Abstract
Pharmacogenetic testing holds great promise to improve health outcomes and reduce adverse drug responses through enhanced selection of therapeutic agents. Since drug responses can be manipulated by verbal suggestions, it is of particular interest to understand the potential impact of pharmacogenetic test results on drug response. Placebo and nocebo-like effects may be possible due to the suggestive nature of pharmacogenetic information that a drug will or will not likely lead to improved health outcomes. For example, pharmacogenetic testing could provide further reassurance to patients that a given drug will be effective and/or cause minimal side effects. However, pharmacogenetic information could adversely affect drug response through negative expectations that a drug will be less than optimally effective or cause an adverse response, known as a nocebo-like effect. Therefore, a patient's perceived value of testing, their understanding of the test results, and the manner in which they are communicated may influence therapeutic outcome. As such, physicians should consider the potential effect of pharmacogenetic test results on therapeutic outcome when communicating results to patients. Studies are needed to investigate the impact of pharmacogenetic information of therapeutic outcome.
Key Words: Adverse outcome, Nocebo effect, Pharmacogenetic testing, Placebo effect
Physicians have long been aware of some patients' improved outcomes following administration of a substance with no known pharmacological properties (i.e., an inert treatment or placebo). Known as the placebo effect, several variables have been found to modulate the degree of this effect including environmental, psychosocial, cognitive, and emotional factors as well as the behavior of healthcare providers [1, 2]. Equally important to the well-known placebo effect is the nocebo effect, whereby negative suggestions following administration of an inert substance result in an adverse outcome [3]. Psychological manipulation of information communicated by a doctor, friend, or family member has been shown to influence outcome [4, 5]. With the advent of pharmacogenetic (PGx) testing, we speculate that patient perceptions and understanding of test results as well as the manner in which they are communicated can influence therapeutic outcome, regardless of the result. While studies are needed to test this hypothesis, physicians should begin to consider the potential power of PGx testing when delivering test results and monitoring therapeutic outcome.
Placebo/Nocebo Effect and the Power of Words
The gold standard of clinical trials research is the double-blind, placebo-controlled, randomized clinical study. The rationale behind this study design is to separate non-specific from specific effects [6]. The existence of a placebo and/or non-specific effects can confound interpretation of clinical trials outcomes. Although some have argued that the placebo effect is part of the expected range of outcomes from the natural course of disease [7, 8] or that it is indistinguishable from patients enrolled in no-treatment arms of a clinical study [9], others have provided support for a psychobiological basis [10]. Evidence of a physiological effect of placebo analgesia was 1st shown in 1978 from stimulation of endogenous opioids [11]. More recently, neurochemical studies have shown that response to placebo analgesics can involve either opioid or non-opioid channels [12,13,14]. Imaging analysis has also demonstrated that neurobiological responses in patients treated with a placebo are similar to that seen in patients treated with an active agent [15].
The lesser-known nocebo effect or response has the opposite outcome of the placebo effect, whereby verbal or other suggestions of a negative outcome with administration of an inert substance result in a poor response. In cases where no inert substance is administered, nocebo-related effects can still result due to expectations of poor outcome based on suggestions [16,17,18], personal beliefs, environmental factors, or emotional states [19]. In addition, knowledge of sickness in other individuals can establish expectations of sickness [20]. It is important to distinguish the nocebo response from non-specific side effects to a placebo, whereby the effect is not attributable to pharmacological actions or to the expectation of a negative outcome [21]. Physiological responses have also been documented in response to negative expectations [22,23,24].
Two general theories have been put forth to account for the placebo/nocebo effect [4]. The conditioning theory, based on the classic Pavlovian conditioning theory, contends that certain things such as places, people, and even pill color linked with an unconditioned stimulus (an effective drug) can elicit a ‘conditioned’ response [25,26,27,28]. The expectation theory postulates that suggestive actions such as encouraging words in conjunction with the administration of a placebo can trigger a physiological response [29,30,31]. Participants' presumption that they have been randomized to the treatment arm in a clinical study has been associated with increased expectations of benefit [32, 33]. Both the conditioning response and expectations can result in the stimulation of measurable physiological responses [4, 31, 34].
Of particular relevance to pharmacogenetics, the context of patient care has also been shown to be associated with placebo effects, including physicians' attitudes, actions, and words [5]. For example, patients who have undergone a complete clinical work-up are more likely to experience a placebo effect since they perceive the physician to be taking control of the illness [1, 35]. Referred to as the ‘placebogenic power’ of the physician, words of encouragement and confidence from a physician can also influence outcome [4, 36]. Differences in response have been noted between the simple phrases ‘it may work’ and ‘it does work’ delivered by a medical professional [37, 38].
Potential Impact and Value of PGx Testing on Outcome
While the purpose of PGx testing is to guide drug selection and dosage, PGx testing could provide further reassurance to patients that a given drug will be effective and/or cause minimal side effects which could lead to increased compliance. The encouraging words of a physician may be reinforced by the test result which could serve as an independent validation. Although a PGx test result can increase a patient's likelihood of responding favorably to a drug, the potential for a nocebo-like effect is equally possible. The latter scenario in which PGx information may adversely affect drug response through negative expectations is of particular concern. For example, PGx testing that reveals that a patient's condition is caused by a mechanism for which there is no target-specific drug available may reduce a patient's confidence in a non-targeted drug. Or a patient found to be a poor metabolizer and thereby requiring a lower dose to avoid drug-induced toxicity may have less confidence in the drug's effectiveness and/or a heightened sensitivity and anxiety about possible adverse effects.
A patient's overall perception of the meaning of their PGx test result and its subsequent potential impact will likely pivot on 2 major factors: (a) the manner in which the physician communicates information about the test and test result, and (b) the degree to which the patient values, perceives, and understands the limitations of the test and the probabilistic implications of the test result. The patient's relationship with their physician is a 3rd factor to consider in their understanding and perception of PGx testing and its impact on outcome [39].
Various interpretations will be applied to the PGx test result, 1st by the testing laboratory, next by the physician, and finally by the patient. Which interpretation is communicated to the patient relies upon the physician's understanding of the test itself as well as its significance for the patient on both clinical and personal levels [40]. Physician preference and comprehension may lead to positive or negative message framing when discussing the implications of a test and/or test result with their patient [41]. Variance in framing has been linked to differences in health/risk perception and health behaviors [42]. In addition, the certainty with which the test is framed in regard to the current state of the science has also been shown to influence patient choice [43]. Others have shown that the influence of message framing is limited to verbal versus written content [44].
The use of clinical tests for patient evaluation can influence non-specific drug responses as it impacts patients' perceived level of care [45]. For instance, patients who underwent diagnostic testing felt that they received ‘better than usual’ care and had a lower incidence of short-term disability [46, 47]. If an informed consent is required for PGx testing, this may signal to the patient an increased importance of this type of test compared to other types of clinical tests. Although testing can increase both physician's and patient's confidence in an initial diagnosis, it can also raise patient anxiety levels despite a normal test result [48, 49]. However, patients who are more aware of their treatment options and potential outcomes tend to feel more empowered about their decision [50].
Communication and understanding of genetic risk information is challenging for the physician and the patient, respectively. Numerous studies have compared patient preferences and understandability of various approaches of risk communication of genetic risks (e.g., numerical, graphical, absolute vs. relative risk) [51,52,53,54]. PGx test results may represent a greater communication challenge if the result is based on a compilation of multiple genetic and non-genetic factors. Prior studies have found that the lack of reassurance following mutation-negative clinical testing is most likely due to failure of providers to clearly communicate the test results to patients [55, 56]. This may be influenced by both the complexity of the test and the patient's innate perceptions.
Patient understanding is influenced on many levels including prior life/health experience, current emotionality of health treatment, and the implied clinical significance of the given test [40]. Both patient anxiety and desire for information have been positively correlated with information recall after a genetic counseling session [57]. Accurate recall, however, may not correspond with correct perceptions of the implications of that information [58]. Even if the results are ‘normal’ or encouraging, additional explanation may be required to provide patients a basis to understand the results and to strengthen the patient-physician relationship [55, 59]. If a PGx test result indicates a high risk of non-response or side effects, addressing patients' concerns directly instead of dismissing them or referring them to other specialists may provide the needed reassurance sought by patients [49]. The potential biasing effects stemming from these factors must be considered in advance with respect to how best to communicate PGx test results.
Testing the Effect of PGx Information
Research is needed to explore the potential impact of PGx information on patients' psychobiological response to the resulting drug prescribed. Such research will be essential to guiding the appropriate communication of test results to reduce harm due to placebo/nocebo effects.
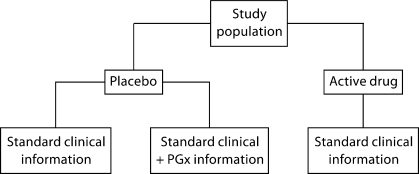
To study the impact of PGx information on either safety or efficacy, a traditional randomized study design could be used to measure the existence of a placebo effect attributed to PGx information. In this scenario, participants are enrolled in a clinical study for a new drug under investigation. To assess the impact of PGx information on efficacy, participants in the placebo arm of the study are randomized into 2 groups – the 1st group receives PGx information indicating the drug is genetically targeted and the 2nd group receives no PGx information (fig. 1). Similarly, to assess the impact of PGx information on safety, participants would instead receive PGx information indicating that the drug should not cause any adverse side effects or no PGx information.
Fig. 1.
Schematic of study design to ascertain impact of placebo effect of pharmacogenetic information.
A more straightforward observational study design could also be used where a placebo is administered to all participants, although they would be led to believe that they were receiving a test drug. Although both study designs require deception, exposing all participants to placebo only while manipulating the information would allow researchers to isolate the situation/context to determine the impact of words (or test results) on response with no specific effect attributable to an active drug [5]. It would further determine whether genetic information provides additional value to standard clinical information. If PGx information can influence placebo/nocebo effects, we would anticipate the informed subjects to have a potentially exaggerated response compared to those who did not receive PGx information.
Several personal factors can contribute to the development of a placebo/nocebo-like effect. One important factor to consider is the special significance often associated with genetic information. Whether culturally constructed or mediated, if an individual considers genetic information to be more significant or predictive of outcome, these preconceptions can contribute to potential placebo/nocebo effects. Therefore, it would be important to assess participants' feelings regarding the significance of genetic information compared to other types of clinical information to determine whether their a priori belief about genetic information is a strong predictor of placebo/nocebo-like effects. Other personal factors that may be predictive of placebo/nocebo-like effects include health and genetic literacy and risk comprehension and, as such, these should be measured in the proposed studies. Lastly, patients' perception of the severity of their illness requiring treatment as well as of the severity of treatment-associated side effects and the impact of non-response to the treatment must be considered particularly within the burgeoning field of PGx-guided cancer treatment.
Another set of studies is needed to investigate health professionals' understanding and delivery of PGx-related messages and how this affects patient response. In particular, qualitative data collected from controlled studies are needed to investigate professionals' interpretation of the test result, the perceived predictive value of the result, the amount of information communicated to the patient, message framing, and method of communication on patients' response. These studies will provide useful data that will enhance delivery of pharmacogenetic information and highlight areas for additional professional education.
Conclusion
The impact of words on drug response and outcome cannot be overlooked with respect to PGx testing. While the overall benefits of PGx testing may far outweigh the risks with respect to improved drug selection and dosage, research is needed to understand how patients will respond to this information and how that response may in turn affect clinical studies of drug efficacy. In the interim, physicians should be sensitive to the potential impact of PGx results, regardless of whether they are considered as positive or negative on their patients' drug response and give special consideration to how best to deliver these test results to minimize adverse responses.
Acknowledgement
Funding for this work was provided by the Duke Institute for Genome Sciences & Policy (SBH and JO) and NIH T32-HD040372 (LW). All work was performed at the Duke Institute for Genome Sciences and Policy.
References
- 1.Chaput de Saintonge DM, Herxheimer A. Harnessing placebo effects in health care. Lancet. 1994;344:995–998. doi: 10.1016/s0140-6736(94)91647-0. [DOI] [PubMed] [Google Scholar]
- 2.Price D, Finniss D, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy WP. The nocebo reaction. Med World. 1961;91:203–205. [PubMed] [Google Scholar]
- 4.Papakostas YG, Daras MD. Placebos, placebo effect and the response to the healing situation: the evolution of a concept. Epilepsia. 2001;42:1614–1625. doi: 10.1046/j.1528-1157.2001.41601.x. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F. How the doctor's words affect the patient's brain. Eval Health Prof. 2002;25:369–386. doi: 10.1177/0163278702238051. [DOI] [PubMed] [Google Scholar]
- 6.Benson H, Friedman R. Harnessing the power of the placebo effect and renaming it ‘remembered wellness.’ Annu Rev Med. 1996;47:193–199. doi: 10.1146/annurev.med.47.1.193. [DOI] [PubMed] [Google Scholar]
- 7.Ernst E, Resch KL. Concept of true and perceived placebo effects. BMJ. 1995;311:551–553. doi: 10.1136/bmj.311.7004.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kienle GS, Kiene H. The powerful placebo effect: fact or fiction? J Clin Epidemiol. 1997;50:1311–1318. doi: 10.1016/s0895-4356(97)00203-5. [DOI] [PubMed] [Google Scholar]
- 9.Hróbjartsson A, G⊘tzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–1602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- 10.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25:10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine JD, Gordon NC, Fields HL. The mechanisms of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 12.Benedetti F. Cholecystokinin type-A and type-B receptors and their modulation of opioid analgesia. News Physiol Sci. 1997;12:263–268. [Google Scholar]
- 13.Zubieta JK, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott D, Stohler C, Egnatuk C, Wang H, Koeppe R, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 15.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia – imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 16.Luparello T, Lyons HA, Bleecker ER, McFadden ER. Influences of suggestion on airway reactivity in asthmatic subjects. Psychosom Med. 1968;30:819–825. doi: 10.1097/00006842-196811000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Lancman ME, Asconape JJ, Craven WJ, Howard G, Penry JK. Predictive value of induction of psychogenic seizures by suggestion. Ann Neurol. 1994;35:359–361. doi: 10.1002/ana.410350319. [DOI] [PubMed] [Google Scholar]
- 18.Dannecker EA, Price DD, Robinson ME. An examination of the relationships among recalled, expected, and actual intensity and unpleasantness of delayed onset muscle pain. J Pain. 2003;4:74–81. doi: 10.1054/jpai.2003.7. [DOI] [PubMed] [Google Scholar]
- 19.Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of US adults. Epidemiology. 1993;4:285–294. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Colligan MJ, Murphy LR. Mass psychogenic illness in organizations: an overview. J Occup Psychol. 1979;52:77–90. [Google Scholar]
- 21.Barsky AJ, Saintfor R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 22.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz J, Hauck M, Paur RC, Nakamura Y, Zimmermann R, Bromm B, Angel AK. Cortical correlates of false expectations during pain intensity judgments: a possible manifestation of placebo/nocebo cognitions. Brain Behav Immun. 2005;19:283–295. doi: 10.1016/j.bbi.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. J Pers Soc Psychol. 1985;48:47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- 26.Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediat. 1992;13:124–125. doi: 10.1097/00004703-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Giang DW, Goodman AD, Schiffer RB, Mattson DH, Petrie M, Cohen N, Ader R. Conditioning of cyclophosphamide-induced leukopenia in humans. J Neuropsychiatry Clin Neurosci. 1996;8:194–201. doi: 10.1176/jnp.8.2.194. [DOI] [PubMed] [Google Scholar]
- 28.Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 29.Swartzman LC, Burkel J. Expectations and the placebo effect in clinical drug trials: why we should not turn a blind eye to unblinding, and other cautionary notes. Clin Pharmacol Ther. 1998;64:1–7. doi: 10.1016/S0009-9236(98)90016-9. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23:4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McRae C, Cherin E, Yamazaki TG, Diem G, Vo AH, Russell D, Ellgring JH, Fahn S, Greene P, Dillon S, Winfield H, Bjugstad KB, Freed CR. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatry. 2004;61:412–420. doi: 10.1001/archpsyc.61.4.412. [DOI] [PubMed] [Google Scholar]
- 33.Bausell RB, Lao L, Bergman S, Lee WL, Berman BM. Is acupuncture analgesia an expectancy effect? Preliminary evidence based on participants' perceived assignments in two placebo-controlled trials. Eval Health Prof. 2005;28:9–26. doi: 10.1177/0163278704273081. [DOI] [PubMed] [Google Scholar]
- 34.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci. 1999;19:484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro AK. Factors contributing to the placebo effect, their implications for psychotherapy. Am J Psychotherapy. 1964;18:73–88. doi: 10.1176/appi.psychotherapy.1964.18.s1.73. [DOI] [PubMed] [Google Scholar]
- 36.Wickramasekera I. A conditioned response model of the placebo effect: predictions from the model. In: White L, Tursky B, Schwartz GE, editors. Placebo: Theory, Research, and Mechanisms. New York: Guilford Press; 1985. pp. 255–287. [Google Scholar]
- 37.Thomas KB. General practice consultations: is there any point in being positive? Br Med J (Clin Res Ed) 1987;294:1200–1202. doi: 10.1136/bmj.294.6581.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollo A, Amanzio M, Arslanian A, Casadio C, Maggi G, Benedetti F. Response expectancies in placebo analgesia and their clinical relevance. Pain. 2001;93:77–84. doi: 10.1016/S0304-3959(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 39.Benson H. The nocebo effect: history and physiology. Prev Med. 1997;26:612–615. doi: 10.1006/pmed.1997.0228. [DOI] [PubMed] [Google Scholar]
- 40.O'Daniel JM, McConkie-Rosell A. Test results: comprehension, communication, and counseling. In: Sharpe NF, Carter RF, editors. Genetic Testing: Care, Consent, and Liability. New York: Wiley Publishing, Inc.; 2006. pp. 355–382. [Google Scholar]
- 41.Zikmund-Fisher BJ, Fagerlin A, Keeton K, Ubel PA. Does labeling prenatal screening test results as negative or positive affect a woman's responses? Am J Obstet Gynecol. 2007;197:528. doi: 10.1016/j.ajog.2007.03.076. e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman AJ, Salovey P, Antone C, Keough K, Drake Martin C. The influence of message framing on intentions to perform health behaviors. J Exp Social Psychology. 1993;29:408–433. [Google Scholar]
- 43.Wragg JA, Robinson EJ, Lilford RJ. Information presentation and decisions to enter clinical trials: a hypothetical trial of hormone replacement therapy. Soc Sci Med. 2000;51:453–462. doi: 10.1016/s0277-9536(99)00477-3. [DOI] [PubMed] [Google Scholar]
- 44.Welkenhuysen M, Evers-Kiebooms G, d'Ydewalle G. The language of uncertainty in genetic risk communication framing and verbal versus numerical information. Patient Educ Couns. 2001;43:179–187. doi: 10.1016/s0738-3991(00)00161-0. [DOI] [PubMed] [Google Scholar]
- 45.McDonald IG, Guyatt GH, Gutman JM, Jelinek VM, Fox P, Daly J. The contribution of a non-invasive test to clinical care. The impact of echocardiography on diagnosis, management and patient anxiety. J Clin Epidemiol. 1988;41:151–161. doi: 10.1016/0895-4356(88)90089-3. [DOI] [PubMed] [Google Scholar]
- 46.Sox HC, Margulies I, Sox CH. Psychologically mediated effects of diagnostic tests. Ann Int Med. 1981;95:680–685. doi: 10.7326/0003-4819-95-6-680. [DOI] [PubMed] [Google Scholar]
- 47.Fitzpatrick R, Hopkins A, Harvard-Watts O. Social dimensions of healing: a longitudinal study of outcomes of medical management of headaches. Soc Sci Med. 1983;17:501–510. doi: 10.1016/0277-9536(83)90057-6. [DOI] [PubMed] [Google Scholar]
- 48.McDonald IG, Daly J, Jelinek VM, Panetta F, Gutman JM. Opening Pandora's box: the unpredictability of reassurance by a normal test result. BMJ. 1996;313:329–332. doi: 10.1136/bmj.313.7053.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fitzpatrick R. Telling patients there is nothing wrong. BMJ. 1996;313:311–312. doi: 10.1136/bmj.313.7053.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connor AM, Fiset V, DeGrasse C, Graham ID, Evans W, Stacey D, Laupacis A, Tugwell P. Decision aids for patients considering options affecting cancer outcomes: evidence of efficacy and policy implications. J Natl Cancer Inst Monogr. 1999;25:67–80. doi: 10.1093/oxfordjournals.jncimonographs.a024212. [DOI] [PubMed] [Google Scholar]
- 51.Lipkus IM, Crawford Y, Fenn K, Biradavolu M, Binder RA, Marcus A, Mason M. Testing different formats for communicating colorectal cancer risk. J Health Commun. 1999;4:311–324. doi: 10.1080/108107399126841. [DOI] [PubMed] [Google Scholar]
- 52.Sachs L, Taube A, Tishelman C. Risk in numbers – difficulties in the transformation of genetic knowledge from research to people – the case of hereditary cancer. Acta Oncol. 2001;40:445–453. doi: 10.1080/028418601750288154. [DOI] [PubMed] [Google Scholar]
- 53.Siegrist M, Orlow P, Keller C. The effect of graphical and numerical presentation of hypothetical prenatal diagnosis results on risk perception. Med Decis Making. 2008;28:567–674. doi: 10.1177/0272989X08315237. [DOI] [PubMed] [Google Scholar]
- 54.Hopwood P, Howell A, Lalloo F, Evans G. Do women understand the odds? Risk perceptions and recall of risk information in women with a family history of breast cancer. Community Genet. 2003;6:214–223. doi: 10.1159/000079383. [DOI] [PubMed] [Google Scholar]
- 55.Kessel N. Reassurance. Lancet. 1979;1:1128–1133. doi: 10.1016/s0140-6736(79)91804-x. [DOI] [PubMed] [Google Scholar]
- 56.Bruster S, Jarman B, Bosanquet N, Weston D, Erens R, Delbanco T. National survey of hospital patients. BMJ. 1994;309:1542–1549. doi: 10.1136/bmj.309.6968.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michie S, French D, Allanson A, Bobrow M, Marteau T. Information recall in genetic counselling: a pilot study of its assessment. Patient Educ Couns. 1997;32:93–100. doi: 10.1016/s0738-3991(97)00068-2. [DOI] [PubMed] [Google Scholar]
- 58.Watson M, Duvivier V, Wade Walsh M, Ashley S, Davidson J, Papaikonomou M, Murday V, Sacks N, Eeles R. Family history of breast cancer: what do women understand and recall about their genetic risk? J Med Genet. 1998;35:731–738. doi: 10.1136/jmg.35.9.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michie S, Thompson M, Hankins M. To be reassured or to understand? A dilemma in communicating normal cervical screening results. Br J Health Psychol. 2004;9:113–123. doi: 10.1348/135910704322778768. [DOI] [PubMed] [Google Scholar]