Abstract
Onset of a movement-related brain potential (lateralized readiness potential, LRP) was used to divide reaction time (RT) into two intervals: (1) stimulus onset to LRP onset, and (2) LRP onset to onset of the overt response. Effects on these intervals of advance information about the to-be-signaled response and of the mapping between fingers and response buttons were examined. These effects were used to reach conclusions about the organization of response preparation and about the identity of the processes influenced by advance information. In the absence of advance information, response preparation involved two steps. First, two of the four possible response alternatives were prepared, then one of these two was prepared further. Which pair of responses was prepared during the first step depended on the spatial arrangement of the fingers on the buttons, rather than on any common anatomical feature. Advance information about the upcoming response allowed the first step to be performed prior to the response signal, thus removing its contribution to RT. The second step, however, remained unaffected.
Keywords: Mental chronometry, Reaction time, Lateralized readiness potential, Precuing paradigm, Response preparation, Response selection
1. Introduction
Among Mike Coles' important contributions to Cognitive Psychophysiology is his pioneering work on mental chronometry. It is fair to say that he bears much of the responsibility for the current use of event-related potentials to draw inferences about the time-course of human information processing. In particular, Coles is one of the originators of a measure now known as the lateralized readiness potential (LRP). Coles and his colleagues (e.g. De Jong et al., 1990; Gratton et al., 1988; Osman et al., 1992) used the LRP to monitor the motor system millisecond-by-millisecond during reaction-time (RT) experiments. This, in turn, enabled them to infer the presence, nature, and time-course of response activation by perceptual and central processes. The work to be reported here grows out of this tradition of LRP-based mental chronometry. So, before turning to this work, the LRP will be described first in some detail.
1.1. Lateralized readiness potential
As its name suggests, the LRP is believed to be related closely to the lateralized portion of the readiness potential (RP; Coles, 1989; Eimer, 1998). The RP is a slow negative potential that precedes spontaneous voluntary movements of the distal limbs (Kornhuber and Deecke, 1965; Vaughan et al., 1968). The later part of the RP is larger over the side of the head contralateral to a moved hand. Both magnetic and intracranial recordings in humans indicate that the lateralized portion of the RP arises mainly from primary motor cortex (Ikeda and Shibasaki, 1992; Lang et al., 1991).
The LRP is measured in choice RT tasks, where a stimulus signals that a response should be made with one of two effectors (usually the hands). Recordings are made from two electrode sites (C3′ and C4′) located, respectively, over the left and right hand areas of primary motor cortex. On every trial, the recording at the site contralateral to the signaled effector is subtracted from the recording at the ipsilateral site. The LRP is the result of this subtraction averaged across trials. Like other ERPs, the LRP is a waveform that represents voltage over time. Let the potentials recorded at the contralateral and ipsilateral sites at time t be denoted as Contra(t) and Ipsi(t). The LRP at time t is then defined as:
| (1) |
The resulting LRP will be positive when there is greater electrical potential (larger negative value) on the side contralateral to the signaled effector and negative when there is greater electrical potential on the side ipsilateral to the signaled effector.
A useful property of the LRP arises from alternative definitions of time (t). The LRP can be either stimulus- or response-locked. Stimulus-locked (S-locked) means that each point in the LRP is based on points from individual trials that follow the response signal (RS) by the same amount of time (t = 0 at RS onset). Response-locked (R-locked) means that each point in the LRP is based on points from individual trials that precede the overt response (RT) by the same amount of time (t = 0 at RT). The interval between the RS and S-locked LRP onset (RS-LRP interval) is related to the duration of the processes that occur before the start of the LRP, and the interval between R-locked LRP onset and RT (LRP-RT interval) is related to the duration of processes that occur after the start of the LRP. By examining which of these two intervals are affected by an experimental manipulation, it is possible to determine whether the manipulation's effects on RT occur before or after the start of the LRP.
1.2. Fractionating RT effects with the LRP
Such LRP fractionation of RT effects has proven extremely useful for mental chronometry. This usefulness derives from the fact that the lengths of the RS-LRP and LRP-RT intervals can be independently manipulated (see Sternberg, 2001 on separate modifiability). Experimental manipulations have been found to affect one of the two intervals without propagating onto the other in numerous studies (e.g. Hackley and Valle-Inclan, 1999; Miller and Low, 2001; Miller et al., 1999; Mueller-Gethmann et al., 2000; Mordkoff et al., 1996; Osman and Moore, 1993; Osman et al., 2000; Smulders et al., 1995; Sommer et al., 2001; Van der Lubbe et al., 2001). Indeed, Smulders et al. (1995) manipulated stimulus quality and response complexity within the same experiment and found the former to affect the RS-LRP interval only, the latter to affect the LRP-RT interval only, and the combined manipulations to affect RT additively.
Because the RS-LRP and LRP-RT intervals can be influenced selectively, they can provide evidence about which stages are responsible for an effect on RT. The identity of the involved stages (or “locus of an effect”), in turn, often bears on the theoretical interpretation of the effect. LRP fractionation has thus far been employed to discover the locus of a wide variety of RT effects, including those due to stimulus intensity (Miller et al., 1999), ancillary and redundant signals (Hackley and Valle-Inclan, 1999; Mordkoff et al., 1996), number of S-R alternatives (Miller and Ulrich, 1998), advance information provided by precues (Leuthold et al., 1996; Mueller-Gethmann et al., 2000; Osman et al., 1995), speed-accuracy tradeoffs (Osman et al., 2000; Van der Lubbe et al., 2001), and the psychological refractory period (Osman and Moore, 1993; Sommer et al., 2001).
A closely related application of LRP fractionation is to use the LRP-RT interval to isolate effects of experimental manipulations on the duration of motor processes. Many experimental manipulations might be expected to influence both motor and nonmotor stages of RT. By examining effects on the LRP-RT interval, it is possible to examine changes in the duration of motor processes alone. It then becomes possible to study these processes in typical chronometric fashion, i.e. through examining the effects of experimental manipulations on their durations. This is the type of methodology and inferential logic used in the current study, wherein LRP fractionation was employed in the context of a precuing paradigm.
1.3. Precuing paradigm
It has long been known that RT in a choice-RT task is speeded by advance information that reduces the number of possible stimulus-response (S-R) alternatives (e.g. Leonard, 1953). This effect is typically studied in the precuing paradigm, where the advance information is provided by a precue signal that precedes the RS by an interval called the foreperiod. During the last two decades, there has been considerable interest in using the effects of such advance information on the duration of motor processes to infer their functional properties and mechanisms. This endeavor has been complicated, however, by the fact that the number of remaining S-R alternatives can also influence non-motoric stages of RT (e.g. LaBerge et al., 1970). Progress has therefore depended on the development of new and better ways to measure selectively the effects of advance information on the duration of motor processes.
One approach has been to use psychophysiological measures. These have provided considerable evidence that precuing can influence the state of the motor system during the foreperiod. For example, single-cell recordings have shown that precuing can affect the activity level of neurons in motor and premotor cortex (e.g. Riehle and Requin, 1989). Similarly, precues identifying response hand can produce an LRP with greater activity contralateral to the precued hand (e.g. De Jong et al., 1988; Hackley and Miller, 1995; Ulrich et al., 1998). Effects on spinal reflexes show that advance information about the responding limb can influence even the most peripheral motor neurons in the spinal cord (Requin et al., 1991).
Unfortunately, while such findings indicate that advance information can affect the motor system, they are nevertheless inconclusive about whether such effects are responsible for the observed shortening of RT. Preparatory activity in the motor system during the foreperiod need not influence RT. That is, changes in RT could result instead from other effects of precuing elsewhere in the nervous system. Nor do foreperiod measures allow us to separate motoric and nonmotoric effects on RT. These latter questions can, however, be addressed by examining precue effects on the LRP-RT interval.
1.4. Precue effects on the LRP-RT interval
Precue effects on the LRP-RT interval have so far been examined in three studies (Leuthold et al., 1996; Mueller-Gethmann et al., 2000; Osman et al., 1995), each of which found an effect. Since the experiment to be reported here extends a previous study by the authors (Osman et al., 1995), this study will be described in some detail. Besides the LRP-RT interval, this study also examined precue effects on the RS-LRP interval, muscle activity (EMG), and the latency of the P300 ERP component (thought to reflect the duration of early RT stages involving “stimulus evaluation”). To divide the effects of an experimental manipulation on RT into its effects before and after LRP onset, it is necessary that the LRP arise only after the RS. When a precue provides information about the upcoming response hand, however, an LRP is already present before the RS. Hence, we were primarily interested in the effects of precues that indicated two responses, each on a different hand.
To minimize lateralized electrical artifacts (e.g. due to eye movements) that can influence LRP measurements, all stimuli and responses occurred at the subject's midline. Each trial began with the presentation of four red stars in a vertical column (warning signal). One half second later, some of the red stars turned yellow (precue). One second later, one of the yellow stars turned green (RS). The location of the green star in the column signaled a spatially compatible press on one of four vertically arranged buttons. Precues indicated either the top two or bottom two buttons, top and bottom or middle two buttons, top and third or second and bottom buttons, or all the buttons. The different precue types and button combinations that each type could signal are shown in Table 1. The arrangement of fingers on the buttons is shown in the very leftmost column (normal mapping).
Table 1.
Arrangement of fingers on buttons for each mapping, and buttons (fingers) signaled by each precue type
| Mapping | Precue type | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Crossed | Up/Down | Inner/Outer | Alternating rows | Noninformative | |||
| RM | RM | * | – | * | – | * | – | * |
| RI | LM | * | – | – | * | – | * | * |
| LM | RI | – | * | – | * | * | – | * |
| LI | LI | – | * | * | – | – | * | * |
Overt responses were faster with informative than noninformative precues. It is this effect on RT that we sought to analyze further using psychophysiological measures. Like RT, P300 latency was shorter for informative than noninformative precues. This suggests that at least part of the effect on RT was due to changes in the duration of stimulus evaluation processes (Magliero et al., 1984). In contrast, precuing had little or no effect on the dynamics (amplitude profile over time) of muscle activity measured relative to RT (R-locked). This rules out effects on the very latest portion of the RT interval, but is not incompatible with effects on the duration of earlier motor processes. As anticipated, an LRP occurred during the foreperiod when the precue provided information about the upcoming response hand. This shows that the precues activated motor cortex but, as noted, it remains possible that the effects on RT reflected changes elsewhere in the nervous system. Finally, the precues indicating fingers on two different hands produced shorter LRP-RT intervals than the noninformative precues.
1.5. Interpreting effects on the LRP-RT interval
Does this last finding mean that the precuing effects on RT were due, at least in part, to changes in the duration of motor processes? The answer depends on whether the LRP-RT interval was determined solely by the duration of motor processes. What determines the LRP-RT interval, in turn, depends on the temporal arrangement of motor and nonmotor components of RT. Consider the case in which the RT interval can be divided into two serial stages1, the earlier containing only nonmotoric processes and the later containing only motoric processes. In the absence of advance information about response hand, the LRP would arise at some point during the later motoric stage. Effects on the LRP-RT interval could thus be attributed solely to changes in the duration of motor processes.
There are, of course, other ways that motor and nonmotor components of RT could be organized. The top panel of Fig. 1 shows one possible scenario for the above precuing task under conditions where the precue does not provide advance information. Here, there are two nonmotoric steps (NM1 and NM2) involving identification of the RS, two motoric steps (M1 and M2) involving response preparation, and a final motoric step (M3) involving execution of the prepared response. In the absence of advance information (noninformative precue), NM1 involves determining whether the green star (the location of which indicates the response finger) occurs in the top or bottom half of the vertical array, and NM2 involves determining which of the two remaining positions it occurs in. Under these circumstances, initial preparation of two fingers on the same hand (and the LRP) could begin as soon as NM1 was complete, and would thus comprise M1. Remaining preparation of the sole responding finger, comprising M2, would have to wait until both nonmotoric steps (NM1 and NM2) were complete. Given this scenario, precue effects on the LRP-RT interval could be caused by changes in the duration or presence of the later set of nonmotoric processes, i.e. if precues signaling two fingers on different hands influenced NM2.
Fig. 1.
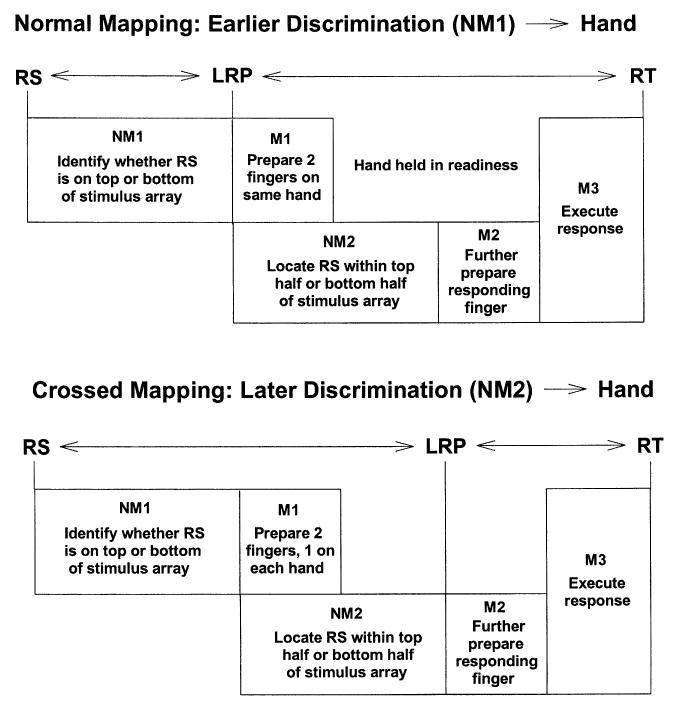
A model of the component processes that determine reaction time (RT) following noninformative precues in the procedure used by Osman et al. (1995) and in the present study. The identity and timing of the component RT processes are shown for the normal mapping condition (both studies) in the top panel and for the crossed mapping condition (present study) in the bottom panel. RS, response signal; LRP, lateralized readiness potential; and RT, reaction time. NM1, NM2, M1, M2, and M3 designate nonmotoric steps 1–2 and motoric steps 1–3. The precue (not shown) appears 1000 ms before the RS.
The above discussion leaves us in a quandary about the interpretation of precue effects on the LRP-RT interval. Given the model in the top panel of Fig. 1, these effects could result from changes in the duration of either motor or nonmotor processes. Yet, even if this model were correct, all need not be lost. The LRP might nevertheless be prevented from arising until both nonmotoric components were complete. Were this the case, then precue effects on the LRP-RT interval could still be attributed unequivocally to changes in the duration of motor processes.
1.6. Present experiment: mapping manipulation
The present experiment sought to prevent the LRP from arising before all nonmotor processes were complete. The procedure was similar to that of Osman et al. (1995), except for the inclusion of an additional mapping manipulation: We varied the arrangement of subjects' fingers on the response buttons. The two finger-button mappings, normal and crossed, are shown in Table 1. The normal mapping provided a replication of Osman et al. The crossed mapping involved a very simple change: The two fingers on the middle two buttons (right index and left middle) were reversed. (For a precuing study with similar stimuli and responses, as well as the same mapping manipulation, see Proctor and Reeve, 1986.)
The mapping manipulation might seem like a pretty minor one, but let's consider the consequences of a crossed mapping for the LRP, which are shown in the bottom panel of Fig. 1. As with the normal mapping (top panel), suppose that, in the absence of advance information, subjects first prepared two of the four possible response fingers (M1) based on whether the green star occurred at the top or bottom of the stimulus array (NM1). This would have produced an LRP in the normal-mapping condition. But, in the crossed-mapping condition, two fingers on opposite hands would be prepared (top, middle fingers; and bottom, index fingers). Since both hands would remain equally prepared (one finger on each), an LRP would not yet arise. The responding finger would be further prepared (M2) following completion of the remaining nonmotor processes (NM2). Only now in the crossed-mapping condition, after NM2, would there be differential preparation of the two hands, and hence an LRP.
The effects of mapping illustrated in Fig. 1 can be generalized to a large class of models in which the duration of nonmotor processes could influence the LRP-RT interval. These models need not involve the parallelism or particular nonmotor processes shown in Fig. 1. For example, the postulated processes might all occur in strict series, or additional processes (e.g. “decision”, “S-R translation”, or “response selection”) might be included within each non-motoric component. What the models have in common are that in the absence of advance information: (1) RT is determined by two separate nonmotor components, one finishing before the other; (2) initial preparation of two of the four possible response fingers can begin as soon as the first nonmotor component is complete, while final preparation of the sole responding finger must wait until both nonmotor components are complete; (3) the first-completed non-motor component depends on the top versus bottom feature of RS location, and the other nonmotor component depends on a different feature of RS location (e.g. center vs. outer edge). If the precuing task is correctly characterized by any model in this class, then the mapping manipulation should have the same effect. That is, the crossed mapping would prevent an LRP until nonmotor processing was complete and, consequently, any precue effects on the LRP-RT interval could be attributed to changes in the duration of motor processes.
How likely is it that such a model applies to the precuing task used by Osman et al. (1995)? Top versus bottom was a salient feature of both the RS and response sets. A top versus bottom discrimination might therefore have finished before discrimination between the middle versus outer or odd versus even rows. Likewise, S-R translation between a top versus bottom feature of the RS and a top versus bottom feature of the response might have had precedence over translation between other features necessary to identify the RS and select a response. (For a discussion of salience in S-R translation, see Proctor and Reeve, 1985.) In any event, the mapping manipulation will provide an empirical test of the above models. According to these models, a crossed mapping should cause the first motor process (M1) and second nonmotor process (NM2) to “move” from the LRP-RT interval to the RS-LRP interval (Fig. 1). All else being equal (i.e. no other effects of mapping), we would therefore expect the mapping manipulation to produce opposite effects on the sizes of these two intervals.
The following experiment had two objectives. The first was to replicate the precue effects on the LRP-RT interval found with the normal mapping. The second was to determine whether these effects would still be present with the crossed mapping. If precue effects on the LRP-RT interval occur regardless of the finger-button mapping, we may be more confident that these effects reflect changes in the duration of motor processes. Suppose, however, that precue effects on the LRP-RT interval vanish with a crossed mapping. This would imply that the durations of processes within this interval under the crossed mapping, including the later two motor processes (M2 and M3), were unaffected. In sum, the mapping manipulation will help identify which motor processes, if any, contribute to the shortening of RT by advance information. It will also provide insights into the general organization of response preparation and inform the LRP fractionation of RT, as we shall see.
2. Method
2.1. Subjects
Sixteen undergraduate students were each tested individually in a single 4-h session. The first 3 h fulfilled a course requirement, and subjects were paid for the remaining hour. All had normal or corrected to normal vision and no apparent sensorimotor or neurological problems.
2.2. Apparatus
Stimulus presentation and data acquisition were controlled by a PC computer. Stimuli were presented on a VGA monitor, and responses were made by depressing one of four vertically arranged buttons on a response box. Approximately 135 g of pressure was required for a response to be registered. To the left and right of each button were labels indicating the finger to be placed on that button in the normal and crossed mappings. Psychophysiological signals were recorded using Ag/AgCl electrodes and a Grass Model 12 Neurodata Acquisition System.
2.3. Stimuli, responses, and trial events
All stimuli consisted of four stars arranged in a vertical column and presented at each subject's midline. At a viewing distance of about 70 cm, each star subtended about 0.4×0.3° of visual angle (dva), and the entire column subtended about 2.5×0.3 dva.
Each trial began with the presentation of four red stars (warning signal). One half second later, some of the red stars turned yellow (precue). There were four types of precue: Up/Down (UD, top two or bottom two stars turned yellow), Inner/Outer (IO, top and bottom stars or two middle stars turned yellow), Alternating (ALT, top star and third-from-the-top star turned yellow or second-from-the-top star and bottom star turned yellow), and Noninformative (NI, all stars turned yellow). One second after precue onset, one of the yellow stars turned green and the other yellow star(s) returned to red (response signal, RS). The RS remained on the screen for 1500 ms, vanishing at the end of the trial. The position of the green star within the column signaled a spatially compatible press on one of four buttons also arranged vertically at the subject's midline (i.e. top star signaled top button, second-from-the-top star signaled second-from-the-top button, etc.).
The precues and RSs indicated buttons. The fingers corresponding to those buttons depended on the arrangement of subjects' fingers on the buttons (finger-button mapping). There were two finger-button mappings: normal and crossed. In the normal mapping, the buttons, from top to bottom, corresponded to the right middle (RM), right index (RI), left middle (LM), and left index (LI) fingers. In the crossed mapping, the buttons, from top to bottom, corresponded to the RM, LM, RI, and LI fingers.
2.4. Design
All precues provided valid information about the upcoming RS. Each type of precue (UD, IO, ALT, and NI) could be followed by an RS indicating any of the four buttons, yielding 16 (4×4) trial types. Each block began with three warmup trials, selected randomly from the different trial types. On practice blocks, the warmup trials were followed by 32 correct trials (two from each trial type) presented in a random order. On experimental blocks, the warmup trials were followed by 80 correct trials (five from each trial type) presented in a random order. Nonwarmup trials resulting in errors were rerun in the same block at a random location in the sequence of remaining trials. Subjects alternated between normal and crossed mappings on successive blocks. Half began with the normal mapping, and half began with the crossed mapping.
2.5. Procedure
Each session consisted of four practice and 14 experimental blocks. During the first two practice blocks, the experimenter observed subjects and offered feedback on their performance. Electrodes for measuring psychophysiological activity were then applied (see Section 2.6). Subjects' electroencephalographic (EEG) and electro-oculographic (EOG) activity were displayed for them on the monitor and the effects of eye movements on EEG recordings demonstrated. Subjects were asked to fixate the column of stars during each trial and to avoid eye movements and blinks while it was present on the screen. Blinks and eye movements were permitted during the intertrial interval, which was indicated by the absence of the column. Next, another two practice blocks were administered to further familiarize subjects with the task and to give them practice minimizing eye movements. During these blocks the experimenter monitored eye movements and offered feedback. Finally, the 14 experimental blocks were administered.
Subjects sat facing the screen with their fingers resting comfortably on the response buttons. After each block, an instruction was presented on the screen reminding the subject of the finger-button mapping for the next block. (Subjects' compliance with the mapping instructions was monitored by means of their EMG activity in each arm.) Each trial lasted 3 s and was followed by an intertrial interval. The intertrial interval lasted 2 s, plus an additional 1/2 s when feedback was presented. When presented, trial feedback occurred immediately after the trial and appeared on the screen just below the location of the star column. Subjects were informed if they made more than one response, no response, an incorrect response, or responded too slowly (> 1 s). Subjects were praised following an RT below their accumulated average for that trial type. After each block, feedback concerning the subject's performance on the previous block was displayed on the screen. This consisted of mean RT for correct responses and the proportion of correct responses. Subjects were allowed to rest for as long as they wished before initiating a new block.
2.6. Recording
EEG, EMG, and EOG activity were recorded on each trial. EEG was recorded unipolarly from midline sites, Fz, Cz, and Pz (International 10/20 System; Jasper, 1958), and referenced to linked mastoids. EEG was recorded bipolarly from electrode sites C3′ and C4′, 4 cm to the left and right of Cz (vertex) along the interaural line. Vertical and horizontal EOG activity was recorded bipolarly from sites above and below the midpoint of the right eye and 2 cm external to the outer canthus of each eye. EMG was recorded bipolarly from the dorsal surface of each forearm, using standard extensor placements (Lippold, 1967). Signals were filtered online with a band pass (half-power cutoff) of 0.01–30 Hz for EEG and EOG and 0.3–30 Hz for EMG. All signals were digitized at 100 Hz, and the recording epoch was 3000 ms, starting with the presentation of the warning signal.
3. Data reduction
3.1. Overt performance
RT was defined as the time between RS onset and the first closure of the microswitch beneath a response button. A correct trial was defined as one on which microswitch closure occurred for the signaled button only and before the end of the trial (RT < 1.5 s). Accuracy was defined as the number of correct trials (140 per subject × precue condition × mapping) divided by the total number of trials.
3.2. Psychophysiological waveforms
Average S- and R-locked waveforms were obtained for the LRP, horizontal EOG (HEOG), and rectified EMG in the responding arm. Also obtained were average S-locked waveforms for the ERP at site Pz (where P300 is maximal). Average waveforms were based on the waveforms from individual correct trials and were obtained for each subject in each precue condition with each finger-button mapping. Each waveform was adjusted by subtracting a baseline voltage from all time points. S-locked waveforms were adjusted by subtracting the average voltage during the 200 ms before the RS. R-locked waveforms were adjusted by subtracting the average voltage during the interval 600–400 ms before RT.
LRPs were obtained from the bipolar recordings of the difference between electrode sites C3′ and C4′ according to Equation 1, where “Ipsi” and “Contra” are with respect to the finger signaled by the RS. To evaluate the contribution of horizontal eye movements to the observed LRPs, bipolar recordings from the HEOG channel were analyzed in a manner analogous to the bipolar recordings from the C3′–C4′ channel. This involved applying Equation 1 to the HEOG channel.
3.3. Timing and amplitude of EMG
Onset latencies were obtained for each subject's average S- and R-locked EMG in each precue × mapping condition. Two criteria were defined for each subject, one for their S-locked EMGs and one for their R-locked EMGs: first, the maximum amplitude was obtained for a subject's S- or R-locked EMGs in each precue × mapping condition (after applying an 8.8 Hz low-pass filter); the maximum amplitudes were then averaged (one average for S-locked and one average for R-locked) and a criterion set at 10% of the average. The onset of a given waveform (unfiltered) was defined as the point at which it began to maintain an amplitude consistently above the criterion. This required that the voltage at onset and the average voltage during the next two 50 ms windows exceed the criterion. The peak amplitude for each R-locked EMG, as well as the time at which this amplitude occurred, were also determined.
3.4. LRP onset
Onset latencies were obtained for each subject's average S- and R-locked LRPs in each of the six mapping × precue conditions in which the precue did not provide advance information about response hand (IO, ALT, and NI for the normal mapping; UD, IO, and NI for the crossed mapping). It was not possible to obtain onset latencies when response hand was precued because the LRP was already present at the time of the RS. LRP onset latencies were obtained in the same manner as EMG onset latencies, but with two exceptions. First, each criterion was based on the average of the maximum amplitudes of three (rather than four) waveforms. Second, the criterion was set at 25% (rather than 10%) of the average.
3.5. P300 latency
P300 latency was obtained from recordings at site Pz that were smoothed using an 8.8 Hz (half power) low-pass filter. P300 latency was defined as the interval between the RS and the maximum positivity occurring from 300 to 800 ms after the RS.
4. Results
Results concerning overt performance (mean RT and error rate), EMG activity, P300 latency, the LRP, and horizontal EOG are each discussed in a separate subsection. Each measure in each precue × mapping condition was obtained separately for each individual subject. All ANOVAs involve repeated measures with replications over subjects. All posthoc comparisons employed the Newman–Keuls test.
4.1. Overt Performance
Mean RT and error rate for each precue × mapping condition are shown in Table 2. Precue type had a highly significant effect on mean RT [F(3,45) = 244; P < 0.001], mostly due to the difference between the noninformative (NI) precue condition and the informative (UD, IO, and Alt) precue conditions. Mean RT was slower with NI precues than with UD, IO, or Alt precues (P < 0.01), but did not differ significantly between any of the latter three precue types (P > 0.05). The mapping manipulation had a small (crossed - normal = 10 ms) effect on mean RT [F(1,15) = 4.889; P < 0.05] and did not significantly interact with precue type [F(3,45) = 1.4; P = 0.255].
Table 2.
Mean RT and error rate for each combination of mapping and precue type
| Precue type | Average | ||||
|---|---|---|---|---|---|
| Up/Down | In/Out | Alternating | Noninformative | ||
| Normal mapping | RI, RM or LI, LM | RI, LM or RM, LI | RM, LM or RI, LI | All fingers | |
| Crossed mapping | RM, LM or RI, LI | RI, LM or RM, LI | RI, RM or LI, LM | All fingers | |
| Mean RT (ms) | |||||
| Normal mapping | 371 | 380 | 389 | 470 | 402 |
| Crossed mapping | 381 | 388 | 395 | 485 | 412 |
| Average | 376 | 384 | 392 | 478 | 406 |
| Error rate (%) | |||||
| Normal mapping | 1.77 | 1.92 | 2.67 | 3.50 | 2.46 |
| Crossed mapping | 1.73 | 2.39 | 2.59 | 3.89 | 2.65 |
| Average | 1.75 | 2.16 | 2.63 | 3.70 | 2.56 |
Fig. 2 shows precue and mapping effects on the entire RT distribution. Shown here are the cumulative distribution functions (CDFs) for each precue type under normal (top panel) and the crossed (bottom panel) mapping conditions. Time is represented on the horizontal axes, and the percentage of RTs equal to or faster than a given time (percentile) is shown on the vertical axes. Each CDF was obtained by Vincintizing the CDFs [averaging the times (X) for each percentile (Y)] from individual subjects.
Fig. 2.
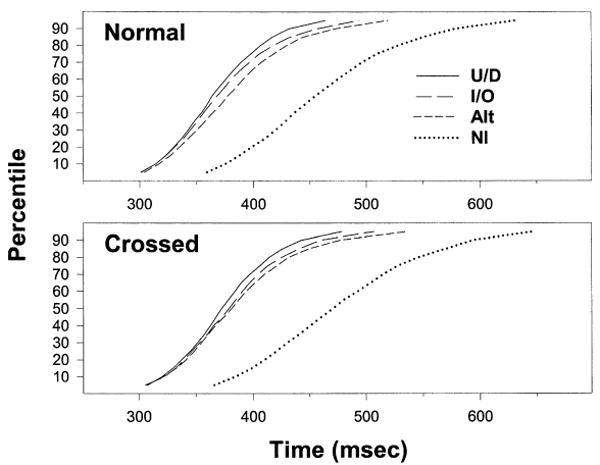
Vincintized cumulative distribution functions of RTs for each type of precue under normal (top panel) and crossed (bottom panel) mappings. U/D, up/down precues; I/O, in/out precues; Alt, precues with alternating rows; and NI, noninformative precues.
The patterns shown in the two panels are quite similar, with the bottom CDFs shifted slightly (about 10 ms) to the right. Perhaps the most salient aspect of both panels is the large difference at all percentiles between the NI CDF and the three CDFs from the informative precue conditions (UD, IO, and Alt). The differences between the latter three CDFs are much smaller and not present at the lower percentiles (i.e. for faster RTs). Another similarity between the two panels is the temporal ordering of the CDFs from the three informative precue conditions. The absence of an effect of mapping on this ordering is interesting because the response parameters signaled by UD and Alt precues (L vs. R hand and I vs. M finger) are swapped across mapping.
Error rates provided no indication of a speed-accuracy tradeoff. There was a main effect of precue type [F(3,45) = 7.64; P < 0.001], but the four precue conditions had the same ordering as they did with RT. Consistent with RT, error rates were slightly lower with the normal mapping than the crossed mapping, but this difference was not significant [F(1,15) = 0.668, P = 0.427] and did not interact with precue type [F(3,45) = 0.386;P = 0.763].
4.2. Electromyographic activity
EMG results are shown for each precue × mapping condition in Table 3 and Fig. 32. The table shows the average (across subjects) onset latency for S-locked EMG and average onset latency, peak latency, and peak amplitude for R-locked EMG. Fig. 3 shows EMG waveforms averaged across subjects (the grandaverage) in each precue × mapping condition. Time is displayed along the X-axes, and voltage is displayed along the Y-axes. The top two panels show S-locked grandaverages. Here, onset of the WS, PC, and RS are indicated by vertical lines. The bottom two panels show R-locked grandaverages. The dashed vertical line in each indicates RT.
Table 3.
EMG results for each combination of mapping and precue type
| Precue type | Average | ||||
|---|---|---|---|---|---|
| Up/Down | In/Out | Alternating | Noninformative | ||
| Normal mapping | RI, RM or LI, LM | RI, LM or RM, LI | RM, LM or RI, LI | All fingers | |
| Crossed mapping | RM, LM or RI, LI | RI, LM or RM, LI | RI, RM or LI, LM | All fingers | |
| S-locked EMG | |||||
| Onset (ms) | |||||
| Normal mapping | 255 | 251 | 260 | 305 | 268 |
| Crossed mapping | 249 | 254 | 252 | 317 | 268 |
| Average | 252 | 253 | 256 | 311 | 268 |
| R-locked EMG | |||||
| Onset (ms) | |||||
| Normal mapping | −60 | −58 | −57 | −64 | −60 |
| Crossed mapping | −61 | −61 | −64 | −63 | −62 |
| Average | −61 | −60 | −61 | −64 | −61 |
| Peak latency (ms) | |||||
| Normal mapping | −20 | −19 | −21 | −18 | −19 |
| Crossed mapping | −18 | −19 | −18 | −17 | −18 |
| Average | −19 | −19 | −20 | −18 | −19 |
| Peak amplitude (uV) | |||||
| Normal mapping | 65.2 | 65.6 | 64.6 | 64.6 | 65.0 |
| Crossed mapping | 60.6 | 61.5 | 61.2 | 61.9 | 61.3 |
| Average | 62.9 | 63.6 | 62.9 | 63.3 | 63.2 |
Fig. 3.
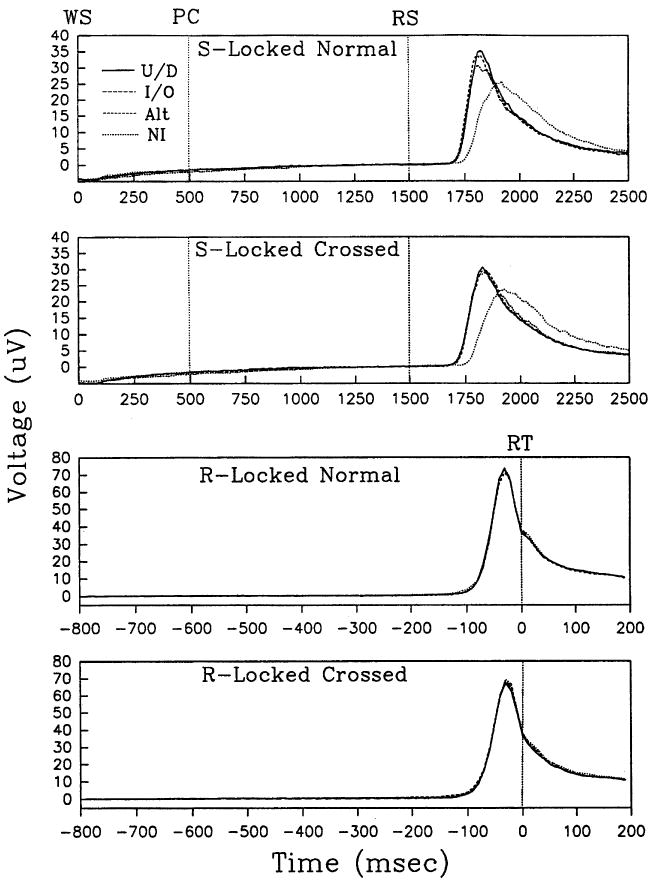
Stimulus (S)-locked and response (R)-locked grandaverages of electromyographic activity in the responding arm for each combination of mapping and precue type. WS, warning signal; PC, precue; RS, response signal; RT, reaction time; U/D, up/down precues; I/O, in/out precues; Alt, precues with alternating rows; and NI, noninformative precues.
The pattern of precue effects on S-locked EMG onset latency was similar to that found for RT. Precue type had a significant effect [F(3,42) = 107; P < 0.001]. EMG activity began later with NI precues than with any of the other three precue types (P < 0.01), and EMG onset did not differ significantly between any of the other three precue types (P > 0.05). Mapping did not produce a significant effect on S-locked EMG onset [F(1,14) = 0.013; P = 0.911], but had a marginally significant interaction with precue type [F(3,42) = 2.198; P = 0.102].
Neither precue type nor mapping had much of an effect on R-locked EMG. The four waveforms in each of the two bottom panels are almost identical, and the two sets of four closely resemble one another (although peak amplitude is slightly higher with the normal mapping). These observations were evaluated statistically by examining three measures: onset latency, peak latency, and peak amplitude. Precue type had no significant effect on onset latency [F(3,42) = 1.429; P = 0.248], peak latency [F(3,42) = 1.525; P = 0.222], or peak amplitude [F(3,42) = 0.401; P = 0.753]. Mapping had no significant effect on peak latency [F(1,14) = 1.112; P = 0.310] or peak amplitude [F(1,14) = 1.109; P = 0.310]. There was a marginally significant effect on onset latency [F(1,14) = 3.006; P = 0.105], but the size (crossed - normal) was only 2.8 ms. Finally, precue type and mapping did not interact significantly on onset latency [F(3,42) = 1.043; P = 0.348], peak latency [F(3,42) = 0.862; P = 0.469], or peak amplitude [F(3,42) = 1.348; P = 0.260].
4.3. P300
Table 4 displays mean P300 latencies, and Fig. 4 shows grandaverage ERPs recorded at Pz, the electrode site where P300 is largest. (Note that positive is plotted down here.) The P300s to the RS are the positive deflections that peak approximately 400–500 ms after the vertical line corresponding to RS onset.
Table 4.
P300 latency for each combination of mapping and precue type
| Precue type | Average | ||||
|---|---|---|---|---|---|
| Up/Down | In/Out | Alternating | Noninformative | ||
| Normal mapping | RI, RM or LI, LM | RI, LM or RM, LI | RM, LM or RI, LI | All fingers | |
| Crossed mapping | RM, LM or RI, LI | RI, LM or RM, LI | RI, RM or LI, LM | All fingers | |
| Latency (ms) | |||||
| Normal mapping | 473 | 438 | 461 | 549 | 480 |
| Crossed mapping | 425 | 444 | 449 | 538 | 464 |
| Average | 449 | 441 | 455 | 544 | 472 |
Fig. 4.
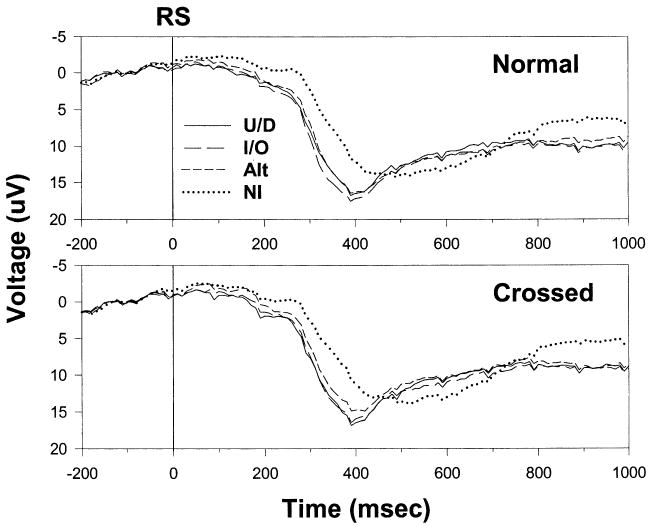
Stimulus-locked grandaverages of ERP recordings from electrode site Pz for each type of precue under normal (top panel) and crossed (bottom panel) mappings. The P300 component in the grandaverages peaks approximately 400–500 ms after presentation of the RS. RS, response signal; U/D, up/down precues; I/O, in/out precues; Alt, precues with alternating rows; and NI, noninformative precues.
The pattern of precue effects on P300 latency resembled that for RT and S-locked EMG. Precue type had a significant effect on P300 peak latency [F(3,45) = 14.3; P < 0.001], due mostly to the difference between informative versus noninformative precues. Peak latency was significantly longer (P < 0.01) for NI precues than for UD, IO, or Alt precues, but did not differ significantly (P > 0.05) between the latter three precue types. As with RT and EMG, mapping did not have much of an effect on P300 latency. It had a small nonsignificant effect [F(1,15) = 2.366; P = 0.145] in the opposite direction to that on RT (normal − crossed = 16 ms) and interacted marginally with precue type [F(3,45) = 2.175; P = 0.104].
4.4. Lateralized readiness potentials
The LRP findings are presented in Table 5 and Fig. 5. We will be concerned here with: (1) the presence of an S-locked LRP during the foreperiod under conditions in which the precues provided advance information about response hand (UD with the normal mapping and Alt with the crossed mapping), and (2) the onset latency of the S- and R-locked LRPs under conditions in which the response hand was not precued (IO, Alt, and NI with the normal mapping; UD, IO, and NI with the crossed mapping).
Table 5.
LRP results for each combination of mapping and precue type
| Precue type | ||||
|---|---|---|---|---|
| Up/Down | In/Out | Alternating | Noninformative | |
| Normal mapping | RI, RM or LI, LM | RI, LM or RM, LI | RM, LM or RI, LI | All fingers |
| Crossed mapping | RM, LM or RI, LI | RI, LM or RM, LI | RI, RM or LI, LM | All fingers |
| S-locked foreperiod (uV) | ||||
| Normal mapping | 2.348 | −0.044 | −0.243 | −0.063 |
| Crossed mapping | −0.026 | −0.112 | 1.781 | −0.485 |
| S-locked onset (ms) | ||||
| Normal mapping | – | 243 | 256 | 276 |
| Crossed mapping | 237 | 250 | – | 335 |
| R-locked onset (ms) | ||||
| Normal mapping | – | −109 | −114 | −153 |
| Crossed mapping | −123 | −113 | – | −120 |
Fig. 5.
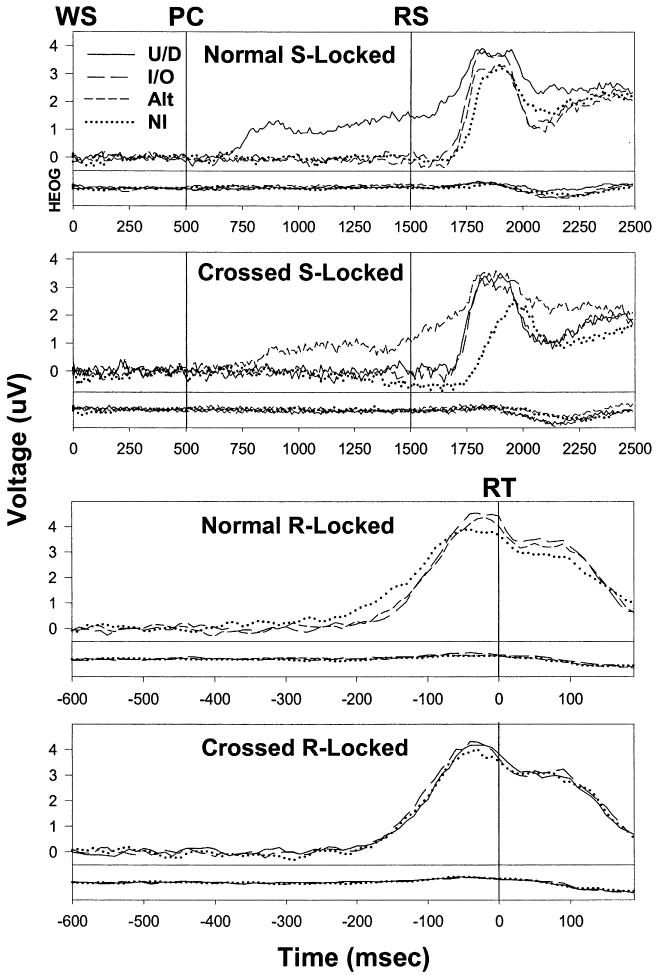
Stimulus (S)-locked and response (R)-locked grandaverages of lateralized readiness potentials (LRPs) and horizontal eye-movement activity (HEOG) for each combination of mapping and precue type. LRPs are shown at the top of each panel, and HEOG is shown at the bottom. Note that the HEOG scale is 1/4 of that for the LRPs (see text for explanation). WS, warning signal; PC, precue; RS, response signal; RT, reaction time; U/D, up/down precues; I/O, in/out precues; Alt, precues with alternating rows; and NI, noninformative precues.
An S-locked LRP arose during the foreperiod when response hand was precued (top two panels of Fig. 5). The area under the LRP in each mapping × precue condition during the 500 ms prior to the RS is displayed in Table 5. Planned tests found this area to be greater than zero following UD precues with the normal mapping [F(1,15) = 17.8; P < 0.001] and Alt precues with the crossed mapping [F(1,15) = 18.9; P < 0.001]. As would be expected (in the absence of precognition), this area was close to zero for the LRPs in the six mapping × precue conditions where advance information about response hand was not provided.
Different patterns of experimental effects were found on the S- and R-locked LRP onsets. Let's first consider the S-locked LRPs. With both mappings, S-locked LRP onset was delayed (i.e. the RS-LRP interval was prolonged) in the NI precue condition, but did not differ between the informative precue conditions. Separate ANOVAs were performed for each mapping on LRP onsets from precue conditions without advance hand information (and the associated foreperiod LRP). Significant effects of precue type were found for both the normal [F(2,30) = 9.647; P < 0.001] and crossed mappings [F(2,30) = 23.676; P < 0.001]. The informative precue conditions did not differ significantly (P > 0.05) from one another with either mapping, and the NI precue condition differed significantly from the informative precue conditions with both mappings (normal: P < 0.01 from IO and P < 0.05 from Alt, crossed: P < 0.01 from UD and IO).
Precue type and mapping interacted in their effects on the RS-LRP interval such that: (1) the difference in LRP onset between the NI and informative precue conditions was larger with the crossed than normal mapping, and (2) mapping had a large effect on LRP onset following NI precues, but little effect following informative precues. These findings were confirmed by a one-way ANOVA performed on the six precue conditions (three from each mapping) in which hand was not precued. While the effect of “condition” was significant [F(5,75) = 16.7; P < 0.001], of more relevance are the posthoc pairwise comparisons. There were no significant differences between the four informative precue conditions (two from each mapping), and onset latency was significantly (P < 0.01) longer in the crossed-NI condition than in any of the other five conditions.
We now turn to R-locked LRP onset, i.e. the interval between LRP onset and RT. With the normal mapping, the pattern of precue effects on R-locked LRP onset was the same as for S-locked LRP onset. Precue type again had a significant effect in the normal mapping condition [F(2,30) = 16.76; P < 0.001]. As with the interval between the RS and S-locked LRP onset, the interval between R-locked LRP onset and RT was significantly (P < 0.01) longer in the NI condition than in the other two precue conditions, but did not differ significantly (P > 0.05) between these latter conditions.
The interaction between mapping and precue type on R-locked LRP onset was opposite to that found for S-locked LRP onset. For the S-locked LRP, the difference between the NI and informative conditions was larger with the crossed than normal mapping. This was not the case for the R-locked LRP. Indeed, there was no significant effect at all of precue type with the crossed mapping [F(2,30) = 0.195; P > 0.824]. As with the S-locked LRP, mapping had little effect on R-locked LRP following informative precues. A one-way ANOVA involving all six relevant (i.e. no foreperiod LRP) precue conditions was significant [F(5,75) = 3.296; P < 0.01], but the four informative precue conditions did not differ significantly (P > 0.05). Nor did the crossed-NI condition differ significantly (P > 0.05) from any of the informative precue conditions. The primary cause for the significant ANOVA was the normal-NI condition, which had a significantly (P < 0.05) longer LRP-RT interval than each of the other five conditions.
The complete pattern of precue and mapping effects on the RS-LRP and LRP-RT intervals can be summarized as follows. The length of the RS-LRP interval did not differ significantly between the four (2 × 2) combinations of mapping and (non-hand) informative precue type. It was longer with NI precues in the normal mapping, and longer still with NI precues in the crossed mapping. The length of the LRP-RT interval did not differ significantly between five of the six (2 × 3) combinations of mapping and (non-hand) precue type, but was longer following NI precues in the normal mapping condition. An explanation of this pattern will be proposed in Section 5.3 below.
4.5. Effects of horizontal eye movements on LRP recordings
HEOG recordings are shown at the bottom of each panel in Fig. 5. Each HEOG waveform was recorded in the same precue condition as one of the LRPs above. HEOG was analyzed in exactly the same way as the LRP (see Section 3.2), but is displayed at a scale 25% that of the LRP. This scale equals a conservative estimate (high side) of the propagation coefficient relating HEOG recorded from the outer canthi (eyes) and HEOG recorded from central electrode sites (Hillyard and Galambos, 1970). In other words, the HEOG is scaled to equal its effects on recordings of the LRP. As can be seen in all four panels, these effects were minimal and cannot account for any of the precue or mapping effects on the S- or R-locked LRPs.
5. Discussion
Precuing information about a to-be-signaled movement's parameters produces savings in the time to begin the movement. What part, if any, of these RT savings is due to a shortening of the motor processes that closely precede overt movement? In order to answer this question, a number of studies have examined the effects of precuing on the segment of RT between LRP onset and the start of overt movement. In one such study (Osman et al., 1995), we found an effect of precuing on the LRP-RT interval, but were unable to exclude the possibility that the interval was influenced by the duration of nonmotor processes. Hence, the precue effects on this interval could not be attributed unequivocally to changes in the duration of motor processes. In the present study, we sought to prevent the contribution of nonmotor processes to the LRP-RT interval. We employed a mapping manipulation that involved two arrangements of fingers on response buttons, a normal mapping which replicated our previous study and a crossed mapping designed to remove nonmotor processes from the LRP-RT interval. Besides elucidating further the motoric effects of advance information, the present findings also bear on the nature of response preparation and the use of the LRP in general to fractionate effects of experimental manipulations on RT.
5.1. Precue effects under the normal mapping conditions
One objective of the present study was to replicate findings from Osman et al. (1995), especially precue effects on the LRP-RT interval. Hence, the normal mapping conditions in the present study were identical to the conditions employed by Osman et al. (1995). Results from the normal mapping conditions that replicate those from our previous study are presented below. These results provide a representative picture of what has been observed to date in precuing studies with LRPs, EMG, and/or the P300.
First, the same pattern was found for all latency measures timed with respect to the RS, including RT. Each was shorter with informative than noninformative precues, but did not depend significantly on which pair of fingers was precued. As noted, an effect on P300 latency suggests that at least part of the RT effect was due to changes in the duration of stimulus evaluation processes (Magliero et al., 1984). This is not surprising, since the informative precues each reduced the number of RS alternatives requiring perceptual discrimination from four to two. Changes in the duration of stimulus evaluation could also have contributed to the S-locked LRP and S-locked EMG effects, since slower stimulus evaluation could delay the start of response-related processes.
Second, precuing had little or no effect on the dynamics (amplitude profile over time) of muscle activity at the end of the RT interval. The timing, amplitude, and shape of the R-locked EMG waveforms were virtually identical in all precue conditions. Similar findings were also reported by Mueller-Gethmann et al. (2000). A small effect (6 ms) of precuing on the interval between EMG onset and RT was, however, found by Possamai et al. (2002). These authors conjectured that the difference between their findings and those of Osman et al. and Gethmann et al. might have been due to more sensitive EMG measures or the greater force required to register a response in their study. In any event, under the present conditions, there appears to be little or no effect of precuing on the very latest portion of RT. This, of course, is not incompatible with effects on the duration of motor processes leading up to EMG onset.
Third, advance information about the upcoming response hand produced an LRP during the foreperiod. Similar findings have been reported in numerous studies (e.g. De Jong et al., 1988; Hackley and Miller, 1995; Leuthold et al., 1996; Mueller-Gethmann et al., 2000; Ulrich et al., 1998). This shows that the hand precues activated motor cortex. The similar RTs for all informative precues suggests that they all produced qualitatively similar effects, including activation of motor cortex. If so, one would still expect the absence of a foreperiod LRP following precues that signaled fingers on opposite hands, due to the activation of both cerebral hemispheres. As noted, activation of the motor system by precues during the foreperiod does not necessarily implicate motor processes in the RT effects.
Finally, precuing affected the interval between LRP onset and RT. Precues indicating two fingers on different hands produced shorter LRP-RT intervals than the noninformative precues. Does this imply that changes in the duration of motor processes contributed to the effects on RT? The answer depends on whether the LRP-RT interval was determined solely by the duration of motor processes. As we saw, there are models of the present precuing procedure (e.g. Fig. 1) in which, under normal mapping conditions, nonmotor processes can contribute to the LRP-RT interval. But we also saw that for a large class of models a crossed finger-button mapping would prevent an LRP from developing until all nonmotor processes are complete. Hence, the second objective of the current study: to examine precue effects on the LRP-RT interval under crossed mapping conditions.
5.2. Effects of the mapping manipulation
If not for the LRP findings, one might easily conclude that the mapping manipulation had little effect on information processing in the precuing task. There was a small main effect of mapping on RT (10 ms), but no significant main effects on P300 latency, S-locked EMG onset, or the R-locked EMG measures. Nor did mapping significantly influence precue effects on any of these measures (although there were marginal, P > 0.1, precue × mapping interactions on P300 latency and S-locked EMG onset).
Mapping and precue interacted in their effects on the timing of the LRP. Moreover, they had different and complementary patterns of interaction on the two LRP intervals. The pattern of effects on the LRP-RT interval is shown in the left panel of Fig. 6, and the pattern of effects on the RS-LRP interval is shown in the right panel. In both panels, the LRP intervals are classified according to the mapping (rows) and whether the precue was informative or noninformative (columns). Each cell indicates the relation between the LRP intervals in that condition and the LRP intervals in the condition with the normal mapping and informative precues (upper left cell).
Fig. 6.
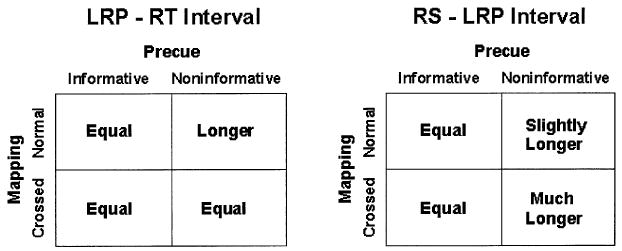
Precue and mapping effects on the LRP-RT (left panel) and RS-LRP (right panel) intervals. LRP intervals are classified in each panel according to mapping (rows) and whether the precue was informative or noninformative (columns). Each cell indicates how the length of the corresponding LRP intervals compares with the length of the intervals obtained with a normal mapping and informative precues (upper left cell).
Let's first consider the pattern of precue and mapping effects on the LRP-RT interval. The LRP-RT intervals are longest in the cell with the normal mapping and noninformative precues and the same length in the remaining three cells. One description of this pattern is that the mapping and precuing manipulations had equal and redundant effects. That is, there were three ways to make the LRP-RT interval shorter than that in the condition with a normal mapping and NI precues: (1) by providing an informative precue, (2) by using a crossed mapping, and (3) both 1 and 2. All three ways shortened the LRP-RT interval by the same amount.
Now, let's turn to the pattern of effects on the RS-LRP interval. These intervals were of the same length for both mappings when the precues were informative, slightly longer with NI precues and the normal mapping, and much longer with NI precues and the crossed mapping. Thus, as with the LRP-RT interval, mapping had no effect on the RS-LRP interval in the informative-precue conditions. Following NI precues, however, mapping produced opposite effects on the two intervals. While the LRP-RT interval was longer with the normal than with the crossed mapping, the RS-LRP interval was longer with the crossed than with the normal mapping.
Returning to the second objective of the study, we see that precues signaling two fingers on different hands affected the length of the LRP-RT interval in the normal mapping condition but not in the crossed mapping condition. Could the absence of a precue effect on the LRP-RT interval in the crossed-mapping condition be due to a lack of nonmotor processes after LRP onset? In other words, does this finding imply that the precue effects on the LRP-RT interval in the normal mapping condition were due solely to changes in the duration of nonmotor processes that occurred after LRP onset? Before attempting to answer this question, let us first consider how a model of the type shown in Fig. 1 might account for the full pattern of precue and mapping effects on the RS-LRP and LRP-RT intervals shown in Fig. 6.
5.3. Interpretation of LRP effects
The entire pattern of precue and mapping effects on the two LRP intervals can be explained by the following scenario, wherein nonmotor processes and initial motor processes (response preparation) each occurred in two serial steps (NM1, M1, NM2, M2) followed by a final motor step (M3). Whether a particular motor (M) or nonmotor (NM) process occurred first or second (1 or 2), and where in the trial individual steps were performed, depended on the particular combination of precue type and mapping condition.
In the absence of advance information (NI conditions), the situation was as described in Fig. 1: (1) NM1 involved determining whether the green star occurred in the top or bottom two positions of the RS array, (2) M1 involved preparing the two fingers resting either on the top or bottom two buttons, (3) NM2 involved narrowing down the position of the green star to a single location, and (4) M2 involved further preparation of whichever finger corresponded to that location. All four steps, plus a final response execution step (M3), occurred after the RS.
With the normal mapping (Fig. 1, top panel), an LRP arose at the start of M1 because the top or bottom two buttons corresponded to fingers on a single hand. With the crossed mapping (bottom panel), the LRP did not arise until the start of M2 because the top or bottom two buttons corresponded to one finger on each hand. It is only during M2 that the fingers on one hand became more prepared than those on the other. Thus, with the normal mapping, NM1 occurred during the RS-LRP interval, while M1, NM2, M2, and M3 occurred during the LRP-RT interval. But, with the crossed mapping, only M2 and M3 occurred during the LRP-RT interval, while NM1, M1, and NM2 occurred during the RS-LRP interval.
Consider now the effects of making the precue informative. An initial nonmotor and motor process (NM1 and M1) could now be performed during the foreperiod, while the remaining nonmotor (NM2) and motor (M2 and M3) processes still had to be performed after the RS. Regardless of mapping, whichever two locations were signaled by the precue were identified (NM1) and the two fingers corresponding to those locations were prepared (M1) in the foreperiod. The RS then provided the information necessary to identify (NM2) and prepare further (M2) one of the two fingers. With an informative precue that did not signal response hand, the LRP arose during M2, as in the case of NI precues and a crossed mapping.
According to the above scenario, the LRP-RT interval should have been the same length for all combinations of precue type and mapping in which the LRP arose during M2 (since it then contained only M2 and M3). This includes all but one of the conditions in which the precue did not signal response hand, the exception being the combination of NI precues and the normal mapping (see Fig. 6, left panel). Likewise, the RS-LRP interval should have been short for all conditions in which the LRP arose during M1 or in which NM1 and M1 occurred during the foreperiod, since it should have then contained only a single nonmotoric step (NM1 or NM2, respectively). This includes all but one condition, the exception being the combination of an NI precue and crossed mapping (see Fig. 6, right panel).
This predicted pattern of effects on the two LRP intervals is exactly the observed pattern shown in Fig. 6, with one exception: The RS-LRP interval is slightly longer in the cell with the normal mapping and NI precues than in the cells with informative precues. According to the proposed scenario, the only difference between the RS-LRP interval in these three cells should be that it contained NM1 following the NI precue with a normal mapping and NM2 following informative precues with both mappings. Perhaps NM1, which involved identifying two among four locations, took longer than NM2, which involved narrowing the two locations down to one.
5.4. Implications for precue effects on RT
Now, back to the question of whether motor processes contributed to the precue effects on RT. Does the absence of a precue effect on the LRP-RT interval in the crossed mapping conditions imply that the precue effects on RT were due entirely to changes in the duration of nonmotor processes? Surprisingly, the larger pattern of findings, in combination with the above scenario, would suggest otherwise. While changes in the duration of late motor processes did not contribute to the RT effects, earlier motor processes were indeed implicated.
Let's first consider which motor processes were unaffected by precuing. These were the motor processes that occurred during the LRP-RT interval with the crossed mapping. Here, the LRP did not arise until step M2, when the sole response finger became the most prepared. Effects on the LRP-RT interval would thus have indicated changes in the duration of processes occurring after the signaled response finger became the most prepared. These latter processes include the final preparation (M2) and execution (M3) of the signaled button press. One might have expected these processes to be expedited by advance information that the executed response was likely to occur (i.e. an increase in probability from 0.25 to 0.5). Such a hypothesis would have been confirmed had we found precue effects on the LRP-RT interval with the crossed mapping. But instead, these late motor processes appear to have been unaffected by advance information.
We did, however, uncover evidence that precues can affect other motor processes occurring during the RT interval. This involves the equivalent and redundant effects of mapping and precuing on the LRP-RT interval (Fig. 6, left panel). Changing from a normal to a crossed mapping in the NI condition shifted LRP onset such that M1 and NM2 moved from the LRP-RT interval to the RS-LRP interval (Fig. 1). An equivalent reduction in the LRP-RT interval from that in the NI normal-mapping condition was obtained by precuing two fingers on different hands. This suggests that the precuing manipulation likewise removed M1 and NM2 from the LRP-RT interval. Moreover, the redundancy involved the lack of a mapping effect on the LRP-RT interval following (nonhand) informative precues. We would not expect this had the LRP-RT interval on these trials still contained M1 under the normal mapping.
In sum, the present findings provide evidence about the effects of advance information on two different sets of motor processes. One set (M2 and M3) occurred after the signaled finger became more prepared than all of the others. These processes involved the final preparation and execution of the signaled button-press, and they occurred during the LRP-RT interval under both mappings. The other set of motor processes (M1) occurred after one pair of fingers became more prepared than the other, but before the signaled finger became the most prepared. These processes occurred during the LRP-RT interval with the normal mapping and during the RS-LRP interval with the crossed mapping. Advance information did not affect the former set of processes, but allowed the latter to be completed during the foreperiod prior to the RS.
5.5. Implications for response preparation
Besides helping to interpret precue effects on RT, the type of model developed here also characterizes several features of response preparation in ways supported by our findings. First, in the absence of advance information, preparation took place in at least two steps. This two-step process, wherein a subset of the possible button-press responses are prepared first and then a subset of those are prepared further, can be contrasted with a single-step process in which only a single finger becomes more prepared than any of the others. In the latter, an LRP would arise only when the sole finger indicated and/or selected by completed nonmotor processes became the most prepared. Were this the case, the mapping manipulation should not have produced the striking reciprocal effects observed on the LRP-RT and RS-LRP intervals. Though this manipulation might still have affected one or both intervals, it should not have shifted the LRP such that part of the preparation process moved from one interval to the other. This, however, would be a natural consequence of a two-step preparation process, wherein mapping determined the step at which the more prepared alternatives favored one hand over the other.
Second, the particular fingers prepared during each step were determined by the spatial location of the fingers, rather than their anatomical identities. In the absence of advance information, the top or bottom two fingers were prepared first, regardless of whether they occurred on the same hand or shared some other anatomical feature. This can be contrasted with always preparing first two fingers that share a particular anatomical feature. For example, if the RS indicated a left index finger response, the two left (or index) fingers might be prepared first regardless of how the fingers were arranged on the buttons. As was the case with single-step preparation, this alternative hypothesis is difficult to reconcile with the reciprocal effect of mapping on the RS-LRP and LRP-RT intervals.
There are at least two possible reasons why, in the absence of advance information, subjects might have prepared first the fingers resting on the top or bottom two buttons. Both assume that perception of RS location involved discrimination of two or more spatial features. One feature indicated whether the RS occurred in the top or bottom half of the array, and the other(s) distinguished between the remaining two possible locations. According to one explanation, these features were discriminated with different latencies, such that the top/bottom feature was always the first available to serve as a basis for response preparation. The other explanation attributes initial preparation of the top or bottom two fingers to S-R translation processes that intervene between perception and response preparation. Translation might have occurred between individual perceptual and response features, with translation between some pairs of features finishing before that of others. Top/bottom half of the array was a very salient perceptual feature, and top/bottom two fingers was a very salient response feature. Perhaps the translation between these two salient features was performed especially quickly (see Proctor and Reeve, 1985).
Finally, the presence of two steps of response preparation suggests that, in the absence of advance information, parts of the RT interval involving response preparation were temporally interleaved with parts involving non-motor processes. That is, the first step of response preparation (M1) began before completion of the non-motor processes (NM2) that provided the basis for further preparation (M2). As mentioned, these non-motor processes could have been perceptual or involved S-R translation. Likewise, they could have occurred in parallel with response preparation (NM2 concurrent with M1) or in strict series (NM1 followed by M1 followed by NM2 followed by M2). Both of these temporal arrangements can be contrasted with one in which motor processes strictly followed non-motor ones (all NM followed by all M). Given this latter arrangement, the RS location would have been identified completely and the signaled response determined uniquely before the start of response preparation. Where this is the case, it is difficult to believe that subjects would have been unable or unwilling to simply prepare the signaled finger in a single step.
5.6. Implications for LRP fractionation of RT
The examination of precue effects on the LRP-RT interval was motivated in part by a more general goal: development of the LRP-RT interval as a tool for studying motor processes in isolation. Under conditions in which this interval can be shown to reflect the duration of motor processes only, experimental effects on the interval can be attributed solely to changes in the duration of motor processes. The LRP-RT interval would then provide a chronometric window on motor processes. An important question raised by the present study is how one can be sure that the LRP-RT interval does indeed reflect the duration of motor processes only.
The interpretation of effects on the LRP-RT interval relies on an important distinction: that between the temporal locus and functional locus of an experimental effect on RT. An effect on the LRP-RT interval provides information about the temporal locus of an effect on RT: it occurs after LRP onset on at least some portion of the trials. We seek to use this information about the temporal locus of an effect to make inferences about its functional locus, i.e. the identity of the affected cognitive process. Unfortunately, such inferences are not always straightforward. For example, it might have seemed self-evident at first that only motor processes would occur between LRP onset and RT. But, as should now be apparent, this belief rests on certain assumptions about the temporal arrangement of motor and nonmotor processes.
Reasoning from the temporal to the functional locus of an effect depends on the temporal arrangement of the component processes that determine RT. This arrangement, in turn, depends on the specific details of an experiment, e.g. the task (Meyer et al., 1985), the stimuli (Miller and Hackley, 1992), and subjects' strategies (Smid et al., 1995). In the present case, we saw how a mapping manipulation, though it barely affected RT, could shift processes between the two LRP intervals. The flexible temporal organization of information processing is a mixed blessing. It certainly complicates the interpretation of effects on the LRP-RT interval, since this interval can reflect the duration of different processes under different conditions. Yet, this flexibility also provides opportunities to select the contents of the LRP-RT interval, e.g. to exclude nonmotor processes or select particular motor processes.
Future research should involve checks on the contents of the LRP-RT interval. One type of check would be to include, along with the experimental manipulation of interest, additional manipulations believed to affect specific nonmotor processes. For example, the clarity of the stimulus or S-R compatibility could be varied to rule out the possibility that the LRP-RT interval reflects, respectively, the durations of perceptual or S-R translation processes (Sanders, 1980). If these latter manipulations were found to affect the RS-LRP interval only, effects of precuing or other manipulations on the LRP-RT interval in the same experiment could be attributed more confidently to changes in the duration of motor processes.
6. Conclusions
Over a decade ago, Mike Coles (1989) predicted that psychophysiological measures would come to play an important role in answering certain questions close to the hearts of cognitive psychologists. Coles believed that both central and autonomic measures would be used increasingly within RT paradigms to identify component cognitive processes, specify their functions, and characterize their organization. His vision has indeed come to pass, in no small part due to his own efforts. Among Coles' major contributions to the synthesis between psychophysiology and mental chronometry is his critical role in the development of the LRP. Coles' accomplishments laid the foundation for a number of new chronometric techniques, including one used in the present study: LRP fractionation of RT effects.
LRP fractionation was used here to reach conclusions about response preparation, the effects of advance information about a to-be-signaled response on RT, and the temporal organization of information-processing. In the absence of advance information, response preparation appears to have involved two steps: first, prepare two of the four possible response alternatives, then prepare further one of these two. It also appears that the pair of responses prepared in the first step depended on the spatial arrangement of the fingers on the buttons, rather than any common anatomical feature. Consequently, the arrangement of fingers on the buttons determined at which step one hand became more prepared than the other, resulting in an LRP. Advance information about the upcoming response allowed the first step to be performed prior to the RS, removing its contribution to RT. The second step, however, was unaffected. These two motor steps were temporally interleaved with perceptual and/or S-R translation processes.
The present study illustrates some of the benefits of and issues surrounding LRP fractionation of RT. The effects of mapping on the organization of response preparation would not have been apparent had we examined RT alone. This manipulation barely affected RT, but instead influenced the point during the RT interval when the LRP first arose. We also saw how the particular processes occurring during the LRP-RT interval depend on experimental conditions and that some of these processes can be non-motoric. It would thus be prudent for future studies using the LRP-RT interval to isolate motoric effects to confirm that the interval does in fact contain motor processes only.
Acknowledgments
Preparation of this article was supported by a grant from the National Institute of Neurological Disorders and Stroke (R01 NS 37528). We thank Monica Fabiani and two anonymous reviewers for their helpful comments.
Footnotes
References
- Coles MGH. Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology. 1989;26:251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- De Jong R, Wierda M, Mulder G, Mulder LJM. The use of partial information in response preparation. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:682–692. doi: 10.1037//0096-1523.14.4.682. [DOI] [PubMed] [Google Scholar]
- De Jong R, Coles MGH, Logan GL, Gratton G. In search of the point of no return: the control of response processes. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:164–182. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Eimer M. The lateralized readiness potential as an on-line measure of central response activiation processes. Behavior Research Methods, Instruments and Computers. 1998;30:146–156. [Google Scholar]
- Gratton G, Coles MGH, Sirevaag EJ, Eriksen CW, Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:331–344. doi: 10.1037//0096-1523.14.3.331. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Miller JO. Response complexity and precue interval effects on the lateralized readiness potential. Psychophysiology. 1995;32:230–241. doi: 10.1111/j.1469-8986.1995.tb02952.x. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclan F. Accessory stimulus effects on response selection: does arousal speed decision making. Journal of Cognitive Neuroscience. 1999;11:321–329. doi: 10.1162/089892999563427. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Galambos R. Eye movement artifacts in the CNV. Electroencephalography and Clinical Neurophysiology. 1970;18:173–182. doi: 10.1016/0013-4694(70)90185-9. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Shibasaki H. Invasive recordings of movement-related cortical potentials in humans. Journal of Clinical Neurophysiology. 1992;90:509–520. doi: 10.1097/00004691-199210000-00005. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Kornhuber H, Deecke L. Hirnpotentialanderungen bei Willkurbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reaffarente Potentiale (Changes in brain potential for voluntary and passive movements in humans: readiness potential and reafferent potential) Pflugers Archiv fur die Gesammte Psychologie. 1965;248:1–17. [PubMed] [Google Scholar]
- LaBerge D, Van Gelder P, Yellot J. A cuing technique in choice reaction time. Perception and Psychophysics. 1970;7:57–62. [Google Scholar]
- Lang W, Cheyne D, Kristeva R, Beisteiner R, Lindinger G, Deeke L. Three-dimensional localization of SMA activity preceding voluntary movement-a study of electric and magnetic fields in a patient with infarction of the right supplementary motor area. Experimental Brain Research. 1991;87:688–695. doi: 10.1007/BF00227095. [DOI] [PubMed] [Google Scholar]
- Leonard JA. Advance information in sensori-motor skill. Quarterly Journal of Experimental Psychology. 1953;5:41–49. [Google Scholar]
- Leuthold H, Sommer W, Ulrich R. Partial advance information and response preparation: Inferences from the lateralized readiness potential. Journal of Experimental Psychology: General. 1996;125:307–323. doi: 10.1037//0096-3445.125.3.307. [DOI] [PubMed] [Google Scholar]
- Lippold OCJ. Electromyography. In: Venables PH, Martin I, editors. A Manual of Psychophysiological Methods. North Holland; Amsterdam: 1967. pp. 249–297. [Google Scholar]
- Magliero A, Bashore TR, Coles MGH, Donchin E. On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology. 1984;21:171–186. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Yantis S, Osman AM, Smith JEK. Temporal properties of human information processing: tests of discrete versus continuous models. Cognitive Psychology. 1985;17:445–518. doi: 10.1016/0010-0285(85)90016-7. [DOI] [PubMed] [Google Scholar]
- Miller JO, Hackley SA. Electrophysiological evidence for temporal overlap among contingent mental processes. Journal of Experimental Psychology: General. 1992;121:195–209. doi: 10.1037//0096-3445.121.2.195. [DOI] [PubMed] [Google Scholar]
- Miller JO, Low K. Motor processes in simple, go/no-go, and choice reaction time tasks: a psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 2001;27:266–289. [PubMed] [Google Scholar]
- Miller J, Ulrich R. Locus of the effect of the number of alternative responses: evidence from the lateralized readiness potential. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1215–1231. [Google Scholar]
- Miller JO, Ulrich R, Rinkenauer G. Effects of stimulus intensity on the lateralized readiness potential. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1454–1471. doi: 10.1037//0096-1523.24.3.915. [DOI] [PubMed] [Google Scholar]
- Mordkoff JT, Miller JO, Roch AC. Absence of coactivation in the motor component: evidence from psychophysiological measures of target detection. Journal of Experimental Psychology: Human Perception and Performance. 1996;22:25–41. doi: 10.1037//0096-1523.22.1.25. [DOI] [PubMed] [Google Scholar]
- Mueller-Gethmann H, Rinkenauer G, Stahl J, Ulrich R. Preparation of response force and movement direction: onset effects on the lateralized readiness potential. Psychophysiology. 2000;37:507–514. [PubMed] [Google Scholar]
- Osman A, Moore CM. The locus of dual-task interference: psychological refractory effects on movement-related brain potentials. Journal of Experimental Psychology: Human Perception and Performance. 1993;19:1–21. doi: 10.1037//0096-1523.19.6.1292. [DOI] [PubMed] [Google Scholar]
- Osman A, Bashore TR, Coles MGH, Donchin E, Meyer DE. On the transmission of partial information: Inferences from movement-related brain potentials. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:217–232. doi: 10.1037//0096-1523.18.1.217. [DOI] [PubMed] [Google Scholar]
- Osman A, Moore CM, Ulrich R. Bisecting RT with lateralized readiness potentials: Precue effects after LRP onset. Acta Psychologica. 1995;90 doi: 10.1016/0001-6918(95)00029-t. in press. [DOI] [PubMed] [Google Scholar]
- Osman A, Lou L, Müller-Gethmann H, Rinkenauer G, Mattes S, Ulrich R. Mechanisms of speed-accuracy tradeoff: evidence from covert motor processes. Biological Psychology. 2000;51:173–199. doi: 10.1016/s0301-0511(99)00045-9. [DOI] [PubMed] [Google Scholar]
- Possamai C, Burle B, Osman A, Hasbroucq T. Partial advance information, number of alternatives, and motor processes: an electromyographic study. Acta Psychologica. 2002;111:125–139. doi: 10.1016/s0001-6918(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Reeve TG. Compatibility effects in the assignment of symbolic stimuli to discrete finger responses. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:623–639. doi: 10.1037//0096-1523.10.4.541. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Reeve TG. Salient-feature coding operations in spatial precuing tasks. Journal of Experimental Psychology: Human Perception and Performance. 1986;12:277–285. [Google Scholar]
- Sanders AF. Stage analysis of reaction processes. In: Stelmach GE, Requin J, editors. Tutorials in Motor Behavior. North-Holland; Amsterdam: 1980. pp. 331–354. [Google Scholar]
- Requin J, Brener J, Ring C. Preparation for action. In: Jennings JR, Coles MGH, editors. Cognitive Psychophysiology: Central and Autonomic Nervous System Approaches. Wiley; Chichester: 1991. pp. 357–448. [Google Scholar]
- Riehle A, Requin J. Monkey primary and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. Journal of Neurophysiology. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- Sommer W, Leuthold H, Schubert T. Multiple bottlenecks in information processing? An electrophysiological examination. Psychonomic Bulletin and Review. 2001;8:81–88. doi: 10.3758/bf03196142. [DOI] [PubMed] [Google Scholar]
- Smid HGOM, Bocker KBE, van Touw DA, Mulder G, Brunia CHM. The selection and use of partial stimulus information in response choice. Acta Psychologica. 1995 doi: 10.1037//0096-1523.22.1.3. [DOI] [PubMed] [Google Scholar]
- Smulders FTY, Kok A, Kenemens JL, Bashore TR. The temporal selectivity of additive factor effects on the reaction process revealed in ERP component latencies. Acta Psychologica. 1995;90:97–109. doi: 10.1016/0001-6918(95)00032-p. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Separate modifiability, mental modules, and the use of pure and composite measures to reveal them. Acta Psychologica. 2001;106:147–246. doi: 10.1016/s0001-6918(00)00045-7. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Leuthold H, Sommer W. Motor programming of response force and movement direction. Psychophysiology. 1998;35:721–728. [PubMed] [Google Scholar]
- Van der Lubbe RHJ, Jaskowski P, Wauschkuhn B, Verleger R. Influence of time pressure in a simple response task, a choice-by-location task, and the Simon task. Journal of Psychophysiology. 2001;15:241–255. [Google Scholar]
- Vaughan H, Costa L, Ritter W. Topography of the human motor potential. Electroencephalography and Clinical Neurophysiology. 1968;25:1–10. doi: 10.1016/0013-4694(68)90080-1. [DOI] [PubMed] [Google Scholar]


