Abstract
The purpose of this project was to assess the validity of a novel “Electroporation and transcutaneous sampling (ETS)” technique for sampling cephalexin from the dermal extracellular fluid (ECF). This work also investigated the plausibility of using cephalexin levels in the dermal ECF as a surrogate for the drug level in the synovial fluid. In vitro and in vivo studies were carried out using hair less rats to assess the workability of ETS. Cephalexin (20mg/kg) was administered i.v. through tail vein and the time course of drug concentration in the plasma was determined. In the same rats, cephalexin concentration in the dermal ECF was determined by ETS and microdialysis techniques. In a separate set of rats, only intraarticular microdialysis was carried out determine the time course of cephalexin concentration in synovial fluid. The drug concentration in the dermal ECF determined by ETS and microdialysis did not differ significantly from each other and so as were the pharmacokinetic parameters. The results provide validity to the ETS technique. Further, there was a good correlation (~0.9) between synovial fluid and dermal ECF levels of cephalexin indicating that dermal ECF levels could be used as a potential surrogate for cephalexin concentration in the synovial fluid.
Keywords: Electroporation, Transcutaneous sampling, Hairless rats, Microdialysis, Pharmacokinetics, Extracellular fluid, Synovial fluid, Transdermal
INTRODUCTION
The most commonly occurring infections in people are skin infections. Particularly, children and elderly people are mostly affected with skin infections due to lack of potent immune system. Cephalosporins, are the most widely used for treatment of skin infections because of their safety profiles.1–3 Cephalexin, a first generation cephalosporin antibiotic is mostly used because of its activity against both the gram-positive and gram negative microorganisms.4 In addition to treatment of skin infections, cephalexin is also commonly used to treat the articular infections.5, 6 Achieving therapeutically active drug levels at the site of infection is vital for any antibiotic therapy. In general, when the infection is situated in the central pharmacokinetic compartment, the activity of the drug is determined by the unbound drug concentration in the plasma. However, in case of infections in the peripheral tissues such as skin and articular region, it is the time course of concentration of unbound antibiotic in the respective tissue fluids which is crucial for successful treatment. In such cases monitoring the drug levels in the plasma may not reflect the actual levels in the affected tissue. Therefore, the time course of antibiotics in the affected tissue needs to be monitored for determining the frequency and dose of drug administration from the safety and efficacy perspectives of the antibiotic therapy.7–11 In case of treatment of skin infections, the cutaneous drug levels could be known by conventional methods of sampling such as skin blister fluid and skin biopsy sampling techniques.12, 13 These techniques are invasive and also the number of samples that could be obtained by these techniques is limited. Microdialysis is a widely used method for sampling drug from tissues, as it is capable of sampling unbound drug from the tissue extracellular fluid (ECF). Microdialysis has been used to sample drugs from skin and synovial fluid as well.14–20 However it is also an invasive technique and has limitations with implementation in routine therapeutic drug monitoring. In this regard, a novel noninvasive technique called electroporation and transcutaneous sampling (ETS) was developed for sampling drugs from the dermal ECF. ETS is a method of reversible permeabilization of stratum corneum and sampling of drugs from the dermal ECF by facilitating reverse diffusion of drug in the direction of dermis to stratum corneum.10, 21, 22 In the current study, using the model antibiotic cephalexin, we seek answer to two questions. First, could ETS be utilized for sampling of cephalexin from dermal ECF? Second, whether the dermal ECF levels can serve as a surrogate for synovial fluid levels of cephalexin?
MATERIALS AND METHODS
Chemicals
Cephalexin hydrate was purchased from Sigma-Aldrich Inc (St.Louis, MO), Phosphate buffered saline (PBS, pH 7.4) premixed powder was obtained from EMD Chemicals (Gibbstown, NJ), and all other chemicals were obtained from Fischer Scientific (Fairway, NJ).
In Vitro studies
The in vitro diffusion studies were carried out in Franz diffusion cells (FDC) (Logan Instruments Ltd, Somerset, NJ) using hairless rat skin excised from the abdomen region. Hairless rat skin is known to be a good model for topical and transdermal drug delivery studies due to the similarity between the rat and human skin with respect to lipid content and water uptake properties.23 Moreover, a good correlation of permeation data between the hairless rat model and human skin models has been reported by several research groups in the past.24 The skin was mounted on the diffusion cell in such a way that the epidermis side of the skin was in contact with upper sampling compartment and dermal side with the lower reservoir compartment. The active diffusion area of FDC was 0.64 cm2. Ag/AgCl electrode wires of 2mm diameter (In Vivo Metric, CA) made in form of circular rings were placed 2mm away from skin in both sampling and reservoir compartments. The sampling compartment and the reservoir compartment were filled with 0.4 and 5ml PBS respectively and the skin was allowed to equilibrate for an hour. The AC electrical resistance of the epidermis was measured by placing a load resistor RL (100 kΩ) in series with the epidermis. The voltage drop across the whole circuit (VO) and across the skin (VS) was measured using an electrical set up consisting of a wave form generator and a digital multimeter (Agilent Technologies, Santa Clara, CA). For measuring resistance, voltage of 100 mv was applied at 10 Hz and the skin resistance in kΩ was approximated from the formula:
| (1) |
Where RS is the skin resistance and RL is the load resistor in kΩ. The piece of skin, which had a resistance greater than 20 kΩ.cm2 was used for the experiment.
Later, the sampling compartment was replaced with fresh 0.4 ml of PBS (pH 7.4) and the reservoir compartment was filled with 5ml of cephalexin solution prepared in PBS (5–40 µg/ml). Thirty square electrical pulses each of 10ms duration at 120V/cm2, 1Hz was applied using ECM 830 Electro Square Porator (BTX Harvard apparatus, Holliston, USA). The electrical resistance was measured immediately after application of electrical pulses to ensure skin permeabilization. PBS from the sampling compartment was withdrawn 15 min after application of electrical pulses and the amount of cephalexin sampled was analyzed by HPLC.26
Ex Vivo plasma protein binding
The blood was collected by cardiac puncture in rats and the plasma was separated by centrifugation at 2000g at 4°C. Rat plasma was spiked with drug to provide concentration ranging from 1–20µg/ml. The spiked plasma samples were thoroughly mixed by vortexing and allowed to equilibrate for 12 h at 4°C. After equilibration, protein free plasma was obtained by using ultra filtration (Millipore Centrifree® filtration units) by centrifugation of 0.5 ml of plasma at 2000g for 20 min.10, 25 The amount of unbound drug present in the filtrate was measured by HPLC after suitable dilution with PBS.
In Vivo studies
The in vivo experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi (Protocol # 07-004). The in vivo studies were carried out in hairless rats (Taconic, Hudson, Newyork) (250–300 g) under ketamine (80mg/kg) and xylazine (10mg/kg) anesthesia administered intraperitoneally.
Plasma sampling, ETS and microdialysis sampling was carried out in the same group of rats (n=6). The samples by all three procedures were obtained at the same time points in each rat simultaneously. Cephalexin solution of 20 mg/kg prepared in sterile isotonic saline was administered by i.v. into tail vein as a bolus injection.
For cutaneous microdialysis, a 20G needle was inserted intradermally through a distance of 1cm in and a linear microdialysis probe of 5mm membrane length and 30kDa cutoff molecular weight (BASi, West Lafayette IN) was inserted through this needle and the needle was withdrawn leaving the probe implanted in the dermal tissue. The inlet tube was connected to an injection pump (BASi, West Lafayette, IN) and PBS was perfused at 2µl/min flow rate for 30 minutes for equilibration. Two samples were collected before drug administration. The drug was injected by i.v through the tail vein after equilibration of the probe. Subsequently the microdialysis samples were collected continuously at every 15 minutes interval including at time points corresponding to ETS and plasma sampling at 30, 60, 120,180,240,300 and 360min.
In case of ETS, prior to the drug administration, a custom made sampling cell was fixed using an adhesive (Krazy glue, Elmers products Inc, Ohio) on the back of the rats (Figure 1). The sampling cell was fitted with an Ag/AgCl electrode and the counter electrode was secured just adjacent to the cell on the surface of the skin using a micropore surgical tape (3M Healthcare, MN). The skin was hydrated with 100µl of saline for 5 minutes before each sampling and was replaced with 100µl of PBS (sampling buffer). One blank sample was collected before drug administration, and subsequent samples were collected at (30, 60,120,180,240,300 and 360min). For ETS procedure thirty electrical pulses each of 10ms duration at 120V/cm2, 1Hz was applied and the sampling fluid remained in the chamber for 15 minutes after pulsing.
Figure 1.
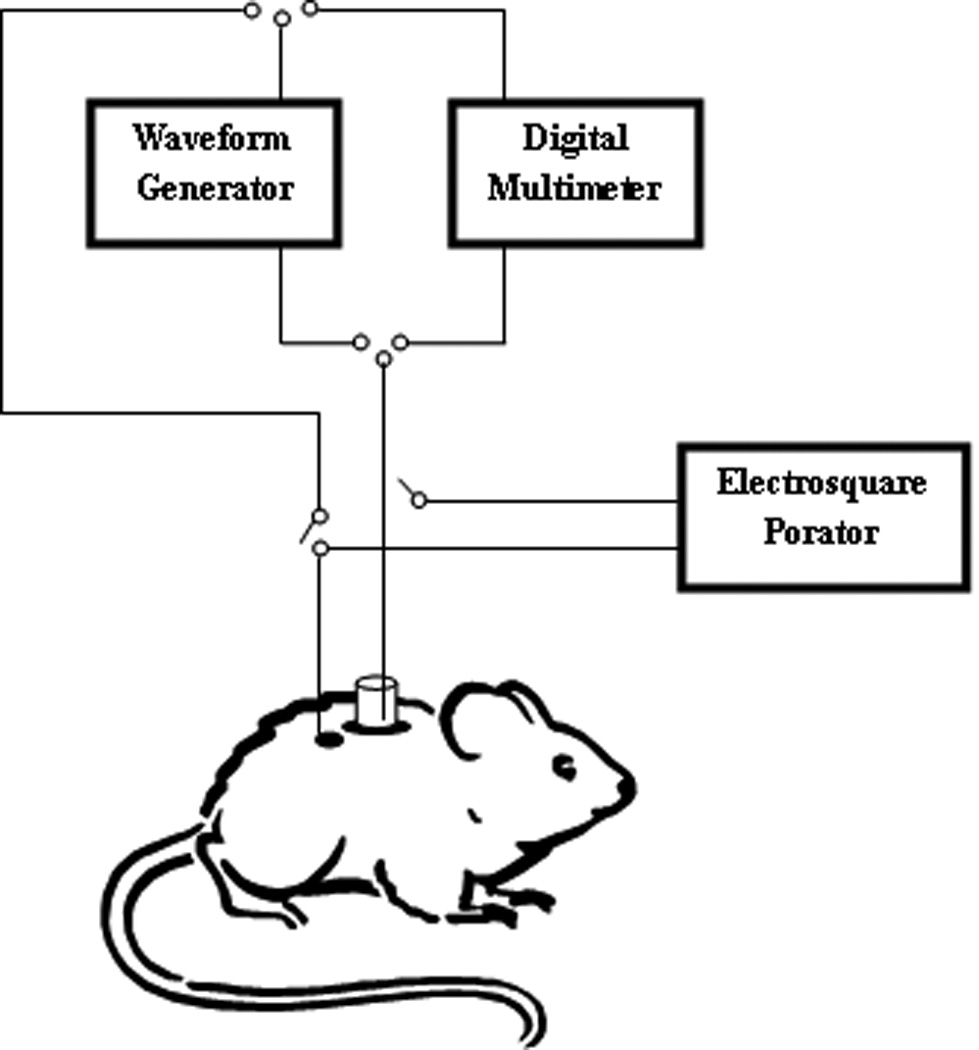
Diagrammatic representation showing an eperimental setup of electroporation and transcutaneous sampling in hairless rats.
For plasma pharmacokinetic studies, one hundred micro liters of blood was collected by retro orbital bleeding before injection of drug and before each episode of transcutaneous sampling and cutaneous microdialysis. The blood samples were diluted with 200µl of PBS and plasma was separated followed by protein precipitation and the plasma drug content was analyzed by HPLC.27, 28
In another set of rats (n=6), intraarticular microdialysis was carried out to determine the amount of cephalexin present in the synovial fluid. After anaesthetizing the rats, the hind limb was held in a fixed position and a 20G needle was passed through the knee joint capsule lateral to the patellar ligament and a microdialysis linear probe of 5mm length and 30kDa cut off molecular weight (BASi, West Lafayette, IN) was inserted through the neeedle.19, 20 The needle was withdrawn leaving the probe implanted in the synovial cavity. PBS was perfused for 30 min prior to drug administration for equilibration at flow rate of 2µl/min. Cephalexin solution (20mg/kg) was administered through the tail vein and microdialysis perfusion was continued for 6 hours with samples collected for 15min interval including at time points corresponding to time points of ETS and cutaneous microdialysis sampling.
In case of both cutaneous and intraarticular microdialysis, the probe recovery was determined in vivo by using retrodialysis method. 29–31 For this, the probe was first equilibrated by perfusing PBS at 2µl/min for 30 min followed by drug solution of known concentration for 30 min. After equilibration dialysate was collected for 15min interval at 15, 30 and 45min and the average recovery of three time points was considered. The in vivo recovery rate was calculated using the formula:
| (2) |
Analytical method
The amounts of cephalexin present in plasma, ETS and microdialysis samples were analyzed by HPLC using Symmetry® C18 column (4.6 × 150mm) with UV detection at 254nm. Mobile phase consisted of a mixture of methanol and 2.5mM sodium phosphate buffer, pH 5.6 (20:80 v/v) and the flow rate was 1 ml/min.23 The sensitivity of the method was 10ng/ml and linearity was between 10–1000ng/ml (R2= 0.99). To the plasma samples, equal volume of acetonitrile was added to precipitate proteins and then centrifuged at 2000g for 10min at room temperature and the supernatant was analyzed for drug content.27, 28 ETS and microdialysis samples were centrifuged and directly injected into HPLC system.
Data analysis
The plasma pharmacokinetic parameters were calculated based on two compartment model represented by the equation:
| (3) |
where A, B are the pre-exponential constants, α is the distribution rate constant, β is the elimination rate constant. The values of α, β, A and B are derived from curve fitting of experimental data. The elimnation half life (t1/2) was calculated using the formula 0.693/β and area under the curve (AUC0–6) was calculated using the trapezoid rule. The pharmacokinetic parameters in case of dermal ECF and sinovial fluid were calculated using non compartmental pharmacokinetic model.
The statistical analysis was carried out using GraphPad Instat 3 software and. The unpaired t-test was selected for comparing the parameters obtained from ETS and microdialysis techniques. p < 0.05 was considered as level of significance. From Pearsons correlation, R2 and p value were calculated using Pearson Correlation (v1.0.3) in Free Statistics Software (v1.1.23-r1).32 The data points shown in graphs are an average of 6 trials with error bars representing standard deviation.
RESULTS AND DISCUSSION
The electrical protocol and sampling time was determined based on our previous studies. 10, 33 Calibration of ETS was carried out in vitro using freshly excised hairless rat skin model. Known concentrations of drug were placed in the receiver compartment and the drug was sampled following electroporation. The amount of drug diffused in 15min following application of electrical pulses was plotted against respective reservoir concentrations (5–40µg/ml) as represented in Figure 2. The linear relationship (R2=0.96) between the amount of drug sampled and the reservoir drug concentration implies that the ETS samples would potentially represent the subdermal drug concentration. The percentage recovery by ETS can be obtained by (slope X 100) from Figure 2 and was found to be 3.06 ± 0.2 %. In control (across the untreated skin) the amount of drug sampled was less than detectable levels. In case of electroporation trials, the resistance of skin dropped ~74±8% whereas in case of control set of experiments, resistance of skin did not change significantly. The recovery of electrical resistance of electroporated skin was insignificant within the sampling duration of 15 min, which is in agreement with our previous reports in case of ETS across the rat skin and porcine epidermis.10, 22
Figure 2.
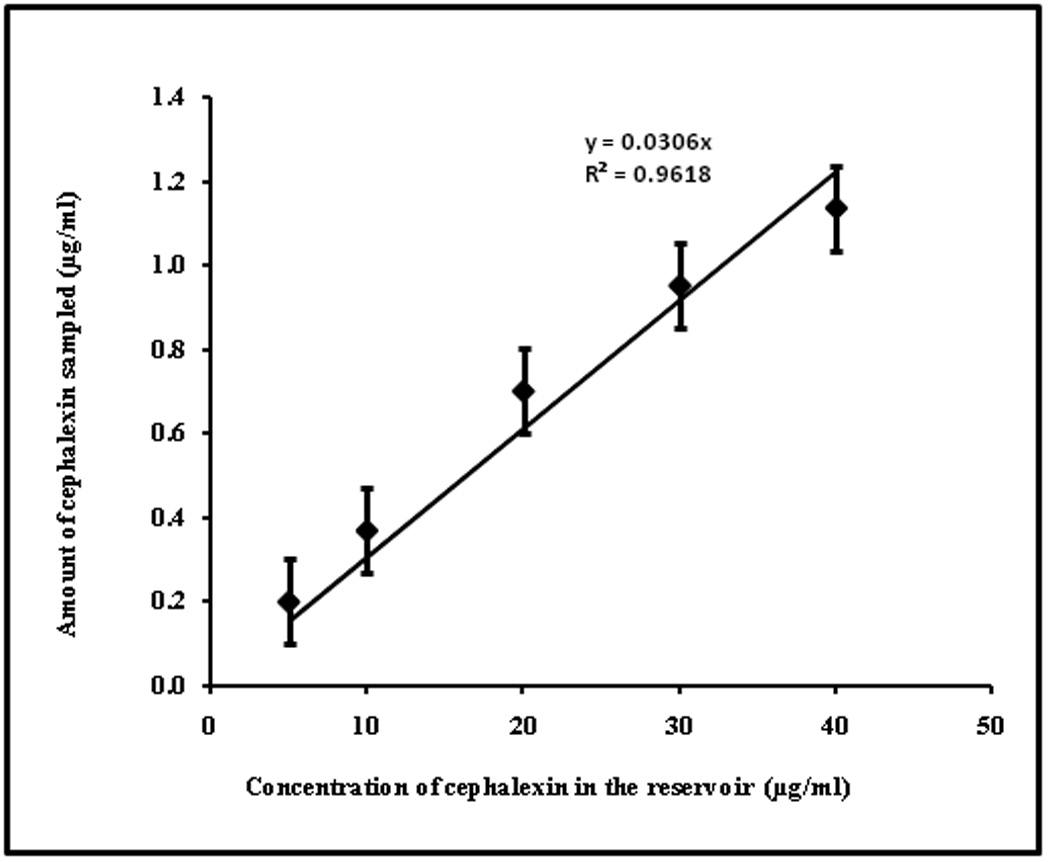
Correlation between cephalexin concentration (5–40µg/ml) in the reservoir compartment and cephalexin sampled by ETS across hairless rat skin in vitro. The data points represent an average of n=6 ± sd.
The plasma protein binding of cephalexin revealed that the fraction of cephalexin bound to plasma was 10.2 ± 2.6% at concentrations between 1–20 µg/ml. Low protein binding of cephalexin is considered to be one of the major reasons for its extensive distribution into the peripheral tissues. The protein binding values were in agreement with 12.4% that was reported by Tsai et al.4
The concentration time profile of cephalexin in plasma and dermal extracellular fluid (determined by ETS and cutaneous microdialysis) samples following i.v. administration of cephalexin (20mg/kg) is shown in Figure 3. The plasma concentration versus time data of cephalexin could be described by a two compartment model.34 The pharmacokinetic parameters calculated for plasma drug concentration- time profile are given in Table 1. The plasma drug concentrations reported in this project are comparable to that reported by Tsai et al in rats considering the difference in dose between the two studies. The plasma elimination half life of cephalexin in the current study was 104.59 ± 28.61 min (1.74 ± 0.47 h) which agrees well with the elimination half life (1.4 ± 0.81 h) reported by Padoin et al.34
Figure 3.
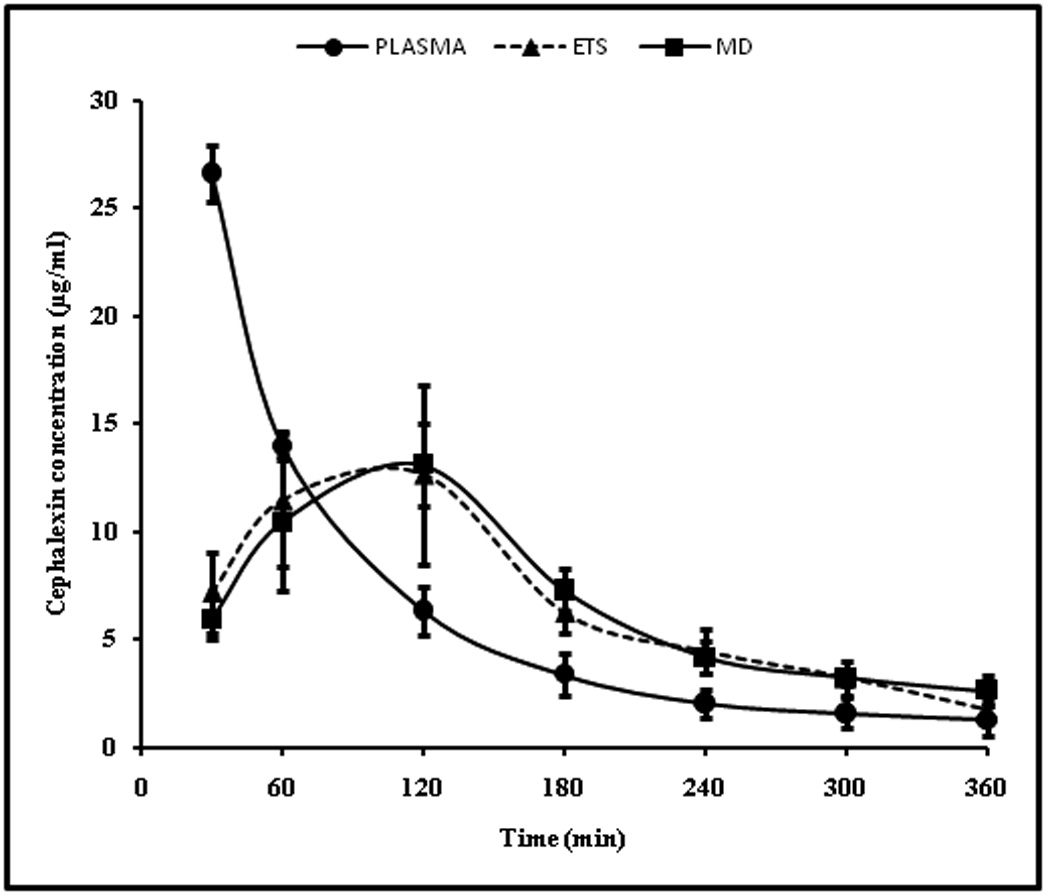
Time course of cephalexin in rat plasma determined by blood sampling and dermal ECF determined by microdialysis and ETS technique following administration of 20mg/kg cephalexin i.v bolus.The data points represent an average of n=6 ± sd. Blood sampling, ETS and microdialysis sampling were carried out simultaneously on each rat at the same time points.
Table 1.
Pharmacokinetic parameters derived from the plasma concentration time data after i.v bolus administration of 20mg/kg of cephalexin in hairless rats (n=6 ± sd).
| Parameter | i.v. bolus |
|---|---|
| A (µg/ml) | 52.35 ± 1.41 |
| B (µg/ml) | 11.89 ± 1.04 |
| t1/2 (min) | 104.59 ± 28.61 |
| α (1/min) | 0.03355 ± 0.004 |
| β (1/min) | 0.00688 ± 0.001 |
| AUC0–6 (min*µg/ml) | 3160.93 ± 250.35 |
The recovery of cephalexin by the microdialysis probe in the cutaneous tissue was found to be 21.14 ± 5.26% whereas the recovery of cephalexin by ETS was only about 3.06 ± 0.2% which is about 7 fold less than that of microdialysis. Although ETS has the advantage of being noninvasive as opposed to microdilaysis, the later has the limitation with the amount of drug that could be sampled from the dermal ECF. Nevertheless, recovery could likely be improved by using more vigorous electrical protocol and/or by increasing the sampling duration. In both microdilaysis as well as ETS techniques, the amount of cephalexin present in the dermal ECF in rats was calculated using the amount sampled and the corresponding recovery values as follows.
| (4) |
In the current study, the point to point comparison of the drug concentration in the dermal extracellular fluid and the pharmacokinetic parameters, i.e. Cmax, Tmax, AUC0–6 and t1/2, determined by ETS and microdialysis techniques did not differ significantly (unpaired t-test, p<0.05) (Table 2). This provides validity to the ETS technique of sampling cephalexin. Further, the percent penetration of cephalexin into the cutaneous tissue (AUC0–6, ECF /AUC0–6, plasma) was found to be 79.48 ± 10.01% and 78.25 ± 8.08% respectively with ETS and cutaneous microdialysis. This is in agreement with the percentage protein binding observed in this study (10.2±2.6%).
Table 2.
Mean pharmacokinetic parameters of cephalexin determined by ETS, microdialysis techniques following administration of 20mg/kg by i.v bolus in hairless rats (n=6 ± sd).
| Parameter | ETS | Microdialysis | P- value |
|---|---|---|---|
| Tmax (min) | 120 | 120 | -- |
| Cmax (µg/ml) | 13.09 ± 1.92 | 12.64 ± 1.90 | 0.39 |
| AUC0–6(min*µg/ml) | 2512.35 ± 250.14 | 2473.66 ± 202.43 | 0.42 |
| t1/2 (min) | 106.61 ± 17.81 | 96.37 ± 12.33 | 0.22 |
The amount of drug present in the synovial fluid is shown in Figure 4 and the pharmacokinetic parameters are given in Table 3. In this case the in vivo microdialysis probe recovery was found to be 10.64 ± 3.44%. The low recovery in synovial fluid compared to cutaneous microdialysis could be due to slow turn over and limited volume of fluid available in the articular region. The percent penetration of cephalexin into synovial fluid (AUCsynovial fluid/ AUCplasma) was 20.69 ± 2.47% as compared to ~79% into cutaneous tissue. The Cmax in case of synovial fluid (3.23±0.58 µg/ml) was four fold less than the dermal ECF (~ 13.09µg/ml). This data suggests that relatively higher doses of cephalexin would be required to achieve effective drug levels in the synovial fluid.
Figure 4.
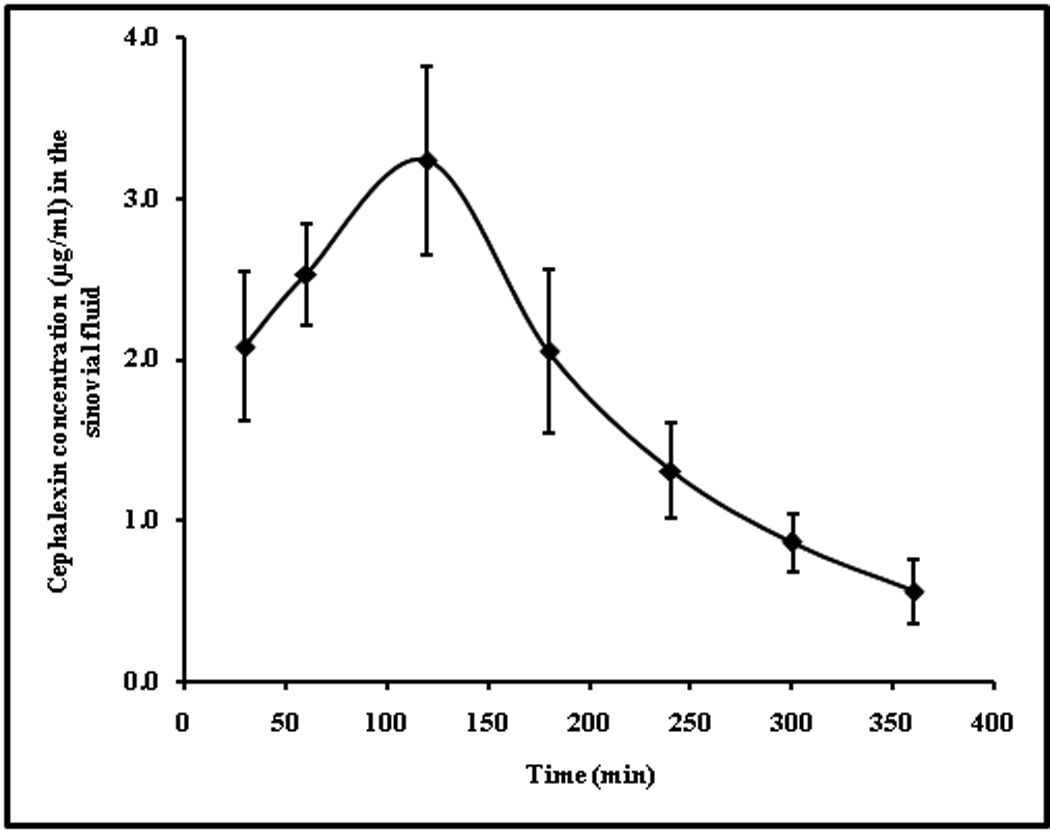
Concentration time profile of cephalexin in synovial fluid, obtained by intraarticular microdialysis after administration of 20mg/kg drug by i.v bolus. The data points represent an average of n=6 ± sd.
Table 3.
Mean pharmacokinetic parameters of cephalexin determined by intraarticular microdialysis in synovial fluid following administration of 20mg/kg by i.v bolus in hairless rats (n=6 ± sd).
| Parameter | Sinovial fluid |
|---|---|
| Tmax (min) | 120 |
| Cmax (µg/ml) | 3.233 ± 0.58 |
| t1/2 (min) | 96.215 ± 8.08 |
| AUC0–6 (min*µg/ml) | 654.10 ± 101.35 |
The drug levels from synovial fluid were plotted against drug levels in dermal ECF obtained by ETS and microdialysis techniques. A good correlation of 0.922 (p=0.00029) and 0.905 (p=0.00047) was observed between the drug levels in synovial fluid and dermal ECF obtained from ETS and microdialysis (Figure 5). From this relationship, it could be said that in rats, the drug level in the skin represents ~3.7X of that in the synovial fluid. Establishing such correlation between the dermal ECF and the concentration of drug in internal tissues would help in monitoring the drug levels of peripheral tissues which are extremely difficult to access. From the results of this experiment it appears that dermal ECF levels of cephalexin could be used as potential surrogate for cephalexin levels in the synovial fluid.
Figure 5.
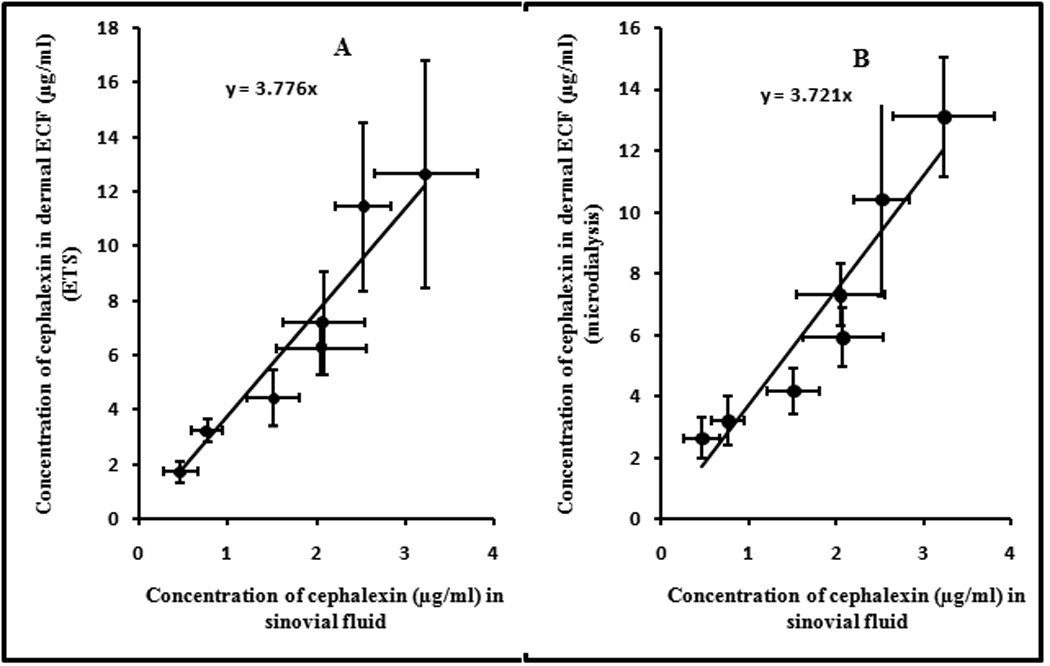
Correlation between cephalexin levels in dermal ECF (A-ETS and B-Microdialysis) with that of synovial fluid drug levels. The data points represent an average of n=6 ± sd.
ETS is a noninvasive method of cutaneous drug sampling and is expected to be relatively safer than microdialysis method. However, there are concerns about potential skin damage due to the application of electrical pulses. Many research groups have evaluated the safety of skin electroporation in animal models and human subjects. Vanbever et al have reported that reversible mild skin reactions occurred following the application of 15 electrical pulses of 250V and 200ms in vivo in hairless rats. Wong et al have shown that electroporation can be carried out in humans without causing pain at 150V, 1ms, 60 pulses by using microelectrode array. The protocol that was applied in current experiments was 120V, 30 pulses each of 10ms duration which is rather mild than the protocols applied on human subjects in other studies.35, 36 The extent of skin damage depends on the applied electrical protocol and the electrode design. Therefore the optimum electrical protocols need to be evaluated in vivo for tolerability, morphological, histological and biochemical changes in the skin before implementation in clinical practice.
CONCLUSION
ETS is a potential noninvasive technique that could be developed for sampling of drugs from the skin tissue. However, the major limitation of the technique is low recovery which limits the application of the technique to drugs which are less protein bound and which are present considerably in high amounts in the dermal ECF. One of the most interesting outcome of the present work was that the dermal ECF concentration of cephalexin correlated well with the concentration in synovial fluid.
ACKNOWLEDGEMENTS
This project described was supported by Grant Number AR053097 from National Institute of Arthritis and Musculoskeletal and Skin Diseases.
REFERENCES
- 1.Del Rosso J. Therapeutic experience with cefdinir in the treatment of uSSSIs. Int J Clin Pract. 2006;60:1313–1316. doi: 10.1111/j.1742-1241.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 2.Davis JL, Salmon JH, Papich MG. Pharmacokintics and tissue fluid distribution of cephalexin in the horse aftyer oral and i.v. administration. J Vet Pharmacol Therap. 2005;28:425–431. doi: 10.1111/j.1365-2885.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 3.Tack KJ, Keyserling CH, McCarty J, Hedrick JA. Study of use of cefdinir versus cephalexin for treatment of skin infections in pediatric patients. Antimicrob Agents Chemother. 1997;41:739–742. doi: 10.1128/aac.41.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai TH, Hung LC, Chang YL, Shum AYC, Chen CF. Simultaneous blood and brain sampling of cephalexin in the rat by microdialysis amd microbore liquid chromatography: application to pharmacokinetic studies. J Chromatogr B. 2000;740:203–209. doi: 10.1016/s0378-4347(00)00078-5. [DOI] [PubMed] [Google Scholar]
- 5.Judd D, Bottoni C, Kim D, Burke M, Hooker S. Infections following arthroscopic anterior cruciate ligament reconstruction. The Journal of Arthroscopic and Related Surgery. 2006;22:375–384. doi: 10.1016/j.arthro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Petersen W, Beske C, Stein V, Laprell H. Arthroscopical removal of a projectile from the intra-articular cavity of the knee joint. Arch Orthop Trauma Surg. 2002;122:235–236. doi: 10.1007/s00402-001-0373-4. [DOI] [PubMed] [Google Scholar]
- 7.Brunner M, Hollenstein U, Delacher S, Jager D, Schmid R, Lackner E, Georgopoulos A, Eichler HG, Muller M. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob Agents Chemother. 1999;43:1307–1309. doi: 10.1128/aac.43.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharbhaiya RH, Shukla UA, Gleason CR, Shyu WC, Wilber RB, Pitman KA. Comparison of cefprozil and cefaclor pharmacokinetics and tissue penetration. Antimicrob Agents Chemother. 1990;34:1204–1209. doi: 10.1128/aac.34.6.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delacher S, Derendorf H, Hollenstein U, Brunner M, Joukhadar C, Hofman S, Georgopoulos A, Eichler HG, Muller M. A combined in vivo pharmacokinetic- in vitro pharmacodynamic approach to simulate target site pharmacodynamics of antibiotics in humans. Journal of Antimicrobial Chemotherapy. 2000;46:733–739. doi: 10.1093/jac/46.5.733. [DOI] [PubMed] [Google Scholar]
- 10.Murthy SN, Zhao YL, Hui SW, Sen A. Electroporation and transcutaneous extraction (ETE) for pharmacokinetic studies of drugs. J Control Release. 2005;105:132–141. doi: 10.1016/j.jconrel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Costa TD, Nolting A, Kovar A, Derendorf H. Determination of free interstitial concentrations of piperacillin-tazobactam combinations by microdialysis. Journal of Antimicrobial Chemotherapy. 1998;42:769–778. doi: 10.1093/jac/42.6.769. [DOI] [PubMed] [Google Scholar]
- 12.Wise R, Lockley RM, Webberly M, Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984;26:208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alguire PC, Mathes BM. Skin biopsy techniques for internist. Journal of General Internal Medicine. 1998;13:46–54. doi: 10.1046/j.1525-1497.1998.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bielecka-Grzela S, Klimo wicz A. Penetration of ciprofloxacin and its desethylenemetabolite into skin in humans after a single oral dose of the parent drug assessed by cutaneous microdialysis. J Clin Pharm Ther. 2005;30:383–390. doi: 10.1111/j.1365-2710.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 15.Ault JM, Lunte CE, Meltzer NM, Riley CM. Microdialysis sampling for the investigation of dermal drug transport. Pharm Res. 1992;9:1256–1261. doi: 10.1023/a:1015892914649. [DOI] [PubMed] [Google Scholar]
- 16.Muller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Pehamberger H, Agneter E, Eichler HG. Characterization of peripheral-compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunner M, Staβ H, Moller JG, Schrolnberger C, Erovic B, Hollenstein U, Zeitlinger M, Eichler HG, Muller M. Target site concentrations of ciprofloxacin after single intravenous and oral dose. Antimicrob Agents Chemother. 2002;46:3724–3730. doi: 10.1128/AAC.46.12.3724-3730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollenstein U, Brunner M, Schmid R, Muller M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. International Journal of Obesity. 2001;25:354–358. doi: 10.1038/sj.ijo.0801555. [DOI] [PubMed] [Google Scholar]
- 19.St. Claire RL, Brouwer KR. Chemical analysis of bis(5-amidino-2-benzimidazolyl) methane in the arthritic rat knee using microdialysis and microcolumn liquid chromatography. J Microcolumn Seperations. 1991;3:531–537. [Google Scholar]
- 20.Liu SH, Wong CH, Chang DM. Incresed monocyte chemoattractant protein-1 in knee joints of rats with adjuvant-induced arthritis: In vivo microdialysis. The Journal of Rheumatology. 2005;32:2205–2211. [PubMed] [Google Scholar]
- 21.Murthy SN, Zhang S. Electroporation and transcutaneous sampling (ETS) of acyclovir. Journal of Dermatological Science. 2008;49:249–251. doi: 10.1016/j.jdermsci.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivasa Murthy S, Siva Ram Kiran V, Mathur SK, Murthy SN. Noninvasive transcutaneous sampling of glucose by electroporation. J Diabetes Sci Technol. 2008;2:250–254. doi: 10.1177/193229680800200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto Y, Hatanaka T, Sugibayashi K, Omiya H. Prediction of skin permeability of drugs: Comparison of human and hairless rat skin. J Pharm Pharmacol. 1992;44:634–639. doi: 10.1111/j.2042-7158.1992.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 24.Wester RC, Maibach HI. Animal Models for Percutaneous Absorption. In: Shah VP, Maibach HL, editors. Topical Drug Bioavailability, Bioequivalence, and Penetration. NY: Plenum Press; 1993. [Google Scholar]
- 25.Schaefer HG, Stass H, Wedgwood J, Hampel B, Fischer C, Kuhlmann J, Schaad UB. Pharmacokinetics of ciprofloxacin in pediatric cystic fibrosis patients. Antimicrob Agents Chemother. 1996;40:29–34. doi: 10.1128/aac.40.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi OK, Song YS. Determination of cefuroxim levels in human serum by micellar electrokinetic capillary chromatography with direct sample injection. J Pharm Biomed Anal. 1997;15:1265–1270. doi: 10.1016/s0731-7085(96)02047-x. [DOI] [PubMed] [Google Scholar]
- 27.Qi M, Wang P, Sun P, Liu X. Liquid chromatographic method for the simultaneous determination of cephalexin and trimethoprim in dog plasma and application to the pharmacokinetic studies of a coformulated preparation. J Chromatogr B. 2006;832:307–312. doi: 10.1016/j.jchromb.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Haginaka J, Yamaoka K, Uno T. High speed liquid chromatographic determination of cephalexin in human plasma and urine. The Journal of Antibiotics. 1978;31:769–775. doi: 10.7164/antibiotics.31.769. [DOI] [PubMed] [Google Scholar]
- 29.Quellec AL, Dupin S, Genissel P, Saivin S, Marchand B, Houin G. Microdialysis probes calibration:Gradient and tissue dependent changes in no net flux and reverse dialysis methods. Journal of Pharmacological and Toxicological Methods. 1994;33:11–16. doi: 10.1016/1056-8719(94)00049-a. [DOI] [PubMed] [Google Scholar]
- 30.Muller M, Staβ H, Brunner M, Moller JG, Lackner E, Eichler HG. Penetration of moxifloxacin into peripheral compartments in humans. Antimicrob Agents Chemother. 1999;43:2345–2349. doi: 10.1128/aac.43.10.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuck EL, Grant M, Derendorf H. Effect of simulated microgravity on the disposition and tissue penetration of ciprofloxacin in healthy volunteers. J Clin Pharmacol. 2005;45:822–831. doi: 10.1177/0091270005276620. [DOI] [PubMed] [Google Scholar]
- 32.Wessa P. Pearson Correlation (v1.0.3) in Free Statistics Software (v1.1.23-rl), Office for Research Development and Education. 2008 URL http://www.wessa.net/rwasp_correlation.wasp/
- 33.Murthy SN, Sen A, Hui SW. Surfactant enhanced transdermal drug delivery by electroporation. J Control Release. 2004;98:307–315. doi: 10.1016/j.jconrel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Padoin C, Tod M, Perret G, Petitjean O. Analysis of the pharmacokinetic interaction between cephalexin and quinapril by a nonlinear mixed-effect model. Antimicrob Agents Chemother. 1998;42:1463–1469. doi: 10.1128/aac.42.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanbever R, Fouchard D, Jaduol A, De Morre N, Preat V, Marty JP. In vivo noninvasive evaluation of hairless rat skin after high -voltage pulse exposure. Skin Pharmacol. Appl. Skin Physiol. 1998;11:23–34. doi: 10.1159/000029805. [DOI] [PubMed] [Google Scholar]
- 36.Wong TW, Chen CH, Huang CC, Lin CD, Hui SW. Painless electroporation with a needle free-microelectrode array to enhance transdermal drug delivery. J Control Release. 2006;110:557–565. doi: 10.1016/j.jconrel.2005.11.003. [DOI] [PubMed] [Google Scholar]


