Abstract
Neural stem cells (NSCs) have some specified properties, but are generally uncommitted and so can change their fate after exposure to environmental cues. It is unclear to what extent this NSC plasticity can be modulated by extrinsic cues and what are the molecular mechanisms underlying neuronal fate determination. Basic fibroblast growth factor (bFGF) is a well known mitogen for proliferating NSCs. However, its role in guiding stem cells for neuronal subtype specification is undefined. Here we report that in vitro expanded human fetal forebrain-derived neural stem cells can generate cholinergic neurons with spinal motor neuron properties when treated with bFGF within a specific time window. bFGF induces NSCs to express the motor neuron marker Hb9, which is blocked by specific FGF receptor inhibitors and bFGF neutralizing antibodies. This development of spinal motor neuron properties is independent of selective proliferation or survival and does not require high levels of MAPK activation. Thus our study indicates that bFGF can play an important role in modulating plasticity and neuronal fate of human NSCs and presumably has implications for exploring the full potential of brain NSCs for clinical applications, particularly spinal motor neuron regeneration.
Keywords: neural stem cell, plasticity, motor neuron, basic fibroblast growth factor, differentiation
INTRODUCTION
Neural stem cells (NSCs) or neural progenitor cells (NPCs) can self renew and differentiate into neurons, astrocytes and oligodendrocytes. One key issue is whether NSCs from different regions or at different developmental stages have the same phenotypic and functional properties (Anderson, 2001). Evidence shows that NSCs are spatially and temporally specified in their gene expression profiles and capacities for self-renewal and differentiation (Abramova et al., 2005; Pearson and Doe, 2004; Seaberg et al., 2005; Temple, 2001). On the other hand, NSCs often lose some of their region-specific properties during in vitro expansion (Anderson, 2001; Gabay et al., 2003; Hitoshi et al., 2002) and the ability of NSCs to become specific cell types seems regulated by extrinsic factors. Identifying such factors and molecular mechanisms underlying NSC plasticity are critical for both understanding the formation of cell diversity during central nervous system (CNS) development and exploring the full potential of NSCs for neural repair.
Basic fibroblast growth factor (bFGF) or FGF-2 is one such extrinsic factor. It belongs to the FGF family (consisting of 22 members) that plays an important role in CNS development, neuronal survival and repair, axonal growth, synapse formation, and learning and memory (Gremo and Presta, 2000; Mason, 2007; Reuss and Bohlen und, 2003). bFGF also stimulates proliferation of NSCs in vitro and induces or inhibits neuronal differentiation from cultured NSCs in a dose-dependent manner (Kilpatrick and Bartlett, 1993; Nelson and Svendsen, 2006; Qian et al., 1997; Ray et al., 1993; Tsai and Kim, 2005). A particular role of bFGF in regulating NSC plasticity has recently been revealed in rodents, showing that bFGF is critical for generation of neurons from adult NSCs derived from nonneurogenic regions (Palmer et al., 1999). Several studies then showed that bFGF ventralizes cultured rodent NSCs/NPCs of dorsal origin, and induces oligodendrocytes from NSCs derived from regions where oligodendrocytes are not present (Gabay et al., 2003; Machon et al., 2005; Naruse et al., 2006).
Olig2 is both necessary and sufficient for generation of motor neurons and oligodendrocytes during development (Lu et al., 2002; Mizuguchi et al., 2001; Zhou and Anderson, 2002). Although bFGF induces the expression of Olig2 in cultured rodent NSCs, its effect on inducing NSCs to become motor neurons remains largely unknown. Previously, we have shown that a 4-day priming procedure, containing bFGF, heparin and laminin (FHL), conditioned human fetal brain-derived NSCs for further differentiation into cholinergic neurons in vitro, and such primed hNSCs acquired a motor neuron phenotype when grafted into adult rodent spinal cords (Gao et al., 2005; Gao et al., 2007; Wu et al., 2002). However, it is unknown whether brain-derived NSCs can differentiate into spinal motor neurons in vitro, and whether bFGF plays a critical role in spinal motor neuron specification. In this study, we report that bFGF plays a major role affecting the plasticity of human brain-derived NSCs and is responsible for caudalizing and ventralizing brain NSCs to specify a spinal motor neuron fate.
MATERIALS AND METHODS
Cell Culture
Human fetal (8–10 weeks) cortical NSCs, lines K048 and K054, were derived and provided by Dr. C.N. Svendsen (Svendsen et al., 1998; Wu et al., 2002). They were expanded as neurospheres in media containing EGF, bFGF and LIF and passaged every 9–10 days as previously described (Tarasenko et al., 2004). Both relatively early (10–20) and late (60–90) passages were used in this study, which mainly used the K048 line of hNSCs unless otherwise specified. For cell priming, ~2×106 hNSCs (small spheres) were plated in T25 flasks pre-coated with 0.01% Poly-D-Lysine and 1 μg/cm2 mouse laminin (Invitrogen/GIBCO), and incubated with either FHL priming media containing 10 ng/ml bFGF, 2.5 μg/ml heparin and 1 μg/ml Laminin (Tarasenko et al., 2004) or ELL media containing 20 ng/ml EGF, 10 ng/ml LIF and 1 μg/ml Laminin. For immunostaining, cells were plated on precoated German glass coverslips (Carolina Biological Supply) at a density of 1–1.5×105 cells/well in 24-well plates.
Treatments
Cell proliferation was inhibited either by treating hNSCs with a chemotherapeutic agent, 1 μM cytosine arabinoside (AraC, Sigma), or with a cell cycle inhibitor, 2.5 μM cyclin-dependent kinase 2 (Cdk2) inhibitor III (EMD Biosciences) for the 4-day period of priming. Specific effects of bFGF were blocked by treating cells with a FGFR specific antagonist PD173074 (a kind gift from Pfizer) at 25 nM, or pretreating FHL priming media for 10 min with 3 μg/ml bFGF neutralizing antibody (US Biologicals) and then followed with antibody treatment every other day. The ERK1/2 MAP kinase pathway was inhibited by treating cells with a MEK1/2 kinase activity inhibitor, U0126 at 5 μM (Cell Signaling Technology); or a MEK1 activation blocker, PD98059 (Promega) at 50 μM, both added daily for 4 days. The optimal dosages of these chemicals were predetermined either by WST-1 Cell Proliferation Assay (Chemicon), BrdU incorporation assay or by Western blot analyses for MAPK phosphorylation.
Semiquantitative and Real-Time Quantitative RT-PCR
Total RNA was extracted using RNAqueous-4PCR kit (Ambion) and followed by reverse transcription (RT) reactions to generate cDNA using an RT kit (Applied Biosystems) according to the manufacturer’s instructions.
Semiquantitative RT-PCR primers for various transcription factors are listed in Table 1 of Supplementary Data. The optimal condition for each individual gene was determined empirically by varying the number of PCR cycles, the amount of cDNA and the concentration of magnesium salt. In most cases, 20 ng cDNA was used in the reaction and the resulting gel images were analyzed using a Chemi-Imager 4400 v5.5 with the Alpha-Ease software (Alpha Innotech Corporation).
Real-time quantitative RT-PCR for Hb9 was performed by the Real-Time PCR Core Facility at UTMB using the Applied Biosystems (ABI) Prism 7000 Sequence Detection System and the 5′ fluorogenic nuclease assay. Chemicals and probes purchased from Applied Biosystems include the Assays-on-Demand™ reagents (#4331182, Assay ID Hs00232128_m1) containing a mix of Hb9 primers and the FAM™ dye-labeled TaqMan® MGB probe for Hb9 gene (ACT TCA ACT CCC AGG CGC AGT CGA A, GeneBank accession # NM_005515.2, X56537.1, AF107457.1), the Eukaryotic 18S rRNA Endogenous Control Probe labeled with VIC™-dye (#4319413E) and the TaqMan® Universal PCR Master Mix (#4304437). For relative quantitation of gene expression, separate-tubes (singleplex) real time PCR was performed with 30 ng cDNA for both Hb9 and the endogenous control. Cycling parameters were uracil N-glycosylase (UNG) activation at 50°C for 2 min, AmpliTaq activation at 95°C for 10 min, denaturation at 95°C for 15 sec and annealing/extension at 60°C for 1 min (repeat 40 times) on an ABI7000 PCR machine. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT(ΔΔCT) method as described by the manufacturer (Applied Biosystems). Values of Hb9 products (2−ΔΔCT) were normalized to the endogenous reference (18S rRNA) and then to a calibrator (one of the experimental samples).
Assays for cell proliferation, survival and death
Cell proliferation and survival were determined by cell counting with the trypan blue exclusion assay, Bromodeoxyuridine (BrdU) labeling and WST-1 based proliferation assay. For manual counting, dissociated cells were treated with 0.1% trypan blue and then counted using a hemocytometer. BrdU (BD Biosciences) pulse labeling was used to monitor hNSC proliferation by adding 0.2 μM BrdU at the beginning of priming and fixing cells 4 days later for immunodetection of BrdU incorporation. Cells were fixed with cold 4% paraformaldehyde for 25 min and then postfixed in 100% Methanol for 20 min at −20°C. After permeablization and DNA uncoiling in 2N hydrochloric acid for 20 min at 37°C and neutralization in Borate Buffer (pH 8.5) for two times and 10 min each at room temperature (RT), cells were blocked with 1% bovine serum albumin/0.5% Tween 20/5% normal goat serum for 1 hr at RT, and then incubated with the primary antibody (see Table 2 in Supplementary Data) for 1 hr at RT. The secondary antibody was conjugated with Alexa Fluor® 568 at the dilution of 1:300 (Molecular Probes). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) at 1:1000. In all experiments, cells stained with secondary antibody only and cells that did not receive BrdU pulse labeling were used as negative controls. The relative numbers of viable cells were also determined by WST-1 assay according to the manufacturer’s instruction [Chemicon, see also (Tarasenko et al., 2004)].
The lactate dehydrogenase (LDH) cell death assay (Roche) was performed according to the manufacturer’s instruction on 100 μl of conditioned media collected from each sample, including a positive control that was treated with 1% Triton X-100. Apoptotic cell death was characterized by caspase-3 activity assay and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).
Caspase-3 activity was quantitatively detected in cell lysates by fluorometric immunosorbent enzyme assay. Twenty μg of protein lysates in triplicates were incubated with a master mix containing reaction buffer, water, 5 mM DTT and 25 μM fluorescently tagged substrate, Z-DEVD-R110. Fluorescent signals were detected by a fluorescent plate reader (excitation at 490 nm and emission at 520 nm) every minute for at least 60 minutes. A positive control included treatment of hNSCs for 4 hours with 1μM Staurosporine (Calbiochem). Values for each triplicate sample were averaged and a linear regression of fluorescence versus time per μg protein was generated with the slope of the line being used to compare caspase activity between samples. A one way ANOVA with Tukey post hoc tests was used to statistically analyze differences between the treatment groups.
TUNEL staining was done using the ApopTag® Fluorescein In Situ Apoptosis Detection Kit (Chemicon) according to our previous description (Jordan et al., 2007).
Immunostaining
Immunofluorescent staining was performed according to our previous descriptions (Tarasenko et al., 2004). Cells were fixed for 30 min in 4% paraformaldehyde for cytoplasmic or surface antigens, and 20 min in 4% paraformaldehyde followed with postfixation for 10 min in 100% methanol at −20°C for transcription factors. Primary antibodies are listed in Table 2 of Supplementary Data. Alexa fluorophore-conjugated secondary antibodies (Molecular Probes) were used at 1:300–400. Images were acquired by Nikon 80i epifluorescent microscope with NIS-Elements imaging software.
MAPK Array and Western Blot Analysis
The Proteome Profiler™ Human Phospho-MAPK Array Kit (R&D Systems, Inc.) was used for an initial parallel determination of the relative levels of MAPK phosphorylation according to the manufacture’s instruction. Briefly, human NSCs were primed for four days either in FHL or ELL. Protein extracts were analyzed using the Human Phospho-MAPK Array Kit (R&D Systems, Inc., Catalog Number ARY002) following the manufacturer instructions. Briefly, cells were extracted in NP40 buffer (20 mM Tris, 137 mM NaCl, 2 mM EDTA, 1 mM NaVO4, 10% glycerol, 1% NP-40, 10 μg/ml Aprotinin, 10 μg/ml Leupeptin). 120 μg of proteins from each priming group were incubated with the arrays overnight followed by addition of the Detection Antibody Cocktail, washes and the addition of Streptavidin-conjugated horseradish peroxidase. The arrays were then exposed to ECL hyperfilm (Amersham Biosciences, UK) for 5 sec – 5 min prior to developing using a standard developer (Kodak).
Further assessments of protein phosphorylation were done using Western blot analyses as previously described (Tarasenko et al., 2004). Specific primary antibodies are listed in Table 2 of Supplementary Data. Horseradish peroxidase-conjugated secondary antibodies were used at dilutions of 1:5000–1:10,000 (Amersham Biosciences). All blots were first probed with the phosphorylated proteins followed by stripping (Restore™; Pierce Biotechnological) and reprobing for the corresponding un-phosphorylated proteins and then for β-actin as a loading control.
Statistical Analysis
All analyses included at least three independent experiments unless otherwise stated. Cells were counted from 10 randomly selected fields of 3–4 coverslips for at least 1000 total cells per treatment. Statistical analyses were performed using Student’s t test or one-way ANOVA with post hoc tests by InStat (GraphPad Software).
RESULTS
FHL priming induces human brain-derived NSCs to express transcription factors for the spinal motor neuron lineage
Previously, we showed that FHL-primed cortical hNSCs acquired a spinal motor neuron fate when grafted into rat spinal cord (Gao et al., 2005; Wu et al., 2002). To determine whether FHL priming was sufficient to drive cortical hNSCs towards spinal motor neuron differentiation in vitro, we first assessed the expression of transcription factors that are involved in motor neuron devolvement. Using semiquantitative reverse transcription polymerase chain reaction (sqRT-PCR), we found that a 4-day FHL priming significantly increased or induced the mRNA expression of Olig2, neurogenin 2 (Ngn2), Islet1, Lim3 and Hb9 when compared to spheres proliferated in the presence of epidermal growth factor (EGF), bFGF and leukemia inhibitory factor (LIF)(EFL) (Fig. 1A). ELL (EGF+LIF+laminin) priming was used as a control since it did not contain bFGF and heparin but otherwise all components in the regular proliferation media. ELL priming showed a slight increase in Islet1 mRNA, a decrease in Hb9 and undetectable levels of Ngn2 and Lim3.
Fig. 1. Expression of transcription factors involved in motor neuron development in FHL-primed hNSCs.
(A) Semiquantitative RT-PCR revealed increased/induced expression of early motoneuron lineage transcription factors in FHL-primed hNSCs as compared to neurospheres and ELL-primed cells. − RT, negative control without reverse transcriptase; + Ctrl, positive control using total RNA from human fetal spinal cord; GAPDH, glyceraldehyde phosphate dehydrogenase as internal control. (B) Semiquantitative RT-PCR revealed the expression levels of various transcription factors in spheres (Sph), ELL- and FHL-primed cells. (C) Olig2 (red) immunoreactivity was detected in the nuclei of ELL- and FHL-primed hNSCs after 4 days of priming. Blue, DAPI nuclear counterstain. Scale bar = 20 μm. (D) Cell counts demonstrated that significantly more FHL-primed hNSCs expressed Olig2 protein than ELL cells (mean ± s.e.m.; n = 3–6; ***p < 0.001).
Gene expression patterns following FHL priming were further characterized on other identity-related transcription factors, including Sox2 (a marker for NSCs)(Ellis et al., 2004), Emx1 (a marker for the dorsal telencephalon)(Simeone et al., 1992), Otx2 (a marker for forebrain and midbrain)(Simeone et al., 1992), Pax3 and Pax7 (markers for dorsal identity) (Wada et al., 1997), Phox2b (a marker for cranial motor neurons)(Pattyn et al., 2000), Pax6 and Irx3 (class I transcription factors in spinal cord) (Briscoe and Novitch, 2008), Nkx2.2 (a Class II ventral marker)(Briscoe and Novitch, 2008) and Gli1 and Gli2 (transcription factors activated by the sonic hedgehog pathway)(Altaba, 1998). No major differences were observed in the mRNA expression of Sox2, Otx2 and Gli2 among the three groups (Fig. 1B), while FHL priming tended to increase Pax6, Nkx2.2 and Gli1 mRNA, which are all related to the development of the oligodendrocyte/motor neuron lineage in the spinal cord (Jessell, 2000). Most of the dorsal markers, Emx1, Pax3 and Pax7, were not detected in any of the groups (data not shown). Both FHL and ELL priming reduced Irx3 expression, a factor required for a spinal interneuron identity. Finally, no cells expressed Phox2b mRNA (a marker for cranial motor neurons, data not shown).
We then focused on Olig2 and Hb9 since they act in the early and late stage of motor neuron development, respectively. FHL-primed hNSCs showed 73% and 90% increases in Olig2 mRNA levels as compared to ELL-primed cells and neurospheres, respectively. The protein level of Olig2 was also significantly upregulated in FHL-primed cells by showing a 1.2-fold increase in the number of Olig2 immunoreactive cells (45.7 ± 2.9%) than for the ELL group (20.6 ± 2.2%) and a 66% increase over spheres (27.5 ± 2.5%) (Fig. 1C–D). Furthermore, ELL cells often showed weak Olig2 labeling whereas most FHL cells were strongly labeled for Olig2.
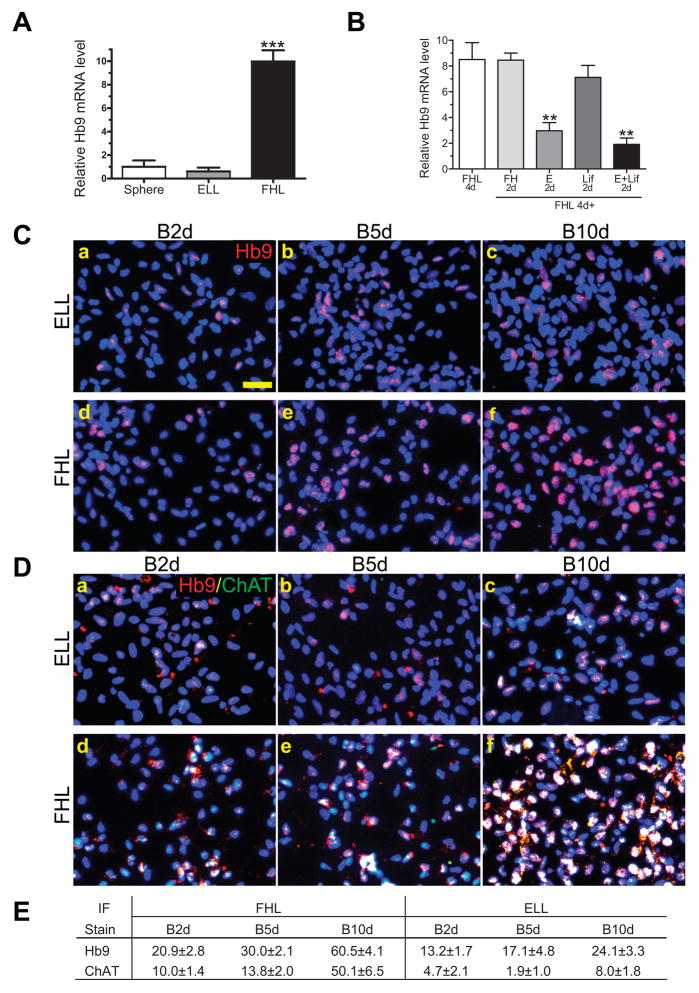
Hb9 is a homeodomain transcription factor established as a marker for pre- and post-mitotic spinal motor neurons (Arber et al., 1999; Thaler et al., 1999). Using sqRT-PCR, we detected substantial increases of Hb9 mRNA in FHL-primed hNSCs, 8.9- and 15-fold higher when compared to unprimed spheres and ELL-primed cells, respectively (Fig. 2A). This pattern of Hb9 expression was confirmed by real time quantitative RT-PCR (data not shown), whereas ELL priming decreased Hb9. The inhibitory effect of ELL priming was further characterized by treating cells with FHL for 4 days followed by a 2-day treatment with FHL (FH), EGF/laminin (E), LIF/laminin (Lif) or E/LIF/laminin (E+Lif). FHL-mediated Hb9 up-regulation was maintained following 2 more days of FHL treatment, whereas cells exposed to EGF or EGF plus LIF for additional 2 days down-regulated Hb9 by 65% or 78%, respectively (Fig. 2B). Furthermore, FHL-increased Hb9 mRNA was not limited to a certain cell line or passage number. Both K048 and K054 lines of cortical hNSCs responded to FHL-priming by increasing Hb9 expression (data not shown). Time in culture also had little effects on Hb9 as both early (< 20) and late (> 60) passages showed similar patterns of Hb9 expression in response to FHL- and ELL-priming (data not shown).
Fig. 2. Differentiation of Hb9 motor neurons in FHL- or ELL-primed hNSCs.
(A) Semiquantitative RT-PCR showed increased Hb9 transcripts in 4-day FHL-primed cells as compared to neurospheres and ELL (mean ± s.e.m.; n = 3; ***p < 0.01). Hb9 values were normalized to the GAPDH internal control and then to spheres. (B) Semiquantitative RT-PCR showed that hNSCs primed with FHL for 4 days had high levels of Hb9 transcripts while those exposed to FHL for 4 days followed by a 2-day treatment with either EGF alone or EGF+Lif showed a down-regulation of Hb9 (mean ± s.e.m.; **p < 0.01 vs. FHL4d). (C) Immunostaining for Hb9 (red) in ELL or FHL primed hNSCs after 4 days of priming followed by 2–10 days of differentiation in B27. Blue, DAPI nuclear stain. (D) Double immunofluorescent staining for Hb9 (red) and choline acetyltransferase (ChAT in green). White indicates cells tripled-labeled with Hb9, ChAT and DAPI. Scale bar for C and D = 20 μm. (E) Percentages of ELL or FHL primed hNSCs immunostained for Hb9 and/or ChAT (mean ± s.e.m.; n ≥10 fields/group). Note FHL-primed hNSCs increased Hb9 and ChAT differentiation over time at the levels significantly higher than ELL-primed cells.
Next we determined the protein expression of the Hb9 gene by immunostaining. Hb9 immunoreactivity was not detected in spheres, 4-day FHL-primed or ELL-primed hNSCs (data not shown). However, Hb9 labeling appeared in both groups after withdrawing growth factors and differentiating in B27 medium for 2 days (Fig. 2C). The intensity of Hb9 immunolabeling in individual ELL cells was much lower than in the FHL group. Furthermore, percentages of Hb9 immunoreactive cells in FHL-primed hNSCs increased from 21% at 2 days to 61% at 10 days post-differentiation (Fig. 2E) and was accompanied by stronger nuclear labeling in those Hb9+ cells, especially at the 10-day time point (Fig. 2C, B10d). In contrast, ELL-primed cells showed only slight increases in the percentage of Hb9+ cells (from 13% to 24%) with much weaker staining (Fig. 2C and E).
FHL priming facilitates spinal motor neuron differentiation from human brain-derived NSCs
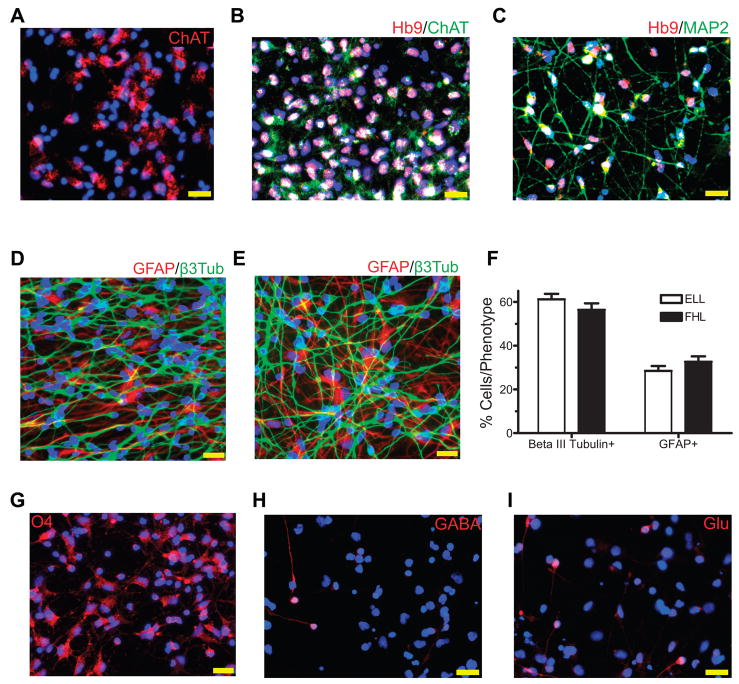
To confirm that FHL priming induced Hb9-expressing spinal motor neurons from in vitro expanded cortical hNSCs, we performed double immunofluorescent staining to detect colocalization of Hb9 and choline acetyltransferase (ChAT) in primed and differentiated cells. About 10% of FHL-primed cells showed double immunoreactivities to Hb9 and ChAT after a 2-day differentiation in B27, increasing to 50% by 10-day differentiation (Fig. 2D–E). Almost all ChAT positive cells colabeled with Hb9. In contrast, only weak staining with much lower percentages of Hb9+/ChAT+ cells (2–8%) were detected in ELL-primed cells (Fig. 2D–E). Longer differentiation did not increase the number of Hb9+/ChAT+ neurons in either group (data not shown). Further characterization revealed that ChAT labeled mainly the cell bodies after a 10-day differentiation (Fig. 2C and 3A), and the labeling expanded into neurites after another 4-day differentiation (Fig. 3B). Some Hb9 positive cells were not double labeled with ChAT, indicating that they were either not fully differentiated or a subpopulation with their identity to be determined. This was further confirmed by the lack of MAP2 (a mature neuronal marker) staining in some of the Hb9 positive cells, whereas the majority of Hb9 cells were colabeled with MAP2 (Fig. 3C).
Fig. 3. Phenotypic differentiation of hNSCs.
(A) Immunostaining of choline acetyltransferase (ChAT, red) in hNSCs after 4-day FHL priming and 10-day further differentiation in B27. (B) Double immunostaining of Hb9 (red) and ChAT (green) in hNSCs after 4-day FHL priming and 14-day further differentiation in B27. (C) Double immunostaining of Hb9 (red) and microtubule-associated protein 2 (MAP2 in green) in cells treated as in (A). Pink, double labeled with red Hb9 and blue DAPI nuclei; White, triple labeled with red Hb9, green ChAT or MAP2 and blue DAPI nuclei. (D – E) Similar degrees of neuronal differentiation detected by type III β-tubulin (β3Tub, green) and astroglial differentiation stained by glial fibrillary acidic protein (GFAP, red) were observed in 14-day B27-differentiated hNSCs after an initial ELL (D) and FHL (E) priming. (F) Quantitative analyses of the immunostaining in D and E; mean ± s.e.m.; n ≥ 10 fields/group. (G) FHL-primed hNSCs differentiated into O4 (red) oligodendrocytes after further 3-week in differentiation media. (H–I) FHL-primed hNSCs treated as in (A) could also differentiate into GABA (H) and glutamate (Glu, I) neurons. Scale bars = 20 μm.
To characterize other differentiation phenotypes, we examined overall neuronal and glial differentiation and found similar degrees of neuronal (about 60% labeled for β3-tubulin) and astroglial (about 30% labeled for glial fibrillary acidic protein or GFAP) differentiation in both FHL- and ELL-primed groups after 14 days in B27 (Fig. 3D–F). O4+ oligodendrocytes were not detectable in these cells at this time, but found in hNSCs after a longer differentiation (more than three weeks) (Fig. 3G). Further neuronal subtype analyses revealed that glutamate and γ-aminobutyric acid (GABA) neuronal subtypes were also generated from hNSCs initially primed with FHL (collectively less than 10% of total cells) or ELL (about 13% of total cells)(Fig. 3H–I).
bFGF induces Hb9 expression in a dose- and time-dependent manner
We next asked what component(s) in FHL induced Hb9 motor neuron differentiation. Heparin and laminin alone did not significantly increase Hb9 transcripts. However, increasing the amount of bFGF (up to 20 ng/ml) resulted in dose-dependent increases of Hb9, while a higher dose (100 ng/ml) failed to enhance Hb9 expression (Fig. 4A). These studies indicated that bFGF was the critical component in FHL to induce Hb9 gene expression in a dose dependent manner. bFGF-induced Hb9 expression also seemed to be time-dependent (Fig. 4B). Hb9 mRNA was significantly increased after 2-day FHL priming and continued to increase throughout the 4-day period. Surprisingly, the Hb9 increase continued even after withdrawal of FHL and peaked at 3 days in B27 differentiation media, which then stabilized at a level significantly higher than spheres. However, continuous priming in FHL for 7–14 days completely abolished Hb9 expression (data not shown).
Fig. 4. Dose and time dependency of bFGF-induced Hb9 expression in hNSCs.
(A) Semiquantitative RT-PCR demonstrated an enhancement of Hb9 transcripts in hNSCs primed with increasing bFGF concentrations for 4 days (mean ± s.e.m.; n ≥ 3/group; **p < 0.01 vs. spheres). Note the high dose of bFGF (100 ng/ml) reduced Hb9 expression. (B) Semiquantitative RT-PCR showed increasing Hb9 expression in FHL-primed hNSCs over the 4-day period, peaked at 3 days after removing FHL and maintained through 14 days of differentiation in B27 (B14d) (mean ± s.e.m.; n ≥ 3/group; *p < 0.05, **p < 0.01 and ***p < 0.001 vs. spheres). (C) Blockade of bFGF signaling by an FGF receptor antagonist, 25nM PD173074 (PD), or 3 μg/ml neutralizing-bFGF antibody (anti-bFGF) significantly decreased Hb9 transcript levels in FHL-primed hNSCs (mean ± s.e.m.; n ≥ 3; *p < 0.05 as compared to vehicle or antibody controls). Values were normalized to GAPDH internal controls and then to spheres.
To further confirm that bFGF was the factor inducing the temporal changes of Hb9 expression in FHL-primed hNSCs, we blocked bFGF signaling via two mechanisms. First, we used a specific FGF receptor (FGFR) antagonist, PD170374, that blocks all FGF receptor subtypes. This experiment was based on our initial finding that FHL priming increased the mRNA expression of several FGFRs, particular FGFR2 and R3 (data not shown). Relatively low concentrations of PD173074, 25–50nM, were chosen to ensure specific inhibitory effect only on FGFRs. Second, we applied a bFGF specific neutralizing antibody. The doses used for PD173074 and the bFGF neutralizing antibody were titrated based on their maximal effects to inhibit bFGF-induced cell proliferation (data not shown). Both bFGF neutralizing antibody and PD173074 significantly down-regulated Hb9 expression by more than 40% as compared to IgG-and vehicle (DMSO) controls, respectively (Fig. 4C).
Cell proliferation and survival are not required for bFGF-induced Hb9 expression
To determine how bFGF up-regulated Hb9 expression in hNSCs, we first asked whether bFGF-enhanced Hb9 was solely due to its mitogenic effect. Both FHL- and ELL-priming for 4 days increased total cell numbers by approximately 130% and 200%, respectively (Fig. 5C), as compared to the initial number of cells seeded as spheres. Bromodeoxyuridine (BrdU) labeling further confirmed that both groups underwent significant proliferation, with a higher percentage of BrdU incorporation in ELL-primed cells (71 ± 2.9%) than that in FHL (60 ± 3.1%)(Fig. 5A–B). Addition of PD173074 to FHL-primed cells decreased the total cell number by 20% and BrdU labeled cells by 53% (Fig. 5A–B). Since ELL also stimulated proliferation yet inhibited Hb9 expression, the bFGF-mediated cell proliferation may not be related to Hb9 enhancement. To exclude the possibility that bFGF may cause selective proliferation of the Hb9-expressing population, we used two proliferation inhibitors, cytosine arabinoside (AraC) and cyclin-dependent kinase 2 inhibitor (CDK2i). Both completely abolished cell proliferation during FHL-priming as shown by the lack of BrdU incorporation (Fig. 5A–B). However, these two inhibitors had different effects on FHL-mediated Hb9 expression in that AraC significantly down-regulated Hb9 mRNA whereas CDK2i had no effects (Fig. 5D–E). Since AraC is incorporated into DNA of replicating cells and inhibits both DNA and RNA polymerases, it is possible that AraC-mediated Hb9 down-regulation was due to its disruption of DNA transcription rather than inhibition of cell proliferation per se. On the other hand, CDK2i inhibits cell proliferation by blocking CDK2 binding to Cyclin E and thus preventing cell progression from G1 to S phase. Taken together, these data indicated that bFGF-induced Hb9 expression unlikely requires cell proliferation.
Fig. 5. Proliferation-independent Hb9 induction by bFGF priming in hNSCs.
(A) Representative images of BrdU labeling (red) in hNSCs primed either by ELL, FHL alone or plus treatment with FGFR inhibitor PD173074 (FHL+PD), mitotic inhibitor cytosine arabinoside (FHL+AraC) or cyclin-dependent kinase 2 inhibitor (FHL+CDK2i) for 4 days. Blue, DAPI nuclear stain. Scale bar = 20 μm. (B) Cell counts of BrdU+ cells showed the highest level of proliferation in ELL-primed cells followed by FHL-primed, FHL-primed plus PD173074 and the absence of proliferation with the addition of AraC or CDK2i (mean ± s.e.m.; n ≥ 10 fields/group; *p < 0.05 and **p < 0.01 vs. FHL). (C) Total cell counts at the beginning (Spheres) or the end of 4-day priming showed the fastest growth rate in ELL cells (mean ± s.e.m.; n = 3; **p < 0.01 and ***p < 0.001). (D) A representative semiquantitative RT-PCR gel of Hb9 products from spheres (Sph), FHL-primed cells (FHL) alone or plus further treatments with either vehicle (DMSO), AraC or CDK2i. (E) Densitometric analyses of semiquantitative RT-PCR for Hb9 mRNA expression. All values were normalized to GAPDH internal controls and then to spheres. AraC but not CDK2i significantly down-regulates Hb9 as compared to controls (mean ± s.e.m.; n ≥ 3; **p < 0.01 vs. DMSO control).
A second possibility for bFGF-mediated Hb9 enhancement in hNSCs was selective survival of Hb9 cells. To address this issue, we first determined whether the overall cell death was different among treatments using the lactate dehydrogenase (LDH) assay. Spheres as well as FHL-primed and ELL-primed hNSCs all exhibited only minimal cell death with no differences among groups (Fig. 6A). Furthermore, FHL-primed cells treated with PD170374 did not significantly increase cell death as compared to all other groups. We next asked whether apoptotic cell death was different among groups through assessment of the enzymatic activity of caspase 3 and detection of DNA fragmentation by TUNEL staining. Caspase 3 activity did not differ significantly among spheres and ELL- and FHL-primed cells at 2 and 4 days post-priming (Fig. 6B). Moreover, PD173074 did not alter caspase 3 activity in FHL-primed cells. TUNEL staining confirmed that apoptosis was minimal in all groups, showing less than 6% TUNEL+ cells. In particular, there were no significant differences in the numbers of TUNEL positive cells among spheres, ELL- and FHL-primed hNSCs (Fig. 6C–D). FHL-primed hNSCs treated with PD173074 showed a ~2.5-fold increase in apoptotic cells as compared to FHL-priming alone, indicating a survival role of bFGF for hNSCs. However, the 2.5-fold difference in apoptotic cell death did not correlate to PD173074-mediated 44% reduction of Hb9 mRNA expression. Together three measures of cell death indicated that the survival of FHL-primed cells was similar to ELL-primed cells and spheres, and it was unlikely that FHL-enhanced Hb9 expression was mediated through increasing Hb9 cell survival.
Fig. 6. Survival-independent Hb9 induction by bFGF priming in hNSCs.
(A) Lactate dehydrogenase (LDH) cytotoxicity assay showed similar low levels of cell death in spheres, ELL-primed, FHL-primed alone or plus PD173074 (FHL+PD). (B) Caspase 3 activity assay showed no significant activity differences among different treatment groups at different time points (2 or 4 days). High levels of caspase 3 activity was revealed in positive controls (Pos Ctrl), in which hNSCs were treated with staurosporine (mean ± s.e.m.; n = spheres and n = 6 for all other groups, ***p < 001, Pos Ctrl vs. all other groups). (C) and (D) Representative images and cell counts of TUNEL immunostaining (red) in spheres, ELL, FHL or FHL-primed plus PD173074, confirmed the presence of few (< 6%) apoptotic cells under any of the culture conditions tested (mean ± s.e.m.; n ≥ 10 fields/group, ***p < 0.001). Scale bar = 20 μm.
Activation of MAPK pathways is not specifically responsible for bFGF-induced Hb9 expression
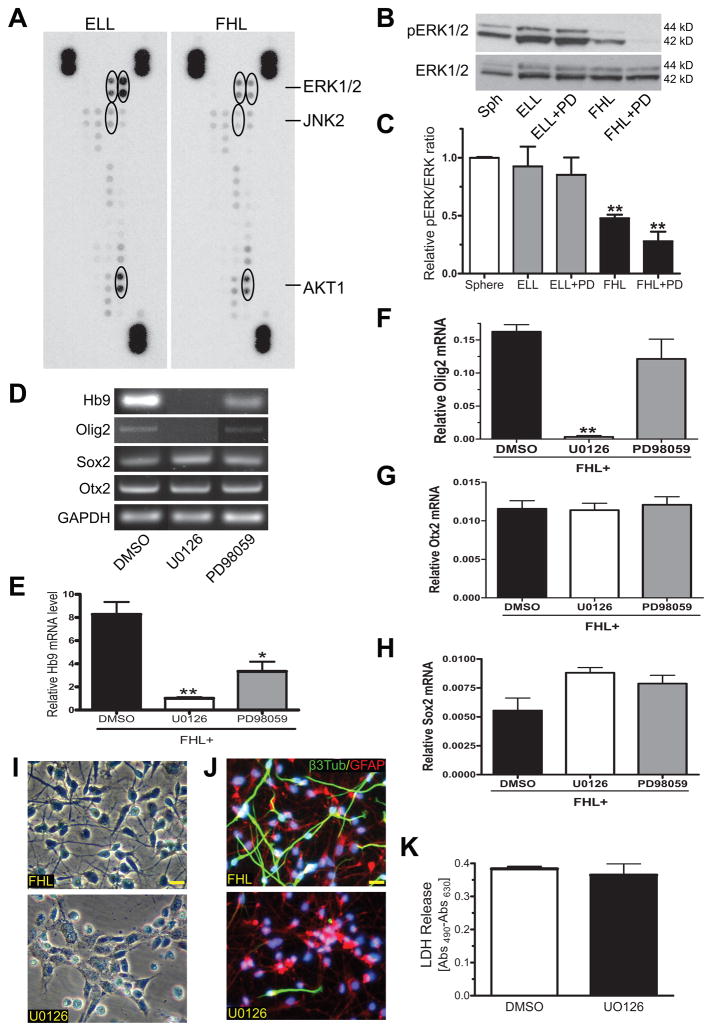
Binding of bFGF to FGFRs activates several canonical signaling pathways, including mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/Akt and phospholipase Cγ(PLCγ)/protein kinase C (PKC). To determine which pathway(s) was responsible for bFGF induction of Hb9, we first focused on MAPK pathways and did an initial screen using a Human Phospho-MAPK Array Kit to compare FHL- and ELL-primed cells. Cells primed for 4 days with FHL had phosphorylated extracellular signaling related kinase 1/2 (ERK1/2) and AKT1, but at levels lower than after ELL-priming (Fig. 7A). In addition, jun N-terminal kinase 2 (JNK2) was unphosphorylated in FHL-primed cells but phosphorylated in the ELL group, which was confirmed by Western blot analyses (data not shown). Further characterization using Western blot analyses also confirmed the presence of phosphorylated ERK1/2 in FHL-primed hNSCs (Fig. 7B). However, the level of phosphoERK1/2 in FHL-primed cells was significantly lower than those in spheres or ELL-primed cells. Treatments with PD173074 or bFGF neutralizing antibody (data not shown) showed a trend to reduce the level of phosphorylation that was statistically insignificant. In contrast, ELL-primed hNSCs exhibited similar levels of phosphorylated ERK1/2 as compared to spheres and was unaltered by PD173074. These data indicated that activation of MAPK, AKT and JNK were probably not responsible for bFGF-induced Hb9 expression.
Figure 7. ERK1/2 activation in FHL-primed hNSCs.
(A) An initial screen using a Human Phospho-MAPK Array Kit showed that the phosphorylation levels of several MAP kinase signals and AKT were lower in FHL-primed cells than ELL-primed. (B–C) Western blot analyses showed similar levels of phosphorylated ERK1/2 (pERK1/2) in spheres (Sph), ELL-primed and ELL plus PD173074 (ELL+PD). FHL-primed and FHL plus PD173074 (FHL+PD) cells had much lower levels of pERK1/2 (mean ± s.e.m.; n = 3, **p < 0.01 vs. spheres). For analysis, pERK1/2 was normalized to loading controls (β-actin) and further to total ERK1/2 levels. (D–H) Semiquantitative RT-PCR analyses revealed that MEK1/2 inhibitors, U0126 and/or PD98059, significantly blocked FHL-induced Hb9 and Olig2 mRNA expression (mean ± s.e.m.; n =3; *p < 0.05 and **p < 0.01 vs. DMSO vehicle control). (I) Phase contrast images of 4-day FHL-primed hNSCs and FHL plus U0126 showed dramatic morphological changes after U-126 treatment. (J) Immunofluorescent staining showed both neuronal (labeled by β3-tubulin in green) and astroglial (glial fibrillary acidic protein or GFAP in red) differentiation in cells primed by FHL for 4 days and followed by 10 days culture in B27. Note that U0126 almost completely blocked neuronal differentiation. Scale bar = 20 μm. (K) U0126 (a MEK1/2 inhibitor) did not cause cell death as measured by LDH cytotoxicity assay (mean ± s.e.m.; n = 3).
Given that there were still some levels of phosphoERK1/2 in FHL-primed hNSCs, we then asked whether blocking the ERK pathway would affect Hb9 expression. Two specific inhibitors targeting the MEK1/2 upstream signals were used, including 5 μM U0126 (MEK1/2 kinase activity inhibitor) and 50 μM PD98059 (MEK1/2 activation blocker). Both inhibitors and particularly U0126 significantly reduced Hb9 and Olig2 mRNAs without affecting Sox2 and Otx2 gene expression in FHL-primed hNSCs (Fig. 7C). No morphological changes were found in PD98059-treated cells (data not shown). However, dramatic changes in morphology were observed in cells treated with U0126 (Fig. 7D), showing flat cell bodies with few processes. When further differentiation was allowed after removing FHL, U0126-treated cells showed reduced neuronal generation (< 2% β3-tubulin-labeled cells) (Fig. 7E). An LDH cytotoxicity assay detected no differences between U0126-treated or non-treated cells (Fig. 7F), indicating that the low dose U0126-mediated neuron reduction was not related to cytotoxicity.
DISCUSSION
In this study, we demonstrate for the first time that human fetal cortical NSCs, which normally generate glutamatergic and GABAergic neurons in vivo, can be induced to express spinal motor neuron characteristics in vitro, and that the critical player in this differentiation is bFGF. Our study thus provides new evidence to support the hypothesis that NSCs are uncommitted and highly plastic, as shown by their losing original region-specific transcription factor identities and their ectopic capabilities of generating different subtypes of neurons.
On the other hand, NSCs do have some specificity. For example, rodent and human NSCs derived from the rostral CNS tend to proliferate longer and have a greater capacity for neuronal differentiation in vitro than cells from the caudal regions (Hitoshi et al., 2002; Kim et al., 2006; Ostenfeld et al., 2002). In agreement, both K048 and K054 lines of hNSCs used in this study, derived originally from human fetal forebrain, can be expanded for a prolonged time in media containing EGF, bFGF and LIF after being chemically dissociated. In particular, K048 cells have gone through 91 passages over 812 days and maintain a diploid karyotype. These late passage cells still respond to FHL priming by increasing Hb9 expression and can differentiate into various types of neurons and glia.
Understanding the molecular mechanisms for NSC plasticity is a key to unravel their full potential for clinical applications. Here we report that bFGF changes human cortical NSCs by enhancing the expression of ventral markers such as Olig2. In agreement, rodent NSC/NPCs, isolated from neocortex or dorsal spinal cord that do not generate the oligodendrocyte lineage in vivo, can be induced by bFGF to express Olig2 and NG2, and eventually became oligodendrocytes (Chandran et al., 2003; Dromard et al., 2007; Gabay et al., 2003; Hack et al., 2004). Such a ventralizing role of bFGF was also found when infusing bFGF into E13.5 mouse dorsal forebrains (Naruse et al., 2006). Although Olig2 positive cells should theoretically be able to generate both oligodendrocytes and motor neurons (Lu et al., 2002; Zhou and Anderson, 2002), only one group examined motor neuron markers and reported no Hb9 expression from rodent dorsal spinal cord NPCs (Gabay et al., 2003). Thus we are the first to show that in vitro bFGF-primed human brain-derived cells express transcription factors indicative of motor neuron lineages (Ngn2, Isl1, Lim3 and Hb9) and become spinal motor neurons, at least as indicated by their Hb9-positive but Phox2b-negative cholinergic phenotypes. In addition, however, some of these cortical NSCs produce glutamate and GABA neurons, oligodendrocytes and astroglia. Our study thus indicates that human forebrain-derived NSCs are more primitive in that they have a greater capacity to be expanded in vitro, have a trilineage differentiation, and particularly respond to the ventralizing and caudalizing effects of bFGF to become spinal motor neurons. Such fetal brain-derived NSCs may therefore be suitable candidates for stem cell therapy to treat motor neuron disorders.
bFGF is well known as a mitogen and survival factor in the CNS. However, the role of bFGF in directing stem cell fate and particularly specific neuronal subtypes is much less well defined. Previous studies have shown that several other members of the FGF family, FGF1 or FGF8 and FGF4, played important roles in the fate determination of dopaminergic and serotonergic neurons, respectively (Du and Iacovitti, 1995; Yang et al., 2004; Ye et al., 1998). Thus, different types of FGF may affect different neuronal subtype specification from NSCs/NPCs. Here, we report that bFGF, also called FGF2, in culture media is necessary to keep human fetal brain-derived NSCs in a primitive stage capable of acquiring trilineage fates after further differentiation, and particularly to become what appear to be spinal motor neurons. Although 4-day priming with bFGF induces ventralization and expression of transcription factors for the spinal motor neuron lineage, these bFGF-primed hNSCs are still dividing progenitors and not neurons. The acquisition of a final motor neuron phenotype requires removal of bFGF and further differentiation. This study thus suggests that neuronal subtype specification may start during proliferation prior to pan-neuronal differentiation. Furthermore, our finding of peak Hb9 expression and cholinergic differentiation after removal of exogenous bFGF supports the previously reported dual functions of bFGF, i.e. at high concentrations (> 1 ng/ml) bFGF primarily acts as a mitogen for NSCs/NPCs and inhibits neuronal differentiation (Kilpatrick and Bartlett, 1993; Ray et al., 1993; Tsai and Kim, 2005), while at low concentrations (< 1 ng/ml) it promotes neurogenesis (Nelson and Svendsen, 2006; Palmer et al., 1999; Qian et al., 1997).
Cell fate specification from NSCs may be guided either instructively or permissively. We believe that bFGF acts here as an instructive factor for neuronal subtype determination of hNSCs because 1) these hNSCs are originally derived from human fetal forebrain and thus are not intrinsically programmed to become spinal motor neurons; and 2) bFGF-enhanced Hb9 expression is independent of cell proliferation and survival. The level of Hb9 expression in proliferating neurospheres is very low, probably due to a combined effect of bFGF induction and EGF inhibition. Thus in the context of FHL priming, increased Hb9 expression is most likely a consequence of both the instructive effect of bFGF and a permissive effect through the removal of inhibitory EGF.
Hb9 induction by bFGF is both dose- and time-dependent. For the latter, FHL priming gradually increases Hb9 mRNA in hNSCs from 2 to 4 days. Interestingly, Hb9 mRNA reaches its peak not during priming but 3 days after removing bFGF and culturing in B27 differentiation medium. Prolonged FHL priming (more than 7 days) actually reduces the level of Hb9 transcripts. Furthermore, our previous microarray study comparing gene expression profiles between FHL-primed cells and spheres indicated that many genes were changed particularly at 4 and 5 days after priming (Cai et al., 2006). These findings suggest a critical time window during which bFGF induces Hb9 and commits cortical hNSCs to a spinal motor neuron fate, while prolonged exposure to a high concentration of bFGF may turn on yet unknown signals and/or transcription factors that repress Hb9 expression.
As an initial step to elucidate possible signaling pathway(s) involved in bFGF induction of Hb9, we focused on one of the canonical pathways of FGF signaling, MAP kinases. Our data indicate that a minimum level of ERK activation seems essential for overall neurogenesis as also suggested by others (Milosevic et al., 2006), whereas high levels of activation are not required or may even be inhibitory to Hb9 fate specification. Interestingly, the inhibitor-mediated inactivation of ERK significantly reduces the mRNA levels of the motor neuron/oligodendrocyte lineage markers (Hb9 and Olig2), but does not affect the expression of the NSC marker Sox2 and the forebrain marker Otx2. These data indicate that at least low levels of ERK activation are required for specific motor neuron development from human brain-derived NSCs. However, it remains to be determined whether the low level of ERK1/2 activation is related directly to bFGF induction of Hb9, or indirectly due to its requirement for general neuronal differentiation. In addition, mechanisms underlying differential effects of bFGF vs. EGF on MEK/ERK signaling in hNSCs also warrant further investigation. Furthermore, other pathways in the FGF signaling network such as PLCγ/PKC and PI3K/Akt need to be more intensively examined. Our unpublished observations suggest that PLCγand conventional/novel PKC are not related to the bFGF inductive effect since specific inhibitors for these enzymes show no effects on Hb9 expression. On the other hand, a specific PI3K inhibitor effectively blocks Hb9 transcription (unpublished observation), indicating a role for PI3K in bFGF induction of Hb9. PI3K is known to activate Akt. However, the phosphorylation level of Akt in bFGF-primed hNSCs is slightly lower than EGF-primed cells. This indicates that high levels of Akt activation are not required for bFGF-induced Hb9 expression. Alternatively, PI3K may activate atypical PKCs through the PDK1 pathway (Le Good et al., 1998;Chou et al., 1998). Further studies are required to determine which downstream signalling molecules of PI3K are related to the bFGF-induced motor neuron differentiation.
Finally, it is important to determine to what extent in vitro cultured human NS/PCs mimic the behavior of those cells during development in vivo. Particularly, generation of spinal motor neurons in vivo requires 1) induction of rostral neural progenitors from ectodermal cells through BMP, FGF and Wnt signaling (Munoz-Sanjuan and Brivanlou, 2002), 2) induction of spinal progenitors by the caudalizing effect of retinoic acid (RA) (Durston et al., 1998) and 3) induction of motor neuron progenitors via the ventralizing action of sonic hedgehog (Shh)(Briscoe et al., 2001). Applying RA and Shh sequentially has successfully induced motor neuron differentiation from rodent and human embryonic stem cells in a way recapitulating motor neuron development in vivo (Barberi et al., 2003; Li et al., 2005; Wichterle et al., 2002). In contrast, human cortical NSCs are induced by bFGF toward spinal motor neuron differentiation independently from RA and Shh. The Shh-independent ventralization by FGF signals has also been implicated in the development of the ventral telencephalon (Gutin et al., 2006), in ectopic generation of oligodendrocytes from mouse dorsal forebrain in vivo (Naruse et al., 2006) and NPCs from rodent dorsal spinal cord or neocortex in vitro (Chandran et al., 2003; Kessaris et al., 2004). Apparently, hNSCs cultured in medium containing specific growth factors such as EGF, bFGF and LIF change many of their intrinsic molecular identities, differentiation capacities and responses to extrinsic factors. Thus, their fate specification does not completely mimic motor neuron development in vivo. However, bFGF-induced spinal motor neuron differentiation from human cortical NSCs does promote expression of many transcription factors that are involved in the molecular patterning of motor neuron development in chick and rodent. Along this line, although some intrinsically programmed properties in these in vitro expanded hNSCs are lost, they still offer a simplified system for dissecting molecular mechanisms underlying neural subtype specifications, which in many respects mimics human neural development in vivo.
In conclusion, we demonstrate for the first time that human fetal brain-derived NSCs can change their regional specificities and acquire spinal motor neuron characteristics. Such a phenotypic transition is induced by bFGF through its regulation on the expression patterns of transcription factors necessary for spinal motor neuron development.
Table 1.
Primers used in RT-PCRs.
| Name of gene | Sequence of forward primer | Sequence of reverse primer | Tm | PCR cycle number | Size of product (bp) |
|---|---|---|---|---|---|
| Emx1 | GAGAAGAACCACTACGTGGTGGG | GCCCGTGTCATTAAGAGAGAGAC | 62°C | 40 | 641 |
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC | 52°C | 21 | 226 |
| Gli1 | TCTCAAACTGCCCAGCTTGTC | AGGCTCTTGAACCTCTGGACTC | 58°C | 40 | 432 |
| Gli2 | GACACATCAAAGAGCAAGGATTG | ACCGTGGACAGAATGAGGCT | 55°C | 40 | 294 |
| Hb9 | AGCTGGGCGCCGGCACCTTCC | CCGCCGCCGCCCTTCTGTTTCTC | 65°C | 37 | 348 |
| HoxC9 | GGCAGCAAGCACAAAGAGGAG | GCAGGCTGGGTAGGGTTTAGG | 55°C | 40 | 301 |
| Irx3 | TGTCCAACGTGCTCTCGTC | TCCTCCTCGTCCTCCTCTTC | 62°C | 37 | 517 |
| Isl1 | AGGATGTGGAGGTAGTGAGA | GGAGATCTCAGTGGCTCTT | 54°C | 40 | 356 |
| Lim3 | TCAAGCGCTTCGGGACCAAG | TCCTGAACGCTGTCCTTGTCC | 60°C | 40 | 493 |
| Ngn2 | GAAGACCCGTAGACTGAA GGC | GAAAGGGAACCCACTAAGGC | 54°C | 40 | 537 |
| Nkx2.2 | TCTACGACAGCAGCGACAAC | GAACCAGATCTTGACCTGCG | 55°C | 40 | 335 |
| Olig2 | AAGCTAGGAGGCAGTGGCTTCAAGTC | CCGTCACCAGTCGCTTCATC | 60°C | 28 | 312 |
| Otx2 | ACGACGTTCACTCGGGCGCAG | ACTGCTGCTGGCAATGGTCGG | 58°C | 40 | 312 |
| Pax3 | CACTCGCCTTTCCGTTTCGCC | CGTTGTCACCTGCTTGGGCTTGC | 58°C | 40 | 393 |
| Pax6 | GGCAACCTACGCAAGATGGC | TGAGGGCTGTGTCTGTTCGG | 62°C | 35 | 459 |
| Pax7 | ACGGCTGCGTCTCCAAGATTC | GCTTCAGTGGGAGGTCAGGTTC | 58°C | 40 | 405, 399 |
| Phox2b | GTCCAAGTGGAAAGAGCCAAG | CCGTGGTCCGTGAAGAGTTTG | 61°C | 40 | 495 |
| Sox2 | TACCTCTTCCTCCCACTCCA | ACTCTCCTCTTTTGCACCCC | 55°C | 35 | 269 |
Table 2.
Antibodies used in immunostaining and immunoblotting.
| Name of antibody | Type | Vendor | Dilution |
|---|---|---|---|
| Immunostaining: | |||
| β3-tubulin | Mouse monoclonal | Covance | 1:5000 |
| BrdU | Mouse monoclonal | Sigma | 1:1000 |
| ChAT | Goat polyclonal | Chemicon | 1:50–100 |
| GABA | Rabbit polyclonal | Sigma | 1:1000 |
| GFAP | Rabbit polyclonal | Chemicon | 1:1000–2000 |
| Glutamate | Rabbit polyclonal | Sigma | 1:4000 |
| Hb9 | Mouse monoclonal | Developmental Studies Hybridoma Bank | 1:50–200 |
| MAP2 | Rabbit polyclonal | Chemicon | 1 :1000 |
| Olig2 | Goat polyclonal | R&D Systems | 10 ug/ml |
| O4 | Mouse monoclonal | R&D Systems | 1:500 |
| Immunoblotting: | |||
| β-actin | Mouse monoclonal | Sigma | 1:25000 |
| AKT | Rabbit polyclonal | Cell Signaling Technology | 1:500 |
| Phospho-AKT (Ser473) | Rabbit polyclonal | Cell Signaling Technology | 1:500 |
| p44/42 MAP Kinase | Rabbit polyclonal | Cell Signaling Technology | 1:500 |
| Phospho-p44/42 MAP Kinase (Thr202/Tyr204) | Rabbit polyclonal | Cell Signaling Technology | 1:500 |
| SAPK/JNK | Rabbit polyclonal | Cell Signaling Technology | 1:1000 |
| phospho-SAPK/JNK (Thr183–Tyr185) | Rabbit monoclonal | Cell Signaling Technology | 1:1000 |
BrdU, bromodeoxyuridine; ChAT, choline acetyltransferase; GABA, gamma-aminobutyric acid; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein 2.
Acknowledgments
Drs. P.M.J. and L.D.O. contribute equally to this study. The authors thank Tiffany J. Dunn, Kirsten A Clingman and Long Ma for technical supports, and Drs. Henry Epstein and Richard Coggeshall for critical reading. The study is supported by the National Institute of Health (NS046025 to P.W. and F30NS060387 Ruth L. Kirschestein National Research Service Award to J.R.T.), the TIRR Foundation (P.W.), the John S. Dunn Research Foundation (P.W.) and the Cullen Foundation (P.W.).
References
- Abramova N, Charniga C, Goderie SK, Temple S. Stage-specific changes in gene expression in acutely isolated mouse CNS progenitor cells. Dev Biol. 2005;283:269–281. doi: 10.1016/j.ydbio.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:19–35. doi: 10.1016/s0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, Perrier AL, Bruses J, Rubio ME, Topf N, Tabar V, Harrison NL, Beal MF, Moore MA, Studer L. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Chen Y, Jessell TM, Struhl G. A hedgehog-insensitive form of patched provides evidence for direct long-range morphogen activity of sonic hedgehog in the neural tube. Mol Cell. 2001;7:1279–1291. doi: 10.1016/s1097-2765(01)00271-4. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Novitch BG. Regulatory pathways linking progenitor patterning, cell fates and neurogenesis in the ventral neural tube. Philos Trans R Soc Lond B Biol Sci. 2008;363:57–70. doi: 10.1098/rstb.2006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Wu P, Ozen M, Yu Y, Wang J, Ittmann M, Liu M. Gene expression profiling and analysis of signaling pathways involved in priming and differentiation of human neural stem cells. Neuroscience. 2006;138:133–148. doi: 10.1016/j.neuroscience.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Chandran S, Kato H, Gerreli D, Compston A, Svendsen CN, Allen ND. FGF-dependent generation of oligodendrocytes by a hedgehog-independent pathway. Development. 2003;130:6599–6609. doi: 10.1242/dev.00871. [DOI] [PubMed] [Google Scholar]
- Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- Dromard C, Bartolami S, Deleyrolle L, Takebayashi H, Ripoll C, Simonneau L, Prome S, Puech S, Tran VB, Duperray C, Valmier J, Privat A, Hugnot JP. NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells. Stem Cells (Dayt) 2007;25:340–353. doi: 10.1634/stemcells.2005-0556. [DOI] [PubMed] [Google Scholar]
- Du X, Iacovitti L. Synergy between growth factors and transmitters required for catecholamine differentiation in brain neurons. J Neurosci. 1995;15:5420–5427. doi: 10.1523/JNEUROSCI.15-07-05420.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston AJ, van der WJ, Pijnappel WW, Godsave SF. Retinoids and related signals in early development of the vertebrate central nervous system. Curr Top Dev Biol. 1998;40:111–175. doi: 10.1016/s0070-2153(08)60366-x. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- Gao J, Coggeshall RE, Chung JM, Wang J, Wu P. Functional motoneurons develop from human neural stem cell transplants in adult rats. Neuroreport. 2007 doi: 10.1097/WNR.0b013e3280b10c2c. (in press) [DOI] [PubMed] [Google Scholar]
- Gao J, Coggeshall RE, Tarasenko YI, Wu P. Human neural stem cell-derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience. 2005;131:257–262. doi: 10.1016/j.neuroscience.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Gremo F, Presta M. Role of fibroblast growth factor-2 in human brain: a focus on development. Int J Dev Neurosci. 2000;18:271–279. doi: 10.1016/s0736-5748(99)00095-7. [DOI] [PubMed] [Google Scholar]
- Gutin G, Fernandes M, Palazzolo L, Paek H, Yu K, Ornitz DM, McConnell SK, Hebert JM. FGF signalling generates ventral telencephalic cells independently of SHH. Development. 2006;133:2937–2946. doi: 10.1242/dev.02465. [DOI] [PubMed] [Google Scholar]
- Hack MA, Sugimori M, Lundberg C, Nakafuku M, Gotz M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Tropepe V, Ekker M, van der KD. Neural stem cell lineages are regionally specified, but not committed, within distinct compartments of the developing brain. Development. 2002;129:233–244. doi: 10.1242/dev.129.1.233. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jordan PM, Cain LD, Wu P. Astrocytes enhance long-term survival of cholinergic neurons differentiated from human fetal neural stem cells. J Neurosci Res. 2007 doi: 10.1002/jnr.21460. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Jamen F, Rubin LL, Richardson WD. Cooperation between sonic hedgehog and fibroblast growth factor/MAPK signalling pathways in neocortical precursors. Development. 2004;131:1289–1298. doi: 10.1242/dev.01027. [DOI] [PubMed] [Google Scholar]
- Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993;10:255–265. doi: 10.1016/0896-6273(93)90316-j. [DOI] [PubMed] [Google Scholar]
- Kim HT, Kim IS, Lee IS, Lee JP, Snyder EY, Park KI. Human neurospheres derived from the fetal central nervous system are regionally and temporally specified but are not committed. Exp Neurol. 2006;199:222–235. doi: 10.1016/j.expneurol.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Machon O, Backman M, Krauss S, Kozmik Z. The cellular fate of cortical progenitors is not maintained in neurosphere cultures. Mol Cell Neurosci. 2005;30:388–397. doi: 10.1016/j.mcn.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- Milosevic J, Brandt A, Roemuss U, Arnold A, Wegner F, Schwarz SC, Storch A, Zimmermann H, Schwarz J. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J Neurochem. 2006;99:913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Sugimori M, Takebayashi H, Kosako H, Nagao M, Yoshida S, Nabeshima Y, Shimamura K, Nakafuku M. Combinatorial roles of olig2 and neurogenin2 in the coordinated induction of pan-neuronal and subtype-specific properties of motoneurons. Neuron. 2001;31:757–771. doi: 10.1016/s0896-6273(01)00413-5. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Naruse M, Nakahira E, Miyata T, Hitoshi S, Ikenaka K, Bansal R. Induction of oligodendrocyte progenitors in dorsal forebrain by intraventricular microinjection of FGF-2. Dev Biol. 2006;297:262–273. doi: 10.1016/j.ydbio.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Nelson AD, Svendsen CN. Low concentrations of extracellular FGF-2 are sufficient but not essential for neurogenesis from human neural progenitor cells. Mol Cell Neurosci. 2006;33:29–35. doi: 10.1016/j.mcn.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Ostenfeld T, Joly E, Tai YT, Peters A, Caldwell M, Jauniaux E, Svendsen CN. Regional specification of rodent and human neurospheres. Brain Res Dev Brain Res. 2002;134:43–55. doi: 10.1016/s0165-3806(01)00291-7. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Hirsch M, Goridis C, Brunet JF. Control of hindbrain motor neuron differentiation by the homeobox gene Phox2b. Development. 2000;127:1349–1358. doi: 10.1242/dev.127.7.1349. [DOI] [PubMed] [Google Scholar]
- Pearson BJ, Doe CQ. Specification of temporal identity in the developing nervous system. Annu Rev Cell Dev Biol. 2004;20:619–647. doi: 10.1146/annurev.cellbio.19.111301.115142. [DOI] [PubMed] [Google Scholar]
- Qian X, Davis AA, Goderie SK, Temple S. FGF2 concentration regulates the generation of neurons and glia from multipotent cortical stem cells. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- Ray J, Peterson DA, Schinstine M, Gage FH. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci U S A. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss B, Bohlen und HO. Fibroblast growth factors and their receptors in the central nervous system. Cell Tissue Res. 2003;313:139–157. doi: 10.1007/s00441-003-0756-7. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, Smukler SR, van der KD. Intrinsic differences distinguish transiently neurogenic progenitors from neural stem cells in the early postnatal brain. Dev Biol. 2005;278:71–85. doi: 10.1016/j.ydbio.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Svendsen CN, ter Borg MG, Armstrong RJ, Rosser AE, Chandran S, Ostenfeld T, Caldwell MA. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- Tarasenko YI, Yu Y, Jordan PM, Bottenstein J, Wu P. Effect of growth factors on proliferation and phenotypic differentiation of human fetal neural stem cells. J Neurosci Res. 2004;78:625–636. doi: 10.1002/jnr.20316. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Thaler J, Harrison K, Sharma K, Lettieri K, Kehrl J, Pfaff SL. Active suppression of interneuron programs within developing motor neurons revealed by analysis of homeodomain factor HB9. Neuron. 1999;23:675–687. doi: 10.1016/s0896-6273(01)80027-1. [DOI] [PubMed] [Google Scholar]
- Tsai RY, Kim S. Fibroblast growth factor 2 negatively regulates the induction of neuronal progenitors from neural stem cells. J Neurosci Res. 2005;82:149–159. doi: 10.1002/jnr.20627. [DOI] [PubMed] [Google Scholar]
- Wada H, Holland PW, Sato S, Yamamoto H, Satoh N. Neural tube is partially dorsalized by overexpression of HrPax-37: the ascidian homologue of Pax-3 and Pax-7. Dev Biol. 1997;187:240–252. doi: 10.1006/dbio.1997.8626. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wu P, Tarasenko YI, Gu YP, Huang LYM, Coggeshall RE, Yu YJ. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosc. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- Yang M, Donaldson AE, Marshall CE, Shen J, Iacovitti L. Studies on the differentiation of dopaminergic traits in human neural progenitor cells in vitro and in vivo. Cell Transplant. 2004;13:535–547. doi: 10.3727/000000004783983729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]