Abstract
Background
Current immunosuppression regimens are highly toxic to transplant recipients and, in many cases, acute rejection episodes occur due to escape of donor-reactive lymphocytes from the immunosuppression. T cells are the mediators of acute, cell-mediated graft damage and are hypothesized to use the CXCR3 chemokine axis for migration into the allograft. In the current study, we investigated the effect of CXCR3 blockade using a non-peptide, small molecule inhibitor, AMG1237845, in murine cardiac allograft survival.
Methods
C57BL/6 (H-2b) mice received vascularized cardiac allografts from A/J (H-2a) donors and were treated with the CXCR3 antagonist. Histological and flow cytometric analyses were used to measure infiltration of leukocytes, and qRT-PCR and IFN-γ ELISPOT assays were used to measure donor-specific reactivity.
Results
CXCR3 antagonism modestly prolonged allograft survival compared to vehicle treatment, but at time-matched intervals post-transplant, neutrophil, CD8+, and CD4+ T cell infiltration was indistinguishable. While proliferation of donor-reactive naïve T cells was unaffected by CXCR3 antagonism, the frequency of IFN-γ-producing cells in the recipient spleen was significantly reduced by AMG1237845 treatment. CXCR3 blockade for 30 days synergized with short-term, low-dose anti-CD154 mAb to prolong survival past 50 days in 75% of grafts and past 80 days in 25% of the cases.
Conclusions
These results indicate that in synergy with co-stimulation blockade, CXCR3 is a viable therapeutic target to prevent acute graft rejection.
Keywords: chemokines, acute rejection, chemokine receptor antagonists, IFN-γ
INTRODUCTION
Solid organ transplantation remains the sole treatment option for patients facing end-stage organ failure. Donor-antigen-reactive CD4 and CD8 T cells are the principal mediators of acute cellular rejection (1, 2). Once donor-reactive effector T cells have been primed in secondary lymphoid organs, they must be recruited to the allograft to mediate the effector functions leading to rejection. This and other laboratories have provided evidence supporting an important role for chemokines in recruiting effector T cells to allografts. In rodent transplant models, elevated mRNA and protein levels of CXCL9/MIG (3, 4), CXCL10/IP-10 (5, 6), and CCL5/RANTES (7) are observed in rejecting skin and cardiac allografts. Importantly, these findings are recapitulated in clinical samples from human allografts where elevated chemokine and chemokine receptor gene and protein expression levels are observed during acute rejection episodes (8-13). Additional studies in rodents using neutralizing antibodies or mice with a targeted deletion in the chemokine gene support findings that the IFN-γ-inducible chemokines CXCL9 and CXCL10 play important roles in acute allograft rejection (6, 14).
Current immunosuppression varies in efficacy between patients, but all protocols carry the burden of risk for infection, malignancy, and renal failure (15, 16). Therapeutics targeted at specific arms of the allogeneic response should provide the greatest potential for minimal side-effects and there has been recent interest in chemokine receptor blockade as a pharmacological mechanism to prevent rejection. An early study indicated the long-term survival (mean survival 58 days) of MHC-mismatched heart allografts in CXCR3−/− recipients, implicating a crucial role for the receptor for CXCL9 and CXCL10 in acute rejection of the allografts (17). This prolonged survival of allografts in CXCR3-deficient recipients was extended to islet, small bowel, and bone marrow allograft models (18-20). The marked decreases in cellular graft infiltration in CXCR3−/− recipients supported proposals that CXCR3 expression on effector T cells is required for donor-reactive T cell infiltration into allografts. Two recent studies, however, indicated little to no prolongation in the survival of MHC-mismatched cardiac allografts in CXCR3−/− recipients, calling into question the importance of CXCR3 in T cell infiltration into allografts (21, 22). In contrast, Uppaluri and colleagues recently generated a non-T cell depleting anti-CXCR3 mAb that results in modest prolongation of MHC-mismatched cardiac and islet allografts when given as monotherapy and, when used in combination with subtherapeutic doses of rapamycin, prolongs allograft survival to greater than 100 days (23). These results suggest that reagents directed at neutralization of CXCR3 function may be efficacious in inhibiting acute rejection of MHC-mismatched allografts.
In the current study we have used an alternative approach in testing whether blockade of CXCR3 would be of benefit in preventing acute rejection of murine cardiac allografts. Previously, we reported the discovery and optimization of a family of quinazolinone-derived, small molecule antagonists of CXCR3 that led to the selection of AMG487 for evaluation in clinical studies (24). An analog of AMG487, hereafter referred to as AMG1237845, demonstrated effective inhibition of 125I-CXCL10 and 125I-CXCL11 binding to CXCR3 on human PBMCs and inhibition of 125I-CXCL10 binding to HEK 293 cells transfected with the mouse CXCR3 receptor. Furthermore, AMG1237845 inhibited CXCL9-, CXCL10-, and CXCL11-induced cellular migration in vitro.
Here, we show that while AMG1237845 treatment prolongs allograft survival, the mechanism does not involve inhibition of donor-reactive effector T cell recruitment into the graft (the hypothesized result). Of important note, CXCR3 antagonism synergizes with short-term, low-dose costimulatory blockade to significantly prolong allograft survival compared to costimulatory blockade alone. Taken together, our results indicate that pharmacological antagonism of CXCR3 with AMG1237845 or one of its congeners may be a potent therapeutic agent to prevent acute allograft rejection.
METHODS
In vitro binding assays
Human peripheral blood mononuclear cells (PBMC) were activated with anti-CD3 mAb and recombinant human IL-2 for 14 days. Cells were co-incubated with AMG1237845 and recombinant human [125I]-CXCL10 (50 pM; PerkinElmer, Waltham MA) or [125I]-CXCL11 (50 pM; Amersham, Piscataway NJ) for 2 hours at room temperature. Cells were harvested onto 96-well filter plates (PerkinElmer) and radioactivity was counted on a scintillation counter.
HEK 293 clones were transfected with the mouse CXCR3 gene and 2×105 mouse CXCR3 HEK 293 cells were incubated with 125I-CXCL10 (GE Life Sciences) at a final concentration of 0.05 nM in the presence of AMG1237845 for 3 h at 4°C in binding buffer (25 mM MEPES, 140 mM NaCl, 1 mM CaCl2, 5 mM MgCl2, and 0.2% bovine serum albumin, pH 7.1). Reactions were aspirated onto PEI-treated GF/B glass filters (PerkinElmer). Plates were counted in a Packard Topcount scintillation counter (PerkinElmer).
In vitro cell migration assays
10 ng/ml of human CXCL9, CXCL10, or CXCL11 resuspended in assay buffer (as above) was added to the lower chamber of a migration plate (5 μm ChemoTX, Neuroprobe). Human PBMC were resuspended in assay buffer and placed in the upper chamber of the migration plate. Plates were incubated at 37°C in a humid chamber (5% CO2) for 3 hours, then the upper chamber was removed and the number of cells in the lower chamber was quantified.
Mice
C57BL/6 (H-2b; Kb, IAb, Db) and A/J (H-2a; Kk, IAk, IEk, Dd, Ld) mice (Charles River Laboratories, Wilmington MA), B6.Thy1.1 and DBA/1 (H-2q) mice (The Jackson Laboratory, Bar Harbor ME), and B6.2C TCR transgenic mice (bred at our facility) were used. All experiments used 8-12 week-old male mice, and all animal use procedures were approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Cardiac transplantation and graft harvest
The methods of Corry and coworkers (25) were used for intra-abdominal heterotopic cardiac transplantation. Total operative times averaged 45-50 minutes. Graft survival was monitored by daily abdominal palpation with cessation of beating confirmed by laparotomy. Unless otherwise noted, all transplants were from A/J donors to C57BL/6 recipients. At the time of graft harvest, 10 mL of Ringer's solution was used to flush the recipient circulatory system. Graft pieces and recipient spleens were then removed and placed in digestion media or were snap-frozen in liquid nitrogen.
AMG1237845 and in vivo antibody treatments
Preliminary testing determined that treating cardiac graft recipients with 32 mg/kg AMG1237845 subcutaneously, twice daily until rejection or until day 30 post-transplant generates serum drug levels that are comparable to in vitro IC50 values. In all experiments, serum samples from treated mice were collected to confirm therapeutic drug levels, and control groups were treated with equivalent volumes of vehicle (15% hydroxypropyl-beta-cyclodextrin). In some experiments, groups of allograft recipients were treated with anti-CD40L mAb (MR1), 0.4 mg i.p. on days 0 and +1 (Bio Express, West Lebanon NH) or with a CD8 depletion cocktail (1:1 mixture of anti-CD8 mAbs YTS169 and TB-150), 0.2mg i.p. on days −3, −2, −1, +4, and every 4 days until rejection.
Immunohistochemistry
At the time of harvest, mid-ventricular transverse sections of graft tissue were snap frozen in OCT compound and 6-8 μm thick sections were prepared. Graft sections were stained with anti-CD4 (GK1.5), anti-CD8 (53-6.7), or anti-Gr-1 (RB6.8C5) primary mAbs (BD Biosciences, San Jose CA) and biotinylated secondary Ab. Positive staining was detected with SA-HRP and DAB development substrate (Vector Labs, Burlingame CA). Antibody-stained sections were counter-stained with eosin (Fisher Scientific, Pittsburgh PA).
Flow cytometry
Detection of graft-infiltrating leukocytes was carried out using a modified method of that published by Afanasyev and colleagues (26). Graft tissue was weighed following harvest and incubated at 37°C in RPMI plus Type II collagenase (Sigma, St. Louis MO). Following incubation, graft tissue was crushed and passed through a 40 μm filter, washed with RPMI, and cells were counted using a hemacytometer. Surface markers were stained using standard methods and commercially available antibodies (eBioscience, San Diego CA and BD Biosciences, San Jose CA).
IFN-γ ELISPOT
Responder CD8 T cells were column-purified (R&D Systems, Minneapolis MN) from recipient spleens, and self, donor, and third-party stimulator splenocytes were depleted of T cells using magnetic beads (Invitrogen, Carlsbad CA). Stimulator and responder cell populations were co-cultured in serum-free HL-1 media for 24 hours at 37°C in 96-well plates coated with anti-IFN-γ capture Ab (R4-6A2, BD Biosciences, San Jose CA). Cells were then washed from the plate, and biotinylated anti-IFN-γ detecting Ab (XMG1.2, BD Biosciences, San Jose CA) was added followed by anti-biotin alkaline phosphatase. Following development, total spots per well were quantified using an ImmunoSpot Series 2 Analyzer (Cellular Technology Ltd., Shaker Heights OH).
RNA Purification and qRT-PCR
Graft tissue was snap-frozen in liquid nitrogen at the time of harvest. Graft pieces were crushed, homogenized using Qiashredders (Qiagen, Valencia CA), and RNA was isolated using Fibrous Tissue Kits (Qiagen). Commercially available reagents and probes were used for reverse transcription, and real-time PCR was performed on a 7500 Fast Real-Time thermocycler, all from Applied Biosystems (Foster City CA).
Statistics
Data analysis was performed using GraphPad Prism Pro (GraphPad Software Inc, San Diego CA). Kruskal-Wallis and Mann-Whitney U testing was used to determine significance throughout. Kaplan-Meyer survival curves were generated and log-rank testing was used to determine significance with p < 0.05 being considered significant.
RESULTS
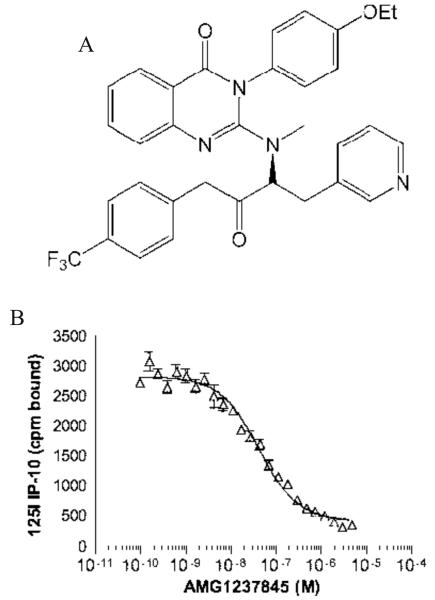
AMG1237845 inhibits CXCR3 ligand binding in vitro
We previously reported that AMG1237845 (structure in Figure 1A) is a potent inhibitor of 125I-CXCL10 binding to CXCR3 on human PBMCs (IC50 = 6.2 ± 3.6 nM) (24). In the current study, further evaluation of this compound demonstrated that it is also a potent inhibitor of 125I-CXCL11 binding to PBMCs (IC50 = 6.8 ± 5.3 nM). Evaluation of AMG1237845 in a cell migration assay demonstrated that the compound is an effective inhibitor of CXCL9 (IC50 = 25 nM), CXCL10 (IC50 = 13 nM), and CXCL11 (IC50 = 12 ± 15 nM) induced cellular migration of human PBMCs in vitro. AMG1237845 was evaluated for its selectivity against CCR5, CXCR1, and CXCR2 and was found to have IC50 > 10 μM against these receptors.
Figure 1.
A. Structure of AMG1237845. B. AMG1237845 inhibits binding of 125I-CXCL10 to the mouse receptor. HEK 293 cells were transfected with the mouse CXCR3 gene. Transfected cells expressing the mouse CXCR3 gene were cultured with AMG1237845 and 125I-CXCL10 and binding was measured with a scintillation counter. AMG1237845 inhibits binding of CXCL10 to the mouse CXCR3 receptor with an IC50 = 53 ± 7 nM. Each concentration of compound was tested in quadruplicate and data were analyzed and plotted using GraphPad Prism.
The sequence of the mouse CXCR3 receptor shares significant homology to the human receptor, and CXCL9, CXCL10, and CXCL11 interact with human and mouse CXCR3 in a similar manner. To test the ability of AMG1237845 to inhibit specific binding of human 125I-CXCL10 to the mouse receptor, HEK 293 cells transfected with mouse CXCR3 were tested in vitro. AMG1237845 inhibited binding of 125I-CXCL10 to the mouse receptor with an IC50 = 53 ± 7 nM (Figure 1B).
CXCR3 blockade prolongs allograft survival
To determine whether CXCR3 antagonism with the small-molecule inhibitor AMG1237845 could prolong allograft survival, groups of C57BL/6 mice received complete MHC-mismatched A/J heart grafts and were treated with AMG1237845 or an equivalent volume of vehicle. Whereas vehicle-treated controls rejected the grafts between days 5-9 post-transplant, treatment with AMG1237845 significantly prolonged allograft survival up to day 16 (p < 0.01, Figure 2A).
Figure 2.

CXCR3 blockade with AMG1237845 prolongs allograft survival but does not affect infiltration of leukocytes. A. Groups of allografts were untreated, were treated with vehicle, or were treated with AMG1237845, as described (n = 5-7/group). CXCR3 blockade significantly prolongs graft survival to median survival of 12.5 days compared to vehicle-treated controls (MST = 8 days, p < 0.01). B. Groups of allograft recipients were treated with AMG1237845, anti-CD8 depleting cocktail, or both as described (n = 5-6/group). CD8 T cell depletion abolishes the graft-protective effect of CXCR3 antagonism. C-E. Grafts were harvested on day 7 post-transplant from vehicle (upper panels) or AMG1237845 (lower panels) treated mice. Hematoxylin and eosin sections (C) demonstrate intense lymphocytic infiltrate in both groups coincident with signs of myocyte damage and graft rejection. Neutrophil (D), CD8+ T cell (E), and CD4+ T cell (F) infiltration appears qualitatively identical regardless of treatment (images representative of 4 sections each from 6 grafts/group, 200 × magnification). G. Grafts were harvested on day 7 post-transplant from vehicle- or AMG1237845-treated groups (n = 6-8/group). Flow cytometry was used to quantify numbers of graft-infiltrating neutrophils, CD8+ T cells, and CD4+ T cells. Regardless of treatment, the numbers infiltrating cells did not differ significantly at the 95% confidence interval.
Since CXCR3 is expressed on CD4 and CD8 T cells (27-29), the effect of CXCR3 antagonism on graft survival following CD8 depletion was directly tested. Groups of allograft recipients were treated with AMG1237845 alone, with depleting anti-CD8 mAb cocktail alone, or with AMG1237845 plus CD8 depletion. AMG1237845 treated recipients rejected grafts within 16 days, but depletion of CD8 T cells abolished this prolongation and grafts rejected within 9 days (MST = 8 days, p < 0.001; Figure 2B). These results suggest that CXCR3 antagonism with AMG1237845 prolongs allograft survival through a CD8-dependent mechanism.
CXCR3 antagonism does not inhibit leukocyte infiltration into cardiac allografts
To begin to understand how CXCR3 blockade with AMG1237845 prolongs allograft survival, leukocyte infiltration at day 7 post-transplant was assessed. Immunohistochemical staining of frozen graft sections indicated no clear difference in neutrophil (Figure 2D), CD8+ T cell (Figure 2E), or CD4+ T cell (Figure 2F) infiltration between AMG1237845-treated and vehicle-treated groups.
To quantify this result, grafts were harvested from vehicle- or AMG1237845-treated recipients at day 7 post-transplant and infiltrating cells were subjected to flow cytometry analysis. Regardless of treatment, neutrophil, CD8+ T cell, and CD4+ T cell infiltration did not differ significantly at day 7 post-transplant (Figure 2G). Thus, while CXCR3 antagonism with AMG1237845 prolonged allograft survival, it did not prevent infiltration of CD4+ or CD8+ T cells or neutrophils into the allografts.
Ex vivo-primed effector cells infiltrate allografts despite blockade of CXCR3 with AMG1237845
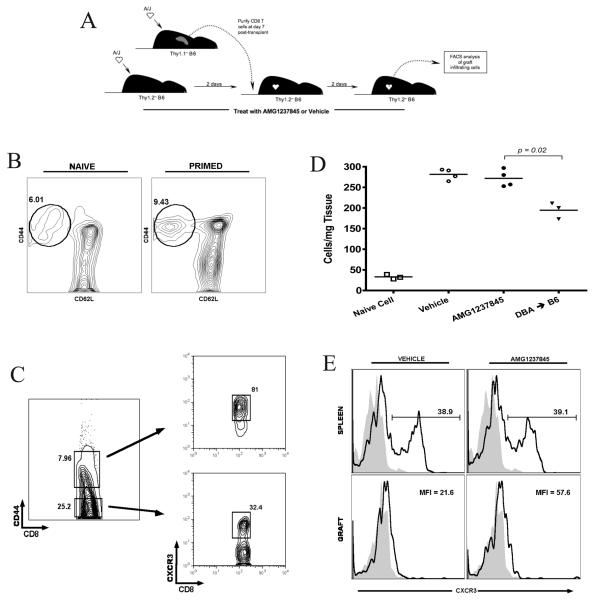
To directly assess the effect of CXCR3 blockade on donor-reactive effector CD8+ T cell recruitment to allograft tissue from any possible effects at the site of priming, an adoptive transfer strategy was developed. CD8+ T cells were purified from Thy1.1+ B6 recipients of A/J heart grafts on day 7 post-transplant (Figure 3A). Flow cytometry analysis of these primed CD8 T cells indicated an increase in cells expressing markers consistent with activation, CD44hiCD62Llo, compared to CD8+ T cells from unmanipulated mice (Figure 3B). As expected, CD8+CD44hi (activated/primed) T cells expressed high levels of CXCR3 when compared to naïve CD8+CD44lo T cells (Figure 3C).
Figure 3.
CXCR3 antagonism does not inhibit infiltration of primed effector CD8 T cells into cardiac allografts. A. B6.Thy1.1 mice received A/J heart grafts and primed CD8 effectors were purified from the spleens on day 7 post-transplant. 5×106 of these Thy1.1+ effectors were transferred to B6.Thy1.2 recipients of A/J heart grafts on day +2. B6.Thy1.2 recipients were treated with vehicle or AMG1237845, grafts were harvested on day +4, and infiltrating cells were subjected to flow cytometry analysis. B. Surface marker expression analysis by flow cytometry of naïve and primed Thy1.1+ cells. C. CXCR3 expression on primed effector (CD44hi) and naïve (CD44lo) CD8+ T cells. D. Quantification of infiltrating Thy1.1+CD8+ T cells in vehicle- and AMG1237845-treated B6 recipients of A/J grafts, B6 recipients of DBA grafts, and naïve Thy1.1 infiltration in B6 recipients of A/J grafts (n = 3-4/group). E. Surface expression of CXCR3 on transferred effectors in recipient spleen and in the grafts at the time of harvest (representative of 4/group;  Isotype,
Isotype,  CXCR3).
CXCR3).
The A/J-primed Thy1.1+CD8+ T cells were transferred to A/J allograft recipients on day 2 post-transplant and the recipients were treated with vehicle or AMG1237845. Grafts were harvested 2 days later and graft infiltrating cells expressing Thy1.1 were quantified. CXCR3 antagonism did not prevent infiltration of the exogenously primed CD8+ T cells (Figure 3D). These A/J-primed cells did not infiltrate third-party DBA allografts as well as they did A/J grafts, demonstrating that this infiltration was specific for A/J allografts. When naïve Thy1.1+ cells were transferred to A/J allograft recipients over the same time course, they did not infiltrate the graft parenchyma in significant numbers, indicating that this protocol did not allow priming of the transferred cells.
Finally, treatment with AMG1237845 did not alter the surface expression of CXCR3 on the transferred cells (Figure 3E). In the recipient spleen, CXCR3 expression on transferred Thy1.1+ cells remained high, near the level of receptor expression at the time of transfer. In the graft, CXCR3 expression on the transferred cells remained low, regardless of treatment.
CXCR3 antagonism does not alter the proliferative capacity of donor-reactive CD8+ T cells
To test alternative mechanisms underlying prolonged allograft survival in the face of CXCR3 antagonism with AMG1237845, the in vivo proliferation of donor-specific CD8+ T cells was measured. Ld-reactive, 2C CD8 T cells were labeled with CFSE and were transferred to C57BL/6 mice which then received an Ld-expressing A/J cardiac allograft one day later. On day 5 post-transplant, grafts and recipient spleens were retrieved and proliferation of A/J-reactive 2C T cells was measured by flow cytometry. In recipient spleens, the number of rounds of proliferation of donor-reactive, 2C CD8 T cells was notably depressed with CXCR3 antagonism compared to vehicle-treated controls although the overall percent of divided 2C cells was not significantly different between groups. This was not recapitulated in the grafts as 2C T cells infiltrating the allograft expressed equivalent dilutions of CFSE and an equal percent of 2C cells had divided (Figure 4).
Figure 4.
AMG1237845 does not alter allograft-driven CD8 T cell proliferation. Ld-reactive 2C TCR transgenic CD8 T cells (B6 background) were labeled with CFSE and 8×106 were adoptively transferred to allograft recipients on day -1. Mice received Ld-expressing A/J heart grafts and were treated with vehicle or AMG1237845 until grafts and spleens were harvested on day +5. CFSE dilution was monitored by flow cytometry. Flow plots are representative of 5-6 mice per group. The percent of splenic and graft-infiltrating 2C T cells dividing is shown and is not statistically different between the two treatment groups at the 95% confidence interval.
Generation of IFN-γ-producing CD8 T cells is depressed by CXCR3 antagonism
In response to cardiac allografts, a large proportion of the donor-reactive T cell repertoire develops to IFN-γ-producing cells. To determine if CXCR3 antagonism alters this development, ELISPOT was used to determine the frequency of donor-reactive CD8 T cells producing IFN-γ in the spleens of allograft recipients treated with vehicle or AMG1237845. On day 7 post-transplant, the peak of T cell priming in the spleen, the frequency of IFN-γ-producing donor-reactive CD8+ T cells was significantly lower in the group treated with AMG1237845 as compared to vehicle-treated controls (Figure 5A). Thus, while CXCR3 blockade did not inhibit effector cell recruitment to the graft, AMG1237845 treatment significantly reduced the frequency of IFN-γ-producing CD8+ T effector cells that were generated in response to the cardiac allograft.
Figure 5.
CXCR3 blockade decreases the number of donor-reactive, IFN-γ-producing CD8+ T cells in the recipient spleen but does not alter IFN-γ expression in the graft. A. CD8+ T cells from naïve B6 mice, vehicle-, and AMG1237845-treated allograft recipient mice were purified on day 7 post-transplant (n = 4/group) and were subjected to IFN-γ ELISPOT. Representative data from one of three repeated experiments is shown. B. mRNA was purified from isografts, vehicle-, and AMG1237845-treated allografts on day 7 post-transplant. Quantitative RT-PCR results for IFN-γ and the IFN-γ-inducible chemokine CXCL9 were normalized to isograft expression levels. There was no significant difference in expression of either gene between vehicle-treated and AMG1237845-treated allograft groups at the 95% confidence interval.
To determine how this depression in priming to an IFN-γ-producing phenotype corresponds to effector function in the graft parenchyma, qRT-PCR analysis of RNA isolated from graft tissue on day 7 post-transplant was performed. IFN-γ mRNA levels did not significantly differ between vehicle- and AMG1237845-treated allograft groups (Figure 5B). Expression of an IFN-γ-inducible gene, CXCL9, also did not differ between treatment groups. Moreover, intragraft expression levels of other effector molecules (IL-17, perforin, and granzyme B) did not significantly differ between vehicle- and AMG1237845-treated groups (data not shown).
Sub-therapeutic costimulatory blockade synergizes with CXCR3 antagonism to prolong allograft survival
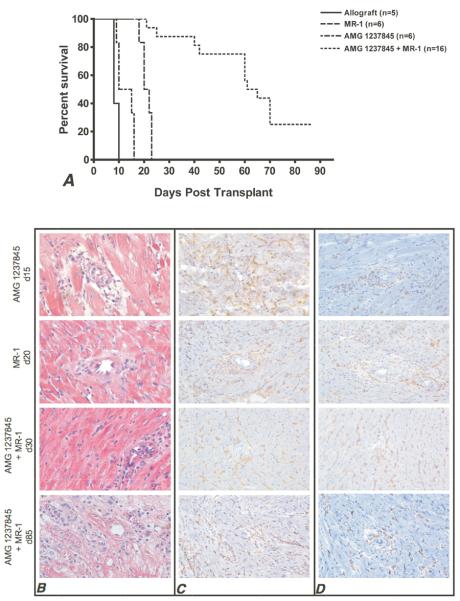
Finally, the potential synergy of CXCR3 antagonism with low-dose costimulatory blockade was investigated. Low-dose anti-CD154 mAb, MR1, given alone on days 0 and +1 prolonged allograft survival greater than AMG1237845 given alone (MST = 21 days versus 12.5 days, respectively, Figure 6A). When short-term anti-CD154 mAb was used in combination with AMG1237845 given until day 30 post-transplant, 75% of allografts survived past 50 days and approximately 25% survived past 80 days.
Figure 6.
Sub-therapeutic costimulatory blockade synergizes with AMG1237845 to significantly prolong allograft survival. A. Allografts (n ≥ 5/group) were treated with MR1 (0.4 mg i.p., days 0 and +1), AMG1237845 (800 μg s.c., bid, until rejection or day +30), or MR1 plus AMG1237845. Combined therapy prolongs allograft survival to median 63 days with 25% of grafts surviving past 80 days. B-D. Histological analysis of graft tissue harvested at the noted times post-transplant is shown. H and E (B), CD8+ T cells (C), and CD4+ T cells (D) were stained and imaged at 200 ×. Images are representative of 4 sections each from 6 grafts per group.
Histological analysis of these grafts (Figure 6B-D) demonstrated that CD4+ and CD8+ T cell infiltration as well as graft tissue necrosis was nearly identical between single-therapy groups at the time of rejection (AMG1237845 at day 15 post-transplant and MR1 at day 20 post-transplant). Conversely, grafts recovered at day 30 post-transplant in the dual-treatment group showed intact cardiac architecture with decreased numbers of CD8+ T cells and similar numbers of CD4+ T cells when compared to single-treatment groups. Graft sections taken at day 85 post-transplant in the dual-treatment group showed marked tissue necrosis and T cell infiltration.
DISCUSSION
Even in the face of significant immunosuppression, many transplant recipients suffer episodes of clinically identified acute rejection as a result of effector T cell escape from immunosuppression. These donor-reactive T cells traffic to the graft parenchyma and express the effector functions that mediate tissue damage. Given the adverse effects of currently used non-specific immunosuppressive therapies, there is a clear role for pharmacological agents that will specifically inhibit donor-reactive T cell functions. Based on elevated expression of the CXCR3-binding chemokines in clinical acute rejection samples and results from murine models of transplantation where this chemokine axis was genetically manipulated, a prevailing hypothesis in the field is that CXCR3 and its ligands are instrumental in recruiting effector T cells to the graft (3-7, 9-13, 30).
We tested the efficacy of AMG1237845, a non-protein, small-molecule antagonist of the chemokine receptor CXCR3 in acute allograft rejection using a murine model of vascularized cardiac transplantation. We previously demonstrated that antagonism of CXCR3 in vivo with analogs of AMG1237845 inhibits cellular recruitment to the lung in a mouse bleomycin model (24) and breast cancer cell metastasis to the lung (31). Thus, small-molecule antagonism of CXCR3 is proving efficacious in preclinical models of other diseases, making this mechanism an attractive target in the transplant setting.
Based on published work, we anticipated that AMG1237845 would prolong allograft survival principally by blocking primed, donor-reactive effector recruitment to the allograft. Treatment of allograft recipients with AMG1237845 prolonged allograft survival compared to vehicle-treated controls, but there was no difference in CD8 or CD4 T cell infiltration into the grafts between groups. CXCR3 blockade also did not prevent infiltration of exogenously primed effector T cells in adoptive transfer experiments. These data demonstrate that blockade of CXCR3 with AMG1237845 in wild-type allograft recipients does not measurably inhibit leukocyte infiltration. This finding is supported by other groups using gene deletion mutants as allograft recipients (32) and by treating recipients with blocking anti-CXCR3 mAb or other small-molecule inhibitors (22, 23). Recent unpublished work in our group suggests that the CXCR3-binding chemokine CXCL9/MIG may not function solely as a recruitment factor for graft-infiltrating donor-reactive T cells, and evidence from this study (Figure 2) supports this implication.
While the mechanism for prolonged graft survival by AMG1237845 remains unclear, several factors are evident. IFN-γ has been shown by others to be dichotomous in its roles in transplantation, being both a protective and pro-rejection factor (33, 34). IFN-γ production by splenic effector and graft infiltrating T cells is commonly used as a marker of effector priming to alloantigen, and we show that CXCR3 antagonism in cardiac allograft recipients depresses the frequency of donor-specific IFN-γ-producing CD8 T cells in the recipient spleen. Growing evidence using in vitro models and human allergy models indicate that a feedback loop exists between CXCR3 and the TCR, resulting in a skewed T cell effector response promoting IFN-γ production upon CXCR3 ligation (35, 36). Depressed IFN-γ, however, is not apparent in the graft parenchyma itself. We postulate that if CXCR3 ligation is necessary for optimal development of IFN-γ-producing effector T cells, CXCR3 blockade at the site of priming would depress the IFN-γ response. Those effectors that do develop to an IFN-γ-producing phenotype would be unaffected in the graft parenchyma as CXCR3 is not required for expression of effector function after the initial priming event.
Several groups have shown that combined blockade of CXCR3 and CCR5 with a single small molecule antagonist (37) or with specific monoclonal antibodies (38) indefinitely prolongs cardiac allograft survival. In light of our recent findings, we propose that CXCR3 antagonism alone modestly prolongs allograft survival via inhibiting donor-reactive CD8 T cell development to an IFN-γ-producing phenotype and that graft infiltration is not measurably affected by CXCR3 antagonism alone. However, combined blockade of CXCR3 and CCR5 potentially prolongs allograft survival as a result of depressed priming and impaired infiltration as there would be no significant compensatory recruitment pathways available to effector CD8 T cells with combined antagonism.
Other mechanisms responsible for prolonged graft survival in our model do not appear to be significant. Plasma cells respond to CXCL9 production by synovial fibroblasts, and CXCR3 expression is required for memory B cell differentiation into plasma cells (39-41). In the current study, donor-specific serum antibody (IgG and IgM) titers were not significantly different between AMG1237845 and vehicle-treated groups at day 7 or 15 post-transplant (data not shown). Additionally, when allograft recipients treated with AMG1237845 were depleted of CD8 T cells using anti-CD8 mAb cocktail, the prolongation of allograft survival was ablated (Figure 2B), suggesting that AMG1237845 prolongs allograft survival via targeting the CD8 T cell response.
Of significant note in this study was the fact that CXCR3 antagonism synergizes with short-term, low-dose costimulatory blockade. In our studies, although many grafts survive long-term, there is a late rejection period around 60 days post-transplant when several grafts cease to function. While we have been unable to elucidate a definitive mechanism for this late rejection, it is clear that grafts reject after cessation of AMG1237845 treatment. When given as monotherapy, low-dose costimulatory blockade prolongs allograft survival to approximately 21 days, thus combined treatment with AMG1237845 is at least additive and is possibly synergistic. The fact that CXCR3 antagonism with AMG1237845 apparently functions primarily though CD8-dependent pathways is supported by the synergism with anti-CD40L mAb. While CD40-CD40L blockade would principally inhibit activation of CD4 T cells and prevent their ability to provide help to generate donor-reactive CD8 T cells, the addition of AMG1237845 directly inhibits the CD8 T cell response through inhibition of priming to an IFN-γ-producing effector cell.
Recently published evidence by several independent groups clearly demonstrates that cardiac allograft recipients genetically deficient in CXCR3 do not have reduced risks for acute rejection as compared to wild-type controls (21-23). However, in agreement with our data, if CXCR3 was blocked with specific mAbs or with a small-molecule inhibitor, graft survival was modestly prolonged. Most promising is that CXCR3 blockade synergizes with low doses of other immunosuppressive agents: cyclosporine A (21), rapamycin (23), and anti-CD40L in our hands. In fact, our data with both monotherapy and combined CXCR3 antagonism with co-stimulation blockade closely matches the findings of Uppaluri and colleages who used a CXCR3 blocking mAb (23). However, when cost of production and the potential of the treated recipient to generate anti-mAb antibodies are taken into consideration, use of a pharmacological CXCR3 antagonist may be a more attractive strategy for clinical implementation. Although the exact mechanism by which CXCR3 blockade attenuates allograft rejection remains elusive, our results and those of other groups using a dual therapy approach warrant further investigation.
Taken together, the findings from this study indicate that targeting the CXCR3 receptor may be a promising component of efficacious strategies to prevent acute allograft rejection; however, the mechanism for prolonged survival is not as anticipated. While chemokines and chemokine receptors certainly play a role in recruiting primed effectors to the graft parenchyma, we demonstrate that CXCR3 function is not an absolute requirement. Thus, future anti-rejection strategies should take into account the implication that chemokines are important factors in the priming process and their blockade may not directly inhibit early infiltration of effector T cells into allograft tissue.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Austin Schenk for his critical review of this manuscript.
Abbreviations
- B6
C57BL/6 mice
- IFN-γ
interferon gamma
- mAb
monoclonal antibody
- MST
median survival time
Footnotes
This work was supported by an Amgen sponsored research agreement and an NIH grant RO1 A151620 (R.F.); NIH grant T32 GM07250 and the Case Medical Scientist Training Program (J.R.).
REFERENCES
- 1.Hall BM, Dorsch SE. Cells mediating allograft rejection. Immunol Rev. 1984;77:31. doi: 10.1111/j.1600-065x.1984.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 2.Hayry P, von Willebrand E, Parthenais E, et al. The inflammatory mechanisms of allograft rejection. Immunol Rev. 1984;77:85. doi: 10.1111/j.1600-065x.1984.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 3.Koga S, Auerbach MB, Engeman TM, Novick AC, Toma H, Fairchild RL. T cell infiltration into class II MHC-disparate allografts and acute rejection is dependent on the IFN-gamma-induced chemokine Mig. J Immunol. 1999;163(9):4878. [PubMed] [Google Scholar]
- 4.Miura M, Morita K, Kobayashi H, et al. Monokine induced by IFN-gamma is a dominant factor directing T cells into murine cardiac allografts during acute rejection. J Immunol. 2001;167(6):3494. doi: 10.4049/jimmunol.167.6.3494. [DOI] [PubMed] [Google Scholar]
- 5.Agostini C, Calabrese F, Rea F, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. Am J Pathol. 2001;158(5):1703. doi: 10.1016/S0002-9440(10)64126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193(8):975. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sawy T, Fahmy NM, Fairchild RL. Chemokines: directing leukocyte infiltration into allografts. Current Opinion in Immunology. 2002;(14):562. doi: 10.1016/s0952-7915(02)00382-5. [DOI] [PubMed] [Google Scholar]
- 8.Hancock WW. Chemokine receptor-dependent alloresponses. Immunol Rev. 2003;196:37. doi: 10.1046/j.1600-065x.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 9.Fahmy NM, Yamani MH, Starling RC, et al. Chemokine and receptor-gene expression during early and late acute rejection episodes in human cardiac allografts. Transplantation. 2003;75(12):2044. doi: 10.1097/01.TP.0000069601.73079.94. [DOI] [PubMed] [Google Scholar]
- 10.Fahmy NM, Yamani MH, Starling RC, et al. Chemokine and chemokine receptor gene expression indicates acute rejection of human cardiac transplants. Transplantation. 2003;75(1):72. doi: 10.1097/00007890-200301150-00013. [DOI] [PubMed] [Google Scholar]
- 11.Fairchild RL, VanBuskirk AM, Kondo T, Wakely ME, Orosz CG. Expression of chemokine genes during rejection and long-term acceptance of cardiac allografts. Transplantation. 1997;63(12):1807. doi: 10.1097/00007890-199706270-00018. [DOI] [PubMed] [Google Scholar]
- 12.Kondo T, Novick AC, Toma H, Fairchild RL. Induction of chemokine gene expression during allogeneic skin graft rejection. Transplantation. 1996;61(12):1750. doi: 10.1097/00007890-199606270-00015. [DOI] [PubMed] [Google Scholar]
- 13.Melter M, Exeni A, Reinders ME, et al. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104(21):2558. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 14.Nozaki T, Amano H, Bickerstaff A, et al. Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients. J Immunol. 2007;179(8):5238. doi: 10.4049/jimmunol.179.8.5238. [DOI] [PubMed] [Google Scholar]
- 15.Ajithkumar TV, Parkinson CA, Butler A, Hatcher HM. Management of solid tumours in organ-transplant recipients. Lancet Oncol. 2007;8(10):921. doi: 10.1016/S1470-2045(07)70315-7. [DOI] [PubMed] [Google Scholar]
- 16.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357(25):2601. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 17.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192(10):1515. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker MS, Chen X, Rotramel AR, et al. Genetic deletion of chemokine receptor CXCR3 or antibody blockade of its ligand IP-10 modulates posttransplantation graft-site lymphocytic infiltrates and prolongs functional graft survival in pancreatic islet allograft recipients. Surgery. 2003;134(2):126. doi: 10.1067/msy.2003.213. [DOI] [PubMed] [Google Scholar]
- 19.Duffner U, Lu B, Hildebrandt GC, et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp Hematol. 2003;31(10):897. doi: 10.1016/s0301-472x(03)00198-x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Kaptanoglu L, Tang Y, et al. IP-10-induced recruitment of CXCR3 host T cells is required for small bowel allograft rejection. Gastroenterology. 2004;126(3):809. doi: 10.1053/j.gastro.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Kwun J, Hazinedaroglu SM, Schadde E, et al. Unaltered graft survival and intragraft lymphocytes infiltration in the cardiac allograft of Cxcr3−/− mouse recipients. Am J Transplant. 2008;8(8):1593. doi: 10.1111/j.1600-6143.2008.02250.x. [DOI] [PubMed] [Google Scholar]
- 22.Zerwes HG, Li J, Kovarik J, et al. The chemokine receptor Cxcr3 is not essential for acute cardiac allograft rejection in mice and rats. Am J Transplant. 2008;8(8):1604. doi: 10.1111/j.1600-6143.2008.02309.x. [DOI] [PubMed] [Google Scholar]
- 23.Uppaluri R, Sheehan KC, Wang L, et al. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation. 2008;86(1):137. doi: 10.1097/TP.0b013e31817b8e4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson M, Li AR, Liu J, et al. Discovery and optimization of a series of quinazolinone-derived antagonists of CXCR3. Bioorg Med Chem Lett. 2007;17(12):3339. doi: 10.1016/j.bmcl.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 25.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16(4):343. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Afanasyeva M, Georgakopoulos D, Belardi DF, et al. Quantitative analysis of myocardial inflammation by flow cytometry in murine autoimmune myocarditis: correlation with cardiac function. Am J Pathol. 2004;164(3):807. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Curr Top Dev Biol. 2005;68:149. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- 28.Lu B, Humbles A, Bota D, et al. Structure and function of the murine chemokine receptor CXCR3. Eur J Immunol. 1999;29(11):3804. doi: 10.1002/(SICI)1521-4141(199911)29:11<3804::AID-IMMU3804>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101(4):746. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168(7):3195. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 31.Walser TC, Rifat S, Ma X, et al. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66(15):7701. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 32.Kwun J, Hu H, Schadde E, et al. Altered distribution of H60 minor H antigen-specific CD8 T cells and attenuated chronic vasculopathy in minor histocompatibility antigen mismatched heart transplantation in Cxcr3−/− mouse recipients. J Immunol. 2007;179(12):8016. doi: 10.4049/jimmunol.179.12.8016. [DOI] [PubMed] [Google Scholar]
- 33.Hidalgo LG, Halloran PF. Role of IFN-gamma in allograft rejection. Crit Rev Immunol. 2002;22(4):317. [PubMed] [Google Scholar]
- 34.Pirenne J, Pirenne-Noizat F, de Groote D, et al. Cytokines and organ transplantation. A review. Nucl Med Biol. 1994;21(3):545. doi: 10.1016/0969-8051(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 35.Campbell JD, Gangur V, Simons FE, HayGlass KT. Allergic humans are hyporesponsive to a CXCR3 ligand-mediated Th1 immunity-promoting loop. Faseb J. 2004;18(2):329. doi: 10.1096/fj.02-0908fje. [DOI] [PubMed] [Google Scholar]
- 36.Gangur V, Simons FE, Hayglass KT. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. Faseb J. 1998;12(9):705. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- 37.Akashi S, Sho M, Kashizuka H, et al. A novel small-molecule compound targeting CCR5 and CXCR3 prevents acute and chronic allograft rejection. Transplantation. 2005;80(3):378. doi: 10.1097/01.tp.0000166338.99933.e1. [DOI] [PubMed] [Google Scholar]
- 38.Schnickel GT, Bastani S, Hsieh GR, et al. Combined CXCR3/CCR5 blockade attenuates acute and chronic rejection. J Immunol. 2008;180(7):4714. doi: 10.4049/jimmunol.180.7.4714. [DOI] [PubMed] [Google Scholar]
- 39.Hauser AE, Debes GF, Arce S, et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J Immunol. 2002;169(3):1277. doi: 10.4049/jimmunol.169.3.1277. [DOI] [PubMed] [Google Scholar]
- 40.Muehlinghaus G, Cigliano L, Huehn S, et al. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105(10):3965. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 41.Tsubaki T, Takegawa S, Hanamoto H, et al. Accumulation of plasma cells expressing CXCR3 in the synovial sublining regions of early rheumatoid arthritis in association with production of Mig/CXCL9 by synovial fibroblasts. Clin Exp Immunol. 2005;141(2):363. doi: 10.1111/j.1365-2249.2005.02850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]