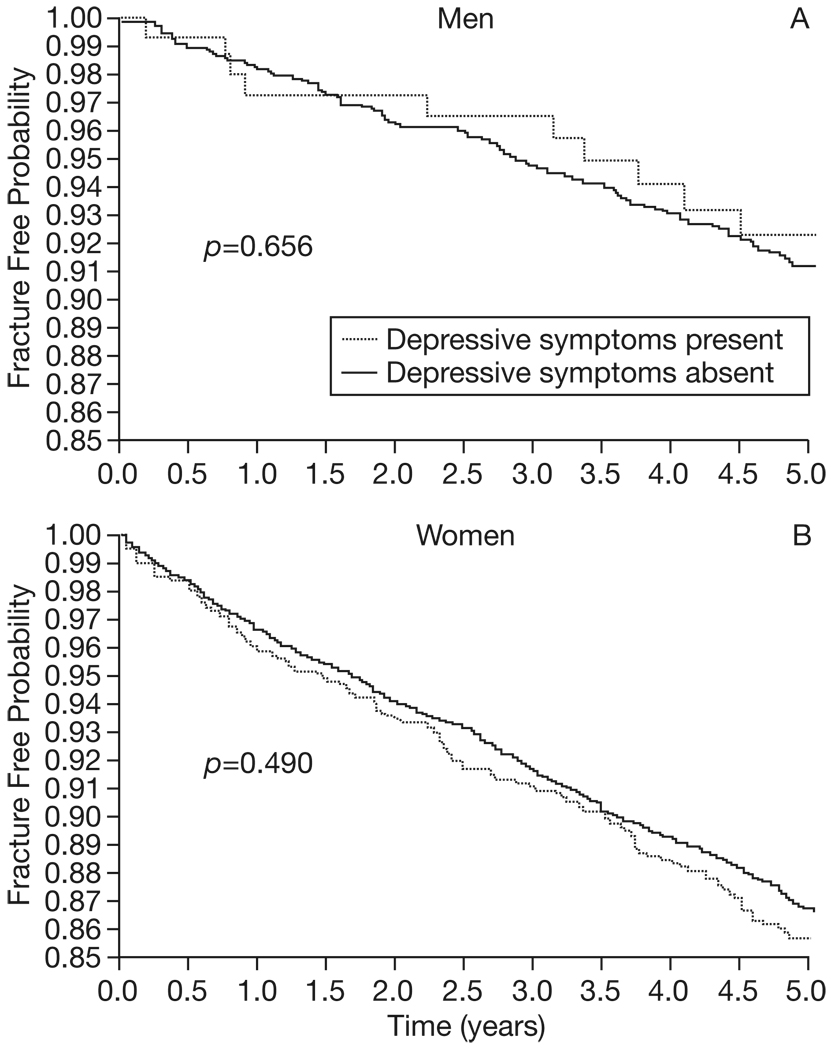
During the five year study period, 134 men and 538 women experienced a radiographically-confirmed clinical fragility fracture. Depressive symptomatology at baseline was not a predictor of time to first fracture in unadjusted or adjusted models (Table 2). It was noted that 192 men (11.6%) and 523 women (12.5%) were missing BMD data at baseline. The participants with missing BMD data were older, less educated, and suffered more chronic diseases than participants who received DXA scans. In order to ensure that including this variable, and thus excluding participants with missing BMD data, did not bias the results of the adjusted analysis, the proportional hazards models were repeated with all the prespecified covariates except femoral neck BMD: results were unchanged (data not shown). Similarly, the models were repeated, including all covariates except fall history; there remained no association between depressive symptomatology and fracture risk. Finally, models were repeated including interaction terms for depressive symptoms*BMD and depressive symptoms *psychoactive medication use. The interactions were not significant and the primary outcome was unchanged. Kaplan-Meier curves illustrating the probability of remaining fracture free over time are presented, for participants with and without depressive symptoms (Fig. 1). No significant differences in estimated fracture rate existed among participants with and without depressive symptoms (Table 3).
Table 2.
Depressive symptoms at baseline were not a significant predictor of time to first fracture. Unadjusted and adjusted proportional hazards models vielded similar results in men and women.
| Men | Women | |
|---|---|---|
| Predictor of time to first fracture | Hazard Ratio (95% CI) | Hazard Ratio (95% CI) |
| Depressive symptoms, Unadjusted | 0.86 | 1.09 |
| (0.45–1.65) | (0.86–1.39) | |
| n=1652 | n=4175 | |
| Depressive symptoms, Adjusted* | 0.77 | 0.93 |
| (0.39–1.53) | (0.70–1.22) | |
| n=1458 | n=3637 |
Adjusted model controls for age, bone mineral density at femoral neck, body mass index, steroid use, smoking status, history of falls, modified Charlson comorbidity index, history of previous minimal trauma fracture, and psychoactive medication use. Observations with missing values were excluded from each analysis.
Fig. 1.
Fracture risk over time in men and women, stratified by the presence or absence of depressive symptoms. Kaplan Meier curves were created for men (Panel A) and women (Panel B) to reflect fracture risk. The curves demonstrate that the probability of remaining fracture free over the five year study was similar, regardless of whether or not a articipant exhibited depressive symptomatology at baseline.
Table 3.
Kaplan-Meier estimates of fracture rate did not differ significantly based on whether or not depressive symptoms were present at baseline.
| Men |
Women |
|||
|---|---|---|---|---|
| Depressive symptoms present | Depressive symptoms absent | Depressive symptoms present | Depressive symptoms absent | |
| Through Year 1 | ||||
| Fracture rate | 2.7% | 1.8% | 4.0% | 3.4% |
| 95% CI | 1.0–7.0 | 1.2–2.6 | 2.7–6.0 | 2.8–4.0 |
| Through Year 5 | ||||
| Fracture rate | 7.7% | 8.8% | 14.4% | 13.4% |
| 95% CI | 4.2–14.0 | 7.4–10.4 | 11.7–17.7 | 12.3–14.5 |
CI= confidence interval.
Women who endorsed depressive symptomatology were more likely to report recent falls (OR 1.52, 95% CI 1.12–2.06, p=0.01). In men, however, the association between depressive symptoms and recent fall history was not statistically significant (OR 1.71, 95% CI 0.96–3.04, p=0.07). Depressive symptomatology was not predictive of bone mineral density at the femoral neck or the lumbar spine in men or women, whether or not the analysis controlled for potential confounders (age, body mass index, steroid use, psychoactive medication use, smoking status, fall history, history of prior fracture, and modified Charlson Index). When the adjusted BMD of men with depressive symptoms is compared to the adjusted BMD of men without depressive symptoms, the estimated percent difference is −1.3% (95% CI −4.0% to 1.4%) at the femoral neck and −0.8% (95% CI −3.9% to 2.2%) at the lumbar spine. In women, the estimated percent difference in adjusted BMD is −0.7% (95% CI −2.1% to 0.7%) at the femoral neck and 0.6% (95% CI −1.1% to 2.3%) at the lumbar spine.
DISCUSSION
In this population-based sample of older adults, depressive symptoms at baseline were not significantly associated with incident clinical fragility fractures during the five year study period, nor were depressive symptoms associated with a significant difference in bone mineral density at baseline. The primary finding contradicts our pre-study hypothesis, which was that depressive symptomatology would predict incident fractures. Although the absence of a statistically significant association does not prove the null hypothesis, the findings of this study are compelling in that multiple models yielded consistent results. In both men and women, the point estimate for the hazard ratio was close to one, regardless of whether the model adjusted for potential confounders. This cohort study has 80% statistical power to detect a minimal hazard ratio for fracture of approximately 2.0 in men and 1.4 in women; thus, it is unlikely that a large effect was missed by this analysis.
Two previous studies have reported an association between depressive symptoms and incident fractures (16, 17), but the present study differs from those in important ways which may explain the disparate result. The study conducted by Mussolino relied on administrative data to detect hip fractures (17), a method that may favor the detection of fractures in depressed people, because people with depressive symptoms access the healthcare system more frequently (32). The study by Whooley et al. did not account for anti-depressant use, and only the association between depressive symptoms and vertebral fractures remained significant after controlling for falls (16). Because CaMos focused on the outcome of clinical fractures, it is likely that asymptomatic or undiagnosed vertebral fractures were undetected in this study. Finally, the impact of confounding may differ substantially in each study, since the populations are quite disparate and the analyses rely on different instruments to detect depressive symptoms and therefore may assess different degrees of depression or other mental health states (16, 17).
It should be emphasized that the current study assessed the association between fracture risk and depressive symptoms, not major depressive disorder (MDD). To our knowledge, no large study has evaluated the fracture risk associated with major depressive disorder, as diagnosed by Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. Depressive symptomatology in this study was not associated with lower BMD, which is consistent with the results of a previous study (16). By contrast, a small study comparing femoral neck BMD of 24 women with MDD to 24 matched controls found that the depressed women had a 13.6% decrease in BMD (12). Thus, patients who suffer from MDD may be at considerably higher risk of fracture compared to individuals who merely exhibit depressive symptoms. The fact that the large CaMos cohort revealed no significant associations between depressive symptomatology and baseline BMD or 5-year hazard of clinical fracture suggests that affective changes per se are not a major risk factor in unselected populations.
This finding may have important implications regarding underlying causes of lower bone mineral density in patients with MDD. For example, it is probable that MDD patients suffer more profound dysregulation in neuro-endocrine systems than individuals who merely exhibited depressive symptomatology at one point in time, and such dysregulations could impact bone metabolism. It is also possible that patients with MDD may have greater exposure to medications with deleterious effects on bone. It is notable that both TCAs and SSRIs have been associated with fracture risk (8, 9), and in the CaMos cohort only 14.9% of men and 17.5% of women with depressive symptomatology reported daily use of one of these medications. This low rate of pharmacologic anti-depressant therapy among individuals with depressive symptoms is consistent with the findings of other large-scale studies in elderly populations (33, 34). Finally, it is possible that complications of low bone mineral density worsen the disease course of depression. A recent study examined 3798 women with low BMD and found that the prevalence of depressive symptoms and probable depression was significantly higher among those with prevalent vertebral fractures compared to those with no prevalent vertebral fracture (35). Of course, the causality of the association cannot be inferred from cross-sectional data, but the authors posit that patients with the dual diagnosis of depression and osteoporosis may suffer worse health outcomes (35). Future studies that seek to determine whether MDD is associated with increased fracture risk should be designed such that investigators can explore the role of potential mediating factors such as medications and endogenous modulators of bone health, as well as the causal direction of the association.
The current study has several limitations. First, the study relies on the mental health inventory-5 (MHI-5) and the mental component score (MCS) of the short form 36 questionnaire (SF-36) to detect depressive symptoms. Although these tools were not specifically designed to measure depressive symptomatology, both instruments are validated measures of affective health which correlate strongly with depression (19–23). Second, depressive symptoms were assessed only once; therefore, the study is unable to determine how duration of depressive symptoms might impact fracture risk. A third limitation in this observational study is the possibility that important confounders were not considered. For example, the cross-sectional association between depressive symptoms and recent falls, which was observed in women, could reflect the fact that both falls and depressive symptoms occur more commonly in winter months. For the primary analysis, covariates were specified a priori with an intention to include the most important potential confounders while avoiding overcrowding the models. The covariates were chosen by consensus of three authors [HEW, DTG, KWL]. It is unlikely that failure to control for a confounder would change the primary result of this study, because no association was observed between the independent variable of interest (depressive symptomatology) and the primary outcome (time to first fracture). Finally, CaMos lacks confirmed death data, so censoring for death was not possible in the proportional hazards analysis. This is unlikely to have significantly impacted the results, because events (fractures) were reported only at annual follow-up and participants were censored at the time of their last contact with the study.
CONCLUSIONS
This is the first study to address the potential association between depressive symptomatology and clinical fracture risk in a large, population-based sample with radiographically confirmed fracture data and BMD measurements. Although depressive symptoms were associated with a history of recent falls in women, no significant association was observed between depressive symptoms and baseline BMD or five-year fracture risk. These results suggest that depressive symptomatology is not a major risk factor for fracture. Future research is necessary to determine whether fracture risk is substantially elevated in patients with major depressive disorder or prolonged dysthymia, and if so, what factors may mediate the association.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. George Ioannidis for assistance with data acquisition and management, and Dr. Wei Zhou for supplementary statistical analysis. Statistical support for this project was funded in part by a Center for Excellence Grant awarded by the John A. Hartford Foundation (2002–0269). In addition, Dr. Whitson is supported by a Veterans Affairs Special Fellowship in Advanced Geriatrics and Drs. Whitson, Lyles, and Pieper are supported by the Duke Claude D. Pepper Older Americans Independence Center (1P30 AG028716-01). The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research (CIHR); Merck Frosst Canada Ltd.; Eli Lilly Canada Inc.; Novartis Pharmaceuticals Inc.; The Alliance: Sanofi-Aventis & Procter and Gamble Pharmaceuticals Canada Inc.; The Dairy Farmers of Canada; and The Arthritis Society. Drs. Whitson, Gold, Lyles, Adachi, and Papaioannou report having consulted, served on advisory boards, conducted trials with, or received honoraria from at least one of the companies listed above. These funding sources had no role in the conception of this analysis, statistical methods or interpretation of the data.
REFERENCES
- 1.Lyles K. Osteoporosis and depression: shedding more light upon a complex relationship. J Am Geriatr Soc. 2001;49:827–828. doi: 10.1046/j.1532-5415.2001.49162.x. [DOI] [PubMed] [Google Scholar]
- 2.Cizza G, Ravn P, Chrousos GP, et al. Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab. 2001;12:198–203. doi: 10.1016/s1043-2760(01)00407-6. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti M, Williams TF, Mayewski R. Fall risk index for elderly patients based on number of chronic disabilities. Am J Med. 1986;80:429–434. doi: 10.1016/0002-9343(86)90717-5. [DOI] [PubMed] [Google Scholar]
- 4.Nevitt MC, Cummings SR, Kidd S, et al. Risk factors for recurrent nonsyncopal falls: a prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- 5.Granek E, Baker SP, Abbey H, et al. Medications and diagnoses in relation to falls in a longterm care facility. J Am Geriatr Soc. 1987;35:503–511. doi: 10.1111/j.1532-5415.1987.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 6.Ensrud KE, Blackwell TL, Mangione CM, et al. Central nervous system - active medications and risk for falls in older women. J Am Geriatr Soc. 2002;50:1629–1637. doi: 10.1046/j.1532-5415.2002.50453.x. [DOI] [PubMed] [Google Scholar]
- 7.Richards JB, Papaioannou A, Adachi JD, et al. Effect of selective serotonin reuptake inhibitors on the risk of fracture. Arch Intern Med. 2007;167:188–194. doi: 10.1001/archinte.167.2.188. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Anderson G, Mittmann N, et al. Use of selective serotoninreuptake inhibitors or tricyclic antidepressants and risk of hip fractures in elderly people. Lancet. 1998;351:1303–1307. doi: 10.1016/s0140-6736(97)09528-7. [DOI] [PubMed] [Google Scholar]
- 9.Schweiger U, Deuschle M, Korner A, et al. Low lumbar bone mineral density in patients with major depression. Am J Psychiatry. 1994;51:1691–1693. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 10.Schweiger U, Weber B, Deuschle M, et al. Lumbar bone mineral density in patients with depression: evidence of increased bone loss at follow up. Am J Psychiatry. 2000;157:118–120. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- 11.Michelson D, Stratakis C, Hill L, et al. Bone mineral density in women with depression. N Engl J Med. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 12.Halbreich U, Rojansky N, Palter S, et al. Decreased bone mineral density in medicated psychiatric patients. Psychosom Med. 1995;57:485–491. doi: 10.1097/00006842-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Robbins J, Hirsch C, Whitmer R, et al. The association of bone mineral density and depression in an older population. J Amer Geriatr Soc. 2001;49:732–736. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 14.Millikin LA, Wilhelmy J, Martin CJ, et al. Depressive symptoms and changes in body weight exert independent and site-specific effects on bone in post-menopausal women exercising for 1 year. J Gerontol A Biol Sci Med Sci. 2006;61:488–494. doi: 10.1093/gerona/61.5.488. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16.Whooley M, Kip KE, Cauley JA, et al. Depression, falls, and risk of fracture in older women. Arch Intern Med. 1999;159:484–490. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 17.Mussolino ME. Depression and hip fracture risk: the NHANES I epidemiologic follow up study. Public Health Rep. 2005;120:71–75. doi: 10.1177/003335490512000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreiger N, Tenenhouse A, Joseph L, et al. Research notes: The Canadian multicentre osteoporosis study (CaMos): Background, rationale, methods. Can J Aging. 1999;18:376–387. [Google Scholar]
- 19.Ware JE, Jr, Snow K, Kowinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. 1st ed. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 20.Beusterien KM, Steinwald B, Ware JE., Jr Usefulness of the SF-36 Health Survey in measuring health outcomes in the depressed elderly. J Geriatr Psychiatry Neurol. 1996;9:13–21. doi: 10.1177/089198879600900103. [DOI] [PubMed] [Google Scholar]
- 21.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening test. Med Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Rogers WH, Adler DA, Bungay KM, et al. Depression screening instruments made good severity measures in a cross-sectional analysis. J Clin Epidemiol. 2005;58:370–377. doi: 10.1016/j.jclinepi.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Wells KB, Hays RD, Burnam MA, et al. Detection of depressive disorder for patients receiving prepaid or fee-for-service care. Results from the Medical Outcomes Study. JAMA. 1989;262:3298–3302. [PubMed] [Google Scholar]
- 24.Genant HK, Grampp S, Gluer CC, et al. Universal Standardization for Dual X-Ray Absorptiometry – Patient and Phantom Cross-Calibration Results. J Bone Miner Res. 1994;9:1503–1514. doi: 10.1002/jbmr.5650091002. [DOI] [PubMed] [Google Scholar]
- 25.Pearson J, Dequeker J, Henley M, et al. European Semi-Anthropomorphic Spine Phantom for the Calibration of Bone Densitometers – Assessment of Precision, Stability and Accuracy – the European Quantitation of Osteoporosis Study-Group. Osteoporos Int. 1995;5:174–184. doi: 10.1007/BF02106097. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Obrant KJ, Bengner U, Johnell O, et al. Increasing age-adjusted risk of fragility fractures: a sign of increasing osteoporosis in successive generations? Calcif Tissue Int. 1989;44:157–167. doi: 10.1007/BF02556558. [DOI] [PubMed] [Google Scholar]
- 29.Holmberg AH, Johnell O, Nilsson PM, et al. Risk factors for fragility fracture in middle age: a prospective population based study of 33,000 men and women. Osteoporos Int. 2006;17:1065–1077. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 30.Piccinelli M, Wilkinson G. Gender differences in depression: critical review. Br J Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- 31.Henderson JG, Jr, Pollard CA. Prevalence of various depressive symptoms in a sample of the general population. Psychol Rep. 1992;71:208–210. doi: 10.2466/pr0.1992.71.1.208. [DOI] [PubMed] [Google Scholar]
- 32.Barbui C, Motterlini N, Garattini L, et al. Health status, resource consumptions, and cost of dysthymia: A multicenter, two-year longitudinal study. J Affect Disord. 2006;90:181–186. doi: 10.1016/j.jad.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 33.Sonnenberg C, Beekman A, Deeg D, et al. Drug treatment in depressed elderly in the Dutch community. Int J Geriatr Psychiatry. 2003;18:99–104. doi: 10.1002/gps.771. [DOI] [PubMed] [Google Scholar]
- 34.Montagnier D, Barberger-Gateau P, et al. Evolution of prevalence of depressive symptoms and antidepressant use between 1988 and 1999 in a large sample of older French people: results from the Personnes Agées Quid Study. J Am Geriatr Soc. 2006;54:1839–1845. doi: 10.1111/j.1532-5415.2006.00969.x. [DOI] [PubMed] [Google Scholar]
- 35.Silverman SL, Shen W, Minshall ME, Xie S, Moses KH. Prevalence of depressive symptoms in post-menopausal women with low bone mineral density and/or prevalent vertebral fracture: Results from the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Rheumatol. 2007;34:140–144. [PubMed] [Google Scholar]