Abstract
Neural stem cells (NSCs) are present not only during the embryonic development but also in the adult brain of all mammalian species, including humans. Stem cell niche architecture in vivo enables adult NSCs to continuously generate functional neurons in specific brain regions throughout life. The adult neurogenesis process is subject to dynamic regulation by various physiological, pathological and pharmacological stimuli. Multipotent adult NSCs also appear to be intrinsically plastic, amenable to genetic programming during normal differentiation, and to epigenetic reprogramming during de-differentiation into pluripotency. Increasing evidence suggests that adult NSCs significantly contribute to specialized neural functions under physiological and pathological conditions. Fully understanding the biology of adult NSCs will provide crucial insights into both the etiology and potential therapeutic interventions of major brain disorders. Here we review recent progress on adult NSCs of the mammalian central nervous system, including topics on their identity, niche, function, plasticity, and emerging roles in cancer and regenerative medicine.
Keywords: Adult neurogenesis, neural stem cells, stem cell niche, plasticity, regeneration, reprogramming, cancer stem cells, hippocampus, olfactory bulb
Introduction
The discovery of adult mammalian neural stem cells (NSCs) marks a milestone in the odyssey of our contemporary understanding of adult brain plasticity. Early in the twentieth century, influential histological and anatomic studies of Koelliker, His, Bizzozero and Cajal had established that the adult mammalian brain remained structurally constant after birth and no new neurons could be conceivably generated in adulthood [1–3]. In his masterpiece [1], Cajal commented “Once the development was ended, the founts of growth and regeneration of the axons and dendrites dried up irrevocably. In the adult centers, the nerve paths are something fixed, ended, and immutable. Everything may die, nothing may be regenerated. It is for the science of the future to change, if possible, this harsh decree.” The stability of neural circuits was also thought to be essential for higher brain functions, such as storing long-term memory [4, 5]. Over the second half of the twentieth century, the notion of activity-dependent neuronal synaptic modification had gained steam and significantly enriched our understanding of the plastic nature of the mammalian brain [6, 7]. Structural plasticity at the cell population level, however, remained less apparent. Scattered evidence suggested the presence of dividing cells in the postnatal and adult brain [8–10], yet little attention was given to those studies since the neuronal fate of those cells and the extent of such phenomena were not immediately clear. Within the past two decades, technical advances, particularly the fate mapping method using 5-bromo-2-deoxyuridine (BrdU) in animals, have allowed researchers to demonstrate that a large number of newly generated cells in the adult brain were indeed neurons [11, 12]. Meanwhile, the identification of trophic and mitogenic actions of growth factors, including fibroblast growth factors (FGF) and epidermal growth factor (EGF) family proteins, paved the way to culture and maintain a variety of neural cells in vitro [13, 14]. In early 1990s, it was demonstrated that neural cells derived from the adult rodent brain were capable of self-replicating and giving rise to both neurons and glia in culture [15, 16]. Adult NSCs with similar properties were subsequently found to be present in many other brain regions of mammals [17–21].
Rapid progress in the field has since led to the general acceptance that adult NSCs are present specifically in the subventricular zone (SVZ) of the lateral ventricle wall and the subgranular zone (SGZ) of the hippocampal dentate gyrus [21–27]. It is believed that the unique niche architectures present in these regions permit functional neurogenesis from NSCs in vivo [22, 23, 28]. Interestingly, NSCs have also been derived from a variety of adult brain regions [18–20, 29], yet it remains controversial whether those regions harbor similar NSCs and enable neurogenesis under physiological conditions in vivo. Once isolated and grown in culture, adult NSCs are better defined in terms of the capacity to self-renew and generate multiple neural lineages, including neurons, astrocytes and oligodendrocytes. Intrinsically, adult NSCs appear to be plastic in their fate programming and reprogramming in certain conditions, giving rise to a variety of lineages not expected from their in vivo counterparts. Perhaps in part due to the plastic nature of adult NSCs, it is becoming increasingly appreciated that many forms of neoplastic conditions in brain cancers might result from dysregulation of adult NSCs in vivo. Advances in derivation and culture of multipotent adult NSCs in vitro also fuel the hope for therapeutic intervention in combating a cohort of neurodegenerative diseases that elicit irreversible loss of neurons and glia. Here we discuss a range of topics related to adult NSCs in the mammalian central nervous system (CNS), including their identity, niche, function, plasticity, application and emerging relevance to brain cancer and neural degenerative diseases.
Adult neural stem cells: identity and properties
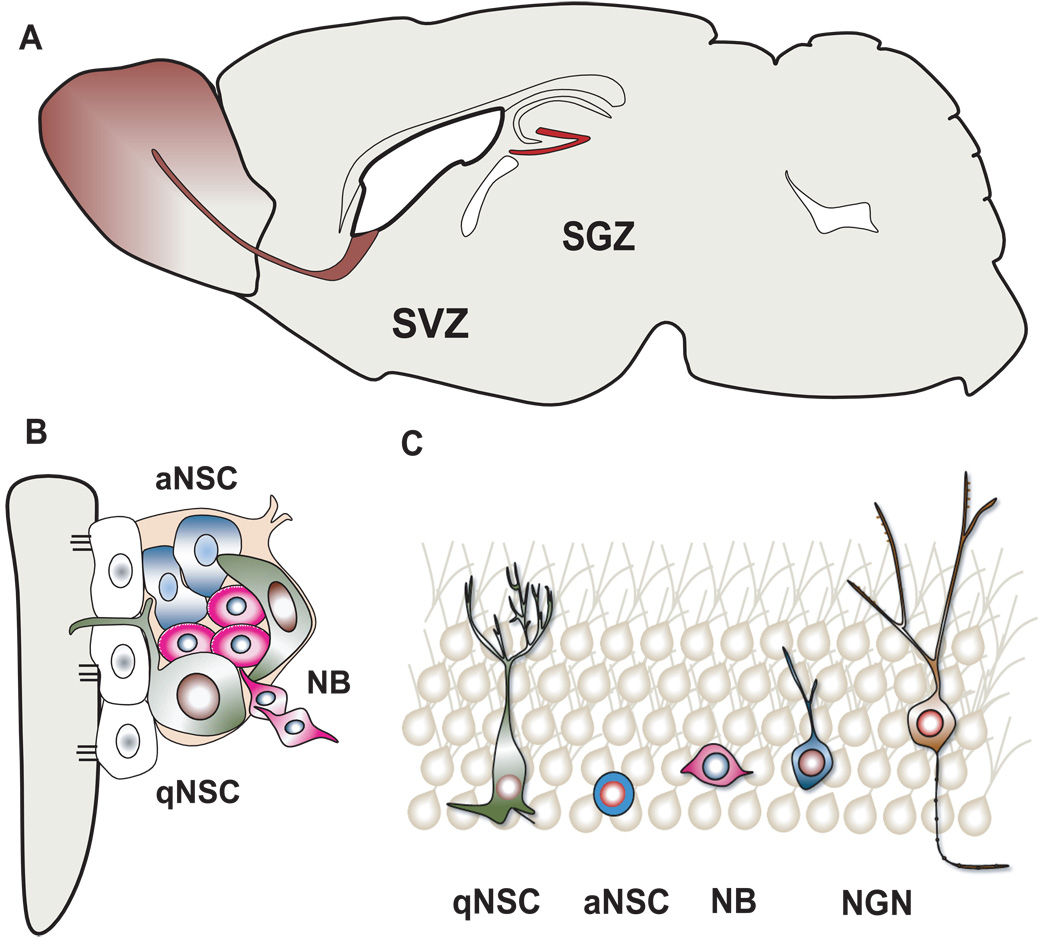
NSCs are self-renewing, multipotent progenitors residing in the nervous system. In the adult brain, NSCs are primarily located in the SVZ of the lateral ventricle and the SGZ of the hippocampal dentate gyrus (Figure 1A)[22, 23]. In the currently prevalent view, primary adult SVZ NSCs in vivo are slowly dividing, long-term BrdU-retaining progenitors that exhibit several common features of subventricular radial glia-like astrocytes and ventricular ependymal cells, including morphological characteristics and expression of the glial fibrillary acidic protein (GFAP) and the glycoprotein CD133. A double nucleotide thymidine analogue-labeling paradigm has been used to identify adult NSCs, based on their ability to re-enter cell cycle after long-term retention of thymidine analogues. Anatomical features and a comprehensive panel of immunohistochemical markers also help ascertain their identity. In the adult SVZ, the quiescent adult NSC population is believed to locate underneath the ependymal layer but contact the ventricle through their apical surfaces (Figure 1B). A subset of these cells is characterized as positive for LeX, CD133, GFAP, and Nestin while negative for differentiated cell markers CD24, O4, NeuN and S100β. Adult SVZ NSCs give rise to Dlx2+Mash1+ transient amplifying progenitors. The majority of these intermediate progenitor cells, in turn, give rise to PSA-NCAM+ doublecortin+ (DCX+) neuroblasts that migrate towards the olfactory bulb (OB) through the rostral migratory stream (RMS) in rodents; recently this has also been proposed to occur in humans through an anatomically distinct migratory stream [30, 31]. In the SGZ of the dentate gyrus, a similar subset of GFAP+, S100β−, Sox2+, Nestin+ radial cells corresponds to quiescent or dormant NSCs (Figure 1C). These NSCs may co-exist with the actively self-renewing population of GFAP−, S100β−, Sox2+, Nestin+ adult NSCs that generate GFAP+, S100β+ mature astrocytes and DCX+ neuroblasts. Unlike the interneuron lineage differentiation in the OB, adult NSCs in the SGZ of the hippocampus predominantly give rise to local glutamatergic excitatory dentate granule cells. The Sox2+ SGZ cells have recently been proposed to possess self-renewal capacity and multipotentiality [32], while these properties have not been strictly tested in vivo for SVZ NSCs under physiological conditions. Nonetheless, it remains unclear whether a single adult NSC undergoes extensive self-renew and generates progeny of multiple neural lineages in vivo.
Figure 1.
Adult NSCs in the SVZ and SGZ of the mammalian brain. (A) A schematic illustration of the adult mammalian brain in mice. Adult NSCs are primarily present in two germinal regions: the subventricular zone (SVZ) of the lateral ventricle wall and the subgranular zone (SGZ) of the hippocampal dentate gyrus. (B). Adult NSCs in the SVZ. Quiescent or dormant adult SVZ NSCs (dNSC) correspond to a unique type of cell population with cell bodies in the SVZ while contacting the ventricle through apical surfaces. They also share several common features of GFAP+ astrocytes and CD133+ ependymal cells. Actively self-renewing adult SVZ NSCs (sNSC) are located in the SVZ and give rise to neuroblasts that migrate toward the olfactory bulb. (C) Adult NSCs in the SGZ. Quiescent or dormant adult SGZ NSCs correspond to radial glia-like cells, some of which might transit to actively self-renewing adult SGZ NSCs and give rise to neuroblasts (NB) and newly generated neurons (NGN).
Despite general acceptance of their existence, the exact identity and location of adult NSCs in vivo have long been controversial [33]. In the lateral ventricle, extensive early efforts had shown that the adult NSCs are mainly located in the SVZ sub-ependymal layers [19, 34]. The findings are particularly intriguing since during development, the SVZ is occupied by intermediate progenitors while embryonic NSCs, radial glia, are located in the ventricular zone. A subsequent study, nevertheless, suggested that bona fide adult NSCs could be identified from the ventricular zone ependymal layer [35]. These controversies have prompted further examination of the exact location of adult NSCs, yet follow-up studies in different labs did not reach consensus [36–38]. The most recent results may provide a unifying hypothesis on this issue: the cell bodies of adult NSCs are located in the SVZ while they contact the ventricles through ependymal cell-like apical surfaces [39]. In the SGZ of the dentate gyrus, two populations of adult NSCs likely co-exist as discussed above, yet their lineage relationship, respective self-renewal properties, and developmental potentials are to be further examined in the future. Rigorous future study of adult NSCs in vivo will require both structural and functional characterization using multiple complementary approaches.
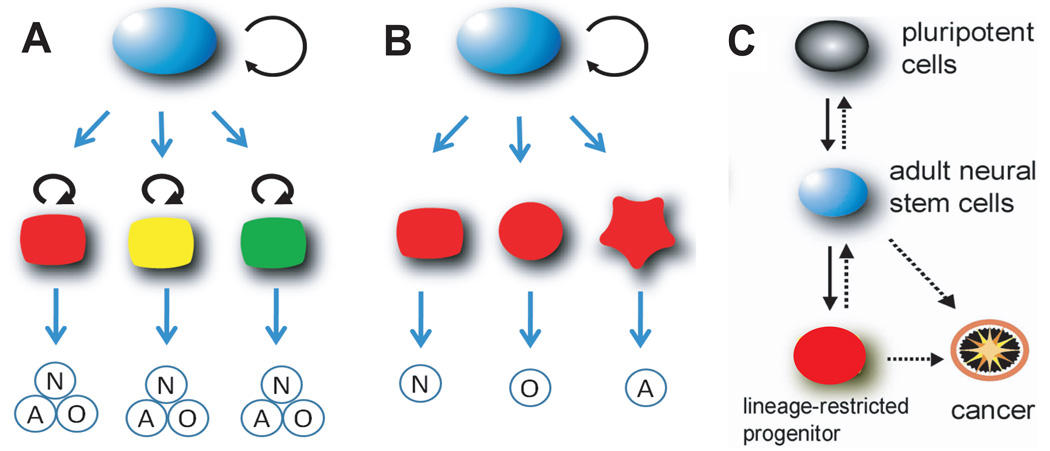
During CNS ontogenesis, adult NSCs appear to descend from their region-specific embryonic counterpart, radial glia [40]. Radial glia of different regions, when labeled during the neonatal period, produced different types of neurons in adulthood, suggesting that postnatal NSCs may be regionally specified according to their locations [41]. Although this study suggested interesting similarity between embryonic and postnatal NSCs, the true diversity of adult NSCs was not directly addressed. It also remains an intriguing possibility that adult NSCs may give rise to a diverse type of lineage-restricted progenitors and neuroblasts that are heterogeneous and regionally specified (Figure 2A, B). In support of this notion, dopaminergic periglomerular neurons, but not granule neurons, in the OB originate mainly from the RMS [42]. In addition, proliferating adult SVZ NSCs express the transcription factor Pax6, but only a small subset of neuroblasts and new OB interneurons derived from these progenitors retains Pax6 expression [43]. In the SGZ, while genetic evidence suggests that radial GFAP+ NSCs correspond to the ancestor population of the majority of new neurons [44], non-radial Sox2+ NSCs may also self-renew and give rise to neurons and astrocytes [32]. Collectively, these findings indicate that adult NSCs and their progeny may exhibit a significant degree of functional diversity resulting from their regional distribution and developmental origins. It will be interesting to investigate how such functional diversity of adult NSCs and their progeny may contribute to, and can be manipulated for specific benefits of brain plasticity under physiological and pathological settings.
Figure 2.
The lineage model of adult NSCs in the mammalian brain. (A). In one lineage model, adult NSCs (red, green, yellow) generated from primitive NSCs (blue) are intrinsically diverse, exhibiting vastly different developmental potential depending on their regions of distribution and developmental origins. (B). In an alternative model, adult NSCs are relatively homogenous (blue) and give rise to a heterogeneous population of lineage-restricted progenitors. (C). Under normal conditions, adult NSCs differentiate into lineage-restricted progenitors and mature neurons and glia. Lineage-restricted progenitor may revert to adult NSCs, which can be further reprogrammed into a pluripotent state under epigenetically altered conditions. Pathologically, adult NSCs or lineage-restricted progenitors may undergo genetic and epigenetic changes, transforming into tumorigenic cancer stem cells.
Given better-defined culture conditions, adult NSCs have been widely studied in vitro. Both the SVZ and SGZ-derived NSCs can be expanded continually as free-floating cell cluster, termed “neurosphere”, exhibiting self-renewal and multi-lineage neural potentiality, defining hallmarks of NSCs [19, 45]. Intriguingly, such adult NSCs in vitro seem to regain some glial characteristics when cultured as neurospheres [46], and may similarly exhibit certain diversity as in vivo [47]. Distinct from lineage-restricted progenitors, adult NSCs are capable of serial neurosphere formation while maintaining multipotentiality at the clonal level [48]. The adult SGZ-derived NSCs can also be expanded as monolayer for a prolonged period of time [17]. In the presence of FGF-2, they can be clonally derived, proliferate while maintaining an undifferentiated state, and capable of differentiating into neurons and glia both in vitro and after transplantation back into the CNS [17, 18, 49–51]. Adult NSCs grown in culture thus provide an advantageous system to study their cellular properties, including self-renewal and multipotency, and may also serve as a valuable model and substrate for developing therapeutic strategies.
Adult neural stem cell niche: architecture and signals that regulate adult neurogenesis
The “niche” is defined as the microenvironment that intimately supports and tightly regulates stem cell behaviors, including their maintenance, self-renewal, fate specification and development [52, 53]. While dormant NSCs might be present and can be derived from multiple regions of the adult brain, unique local niche structure seems to restrict active neurogenesis from adult NSCs to two discrete regions: SVZ and SGZ [22, 23]. The overall structure of SVZ and SGZ niches has been extensively characterized [23, 54, 55]. In the SVZ, the NSC niche spreads extensively from the lateral ventricle along the RMS to the OB to accommodate local generation of new neurons in the OB [31]. In the adult SGZ, the niche is less structurally apparent and largely confined within the SGZ hilus region [55]. These distinguishing features of each niche structure may thus allow regulation of adult SVZ or SGZ NSCs in a region-specific manner, exemplified by a varying degree of modulation by external cues through neuronal activity [56–58]. Though the specific niche architecture in the SVZ and SGZ is distinct, there are common features, including their cellular niche components and extra-cellular niche signals that regulate behavior of adult NSCs and their development.
Astroglia, ependymal cells, vascular cells, NSC progeny and mature neurons are among major cellular components of the neurogenic niche. Ample in vitro and in vivo evidence suggests pivotal roles of astroglia in regulating almost every developmental process of adult neurogenesis, including self-renewal, fate specification of adult NSCs, migration, differentiation and final synaptic integration of new neurons [22, 50, 59, 60]. Mature ependymal cells seem to mainly regulate the quiescence and self-renewal of adult NSCs in the SVZ by direct cell-cell contact and diffusible signals including the pigment epithelium-derived factor [61]. Through oriented cilia beating and formation of gradient guidance cues, ependymal cells also promote neuroblasts migration along the RMS [62]. On the other hand, SGZ neurogenesis is known to be particularly sensitive to the surrounding neuronal activity. Thus, mature neurons near the neurogenic site are suited to function as niche cells, providing spatiotemporal regulation of adult neurogenesis in response to neuronal activity [56, 58, 63]. Accumulating evidence also points to prominent roles of vascular cells in regulating the proliferation of adult NSCs, with early studies focused on particularly the SGZ [64]. Recent three-dimensional imaging techniques have revealed that the SVZ vasculature comprises an extensive network of planar interconnected blood vessels [65, 66]. The contacts between adult SVZ NSCs and vessels are unusually permeable and frequently devoid of astrocytic and pericyte interferences, suggesting that blood-derived cues are gaining access to regulate adult NSCs. Though these individual cellular niche components have been well described so far, their exact modes of regulation, relative importance in different developmental processes of the adult NSCs, and their mutual cross-talk and coordination remain under intensive investigation.
Recent advances have led to the identification of major molecular niche signals for adult NSCs [67]. A plethora of developmental cues and physiological humoral factors have been shown to promote progenitor proliferation and maintenance, including Wnt [68], Sonic Hedgehog (Shh) [69], Bone Morphogenic Protein (BMP) antagonists [45], membrane-associated Notch signaling [70], leukemia inhibitory factor (LIF) [71], transforming growth factor-alpha (TGF-a) [72] and cytokines [73, 74]. Growth factors, including FGFs and neurotrophins such as brain-derived neurotrophic factor (BDNF), also significantly contribute to proliferation, survival and dendritic development of newborn neurons in the adult brain [75–78]. The extracellular matrix (ECM) provides a platform for presentation of molecular cues and cellular interaction within neurogenic niches [79]. Most of these factors exert specific effects on both SVZ and SGZ NSCs in the adult brain, reminiscent of their roles in regulating NSCs in the developing nervous system. The cellular environments of NSCs in the adult versus developing nervous system, however, are strikingly different. The regulated production of diffusible factors from niche cells, therefore, may serve to translate a myriad of physiological milieu into precise regulation of adult NSCs and strategic addition of new neurons into the existing neuronal circuitry.
Functions of adult neural stem cells
Most types of adult stem cells outside of the nervous system, especially those from epithelial origins, function to maintain tissue homeostasis by providing a continual replacement for lost cells during physiological cell turnover or upon injury [52]. This general “cell replacement” view of adult stem cells is also part of the initial skepticism of the functional relevance of adult NSCs and new neurons for any significant higher brain functions. Accumulating evidence has clearly shown that a large number of newborn neurons can be generated from adult NSCs, and integrate into pre-existing neural circuits [80]. Adult neurogenesis in either the SVZ or the SGZ is also highly sensitive to environmental cues, physiological stimuli and neuronal activity [24, 56, 81, 82], suggesting that the tailored addition of new neurons might serve specific neuronal functions. Direct functional evidence for adult NSCs and newly generated neurons, however, has not been obtained until recently.
Early efforts attempted to use anti-mitotic drugs or X-irradiation to assess the contribution of adult NSCs to animal behavior [83, 84]. Though certain learning deficits have been shown in early studies, the specificity of manipulation was called into question because other proliferating precursor cells and mature cell types were affected. Using elegant mouse genetic approaches, Zhang et al. demonstrated that removal of a crucial regulator of adult NSC proliferation, the transcription factor TLX [85], specifically from the adult NSCs resulted in marked deficits in spatial learning [86]. In contrast, suppressed adult neurogenesis does not affect contextual fear conditioning, locomotion or diurnal rhythmic activities, indicating a selective contribution of adult NSCs to specific cognitive functions. More recent results based on cell type-specific and temporally controlled genetic ablation of adult NSCs or neurogenesis seem to suggest distinct modes for adult SVZ and SGZ contribution to brain functions [87]. While the SVZ NSCs and neurogenesis are essential for the maintenance of the olfactory bulb, the hippocampal SGZ NSCs and neurogenesis provide a substrate for additional brain plasticity and are crucial for spatial learning and memory [87]. By maintaining the high rate of cellular turnover in the OB from the SVZ NSCs, a complement of new young neurons may confer on the sensory organ with a privilege in behavior adaptation, such as olfactory learning of novel odorants. Supporting this notion, OB neurogenesis is functionally correlated with olfactory discrimination learning, and new OB neurons are preferentially recruited during olfactory behavior [88, 89]. Unlike other adult somatic tissues, the dentate gyrus appears to increase its volume over the lifetime of an animal through continued addition of new neurons. Ablation of adult Nestin+ progenitors blocked the increase of its volume over time and resulted in behavior deficits in spatial learning and memory [87]. At the circuitry level, new neurons from adult SGZ NSCs possess unique physiological properties with enhanced plasticity during specific time windows and are preferentially recruited for information processing in hippocampus-dependent learning behaviors [90–92]. Thus, adult SGZ NSCs are essential for neuronal addition and hippocampal growth, potentially contributing to new memory formation and an extra cellular level of neural plasticity throughout life.
In addition to expanding plasticity for the hippocampus, adult NSCs from the SGZ have also been suggested to play a significant role in mood regulation [93, 94]. On one hand, stress and various antidepressant treatments can profoundly affect adult hippocampal neurogenesis [93, 95]. On the other hand, disrupting antidepressant-induced adult SGZ neurogenesis blocks behavioral responses to antidepressants [94]. Furthermore, “learned safety”, a physiological paradigm mimicking anti-depressant treatment, promotes the survival of new neurons from adult SGZ NSCs and its antidepressant effects are abolished in mice with ablated adult hippocampal NSCs and neurogenesis [96]. Such mood regulation appears to be uniquely attributed to the SGZ NSCs, and might be explained by the convergent role of adult SGZ NSCs in expanding plasticity for the hippocampus: new neurons generated from the SGZ NSCs may gate contextually appropriate new memory formation to prefrontal cortical–striatal circuits in alleviating depression. The exact manner by which adult SGZ NSCs and their progeny participate in alleviating depression or learning and memory remains a fascinating issue to be explored.
Cellular plasticity of adult neural stem cells
Under physiological conditions, adult NSCs follow a highly stereotypic differentiation path to generate predominantly inhibitory granule/periglomerular interneurons in the OB and excitatory granule neurons in the dentate gyrus. While the local niches significantly shape such stereotyped neuronal differentiation, multipotent adult NSCs appear to be intrinsically plastic in their neural fate programming in vivo. In the adult SVZ, transient amplifying progenitors may revert to an adult NSC-like state when situated in certain conditions, such as heightened EGF receptor signaling [97]. Over-expression of the transcription factor Olig2 diverts the neuronal fate of transient progenitors from adult NSCs towards oligodendrocytes that migrate away from the SVZ to the corpus callosum [42]. Similarly, the neuronal subtype differentiation in the OB is not completely fixed since high Pax6 maintenance leads to almost complete conversion of all precursors in the RMS towards a periglomerular neuron fate [42]. In the SGZ, retrovirus-mediated over-expression of the transcription factor Ascl1 redirected the fate of the adult NSCs predominately to oligodendrocytes [98]. Such role of Ascl1 is region-specific since no fate switch and oligodendrocyte differentiation of SVZ progenitors occurred. In certain pathological conditions, such as brain injury, new neurons appear to be also generated in local non-neurogenic sites, including the cortex [60, 99]. For example, non-neurogenic, ventricular ependymal cells can be activated during stroke and surprisingly generate both neuroblasts and glia [100]. In the SGZ, loss of Disrupted-In-Schizophrenia 1 (DISC1) during NSC differentiation causes accelerated neuronal integration and mis-positioning of new neurons in a cell-autonomous fashion [101]. These findings indicate that adult NSCs might be highly plastic in nature and are subject to a combination of extrinsic and intrinsic instructions during neural differentiation.
Consistent with their plastic nature in vivo, adult NSCs are also amenable to epigenetic reprogramming in vitro [102]. Adult NSCs co-cultured with endothelial cells were converted to cells that stably express endothelial markers and form capillary networks, independent of cell fusion [103]. This is particularly surprising because NSCs and endothelial cells are believed to be descendants of the ectoderm and mesoderm, respectively. Adult NSCs may even contribute to the formation of tissues from all three germ layers when injected into early mouse embryo [104], although the possibility of cell fusion events to host cells was not thoroughly examined. Nevertheless, it is an intriguing possibility that specific culture conditions to expand adult NSCs in vitro may reprogram their epigenetic status and partially contribute to expanded capacity of their developmental potential [46, 105–107]. Directed reprogramming of adult NSCs to generate pluripotent embryonic stem cell (ESC)-like cells further demonstrates the striking plasticity of adult NSCs (Figure 2C). Somatic cell nuclear transfer or cell fusion with pluripotent ESCs can reprogram most types of adult somatic cells into pluripotency, yet reprogramming of NSCs using these methods appear to be particularly efficient [108]. In accordance with the epigenetic nature of reprogramming, altering the epigenetic status through DNA or histone modification of adult NSCs can dramatically affect the efficiency of reprogramming [109]. For genetic factor-induced reprogramming of somatic cells [110, 111], only one factor Oct4 is sufficient to reprogram adult NSCs into induced pluripotent stem (iPS) cells whereas most other cell types require three or four factors [111–114]. The reduced requirement for reprogramming of adult NSCs may be due to their close resemblance to ESCs, including unlimited self-renewal and expression of key transcription factors, such as Sox2 and c-myc [115]. These exciting findings suggest that adult NSCs may possess unusually plastic epigenomes that can be manipulated for reprogramming, thus providing an important experimental model for understanding reprogramming mechanisms and for therapeutic applications.
Adult NSCs in brain disorders and therapeutic application
Dysregulation or disruption of endogenous adult NSCs has been implicated in brain disorders. In the SGZ, ectopic integration of the progeny of adult NSCs into epileptogenic networks may directly contribute to mossy fiber sprouting and increased seizure susceptibility [116]. Aberrant regulation of adult NSCs per se has been increasingly studied in the context of brain tumors that are hypothesized to result from subversion of intrinsic properties of adult NSCs [117] (Figure 2C). As functional roles of adult neurogenesis become more defined, adult NSCs may well contribute to the capacity of the brain to maintain physiological tissue homeostasis and to protect the animal against anxiety, depression, learning and memory deterioration.
One major implication of adult NSC study is pertinent to understanding brain tumor formation. Brain tumors are essentially a diverse group of neoplasm conditions that closely resemble most tissue organs in their cellular and functional hierarchy, as the homeostasis is governed by a distinct sub-population of stem-like cells in both situations. There is now increasing evidence that the tumor-initiating cells might arise from endogenous stem cells through accumulated multiple genetic and epigenetic alterations [117]. Cancer stem cells (CSCs) have been isolated from major malignant brain tumors, including medulloblastomas, glioblastoma, and ependymomas, and they share several key properties of adult NSC, such as long-term self-renewal [118–121]. These CD133+ brain CSCs form self-renewing neurosphere-like colonies in vitro, and can differentiate into one or more neural lineages. Unlike adult NSCs, however, CSCs are genetically or epigenetically aberrant with growth factor-independent proliferation and differentiation [122]. Certain CSCs also seem to further acquire the ability to take advantage of the vascular niche structure of normal adult NSCs in order to gain uncontrolled growth in metastatic tissue loci [123]. Importantly, multiple studies have shown that the purified population of CD133+ sphere-forming CSCs expedites tumor formation following transplantation into the immunodeficient mice [119, 121, 124]. It remains to be further investigated whether most brain tumors originate from endogenous adult NSCs. Though endogenous adult NSCs are attractive candidates due to their long-term self-renewal capacity in accumulating mutations, direct evidence is lacking on whether cancer-initiating events occur in NSCs, progenitors or differentiated cells. Clinically, many types of human brain tumors are known to frequently arise deep in the brain near the SVZ region [125]. In animal models, introducing the oncogene constitutively active EGFR and deleting the tumor suppressor gene Ink4a/Arf in NSCs lead to high-grade glioma [126], while mutant mice deficient in p53 and with conditional null allele NF1 or PTEN in a GFAP+ population developed glioblastoma at very high penetrance [127, 128]. In the future, more sophisticated genetic models may clarify the issue of the origin(s) of different types of tumors, and pinpoint novel targets for potential therapeutic interventions against malignant brain cancer.
Discovery of endogenous adult NSCs with unique capacity to expand in culture and diverse developmental potential for neuronal and glial differentiation also opens doors for therapeutic application of these cells. Adult NSCs provide tools for understanding disease mechanisms and for drug screening using differentiated neural cells from these adult NSCs in vitro [26, 129]. Common neurological disorders and neurodegenerative diseases, including Parkinson's disease, Alzheimer’s disease, spinal cord injury, epileptic seizure, demyelinating diseases, stroke and multiple sclerosis, among others, are caused by, or accompanied with a major irreversible loss of neurons and glial cells [130, 131]. The application of adult NSCs has been quite successful in several animal models and has yielded important insights for potential stem cell-based human trials [130, 132, 133]. Substantial challenges remain before translating animal studies into clinically meaningful human therapy, such as efficient isolation and expansion of human NSCs; tumorigenicity of cells after transplantation; delivery methods for transplantation of cells; full maturation and functional integration of transplanted cells. Ultimately, any effective cell replacement therapy would require disease-specific application of stem cells, and equally important, the basic understanding of mechanisms regulating their in vitro proliferation, differentiation, in vivo integration, and optimization of functional recovery in disease-specific animal models. In addition to directly replacing lost cells in diseases, accumulating evidence suggests that transplanted NSCs may also ameliorate neuronal dysfunction through various other mechanisms, including serving as pumps of neuroprotective agents [134], restoring homeostasis to the surrounding tissue [135], and acting in synergy with other established therapies [136].
Endogenous adult NSCs seem to be involved in tissue repair during pathological conditions, such as brain injury, and thus may be therapeutically useful for self-repair if properly mobilized [137]. For example, induced apoptotic degeneration of corticothalamic neurons in anterior cortex of adult mice was shown to stimulate proliferation of local progenitors, and the generation of new pyramidal neurons that appeared to eventually integrate into the corticothalamic circuit [99]. In a brain ischemic model, endogenous adult NSCs were found to proliferate in response to ischemic stroke, and migrate to the hippocampal area regenerating local pyramidal neurons. This process is inherently limited but greatly enhanced by infusion of mitogens including FGF-2 [138]. Compared with transplantation of adult NSCs, mobilization of endogenous adult NSCs for self-repair has advantages, including reduced immune rejection, better location-specific recruitment, enhanced maturation and functional integration of generated progeny. To realize the clinical potential of such endogenous adult NSCs, however, it is essential to understand modes and mechanisms of adult NSC mobilization, as well as novel means to maximize the extent of such mobilization for self-repair in a highly controlled manner.
Conclusions
After a century-long conceptual assumption on the fixed regenerative capacity of the adult mammalian brain, the surprising discovery of adult mammalian NSCs and continued neurogenesis throughout life heralds a new era for deeper understanding of brain plasticity. Adult NSCs not only contribute to the maintenance of neural tissue, but also offer expanded plasticity to key brain structures that are critical for learning and memory. Isolation and culture of adult NSCs provide an advantageous model for understanding the basic biology regulating self-renewal, developmental potential and reprogramming of stem cells, in addition to serving as a valuable source for cell replacement-based therapy in treating neurological disorders. Knowledge of adult NSCs both in vitro and in vivo also proves to be directly pertinent toward understanding the capacity of the brain for self-repair, as well as yielding novel insights into brain cancer research and treatment.
Rapid and exciting progress in the field has shed light on the biology and clinical potential of adult NSCs. Still more unknowns and uncharted territories awaits further exploration. At the systems level, the exact manner by which adult NSCs and their progeny interact with and exert impact on the host tissue in the adult brain still remains poorly understood. Evolutionarily, the functional significance of adult NSCs extant in mammals remains an open and intriguing question. The clinical promise of adult NSCs is yet to be realized, which requires a comprehensive understanding of the mechanisms of their properties, regulation, and application through long-term collaborative efforts of both basic and translational research. There is no doubt that science of today is “changing the harsh decree of adult (brain) centers” viewed by Cajal a century ago.
Acknowledgement
The research in the laboratories of Ming and Song was supported by the NIH (NS047344, AG024984, MH084018, NS048271), March of Dimes, Sloan, Maryland Stem Cell Research Fund (MSCRF), NARSAD, McKnight Foundation, Packard Center for ALS and MDA. M.A. B. was supported by a postdoctoral fellowship from MSCRF.
References
- 1.Cajal R. Degeneration and Regeneration of the Nervous System. 1913 [Google Scholar]
- 2.Colucci-D'Amato L, Bonavita V, di Porzio U. The end of the central dogma of neurobiology: stem cells and neurogenesis in adult CNS. Neurol Sci. 2006;27(4):266–270. doi: 10.1007/s10072-006-0682-z. [DOI] [PubMed] [Google Scholar]
- 3.Gross CG. Neurogenesis in the adult brain: death of a dogma. Nat Rev Neurosci. 2000;1(1):67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 4.Rakic P. Limits of neurogenesis in primates. Science. 1985;227(4690):1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- 5.Rakic P. DNA synthesis and cell division in the adult primate brain. Ann N Y Acad Sci. 1985;457:193–211. doi: 10.1111/j.1749-6632.1985.tb20806.x. [DOI] [PubMed] [Google Scholar]
- 6.Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86(3):1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- 7.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 8.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124(3):319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 9.Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 13.Morrison RS, Kornblum HI, Leslie FM, Bradshaw RA. Trophic stimulation of cultured neurons from neonatal rat brain by epidermal growth factor. Science. 1987;238(4823):72–75. doi: 10.1126/science.3498986. [DOI] [PubMed] [Google Scholar]
- 14.Nurcombe V, Ford MD, Wildschut JA, Bartlett PF. Developmental regulation of neural response to FGF-1 and FGF-2 by heparan sulfate proteoglycan. Science. 1993;260(5104):103–106. doi: 10.1126/science.7682010. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175(1):1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 17.Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6(5):474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- 18.Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8(6):389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- 19.Morshead CM, Reynolds BA, Craig CG, et al. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13(5):1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 20.Weiss S, Dunne C, Hewson J, et al. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 22.Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15(5):514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41(5):683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 26.Goh EL, Ma D, Ming GL, Song H. Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res. 2003;12(6):671–679. doi: 10.1089/15258160360732696. [DOI] [PubMed] [Google Scholar]
- 27.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18(1):108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan JD, Ma DK, Ming GL, Song H. Cellular niches for endogenous neural stem cells in the adult brain. CNS Neurol Disord Drug Targets. 2007;6(5):336–341. doi: 10.2174/187152707783220866. [DOI] [PubMed] [Google Scholar]
- 29.Seri B, Herrera DG, Gritti A, et al. Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16 Suppl 1:i103–i111. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- 30.Curtis MA, Kam M, Nannmark U, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 31.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271(5251):978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 32.Suh H, Consiglio A, Ray J, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10(2):153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- 34.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 35.Johansson CB, Momma S, Clarke DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 36.Coskun V, Wu H, Blanchi B, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A. 2008;105(3):1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35(5):865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 38.Rietze RL, Valcanis H, Brooker GF, et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature. 2001;412(6848):736–739. doi: 10.1038/35089085. [DOI] [PubMed] [Google Scholar]
- 39.Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3(3):265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2(4):287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 41.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317(5836):381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 42.Hack MA, Saghatelyan A, de Chevigny A, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8(7):865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 43.Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25(30):6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 45.Bonaguidi MA, Peng CY, McGuire T, et al. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28(37):9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dromard C, Bartolami S, Deleyrolle L, et al. NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells. Stem Cells. 2007;25(2):340–353. doi: 10.1634/stemcells.2005-0556. [DOI] [PubMed] [Google Scholar]
- 47.Brazel CY, Limke TL, Osborne JK, et al. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4(4):197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 48.Louis SA, Rietze RL, Deleyrolle L, et al. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells. 2008;26(4):988–996. doi: 10.1634/stemcells.2007-0867. [DOI] [PubMed] [Google Scholar]
- 49.Suhonen JO, Peterson DA, Ray J, Gage FH. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383(6601):624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- 50.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 51.Song HJ, Stevens CF, Gage FH. Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci. 2002;5(5):438–445. doi: 10.1038/nn844. [DOI] [PubMed] [Google Scholar]
- 52.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 54.Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 55.Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478(4):359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 56.Ma DK, Kim WR, Guo L-M, Song H. Activity-dependent extrinsic regulation of adult hippocampal and olfactory bulb neurogenesis. Ann N Y Acad Sci. 2009 doi: 10.1111/j.1749-6632.2009.04373.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Kempermann G, van Praag H, Gage FH. Activity-dependent regulation of neuronal plasticity and self repair. Prog Brain Res. 2000;127:35–48. doi: 10.1016/s0079-6123(00)27004-0. [DOI] [PubMed] [Google Scholar]
- 59.Barkho BZ, Song H, Aimone JB, et al. Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev. 2006;15(3):407–421. doi: 10.1089/scd.2006.15.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiao J, Chen DF. Induction of neurogenesis in nonconventional neurogenic regions of the adult central nervous system by niche astrocyte-produced signals. Stem Cells. 2008;26(5):1221–1230. doi: 10.1634/stemcells.2007-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9(3):331–339. doi: 10.1038/nn1657. [DOI] [PubMed] [Google Scholar]
- 62.Sawamoto K, Wichterle H, Gonzalez-Perez O, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311(5761):629–632. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 63.Ma DK, Jang MH, Guo JU, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma D, Ming GL, Song H. Neurogenic niche in the adult brain. Cold Spring Habor Laboratory Press; 2008. [Google Scholar]
- 68.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 69.Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437(7060):894–897. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- 70.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442(7104):823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 71.Bauer S, Patterson PH. Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J Neurosci. 2006;26(46):12089–12099. doi: 10.1523/JNEUROSCI.3047-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tropepe V, Craig CG, Morshead CM, van der Kooy D. Transforming growth factor-alpha null and senescent mice show decreased neural progenitor cell proliferation in the forebrain subependyma. J Neurosci. 1997;17(20):7850–7859. doi: 10.1523/JNEUROSCI.17-20-07850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller S, Chakrapani BP, Schwegler H, Hofmann HD, Kirsch M. Neurogenesis in the Dentate Gyrus Depends on CNTF and STAT3 Signaling. Stem Cells. 2008 doi: 10.1634/stemcells.2008-0234. [DOI] [PubMed] [Google Scholar]
- 74.Bauer S. Cytokine control of adult neural stem cells. Ann N Y Acad Sci. 2009;1153:48–56. doi: 10.1111/j.1749-6632.2009.03986.x. [DOI] [PubMed] [Google Scholar]
- 75.Bergami M, Rimondini R, Santi S, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105(40):15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Luikart BW, Birnbaum S, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(5–6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 78.Yoshimura S, Takagi Y, Harada J, et al. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98(10):5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Campos LS. Beta1 integrins and neural stem cells: making sense of the extracellular environment. Bioessays. 2005;27(7):698–707. doi: 10.1002/bies.20256. [DOI] [PubMed] [Google Scholar]
- 80.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 81.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 82.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 83.Shors TJ, Miesegaes G, Beylin A, et al. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- 84.Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130(4):843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 85.Shi Y, Chichung Lie D, Taupin P, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427(6969):78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 86.Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 87.Imayoshi I, Sakamoto M, Ohtsuka T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 88.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22(7):2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magavi SS, Mitchell BD, Szentirmai O, Carter BS, Macklis JD. Adult-born and preexisting olfactory granule neurons undergo distinct experience-dependent modifications of their olfactory responses in vivo. J Neurosci. 2005;25(46):10729–10739. doi: 10.1523/JNEUROSCI.2250-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 92.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 94.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 95.Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299(2):401–407. [PubMed] [Google Scholar]
- 96.Pollak DD, Monje FJ, Zuckerman L, et al. An animal model of a behavioral intervention for depression. Neuron. 2008;60(1):149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36(6):1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 98.Jessberger S, Toni N, Clemenson GD, Jr, Ray J, Gage FH. Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci. 2008;11(8):888–893. doi: 10.1038/nn.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405(6789):951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 100.Carlen M, Meletis K, Goritz C, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12(3):259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 101.Duan X, Chang JH, Ge S, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vescovi AL, Snyder EY. Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo. Brain Pathol. 1999;9(3):569–598. doi: 10.1111/j.1750-3639.1999.tb00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wurmser AE, Nakashima K, Summers RG, et al. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature. 2004;430(6997):350–356. doi: 10.1038/nature02604. [DOI] [PubMed] [Google Scholar]
- 104.Clarke DL, Johansson CB, Wilbertz J, et al. Generalized potential of adult neural stem cells. Science. 2000;288(5471):1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 105.Morshead CM, Benveniste P, Iscove NN, van der Kooy D. Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med. 2002;8(3):268–273. doi: 10.1038/nm0302-268. [DOI] [PubMed] [Google Scholar]
- 106.Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron. 2003;40(3):485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- 107.Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19(19):8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blelloch R, Wang Z, Meissner A, et al. Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells. 2006;24(9):2007–2013. doi: 10.1634/stemcells.2006-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma DK, Chiang CH, Ponnusamy K, Ming GL, Song H. G9a and Jhdm2a regulate embryonic stem cell fusion-induced reprogramming of adult neural stem cells. Stem Cells. 2008;26(8):2131–2141. doi: 10.1634/stemcells.2008-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 112.Di Stefano B, Prigione A, Broccoli V. Efficient genetic reprogramming of unmodified somatic neural progenitors uncovers the essential requirement of Oct4 and Klf4. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0180. [DOI] [PubMed] [Google Scholar]
- 113.Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 114.Aoi T, Yae K, Nakagawa M, et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321(5889):699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 115.Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26(10):2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 116.Parent JM, Jessberger S, Gage FH, Gong C. Is neurogenesis reparative after status epilepticus? Epilepsia. 2007;48 Suppl 8:69–71. doi: 10.1111/j.1528-1167.2007.01355.x. [DOI] [PubMed] [Google Scholar]
- 117.Dirks PB. Brain tumor stem cells: bringing order to the chaos of brain cancer. J Clin Oncol. 2008;26(17):2916–2924. doi: 10.1200/JCO.2008.17.6792. [DOI] [PubMed] [Google Scholar]
- 118.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 119.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 120.Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39(3):193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 121.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 122.Zhang QB, Ji XY, Huang Q, et al. Differentiation profile of brain tumor stem cells: a comparative study with neural stem cells. Cell Res. 2006;16(12):909–915. doi: 10.1038/sj.cr.7310104. [DOI] [PubMed] [Google Scholar]
- 123.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 124.Yuan X, Curtin J, Xiong Y, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 125.Lewis PD. Mitotic activity in the primate subependymal layer and the genesis of gliomas. Nature. 1968;217(5132):974–975. doi: 10.1038/217974a0. [DOI] [PubMed] [Google Scholar]
- 126.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1(3):269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 127.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8(2):119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zheng H, Ying H, Yan H, et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 2008;455(7216):1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taupin P. Therapeutic potential of adult neural stem cells. Recent Pat CNS Drug Discov. 2006;1(3):299–303. doi: 10.2174/157488906778773670. [DOI] [PubMed] [Google Scholar]
- 130.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441(7097):1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 131.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 132.Shetty AK, Hattiangady B. Concise review: prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25(10):2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Einstein O, Ben-Hur T. The changing face of neural stem cell therapy in neurologic diseases. Arch Neurol. 2008;65(4):452–456. doi: 10.1001/archneur.65.4.452. [DOI] [PubMed] [Google Scholar]
- 134.Behrstock S, Svendsen CN. Combining growth factors, stem cells, and gene therapy for the aging brain. Ann N Y Acad Sci. 2004;1019:5–14. doi: 10.1196/annals.1297.002. [DOI] [PubMed] [Google Scholar]
- 135.Li J, Imitola J, Snyder EY, Sidman RL. Neural stem cells rescue nervous purkinje neurons by restoring molecular homeostasis of tissue plasminogen activator and downstream targets. J Neurosci. 2006;26(30):7839–7848. doi: 10.1523/JNEUROSCI.1624-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JP, Jeyakumar M, Gonzalez R, et al. Stem cells act through multiple mechanisms to benefit mice with neurodegenerative metabolic disease. Nat Med. 2007;13(4):439–447. doi: 10.1038/nm1548. [DOI] [PubMed] [Google Scholar]
- 137.Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. 2003;13(1):127–132. doi: 10.1016/s0959-4388(03)00017-5. [DOI] [PubMed] [Google Scholar]
- 138.Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]