Abstract
In spite of the impact of aneuploidy on human health little is known concerning the molecular mechanisms involved in the formation of structural or numerical chromosome abnormalities during meiosis. Here, we provide novel evidence indicating that lack of PARP-1 function during oogenesis predisposes the female gamete to genome instability. During prophase I of meiosis, a high proportion of Parp-1 (−/−) mouse oocytes exhibit a spectrum of meiotic defects including incomplete homologous chromosome synapsis or persistent histone H2AX phosphorylation in fully synapsed chromosomes at the late pachytene stage. Moreover, the X chromosome bivalent is also prone to exhibit persistent double strand DNA breaks (DSBs). In striking contrast, such defects were not detected in mutant pachytene spermatocytes. In fully-grown wild type oocytes at the germinal vesicle stage, PARP-1 protein associates with nuclear speckles and upon meiotic resumption, undergoes a striking re-localization towards spindle poles as well as pericentric heterochromatin domains at the metaphase II stage. Notably, a high proportion of in vivo matured Parp-1 (−/−) oocytes show lack of recruitment of the kinetochore-associated protein BUB3 to centromeric domains and fail to maintain metaphase II arrest. Defects in chromatin modifications in the form of persistent histone H2AX phosphorylation during prophase I of meiosis and deficient sister chromatid cohesion during metaphase II predispose mutant oocytes to premature anaphase II onset upon removal from the oviductal environment. Our results indicate that PARP-1 plays a critical role in the maintenance of chromosome stability at key stages of meiosis in the female germ line. Moreover, in the metaphase II stage oocyte PARP-1 is required for the regulation of centromere structure and function through a mechanism that involves the recruitment of BUB3 protein to centromeric domains.
Keywords: Meiosis, Pericentric Heterochromatin, Epigenetic Modifications, Aneuploidy, BUB3, Chromatin remodeling, Genome Instability
Introduction
Aneuploidy is a leading cause of pregnancy loss in women and the single most common cause of congenital birth defects in the human species (Hassold et al., 2007; Hassold and Hunt, 2001). However, in spite of the impact of aneuploidy on human health, little is known concerning the molecular mechanisms and/or environmental factors that predispose the female gamete to abnormal chromosome segregation (Hassold et al., 2007). Epigenetic modifications regulate many aspects of chromosome biology and as such, play an important role in the maintenance of genomic stability during meiosis (Bourc’his and Bestor, 2004; Celeste et al., 2002; De La Fuente et al., 2006; Peters et al., 2001; Webster et al., 2005). For example, in several organisms including mammals centromere structure and function is epigenetically regulated and therefore chromatin modifications are critical for the maintenance of a euploid chromosome complement (Choo, 2001; Cleveland, 2003; Dillon and Festenstein, 2002; Henikoff et al., 2001; Karpen and Allshire, 1997; Murphy and Karpen, 1998).
Mammalian species exhibit highly specialized centromeres comprising of a centric heterochromatin region, that is the site of kinetochore formation, as well as a pericentric heterochromatin domain that provides the assembly site for large multiprotein complexes to induce a higher order chromatin structure (Bernard and Allshire, 2002; Choo, 2001; Cleveland, 2003; Karpen and Allshire, 1997; Peters et al., 2001; Pluta et al., 1995). Mammalian centromeres reveal a highly dynamic structure that might be regulated through several histone post-translational modifications as well as large scale chromatin remodeling (Cleveland, 2003; Henikoff et al., 2001; Karpen and Allshire, 1997; Murphy and Karpen, 1998; Pluta et al., 1995; Wiens and Sorger, 1998).
Centromere structure and function can be regulated by histone post-translational modifications such as acetylation (Grunstein, 1997; Taddei et al., 2001), phosphorylation (Gieni et al., 2008; Peterson and Laniel, 2004), methylation (Bannister et al., 2002; Ekwall, 2007; Gieni et al., 2008) and sumoylation (Gill, 2004; Zhang et al., 2008). Although, the poly (ADP-ribose) polymerase protein PARP-1 has been previously shown to localize to pericentric heterochromatin in mammalian somatic cells (Earle et al., 2000; Saxena et al., 2002a; Saxena et al., 2002b) its potential contribution to the maintenance of centromere function and chromosome segregation during meiosis is not known. Importantly, whether PARP-1 plays a role in the establishment of epigenetic modifications involved in centromeric heterochromatin formation during oogenesis remains largely unexplored.
Poly (ADP-ribose) polymerase-1 (PARP-1) is the most abundant member of a large family comprising 18 different proteins sharing a highly conserved catalytic domain. Importantly, PARP-1 accounts for more than 90% of the enzymatic activity responsible for inducing ADP ribosylation of most acceptor proteins identified until now (Burkle, 2005). Poly(ADP-ribosyl)ation involves the covalent attachment of ADP-ribose molecules in the form of linear or branched polymers of 20–200 residues not only to histone molecules but also to multiple proteins with diverse molecular and cellular functions including transcription, DNA repair and recombination, chromatin remodeling, genome stability and the formation of a proper mitotic spindle in somatic cells (Ame et al., 2004; D’Amours et al., 1999; Kim et al., 2004; Kim et al., 2005; Schreiber et al., 2006).
PARP-1 is an abundant and constitutively expressed nuclear protein that becomes activated upon the induction of DNA damage by direct binding to DNA breaks through its zinc finger domains. The subsequent attachment of ADP ribose polymers modulates the activity of acceptor proteins known to coordinate a rapid detection of the type of DNA damage with the induction of the appropriate DNA repair pathway (Ame et al., 2004; Burkle, 2005; D’Amours et al., 1999; Kim et al., 2005).
Notably, studies conducted in Drosophila chromosomes indicate that PARP activation can also take place in response to developmental or environmental stimuli in order to regulate important aspects of chromatin organization at the global scale (Kim et al., 2004; Tulin and Spradling, 2003; Tulin et al., 2002). Here, we provide novel evidence indicating that oocytes from Parp-1 null female mice exhibit abnormal chromatin modifications in the form of persistent histone H2AX phosphorylation in fully synapsed chromosomes at the pachytene stage. Importantly, at this stage, the X-chromosome bivalent is also prone to exhibit incomplete resolution of double strand DNA breaks (DSBs). Parp-1 null females are fertile, however lack of PARP-1 protein function predisposes the female gamete to severe chromosomal instability ex vivo. Defects in chromatin modifications during prophase I of meiosis and deficient sister chromatid cohesion during metaphase II predispose mutant oocytes to premature anaphase onset after a brief exposure to the culture environment. The mechanisms leading to the abnormal centromere cohesion observed and the potential implications for our understanding of the epigenetic and environmental factors contributing to the onset of aneuploidy in mammalian oocytes are discussed.
Materials and methods
Mice
Mice homozygous for a null mutation of the PARP-1 protein (129S-Parp-1tm1Zqw/J) (Wang et al., 1997) and the corresponding control wild type strain of the same genetic background (129S1/SvImJ) were obtained from the Jackson Laboratory (Bar Harbor, ME). Genotyping of Parp-1 (−/−) mice was based on “The Jackson Laboratory Genotyping Protocol for Parp-1tm1Zqw, version 1”. The following primer sequences were used: P13, 5′-CTTGGGTGGAGAGGCTATTC-3′; P14, 5′-AGGTGAGATGACAGGA GATC-3′; P72, 5′-CCAGCGCAGCTCAGAGAAGCCA-3′; P73, 5′-CATGTTCGATG GGAAAGTCCC-3′. Genomic DNA from mouse-tail was extracted by using the DNeasy Blood & Tissue Kit (QIAGEN Sciences, Germantown, MD) following manufacturer’s instructions. The conditions for the PCR reaction were as follows: 94°C for 3 min; 35 cycles of 94°C for 30 sec, 60°C for 1 min and 72 °C for 1 min; followed by 72 °C for 10 min, and maintained at 4°C until gel electrophoresis on 1.5% agarose (Bio-Rad Laboratories, Hercules, CA). The fertility of wild type females was compared with that observed in Parp-1 mutant females following mating 2 month-old males with similarly aged females for a period of up to 13 months.
Analysis of meiotic configuration in Parp-1 (−/−) oocytes
Fetal ovaries were dissected from wild type and Parp-1 (−/−) females on day 18 of embryonic development (E18) and immediately processed for the analysis of chromosome synapsis and meiotic recombination proteins on surface spread oocytes as described (Libby et al., 2002). Unless otherwise indicated, all primary antibodies were used following an overnight incubation at 4°C. The type of meiotic configuration found in wild type and mutant oocytes at the pachytene stage of meiosis was initially determined by immunochemical detection of the lateral elements of the synaptonemal complex protein SYCP3 using a 1:1000 dilution of a rabbit anti-SYCP3 antibody (Lammers et al., 1994). The subcellular localization of the PARP-1 protein in wild type oocytes at prophase I of meiosis was determined using a 1:400 dilution of a goat anti-PARP-1 antibody (R&D Systems, Minneapolis, MN). The anti-phospho histone H2AX (Ser-139) antibody (Upstate, Charlottesville, VA) was used at a 1:500 dilution. The extent of homologous chromosome synapsis present in wild type and mutant oocytes at the pachytene stage was analyzed by the simultaneous staining of the central element of the synaptonemal complex with a rabbit anti-SYCP1 antibody (1:500) and a guinea pig anti-SYCP3 at a 1:250 dilution (Yuan et al., 2002). The mouse monoclonal anti-MLH1 (BD Pharmingen) and mouse polyclonal anti-RAD51 (Oncogene) were both used following an overnight incubation at 37°C at a 1:50 dilution in combination with rabbit anti-SYCP1 antibody (1:500) and rabbit anti-SYCP3 (1:250) respectively.
The secondary antibodies used were Alexa-fluor 555 rabbit anti-goat IgG, Alexa-fluor 488 goat anti-rabbit IgG, Alexa-fluor 555 goat anti-mouse and Alexa-fluor 488 goat anti-guinea pig all used at a 1:500 dilution and purchased from Molecular Probes, Inc (Eugene, OR). After immunostaining, slides were counterstained with 4 μl of antifading medium supplemented with DAPI (Vectashield; Vector Laboratories, Burlingame, CA). Epifluorescence analysis of meiotic chromosomes was conducted on a DMRX/E microscope (Leica Microsystems) using a 100X objective as described (De La Fuente et al., 2004).
Fluorescence in situ hybridization (FISH)
The position of the X chromosome bivalent in control and mutant oocytes at the pachytene stage was determined by FISH analysis following immunochemical localization of synaptonemal complex protein (SYCP3) and RAD51 foci on the same slides using an FITC-conjugated X chromosome paint STARFISH (Cambio). Probe denaturation was conducted at 75 °C, followed by a hybridization step conducted at 40 °C. Stringency washes were conducted in 50% formamide and 2X SSC as described (De La Fuente et al., 2006; De La Fuente et al., 2004). Co-localization of a fully synapsed bivalent with the signal provided by the X chromosome-specific probe was considered indicative of complete homologous pairing for the X bivalent at the pachytene stage.
Collection and culture of fully-grown oocytes
Fully-grown oocytes at the germinal vesicle (GV) stage were obtained from adult wild type and mutant females 48 h after gonadotropin stimulation with 5 IU pregnant mare serum gonadotropin (PMSG; EMD Biosciences, La Jolla, CA). Oocyte-cumulus complexes were collected in 3.0 ml Minimal Essential Medium (MEM) supplemented with 3 mg/ml crystallized BSA (Sigma, St. Louis MO) and 10 μM Milrinone (Sigma) in order to maintain oocytes with an intact GV as described (De La Fuente et al., 2004). Cumulus cells were removed by continuous pipetting and denuded oocytes washed three times with fresh medium supplemented with 10 μM Milrinone (Sigma). In vivo matured metaphase II stage oocytes were obtained from the oviducts of superovulated females 16 hours after an intraperitoneal injection of 5 IU of human chorionic gonadotropin (HCG; EMD Biosciences). Denuded oocytes were cultured in fresh MEM medium supplemented with 3 mg/ml BSA (Sigma) before allocation to different experimental groups. All oocytes were cultured at 37°C under an atmosphere of 5% O2, 5% CO2 and 90% N2. In some experiments, in vivo matured metaphase II eggs were treated with the proteasome inhibitor MG-132 (Biomol, Plymouth Meeting, PA) diluted in cell culture tested DMSO (American Type Culture Collection; Manassas, VA) and used at different concentrations for 3 h at 37°C.
Western Blot Analysis
Groups of 150 oocytes at the GV stage, metaphase I and metaphase II stage were washed in PBS supplemented with protease inhibitors (10 μg/ml leupeptin, pepstatin and aprotinin, 1mM DTT and 1 mM pefabloc; Sigma) and frozen at −80°C in 10 μl of Laemmli sample buffer (Biorad, Hercules, CA) supplemented with 0.71 M β-Mercaptoehtanol (Biorad). Samples were thawed on ice, and subsequently heated to 100°C for 5 minutes. Proteins were separated by electrophoresis in a 4% stacking gel and a 12% polyacrylamide separating gel containing 0.1% SDS and transferred onto a hydrophobic polyvinylidene difluoride (PVDF) membrane (Amersham, Piscataway NJ) for 90 min at 100 volts. The membrane was washed twice in TBS buffer (pH 7.4; Biorad) containing 0.1% Tween 20 (TBST) and then blocked in TBST containing 5% non-fat dry milk for 1 h at room temperature. Immunodetection of the PARP-1 protein was conducted using a goat anti-PARP antibody (1:1000; R&D systems) at 4°C overnight followed by exposure to a horseradish peroxidase rabbit anti-goat secondary antibody (1:4000; Jackson ImmunoResearch, West Grove, PA).
Immunochemistry of chromosomal spreads and whole mount oocytes
For the immunochemical detection of centromeric heterochromatin-binding proteins, chromosomal spreads were prepared from zona-free oocytes at different stages of meiotic maturation (Hodges and Hunt, 2002) with minor modifications. The zona pellucida was removed with 3 mg/ml pronase (Sigma) in PBS. Zona-free oocytes were rinsed twice in MEM medium and immediately processed for cytogenetic preparations. Chromosomal proteins were cross-linked by spreading the oocytes on a wet slide containing 1% paraformaldehyde and 0.15 % Triton X. Slides were allowed to dry at room temperature and stored at −20°C until further use. Chromosomal spreads were immunostained with a 1:50 dilution of a mouse anti-BUB3 antibody (BD Transduction Laboratories, San Jose, CA) and a 1:500 dilution of a human CREST antiserum (a generous gift from Dr. W. Earnshaw) and counterstained with DAPI (Vectashield; Vector Laboratories).
Protein localization in whole mount oocytes at different stages of meiotic maturation was conducted following fixation in 4% paraformaldehyde supplemented with 1% Triton X for 10 minutes at room temperature and subsequently blocked overnight in 10% fetal calf serum in PBS and 0.2% Tween 20 (PBT). Whole mount oocytes were immunostained with a 1:400 dilution of a goat anti-PARP-1 antibody (R&D systems) and a 1:500 dilution of a mouse anti-γ Tubulin antibody (Sigma) or a 1:400 dilution of a mouse anti-Smith antigen antibody (Neomarkers, Fremont, CA). Chromosomes were counterstained with DAPI supplemented in the mounting medium (Vectashield; Vector Laboratories). Oocytes were examined on a Leica DMRX/E inverted microscope (Leica Microsystems).
Statistical Analysis
Data are presented as the mean percentage of at least three independent experiments; variation among replicates is presented as the standard deviation (S.D.). The percentage of oocytes that exhibited different types of meiotic abnormalities as indicated for individual experiments in control wild type and PARP-1 deficient females was analyzed using arcsine transformed data and compared by one-way analysis of variance (ANOVA) as well as following the comparison of all pairs by Tukey-Kramer HSD using JMP Start Statistics (SAS Institute, Inc., Cary, NC). Differences were considered significant when (p<0.05).
Results
Sub-cellular localization of PARP-1 protein during female meiosis
In somatic cells, PARP-1 associates with different nuclear compartments including the nucleolus (Meder et al., 2005) as well as the centromeres of mitotic chromosomes (Saxena et al., 2002a; Saxena et al., 2002b). Moreover, it is also localized to the centrosomes of the mitotic spindle (Kanai et al., 2000). However, whether PARP-1 plays a role during female meiosis is not known. Therefore, experiments were conducted to determine the patterns of expression and sub-cellular localization of the PARP-1 protein during oogenesis. Immunochemical analyses of surface spread oocytes obtained during prophase I of meiosis revealed that PARP-1 is localized throughout the nucleus of oocytes at the leptotene and zygotene stage (Supplemental Figure 1). Similarly, PARP-1 exhibits a diffuse nuclear localization in wild-type pachytene stage oocytes (Figure 1 A). These results indicate that in mammalian oocytes, PARP-1 is constitutively expressed during prophase I of meiosis as a nucleoplasmic protein associated with both euchromatin and heterochromatin domains.
Figure 1. Sub-cellular localization of the PARP-1 protein during prophase I of meiosis in mouse oocytes.
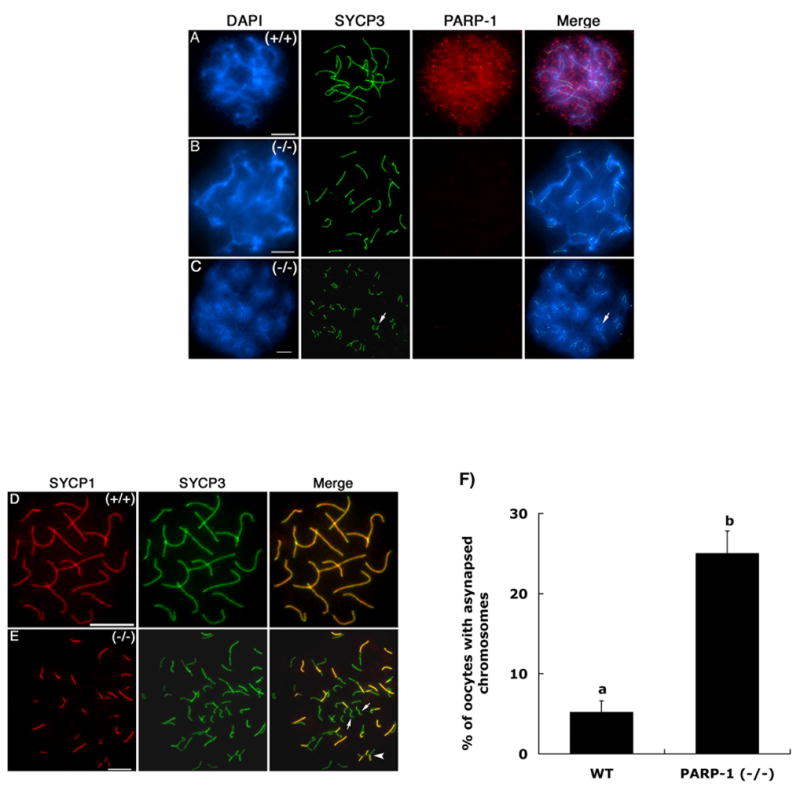
(A) PARP-1 (red) exhibits a diffuse nuclear localization pattern in oocytes showing fully synapsed bivalents at the pachytene stage. The axial/lateral elements of the synaptonemal complex were stained with an antibody against the SYCP3 protein and are shown in green. (B) Pachytene stage oocyte obtained from Parp-1 null females showing fully synapsed bivalents lack detectable levels of PARP-1 protein, thus confirming antibody specificity. (C) A subpopulation of mutant oocytes exhibits a range of meiotic defects including incomplete homologous chromosome synapsis. (D) Co-localization of SYCP-1 and SYCP3 proteins at the pachytene stage confirms full synapsis of homologous chromosomes in wild-type oocytes. (E) The partial co-localization of SYCP1 and SYCP3 proteins observed in mutant oocytes revealed the extent of asynapsis between homologous chromosomes (arrows). (F) Analysis of meiotic configurations in wild type (WT) and Parp-1 (−/−) females revealed a significant increase (p<0.05) in the proportion of mutant oocytes that exhibit asynapsed bivalents. Scale bars=10 μm.
As expected, oocytes obtained from Parp-1 knockout females, lacked detectable levels of the protein (Figure 1 B–C). However, analysis of meiotic configurations in Parp-1 (−/−) oocytes (n=176) revealed a significant increase (p<0.05) in the proportion of cells (25%) showing asynapsed chromosomes (Figure 1 C and 1 F) compared with wild-type oocytes (5.2%; n=162). Mutant oocytes showed a range of meiotic abnormalities including incomplete synapsis in one or more bivalents as determined by simultaneous analysis of the synaptonemal complex proteins SYCP1 and SYCP3 (Figure 1 E, arrows). The patterns of sub-cellular localization observed in wild-type oocytes as well as the higher incidence of meiotic abnormalities in mutant oocytes indicate that PARP-1 may have an important, albeit previously unidentified role during prophase I of meiosis in the female germ line.
Lack of PARP-1 function affects the patterns of H2AX phosphorylation (γ-H2AX) during meiosis in female but not male germ cells
In somatic cells, PARP-1 plays an important role in DSB repair (Althaus et al., 1994). However, whether PARP-1 contributes to the mechanisms involved in the resolution of programmed DSBs during meiotic prophase-I is not known. Formation of double strand breaks during the initial stages of meiosis is associated with phosphorylation of the histone variant H2AX (Mahadevaiah et al., 2001). In turn, the presence of the phosphorylated form of H2AX (γH2AX) at the sites of DNA damage is essential to recruit component molecules of the DNA repair pathway in order to resolve DSBs and ensure proper chromosome synapsis (Burgoyne et al., 2007; Chicheportiche et al., 2007; Mahadevaiah et al., 2001; Moens et al., 2002; Paull et al., 2000; Thiriet and Hayes, 2005). Therefore, experiments were conducted to determine the patterns of γH2AX association with meiotic chromosomes in Parp-1 (−/−) oocytes. Simultaneous analysis of H2AX phosphorylation as well as the extent of chromosome synapsis in mutant oocytes revealed three distinct patterns of γH2AX staining (Figure 2). For example, mid to late pachytene stage oocytes exhibiting full synapsis of homologous chromosomes showed the presence of one or two small γH2AX foci associated with each synapsed bivalent (Figure 2 A; small arrows). In contrast, 32% of mutant oocytes with fully synapsed bivalents at the late pachytene stage showed a striking pattern in which, except for the centromeric domains, γH2AX staining was found associated with the entire length of meiotic chromosomes (Figure 2B; large arrow). Importantly, these patterns of γH2AX nuclear localization were clearly different from those observed in the fraction of mutant oocytes (25%) exhibiting asynapsed bivalents (Figure 2C; arrow-heads) in which γH2AX staining has been shown to result from the activation of the mechanism for meiotic silencing of unpaired chromatin at the pachytene stage (Turner et al., 2004; Turner et al., 2005).
Figure 2. Persistence of γH2AX phosphorylation in late pachytene stage Parp-1 (−/−) oocytes.
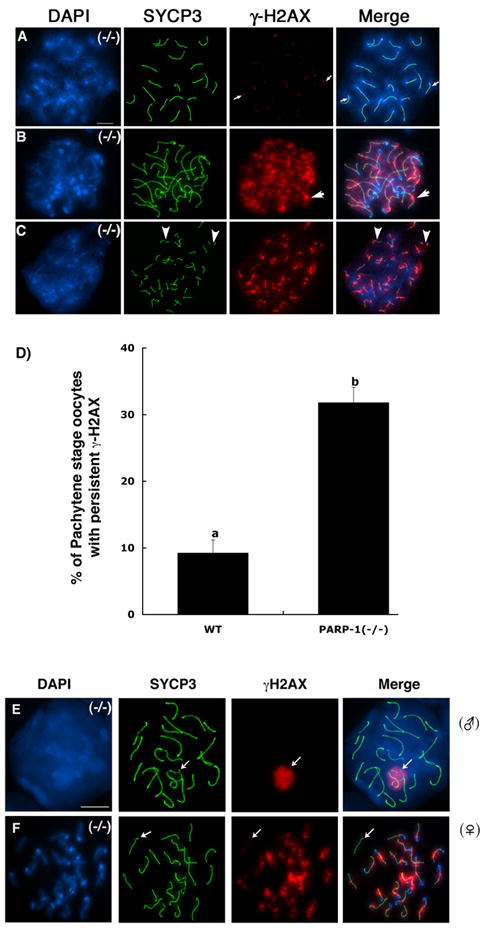
(A) Late pachytene stage oocyte obtained from a Parp-1 (−/−) female showing complete homologous chromosome synapsis (n=20 bivalents). At this stage, γH2AX phosphorylation is only detectable by the presence of a few small foci associated with synapsed bivalents. (B) A subpopulation of Parp-1 (−/−) oocytes at the late pachytene-early diplotene stage exhibits persistence of γH2AX phosphorylation throughout the chromatids of fully synapsed bivalents (arrows). (C) Abnormal meiosis in Parp-1 (−/−) oocytes showing intense γH2AX phosphorylation associated with each asynapsed chromosome and partial interruptions in SYCP3 staining suggesting the presence of chromosome breaks (arrowheads). (D) Proportion of mutant oocytes that exhibit persistent γH2AX phosphorylation in fully synapsed bivalents at the late pachytene-early diplotene stage. In contrast to the meiotic phenotype observed in the female germ line, pachytene stage spermatocytes (E) showed normal chromosome synapsis and γH2AX staining was found exclusively associated with the sex chromosome bivalent (arrow). Simultaneous staining of a mutant oocyte (F) reveals the extent of persistent γH2AX phosphorylation in the majority of chromosomes except for a single synapsed bivalent (arrow). Scale bars=10 μm.
Notably, a direct comparison of male and female mutant meiocytes at the pachytene stage revealed normal patterns of chromosome synapsis as well as H2AX phosphorylation in mutant spermatocytes as indicated by a prominent γ-H2AX signal exclusively associated with the sex bivalent (Figure 2E; arrow). These results indicate that PARP-1 function exhibits a sexual dimorphism during mammalian meiosis and that in contrast with the timely removal of γ-H2AX from the autosomes of mutant spermatocytes, large γ-H2AX foci persist in the fully synapsed chromosomes of Parp-1 null oocytes at the late pachytene and early diplotene stage, potentially reflecting abnormal chromatin modifications during late prophase I or alternatively, a failure to resolve DSBs, and therefore a defect in DNA repair.
Parp-1 (−/−) oocytes exhibit persistent γ-H2AX phosphorylation and fail to resolve DSBs at the X-chromosome bivalent
In order to establish whether persistent γH2AX staining in Parp-1 (−/−) oocytes is due to a failure to repair DSBs at the pachytene stage we set out to determine the pattern of expression and chromosomal localization of the RAD51 protein, a DNA recombination intermediate, known to mark the sites of DSB formation in meiotic chromosomes (Burgoyne et al., 2007; Moens et al., 2002). During prophase I of meiosis at the leptotene and zygotene stage, the RAD51 protein labels the sites of early DNA recombination nodules. However, RAD51 foci progressively disappear, as DSBs are resolved following homologous chromosome synapsis at the pachytene stage (Bannister and Schimenti, 2004; Moens et al., 2002). Accordingly, no RAD51 foci were detected in the majority of control wild type oocytes (n=205) at the pachytene stage indicating a timely repair of DSBs in fully synapsed chromosomes (Figure 3A–C). In contrast, an average of 13.5% of PARP-1 deficient oocytes (n=211) showed a striking accumulation of RAD51 foci associated with a single synapsed bivalent as determined by SYCP3 staining (Figure 3D–F and 3G). Simultaneous analysis of γH2AX staining and RAD51 localization confirmed that unresolved DSBs labeled by RAD51 are co-localized with a prominent γH2AX signal in a single chromosome bivalent (Figure 4A; arrow). Importantly, γH2AX staining also remained associated with most bivalents in which RAD51 foci were no longer detectable (Figure 4A; arrowheads). Furthermore, immuno-FISH analyses indicated that the synapsed chromosome bivalent that failed to resolve RAD51 foci in mutant oocytes (Figure 4C; arrow) corresponded to the X-chromosome bivalent on every meiotic spread analyzed (Figure 4D; arrow). These results suggest that γ-H2AX phosphorylation is due to persistent DSBs only at the X-chromosome bivalent as indicated by the presence of RAD51 foci, at the late pachytene stage. However, Parp-1 null oocytes also exhibit persistent H2AX phosphorylation associated with the majority of synapsed meiotic chromosome cores that is independent of unresolved DSBs.
Figure 3. Double strand DNA breaks (DSBs) persist at a single synapsed bivalent in Parp-1 (−/−) oocytes at the pachytene stage.
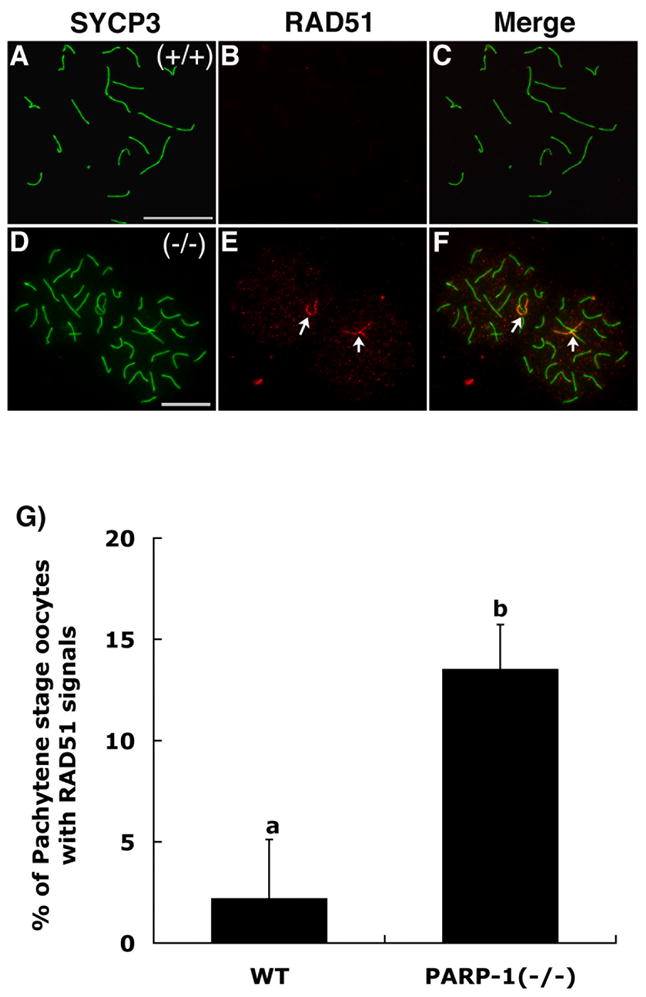
(A) Wild type oocyte showing full synapsis of homologous chromosomes (20 bivalents) as determined by SYCP3 staining. (B) RAD51 foci (DSBs) are progressively resolved and hence become undetectable as meiotic chromosomes reach full synapsis in wild type oocytes. (C) Merge. (D–F) In contrast, persistence of RAD51 foci (red) at synapsed chromosomes (green) confirms the presence of unresolved DSBs at a single synapsed bivalent on each mutant oocyte (arrows). Note that two closely apposed pachytene stage oocytes are shown. (G) Proportion of wild type (WT) and mutant oocytes that exhibit persistent DSBs as labeled by RAD51 foci in a single bivalent (p<0.05). Scale bars=10 μm.
Figure 4. The X-chromosome bivalent fails to resolve DSBs in Parp-1 (−/−) oocytes.
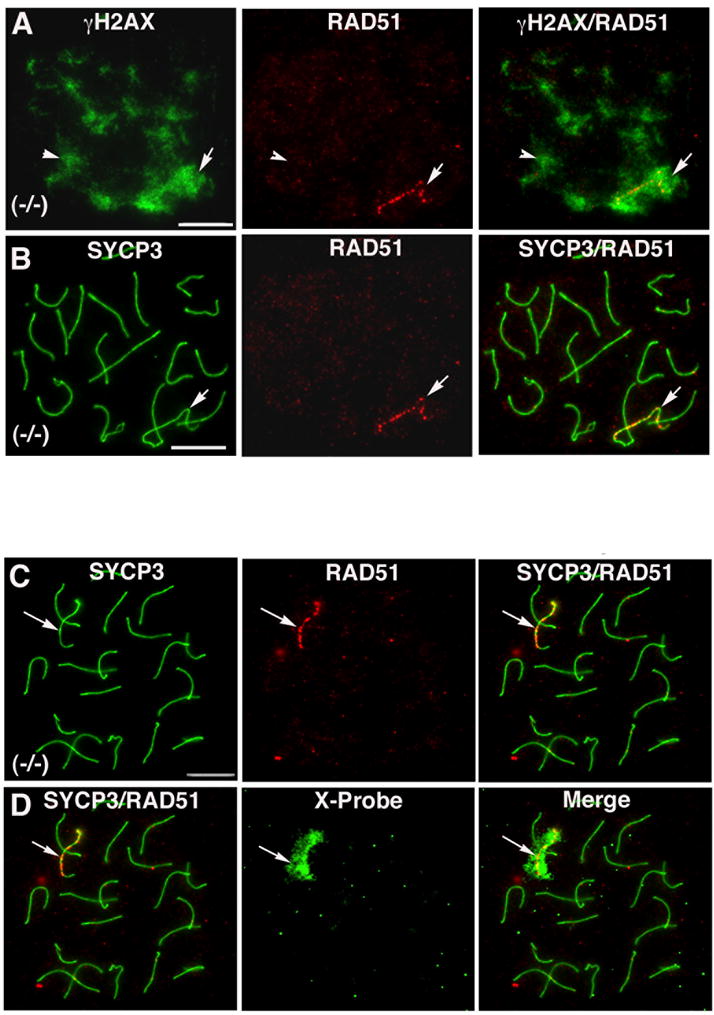
(A) A small proportion of mutant oocytes exhibit both persistent γH2AX phosphorylation (green) and RAD51 foci (red). However, in contrast with the wide distribution of γH2AX staining in the majority of synapsed chromosomes (arrowheads), RAD51 foci remain exclusively associated with a single chromosome bivalent (arrow). (B) Corresponding micrograph showing synapsed bivalents and localization of RAD51 foci in mutant oocytes. Immuno-FISH analysis revealed that in the absence of PARP-1 function, RAD51 foci (C; arrow) remain exclusively associated with the X-chromosome bivalent in mutant oocytes (D; arrow). Scale bars=10 μm.
Next we determined whether the persistence of recombination intermediates or the abnormal chromatin modification patterns in mutant pachytene stage oocytes might interfere with the formation of crossovers between homologous chromosomes. Analysis of the chromosomal distribution of the mismatch repair protein MLH1, a marker for crossover formation (Baker et al., 1996; Bannister and Schimenti, 2004; Moens et al., 2002), revealed that wild type pachytene stage oocytes exhibit one or two MLH1 foci associated with each synapsed bivalent and have an average of 26.6±0.6 foci per oocyte (Supplemental Figure 2). Notably, no significant differences were found in the number of MLH1 foci (27.3±1.3) in Parp-1 null oocytes, suggesting that in the fraction of mutant oocytes that exhibit proper synapsis of homologous chromosomes, establishment of crossovers proceeds as in wild type oocytes.
PARP-1 exhibits a dynamic nuclear localization during mouse oocyte growth and meiotic maturation
The PARP-1 protein has been shown to localize to the centromeres of human somatic cells during mitosis (Saxena et al., 2002a; Saxena et al., 2002b). However, its potential involvement in the functional differentiation of chromatin structure in mammalian oocytes remains to be established. To determine whether PARP-1 has a role during the completion of meiosis in the female gamete, we analyzed the patterns of expression and sub-cellular localization of PARP-1 protein in fully-grown germinal vesicle (GV) stage oocytes and in vivo matured metaphase II stage oocytes obtained from wild type females.
Immuno-chemical analyses conducted in whole mount oocytes at the GV stage indicate that PARP-1 (Figure 5A; green) is an abundant protein that exhibits a diffuse nuclear localization. Notably, PARP-1 also forms a different number of nuclear aggregates (arrows) according to the type of chromatin configuration present in the GV. For example, in transcriptionally active oocytes with decondensed chromatin that exhibit the non-surrounded nucleolus (NSN) configuration (n=31) an average of 6.4± 3.7 nuclear aggregates were present (Figure 5A). In contrast, in transcriptionally inactive oocytes with condensed chromatin showing the surrounded nucleolus (SN) configuration (n=69), these multiple aggregates were found to coalesce into one or two (average=1.7± 0.8) large nuclear bodies that lacked any association with DAPI-stained chromatin (arrow in Figure 5B). Simultaneous analysis with an antibody directed against the Smith (Sm) antigen, a marker for nuclear speckles (Lamond and Spector, 2003), revealed that PARP-1 is co-localized with the Sm-antigen at interchromatin regions as well as the peri-nucleolar region in the GV of transcriptionally active oocytes that exhibit the NSN configuration (Figure 5D). Similar results were observed in transcriptionally inactive oocytes with the SN configuration (Figure 5E). These results indicate that in addition to its diffuse nuclear localization, PARP-1 associates with the peri-nucleolar region and accumulates at nuclear speckles in the GV of fully-grown oocytes.
Figure 5. PARP-1 associates with nuclear speckles in fully-grown oocytes at the GV stage.
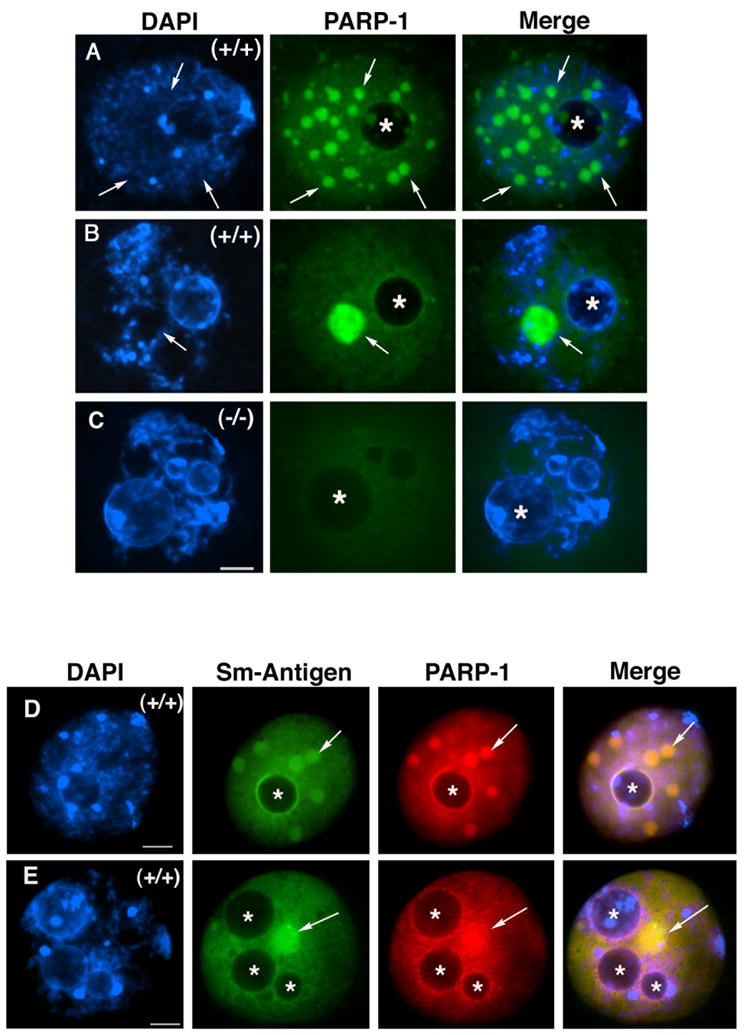
(A) Fully-grown wild type oocyte exhibiting decondensed chromatin typical of the non-surrounded nucleolus (NSN) configuration. In addition to its diffuse nuclear localization, PARP-1 forms several nuclear aggregates that accumulate at regions with no DAPI staining (arrows). The position of the nucleolus is indicated by (*). (B) Upon the transition into the surrounded nucleolus (SN) configuration, these nuclear aggregates coalesce into a prominent nuclear body that shows no association with DAPI-stained chromatin (arrows). (C) Parp-1 (−/−) oocyte showing only background fluorescence used as control for antibody specificity. (D–E) Representative images of wild type oocytes with the NSN configuration (D) and the SN configuration (E) showing diffuse nuclear staining as well as co-localization of PARP-1 protein with the Smith antigen (Sm), a marker for nuclear speckles. Note that PARP-1 can also be observed associated with the perinucleolar region. The position of the nucleolus is indicated by (*). Scale bars=10 μm.
PARP-1 was detected as a single band of approximately 116 kDa at the GV stage and showed no apparent change in electrophoretic mobility in oocytes at the metaphase I or metaphase II stage (Figure 6A). Upon resumption of meiosis, PARP-1 was found associated with the meiotic spindle poles (Figure 6C; arrowheads) where it is also co-localized with γ-Tubulin (Figure 6D; red). Notably, PARP-1 signals were also detectable associated with the chromosomes of metaphase II stage oocytes (Figure 6C; arrow). Furthermore, analysis of chromosome spreads under high resolution, confirmed that PARP-1 is present in the chromatids of metaphase II stage chromosomes and preferentially enriched at pericentric heterochromatin domains (Figure 6G; arrow) where it is found co-localized with the more circumscribed signals detected by the CREST antiserum, a bona fide centromere marker in mammalian chromosomes (Figure 6H–I). These results indicate that PARP-1 shows a dynamic association with distinct nuclear compartments in the GV of pre-ovulatory oocytes and upon meiotic resumption, with critical components of the meiotic spindle as well as pericentric heterochromatin domains in mature metaphase II eggs.
Figure 6. PARP-1 associates with meiotic spindle poles and peri-centric heterochromatin in metaphase II stage oocytes.
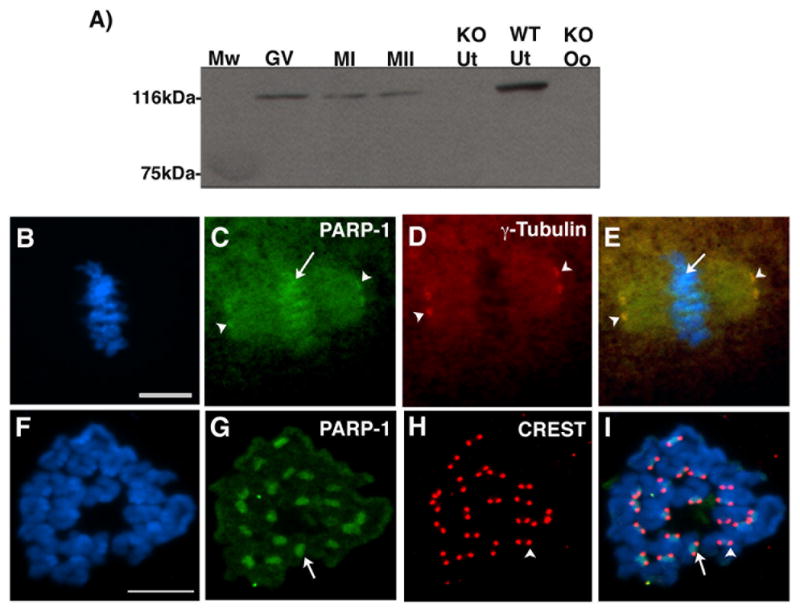
(A) Western blot analysis using wild type oocytes at the germinal vesicle (GV), metaphase I (MI) and metaphase II (MII) stage indicates that PARP-1 is a protein of approximately 116 kDA that shows no detectable change in electrophoretic mobility during oocyte meiotic maturation. Knockout uterus (KO Ut), knockout oocytes (KO Oo) and wild type uterus (WT Ut) were used as negative and positive controls, respectively. (B) Metaphase II stage chromosomes as shown by DAPI staining of whole mount oocytes. (C) PARP-1 is present at spindle poles (arrowheads) as well as the chromosomes of mature oocytes (arrows). (D) Corresponding micrograph showing the localization of γ-Tubulin signals at meiotic spindle poles. (E) Overlay. (F) High resolution chromosome spread at the metaphase II stage. (G) Association of PARP-1 with pericentric heterochromatin (arrow). Although PARP-1 signal can be detected at the chromatids of metaphase II stage chromosomes, the protein is highly enriched at pericentric domains. (H) Corresponding micrograph showing the localization of centromeres as detected by the CREST antiserum (arrowhead). (I) Overlay, note that the PARP-1 signal extends over a larger block of heterochromatin compared with the CREST signal. Scale bars=10 μm.
PARP-1 deficiency predisposes the female gamete to chromosome instability
To determine whether PARP-1 has a role in female fertility, we compared the average litter size observed in control wild-type females maintained on a similar genetic background with that observed over the reproductive lifespan of Parp-1 (−/−) females. A total of 6 wild type females produced 41 litters over a period of 13 months and had an average of 5.9±0.7 pups per litter (Figure 7A). However, in spite of the meiotic abnormalities detected in a high proportion of Parp-1 (−/−) oocytes during prophase I of meiosis, no significant differences were observed in the average litter size (5.4±1.3 pups per litter) in a total of 63 litters obtained from seven Parp-1 (−/−) females evaluated over a period of 14 months (Figure 7A). These results suggest that oocytes with abnormal synapsis or persistence of DSBs might be selectively eliminated early during post-natal oocyte growth.
Figure 7. Evidence for a role of PARP-1 in sister chromatid cohesion in mouse oocytes.
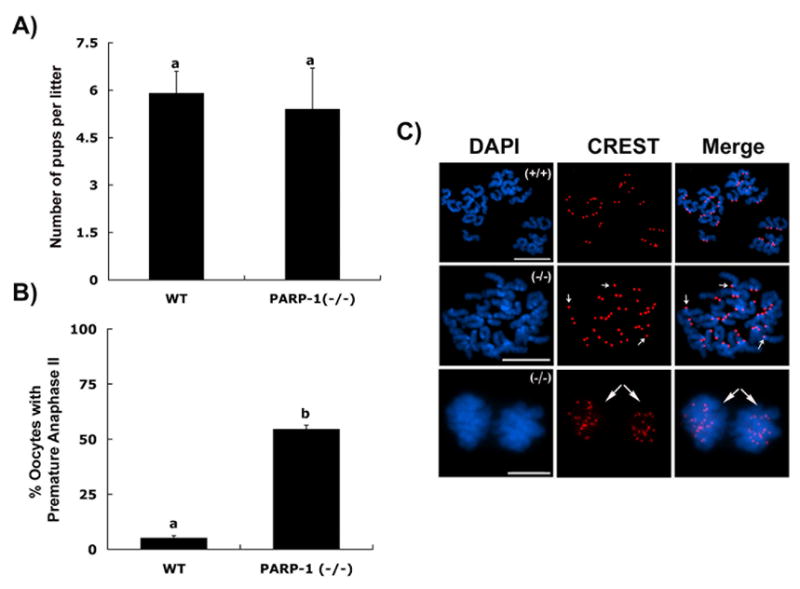
(A) Average litter size (±S.D.) in control and PARP-1 null females; (B) Percentage of oocytes showing premature anaphase-II onset upon removal from the oviductal environment. Different superscripts denote significant differences (p<0.05). (C) Top panel: Wild-type oocyte showing a normal chromosome complement with 40 CREST signals at the metaphase II stage. Middle panel: In vivo matured mutant oocyte showing the presence of several single chromatids (small arrows) at the metaphase II stage. Lower panel: Mutant oocyte showing premature anaphase-II onset ex vivo following exposure to the culture environment for 15 minutes. Long arrows indicate the premature segregation of sister chromatids (20 CREST signals each) into opposite poles. Scale bars=10 μm.
Compelling evidence suggests that in human oocytes, factors that disrupt the normal rates of DNA recombination during prophase I of meiosis might induce high levels of aneuploidy in mature ova (Hassold and Hunt, 2001; Lamb et al., 2005). Therefore, we conducted a detailed cytogenetic analysis of in vivo matured oocytes obtained after superovulation of adult wild type and Parp-1 (−/−) females. Analysis of 97 in vivo derived, metaphase II stage control oocytes in three independent experimental replicates indicated that in this group only 5% of oocytes showed evidence of aneuploidy (Figure 7B). In contrast, analysis of mutant ova (n=88) revealed a significant increase (p<0.05) in the proportion of oocytes (54.5%) with one or more chromosomes showing precocious centromere separation (Figure 7C; middle panel) or, in extreme cases, a complete segregation of sister chromatids into a configuration resembling a premature anaphase II onset in which two distinct sets of single chromatids could be identified (Figure 7B and C; lower panel). These results indicate that lack of PARP-1 function during meiosis might predispose mutant oocytes to chromosome instability.
To gain insight into the potential mechanisms leading to premature anaphase onset in Parp-1 mutant oocytes, we set out to determine whether the cell cycle checkpoint protein (BUB3) is properly recruited to the centromere in the absence of PARP-1 function. Analysis of chromosomal spreads from in vivo matured wild type MII stage oocytes (n=84) indicated that BUB3 (red) is co-localized with CREST signals (green) at centromeric domains in the majority (94%) of oocytes (Figure 8A–B). In contrast, a significant increase (p<0.05) in the proportion of Parp-1 (−/−) oocytes (51%; n=65) with no centromeric BUB3 staining was observed. In this group, centromeric domains were found exclusively labeled with the CREST antiserum (Figure 8A; lower panel and 8B). These results provide critical evidence indicating that the cell cycle checkpoint protein (BUB3) is not properly recruited to the centromere in Parp-1 ( −/−) oocytes at the metaphase-II stage.
Figure 8. Parp-1 (−/−) oocytes fail to recruit BUB3 protein to the centromere at the metaphase-II stage.
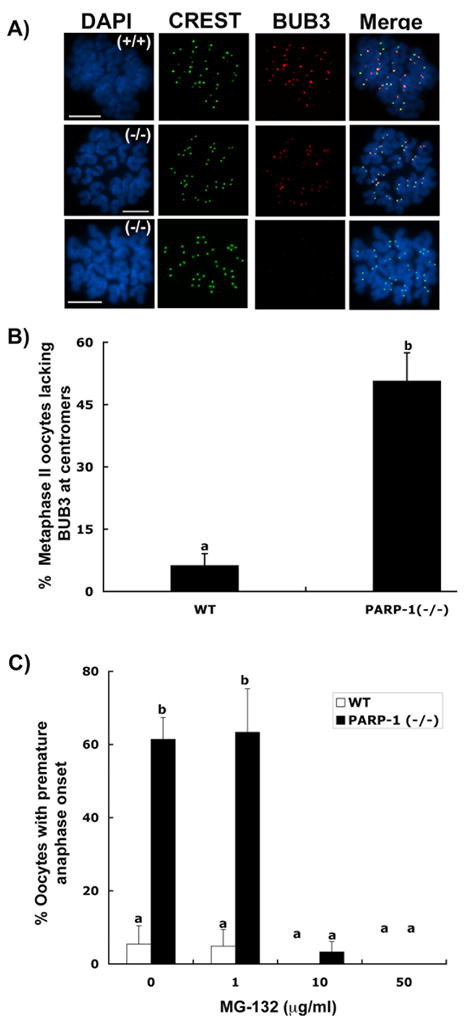
(A) Top panel: Specific co-localization of the CREST signals (green) with the BUB3 protein (red) at the centromeres of wild-type metaphase-II oocytes. Center panel: Similar to wild-type oocytes, some mutant oocytes show correct localization of the BUB3 protein at centromeres. Lower panel: A high proportion of Parp-1(−/−) oocytes fail to recruit BUB3 to meiotic centromeres, yet show proper CREST staining. Scale bars=10 μm. (B) Proportion of metaphase-II oocytes lacking BUB3 protein at centromeres; data are presented as the mean (±S.D.) after three independent experiments (p<0.05). (C) Proportion of wild type (WT) and mutant oocytes that exhibit premature anaphase onset in the presence or absence of the proteasome inhibitor MG-132 (p<0.05).
Next, in vivo matured metaphase II stage oocytes were obtained from the oviducts of gonadotropin stimulated females and maintained in culture with surrounding cumulus cells for 3 h in the presence or absence of different concentrations of the proteasome inhibitor MG-132. Consistent with our previous experiments, exposure to the culture environment induced a significant increase (p<0.05) in the proportion of mutant oocytes (61%) with premature anaphase onset compared with wild type oocytes (5%). Importantly, premature anaphase onset was effectively prevented in mutant oocytes after exposure to 10 and 50 μg/ml of the proteasome inhibitor (Figure 8C). These results indicate that PARP-1 is required for the recruitment of BUB3 protein to centromeric domains in mammalian oocytes. Notably, the presence of single chromatids at metaphase-II indicates that precocious sister chromatid separation in Parp-1 null oocytes takes place through a mechanism involving premature centromere separation and that premature anaphase onset is mediated through abnormal activation of the proteasome pathway.
Discussion
The centromeres of meiotic chromosomes exhibit unique structural and functional properties that are required to ensure the sequential separation of homologous chromosomes and then sister chromatids during metaphase-I and metaphase-II stage of meiosis, respectively (Page and Hawley, 2003; Petronczki et al., 2003; Watanabe, 2005). Importantly, compelling evidence indicates that pericentric heterochromatin is of critical importance to regulate centromere cohesion during mitosis and for the timely separation of sister chromatids during anaphase (Bernard and Allshire, 2002; Dernburg et al., 1996; Guenatri et al., 2004; Peters et al., 2001). However, little is known concerning the epigenetic mechanism(s) involved in the regulation of heterochromatin formation during oogenesis. Here, we provide evidence indicating that PARP-1 exhibits a dynamic subcellular localization pattern in the female germ line. PARP-1 shows a diffuse nucleoplasmic staining during prophase I of meiosis and subsequently becomes associated with nuclear speckles during oocyte growth and differentiation. Moreover, following the resumption of meiosis, PARP-1 exhibits a prominent association with pericentric heterochromatin domains as well as meiotic spindle poles in mature metaphase II stage oocytes.
Although Parp-1 null females are fertile, more than 50% of mutant oocytes exhibit a spectrum of chromosomal defects during prophase I of meiosis, including either incomplete homologous chromosome synapsis or the presence of abnormal chromatin modifications in the form of persistent H2AX phosphorylation in fully synapsed bivalents. Notably, in the absence of functional PARP-1 protein at the pachytene stage, the fully synapsed X chromosome bivalent is also prone to exhibiting persistent DSBs. Furthermore, a high proportion of in vivo matured mutant oocytes showed evidence for the presence of single chromatids at the metaphase II stage. Such defects in centromere cohesion lead to a premature anaphase II onset in >50% of oocytes upon minutes after exposure to the culture environment. Importantly, a similar proportion of mutant metaphase II stage oocytes lacked centromeric BUB3 staining. The premature anaphase onset observed in mutant oocytes was efficiently prevented by simultaneous exposure to the proteasome inhibitor MG-132. Collectively, our results indicate that PARP-1 plays a critical role in the maintenance of chromosome stability at key stages of meiosis in the female germ line. These results are consistent with a sexually dimorphic role for PARP-1 in the control of γ-H2AX phosphorylation patterns during female meiosis. Moreover, PARP-1 is required for the regulation of centromere structure and function through a mechanism that involves the recruitment of BUB3 protein to centromeric domains in the metaphase II stage oocyte.
Persistent H2AX phosphorylation following resolution of DSBs in Parp-1−/− oocytes
During mammalian meiosis, phosphorylation of the histone variant H2AX (γH2AX) is associated with DSBs formation at the leptotene stage (Mahadevaiah et al., 2001; Turner et al., 2005). However, γH2AX foci are progressively eliminated from meiotic chromosome cores following the resolution of DSBs in fully synapsed bivalents at the late pachytene stage (Burgoyne et al., 2007; Chicheportiche et al., 2007; Mahadevaiah et al., 2001; Paull et al., 2000). In contrast, a significant proportion of Parp-1 null oocytes exhibit persistent H2AX phosphorylation in fully synapsed chromosomes at the late pachytene and early diplotene stage. Persistent γ-H2AX staining following chromosome synapsis has been associated with the presence of unresolved DSBs, as determined by the co-localization of γ-H2AX foci with several recombination intermediates including RAD51, in synapsed chromosomes of both male and female germ cells deficient for the synaptonemal complex protein SYCP3 (Wang and Hoog, 2006) as well as the recombination factors MRE11, Trip 13, ERCC1 and MSH2 (Cherry et al., 2007; Li and Schimenti, 2007; Paul et al., 2007), where unresolved DSBs result from structural damage to meiotic chromosomes or impaired meiotic recombination, respectively (Cherry et al., 2007; Li and Schimenti, 2007; Wang and Hoog, 2006). In contrast, in PARP-1 deficient oocytes, γ-H2AX staining associated with RAD51 foci persists only in the X chromosome bivalent. The mechanisms involved in the failure to resolve DSBs exclusively at the X chromosome bivalent in Parp-1 (−/−) oocytes remain to be determined. However, it is conceivable that functional redundancy with PARP-2 (Ame et al., 2004; Kim et al., 2004; Kim et al., 2005; Schreiber et al., 2006) accounts for the resolution of DSBs in the majority of autosomes whereas the presence of unresolved DSBs at the X bivalent might be a reflection of an increased susceptibility of the X chromosome to the formation of superfluous or illegitimate recombination in the absence of PARP-1 function. Interestingly, the higher frequency of unresolved DSBs observed on the X chromosome bivalent in this study may be a contributing factor to explain the previously described X chromosome instability that has been detected in female but not male embryonic fibroblasts of PARP-1+/−/Parp 2−/− double mutant mice (Ménissier de Murcia et al., 2003).
The presence of large γ-H2AX foci in the majority of synapsed autosomes that show no RAD51 accumulation at the late pachytene stage suggests that mechanisms other than unresolved DNA breaks might account for the persistence of H2AX phosphorylation in the absence of a functional PARP-1 protein during late meiosis. Accordingly, persistent γ-H2AX staining might be due to alterations in global chromatin structure and/or aberrant chromatin modifications such as altered histone acetylation or phosphorylation (Fernandez-Capetillo et al., 2004). Consistent with this hypothesis, activation of the ataxia-telangiectasia-mutated (ATM) kinase and subsequent H2AX phophorylation can also take place in response to changes in chromatin structure that is independent of DSB formation (Bakkenist and Kastan, 2003; Haince et al., 2007). Therefore, the presence of large γH2AX clusters that extend over chromatin regions adjacent to the synaptonemal complex following the resolution of DSBs revealed for the first time a defect in the control of histone H2AX phosphoprylation associated with subsequent chromosome instability during meiosis in Parp-1 mutant oocytes. Importantly, our results provide critical evidence to support recent findings indicating that H2AX phosphorylation might have functions other than its traditional role in DNA repair mechanisms (Ichijima et al., 2005; Ismail and Hendzel, 2008; McManus and Hendzel, 2005) and contribute with novel information indicating that PARP-1 function is required to resolve γ-H2AX foci from meiotic chromosomes and hence the establishment of proper chromatin modifications during late meiosis in the female germ line.
A role for PARP-1 in the epigenetic regulation of centromere function in mammalian oocytes
PARP-1 exhibits a striking accumulation at nuclear speckles in the germinal vesicle of fully-grown oocytes. Notably, this pattern of PARP-1 localization seems to be restricted to the oocyte nucleus as no such association has been reported in somatic cells. Therefore it is conceivable that PARP-1 may interact with additional factors that have been previously shown to accumulate at nuclear speckles in meiotically competent oocytes such as transcription or splicing factors (Truchet et al., 2004), members of the minichromosome maintenance complex (MCM) (Swiech et al., 2007) or the SUMO-conjugating enzyme (UBE2I) (Ihara et al., 2008) and might thus contribute to the establishment of post-translational modifications in the oocyte genome.
Interestingly, PARP-1 exhibits a striking redistribution from its original location at nuclear speckles in fully-grown oocytes towards centromeric heterochromatin as well as meiotic spindle poles upon meiotic resumption suggesting that even in the absence of any detectable changes in electrophoretic mobility during meiosis, PARP-1 is co-localized with major components of the metaphase II spindle. Both PARP-1 and PARP-2 proteins have been previously shown to localize to pericentric heterochromatin as well as the centromeres of mammalian somatic cells, respectively (Earle et al., 2000; Saxena et al., 2002; Saxena et al., 2002). Moreover, PARP-1 and PARP-2 exhibit a physical association with constitutive centromeric proteins such as Cenp-A and Cenp-B as well as the mitotic checkpoint protein BUB3 (Saxena et al., 2002; Saxena et al., 2002). Our results demonstrate for the first time that PARP-1 function is essential to recruit BUB3 to centromeric heterochromatin in mammalian oocytes and underscore a critical role for PARP-1 in the regulation of centromere structure and function and hence the control of proper chromosome segregation and maintenance of genome stability in the female gamete. Consistent with this notion, a novel poly(ADP-ribose)-binding zinc finger (PBZ) motif has been recently shown to be required for poly(ADP-ribosyl)ation of the cell cycle checkpoint protein CHFR, independent of DNA damage, in somatic cells suggesting that basal levels of automodified PARP-1 protein may be critical to recruit factors with the PBZ motif including checkpoint regulatory proteins to centromeric domains (Ahel et al., 2008).
PARP-1 is required to maintain sister chromatid cohesion in mature metaphase II stage oocytes
The fertility of PARP-1 null females observed in this study might be due to the presence of a previously reported functional redundancy with PARP-2 (Ménissier de Murcia et al., 2003; Yelamos et al., 2008). Moreover, previous studies have reported the presence of a normal litter size in female mice heterozygous for a missense mutation of DMC1 which exhibit a high incidence (60%) of meiotic defects at the pachytene stage, suggesting the existence of an ovarian compensatory mechanism (Bannister et al., 2007). Our results however, demonstrate that lack of PARP-1 function alone, renders the female gamete extremely susceptible to chromosome instability during completion of meiosis in response to environmental conditions. PARP-2 null spermatocytes exhibit normal autosome synapsis at prophase I of meiosis but show synaptic defects of the X and Y chromosome associated with lack of sex chromosome inactivation. In addition, approximately 36% of PARP-2 null spermatocytes at metaphase I exhibit univalent chromosomes (Dantzer et al., 2006). In contrast, our results indicate that synapsis of the sex chromosome bivalent in Parp-1 null spermatocytes is normal. This is consistent with previous suggestions indicating that PARP-1 and PARP-2 might function as heterodimers showing both, functional redundancy as well as critical non-redundant functions in the maintenance of genome stability (Ménissier de Murcia et al., 2003).
Specifically, our studies revealed that lack of PARP-1 function at pericentric heterochromatin predisposes the female gamete to premature sister chromatid separation at the metaphase II stage through a mechanism involving the lack of recruitment of BUB3 to centromeric domains. It is well established that BUB3 inhibits the anaphase promoting complex/cyclosome (APC/C) pathway from targeting cell cycle regulatory proteins for proteolytic degradation and hence controls the onset of anaphase (Kalitsis et al., 2000; Sudakin et al., 2001). BUB3 has also been shown to establish a functional interaction with MAD2 during mitosis (Sudakin et al., 2001). Notably, MAD2 is present at the kinetochore regions of in vivo matured metaphase II stage oocytes where it might be required to maintain metaphase II arrest and the timely transition to anaphase (Kallio et al., 2000). Therefore in the absence of BUB3, PARP-1 mutant oocytes might exhibit a premature activation of the anaphase promoting complex following exposure to the culture environment, a notion supported by the efficient prevention of spontaneous anaphase II onset following inhibition of the proteasome pathway with MG-132 in this study. These results suggest the presence of a potential link between PARP-1 function and sister centromere cohesion in the mammalian oocyte.
What is the role of pericentric heterochromatin in protecting centromere cohesion during meiosis? In several organisms including mammals, pericentric heterochromatin has been shown to be essential for centromere cohesion and the timely separation of individual chromatids during mitosis (Bernard and Allshire, 2002; Dernburg et al., 1996; Guenatri et al., 2004). Importantly, targeted deletion of several chromatin remodeling proteins has provided critical evidence indicating that the formation of both centric and pericentric heterochromatin domains is required for the modulation of homologous chromosome synapsis during male (Bourc’his and Bestor, 2004; Peters et al., 2001; Webster et al., 2005) and female meiosis (De La Fuente et al., 2006) as well as for proper chromosome segregation during spermatogenesis (Dantzer et al., 2006; Peters et al., 2001). Importantly, our studies revealed a role for PARP-1 in the recruitment of BUB3 to meiotic centromeres in the female germ line. Further studies will be aimed at establishing whether PARP-1 is involved in the recruitment of component molecules of the cohesin complex required to protect meiotic centromeres from premature separation (Bernard and Allshire, 2002; Lee et al., 2008; Llano et al., 2008).
Supplementary Material
Supplemental Figure 1. PARP-1 exhibits a diffuse nuclear localization throughout the different stages of meiotic prophase I in the female germ line
A) Wild-type (WT) oocyte at the leptotene stage showing initial formation of the lateral elements of the synaptonemal complex stained with anti-SYCP3 antibody (green). PARP-1 (red) is an abundant protein that exhibits diffuse nucleoplasmic staining. (B) Representative micrograph of a WT oocyte showing the chromosome morphology typical of the zygotene stage as detected by SYCP3 staining (green) and prominent nucleoplasmic PARP-1 staining (red). (C) Parp-1 (−/−) oocyte at the zygotene stage, as detected by the patterns of SYCP3 staining (green), used as a negative control lacks PARP-1 staining and thus confirms antibody specificity. Scale bar=10 μM.
Supplemental Figure 2. Establishment of crossovers in Parp-1 (−/−) oocytes showing complete synapsis of homologous chromosomes
A) Comparison of the chromosomal distribution of the mismatch repair protein MLH1 (red) in wild type oocytes at the pachytene stage revealed no significant differences in the establishment of crossovers in the fraction of Parp-1 (−/−) oocytes showing complete synapsis of homologous chromosomes as determined by SYCP1 staining (green). One or two MLH1 foci (white arrows) can be detected on each bivalent. Scale bar=10 μM. B) The average number of MLH1 foci per oocyte at the pachytene stage showed no significant differences between wild type (n=36) and Parp-1 (−/−) oocytes (n=42); data are presented as the mean (±S.D.) after three independent experiments.
Acknowledgments
We are grateful to Dr. M. M. Viveiros for helpful discussions and comments during manuscript preparation and to Drs. W. Earnshaw, C. Heyting and C. Hoog for the gift of antibodies. This research was supported by Funds from the University of Pennsylvania Research Foundation and a grant from the National Institute of Child Health and Human Development (NICHD) National Institutes of Health (HD042740) to R. De La Fuente.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahel I, Ahel D, Matsusaka T, Clark AJ, Pines J, Boulton SJ, West SC. Poly(ADP-ribose)-binding zinc finger motifs in DNA repair/checkpoint proteins. Nature. 2008;451:81–5. doi: 10.1038/nature06420. [DOI] [PubMed] [Google Scholar]
- Althaus FRHL, Kleczkowska HE, Malanga M, Naegeli H, Panzeter PL, Realini CA. Histone shuttling by poly ADP-ribosylation. Mol Cell Biochem. 1994;138:53–9. doi: 10.1007/BF00928443. [DOI] [PubMed] [Google Scholar]
- Ame JC, Spelenhauer C, de Murcia G. The PARP superfamily. Bioessays. 2004;26:882–93. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A, Ashley T, Liskay RM. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet. 1996;13:336–42. doi: 10.1038/ng0796-336. [DOI] [PubMed] [Google Scholar]
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109:801–6. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- Bannister L, Schimenti J. Homologous recombinational repair proteins in mouse meiosis. Cytogenet Genome Res. 2004;107:191–200. doi: 10.1159/000080597. [DOI] [PubMed] [Google Scholar]
- Bannister LA, Pezza RJ, Donaldson JR, de Rooij DG, Schimenti KJ, Camerini-Otero RD, Schimenti JC. A dominant, recombination-defective allele of Dmc1 causing male-specific sterility. PLoS Biol. 2007;5:e105. doi: 10.1371/journal.pbio.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard P, Allshire R. Centromeres become unstuck without heterochromatin. Trends Cell Biol. 2002;12:419–24. doi: 10.1016/s0962-8924(02)02344-9. [DOI] [PubMed] [Google Scholar]
- Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–9. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Turner JM. The management of DNA double-strand breaks in mitotic G(2), and in mammalian meiosis viewed from a mitotic G(2) perspective. Bioessays. 2007;29:974–86. doi: 10.1002/bies.20639. [DOI] [PubMed] [Google Scholar]
- Burkle A. Poly(ADP-ribose). The most elaborate metabolite of NAD+ Febs J. 2005;272:4576–89. doi: 10.1111/j.1742-4658.2005.04864.x. [DOI] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–7. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry SM, Adelman CA, Theunissen JW, Hassold TJ, Hunt PA, Petrini JH. The Mre11 complex influences DNA repair, synapsis, and crossing over in murine meiosis. Curr Biol. 2007;17:373–8. doi: 10.1016/j.cub.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicheportiche A, Bernardino-Sgherri J, de Massy B, Dutrillaux B. Characterization of Spo11-dependent and independent phospho-H2AX foci during meiotic prophase I in the male mouse. J Cell Sci. 2007;120:1733–42. doi: 10.1242/jcs.004945. [DOI] [PubMed] [Google Scholar]
- Choo KH. Domain organization at the centromere and the neocentromere. Developmental Cell. 2001;1:165–177. doi: 10.1016/s1534-5807(01)00028-4. [DOI] [PubMed] [Google Scholar]
- Cleveland DWMY, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–21. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- D’Amours DDS, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem J. 1999;342:249–68. [PMC free article] [PubMed] [Google Scholar]
- Dantzer F, Mark M, Quenet D, Scherthan H, Huber A, Liebe B, Monaco L, Chicheportiche A, Sassone-Corsi P, de Murcia G, Menissier-de Murcia J. Poly(ADP-ribose) polymerase-2 contributes to the fidelity of male meiosis I and spermiogenesis. Proc Natl Acad Sci U S A. 2006;103:14854–9. doi: 10.1073/pnas.0604252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente R, Baumann C, Fan T, Schmidtmann A, Dobrinski I, Muegge K. Lsh is required for meiotic chromosome synapsis and retrotransposon silencing in female germ cells. Nat Cell Biol. 2006;8:1448–54. doi: 10.1038/ncb1513. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Viveiros M, Wigglesworth K, Eppig JJ. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev Biol. 2004;275:447–58. doi: 10.1016/j.ydbio.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Dernburg AF, Sedat JW, Hawley RS. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell. 1996;86:135–46. doi: 10.1016/s0092-8674(00)80084-7. [DOI] [PubMed] [Google Scholar]
- Dillon N, Festenstein R. Unravelling heterochromatin: competition between positive and negative factors regulates accessibility. Trends Genet. 2002;18:252–8. doi: 10.1016/s0168-9525(02)02648-3. [DOI] [PubMed] [Google Scholar]
- Earle E, Saxena A, MacDonald A, Hudson DF, Shaffer LG, Saffery R, Cancilla MR, Cutts SM, Howman E, Choo KH. Poly(ADP-ribose) polymerase at active centromeres and neocentromeres at metaphase. Hum Mol Genet. 2000;9:187–94. doi: 10.1093/hmg/9.2.187. [DOI] [PubMed] [Google Scholar]
- Ekwall K. Epigenetic control of centromere behavior. Annu Rev Genet. 2007;41:63–81. doi: 10.1146/annurev.genet.41.110306.130127. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo Allis CD, Nussenzweig A. Phosphorylation of histone H2B at DNA double-strand breaks. J Exp Med. 2004;199:1671–7. doi: 10.1084/jem.20032247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gieni RS, Chan GK, Hendzel MJ. Epigenetics regulate centromere formation and kinetochore function. J Cell Biochem. 2008;104:2027–2039. doi: 10.1002/jcb.21767. [DOI] [PubMed] [Google Scholar]
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–59. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–52. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- Guenatri M, Bailly D, Maison C, Almouzni G. Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. J Cell Biol. 2004;166:493–505. doi: 10.1083/jcb.200403109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haince JF, Kozlov S, Dawson VL, Dawson TM, Hendzel MJ, Lavin MF, Poirier GG. Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents. J Biol Chem. 2007;282:16441–53. doi: 10.1074/jbc.M608406200. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hall H, Hunt P. The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet. 2007;16(Spec No 2):R203–8. doi: 10.1093/hmg/ddm243. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: The genesis of human aneuploidy. Nature Reviews Genetics. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293:1098–102. doi: 10.1126/science.1062939. [DOI] [PubMed] [Google Scholar]
- Hodges CA, Hunt PA. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma. 2002;111:165–169. doi: 10.1007/s00412-002-0195-3. [DOI] [PubMed] [Google Scholar]
- Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun. 2005;336:807–12. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- Ihara M, Stein P, Schultz RM. UBE2I (UBC9), a SUMO-Conjugating Enzyme, Localizes to Nuclear Speckles and Stimulates Transcription in Mouse Oocytes. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.070474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail IH, Hendzel MJ. The gamma-H2A.X: is it just a surrogate marker of double-strand breaks or much more? Environ Mol Mutagen. 2008;49:73–82. doi: 10.1002/em.20358. [DOI] [PubMed] [Google Scholar]
- Kalitsis P, Earle E, Fowler KJ, Choo KH. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 2000;14:2277–82. doi: 10.1101/gad.827500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M, Eriksson JE, Gorbsky GJ. Differences in spindle association of the mitotic checkpoint protein Mad2 in mammalian spermatogenesis and oogenesis. Dev Biol. 2000;225:112–23. doi: 10.1006/dbio.2000.9818. [DOI] [PubMed] [Google Scholar]
- Kanai MUM, Hanai S, Uematsu N, Uchida K, Miwa M. Poly(ADP-ribose) polymerase localizes to the centrosomes and chromosomes. Biochem Biophys Res Commun. 2000;278:385–9. doi: 10.1006/bbrc.2000.3801. [DOI] [PubMed] [Google Scholar]
- Karpen GH, Allshire RC. The case for epigenetic effects on centromere identity and function. Trends Genet. 1997;13:489–96. doi: 10.1016/s0168-9525(97)01298-5. [DOI] [PubMed] [Google Scholar]
- Kim M, Mauro S, Gevry N, Lis JT, Kraus WL. NAD(+)-Dependent Modulation of Chromatin Structure and Transcription by Nucleosome Binding Properties of PARP-1. Cell. 2004;119:803–14. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Kim MY, Zhang T, Kraus WL. Poly(ADP-ribosyl)ation by PARP-1: ‘PARlaying’ NAD+ into a nuclear signal. Genes Dev. 2005;19:1951–67. doi: 10.1101/gad.1331805. [DOI] [PubMed] [Google Scholar]
- Lamb NE, Sherman SL, Hassold TJ. Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet Genome Res. 2005;111:250–5. doi: 10.1159/000086896. [DOI] [PubMed] [Google Scholar]
- Lammers J, Offenberg HH, van Aalderen M, Vink AC, Dietrich AJ, Heyting C. The gene encoding a major component of the lateral elements of synaptonemal complexes of the rat is related to X-linked lymphocyte-regulated genes. Mol Cell Biol. 1994;14:1137–46. doi: 10.1128/mcb.14.2.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond AI, Spector DL. Nuclear speckles: a model for nuclear organelles. Nat Rev Mol Cell Biol. 2003;4:605–12. doi: 10.1038/nrm1172. [DOI] [PubMed] [Google Scholar]
- Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol. 2008;10:42–52. doi: 10.1038/ncb1667. [DOI] [PubMed] [Google Scholar]
- Li X, Schimenti JC. Mouse pachytene checkpoint 2 (trip13) is required for completing meiotic recombination but not synapsis. PLoS Genet. 2007;3:e130. doi: 10.1371/journal.pgen.0030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby BJ, De La Fuente R, O’Brien MJ, Wigglesworth K, Cobb J, Inselman A, Eaker S, Handel MA, Eppig JJ, Schimenti JC. The mouse meiotic mutation mei1 disrupts chromosome synapsis with sexually dimorphic consequences for meiotic progression. Dev Biol. 2002;242:174–87. doi: 10.1006/dbio.2001.0535. [DOI] [PubMed] [Google Scholar]
- Llano E, Gomez R, Gutierrez-Caballero C, Herran Y, Sanchez-Martin M, Vazquez-Quinones L, Hernandez T, de Alava E, Cuadrado A, Barbero JL, Suja JA, Pendas AM. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400–13. doi: 10.1101/gad.475308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27:271–6. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Mol Biol Cell. 2005;16:5013–25. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder VS, Boeglin M, de Murcia G, Schreiber V. PARP-1 and PARP-2 interact with nucleophosmin/B23 and accumulate in transcriptionally active nucleoli. J Cell Sci. 2005;118:211–22. doi: 10.1242/jcs.01606. [DOI] [PubMed] [Google Scholar]
- Ménissier de Murcia J, Ricoul Michelle, Tartier Laurence, Niedergang Claude, Huber Aline, Dantzer Françoise, Schreiber Valérie, Amé Jean-Christophe, Dierich Andrée, LeMeur Marianne, Sabatier Laure, Pierre Chambon, Gilbert de Murcia. Functional interaction between PARP-1 and PARP-2 in chromosome stability and embryonic development in mouse. EMBO Journal. 2003;22:2255–2263. doi: 10.1093/emboj/cdg206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P, Kolas NK, Tarsounas M, Marcon E, Cohen PE, Spyropoulos B. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J Cell Sci. 2002;115:1611–22. doi: 10.1242/jcs.115.8.1611. [DOI] [PubMed] [Google Scholar]
- Murphy TD, Karpen GH. Centromeres take flight: alpha satellite and the quest for the human centromere. Cell. 1998;93:317–20. doi: 10.1016/s0092-8674(00)81158-7. [DOI] [PubMed] [Google Scholar]
- Page S, Hawley R. Chromosome choreography: the meiotic ballet. Science. 2003;301:785–9. doi: 10.1126/science.1086605. [DOI] [PubMed] [Google Scholar]
- Paul C, Povey JE, Lawrence NJ, Selfridge J, Melton DW, Saunders PT. Deletion of genes implicated in protecting the integrity of male germ cells has differential effects on the incidence of DNA breaks and germ cell loss. PLoS ONE. 2007;2:e989. doi: 10.1371/journal.pone.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull T, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–95. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–37. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004;14:R546–51. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Siomos MF, Nasmyth K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–40. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Pluta AF, Mackay AM, Ainsztein AM, Goldberg IG, Earnshaw WC. The centromere: hub of chromosomal activities. Science. 1995;270:1591–4. doi: 10.1126/science.270.5242.1591. [DOI] [PubMed] [Google Scholar]
- Saxena ASR, Wong LH, Kalitsis P, Choo KH. Centromere proteins Cenpa, Cenpb, and Bub3 interact with poly(ADP-ribose) polymerase-1 protein and are poly(ADP-ribosyl)ated. J Biol Chem. 2002a;277:26921–6. doi: 10.1074/jbc.M200620200. [DOI] [PubMed] [Google Scholar]
- Saxena A, Wong LH, Kalitsis P, Earle E, Shaffer LG, Choo KHA. Poly (ADP-ribose) polymerase 2 localizes to mammalian active centromeres and interacts with PARP-1, Cenpa, Cenpb and Bub3 but not Cenpc. Human Molecular Genetics. 2002b;11:2319–2329. doi: 10.1093/hmg/11.19.2319. [DOI] [PubMed] [Google Scholar]
- Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- Sudakin V, Chan GK, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J Cell Biol. 2001;154:925–36. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiech L, Kisiel K, Czolowska R, Zientarski M, Borsuk E. Accumulation and dynamics of proteins of the MCM family during mouse oogenesis and the first embryonic cell cycle. Int J Dev Biol. 2007;51:283–95. doi: 10.1387/ijdb.062239ls. [DOI] [PubMed] [Google Scholar]
- Taddei A, Maison C, Roche D, Almouzni G. Reversible disruption of pericentric heterochromatin and centromere function by inhibiting deacetylases. Nat Cell Biol. 2001;3:114–20. doi: 10.1038/35055010. [DOI] [PubMed] [Google Scholar]
- Thiriet C, Hayes JJ. Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–22. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Truchet S, Chebrout M, Djediat C, Wietzerbin J, Debey P. Presence of permanently activated signal transducers and activators of transcription in nuclear interchromatin granules of unstimulated mouse oocytes and preimplantation embryos. Biol Reprod. 2004;71:1330–9. doi: 10.1095/biolreprod.104.029405. [DOI] [PubMed] [Google Scholar]
- Tulin A, Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–2. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- Tulin A, Stewart D, Spradling AC. The drosophila heterochromatic gene encoding poly (ADP-ribose) polymerase (PARP) is required to modulate chromatin structure during development. Genes and Development. 2002;16:2108–2119. doi: 10.1101/gad.1003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GV, Barrett JC, Burgoyne PS, Deng CX. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14:2135–42. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Turner J, Mahadevaiah SK, Fernandez-Capetillo O, Nussenzweig A, Xu X, Deng CX, Burgoyne PS. Silencing of unsynapsed meiotic chromosomes in the mouse. Nat Genet. 2005;37:41–7. doi: 10.1038/ng1484. [DOI] [PubMed] [Google Scholar]
- Wang H, Hoog C. Structural damage to meiotic chromosomes impairs DNA recombination and checkpoint control in mammalian oocytes. J Cell Biol. 2006;173:485–95. doi: 10.1083/jcb.200512077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Stingl L, Morrison C, Jantsch M, Los M, Schulze-Osthoff K, Wagner EF. PARP is important for genomic stability but dispensable in apoptosis. Genes Dev. 1997;11:2347–58. doi: 10.1101/gad.11.18.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y. Sister chromatid cohesion along arms and at centromeres. Trends Genet. 2005;21:405–12. doi: 10.1016/j.tig.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Webster KE, O’Bryan MK, Fletcher S, Crewther PE, Aapola U, Craig J, Harrison DK, Aung H, Phutikanit N, Lyle R, Meachem SJ, Antonarakis SE, de Kretser DM, Hedger MP, Peterson P, Carroll BJ, Scott HS. Meiotic and epigenetic defects in Dnmt3L-knockout mouse spermatogenesis. Proc Natl Acad Sci U S A. 2005;102:4068–73. doi: 10.1073/pnas.0500702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens GR, Sorger PK. Centromeric chromatin and epigenetic effects in kinetochore assembly. Cell. 1998;93:313–6. doi: 10.1016/s0092-8674(00)81157-5. [DOI] [PubMed] [Google Scholar]
- Yelamos J, Schreiber V, Dantzer F. Toward specific functions of poly(ADP-ribose) polymerase-2. Trends Mol Med. 2008;14:169–78. doi: 10.1016/j.molmed.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Yuan L, Liu JG, Hoja MR, Wilbertz J, Nordquist K, Hoog C. Female germ cell aneuploidy and embryo death in mice lacking the meiosis-specific protein SCP3. Science. 2002;296:115–118. doi: 10.1126/science.1070594. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Goeres J, Zhang H, Yen TJ, Porter AC, Matunis MJ. SUMO-2/3 modification and binding regulate the association of CENP-E with kinetochores and progression through mitosis. Mol Cell. 2008;29:729–41. doi: 10.1016/j.molcel.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. PARP-1 exhibits a diffuse nuclear localization throughout the different stages of meiotic prophase I in the female germ line
A) Wild-type (WT) oocyte at the leptotene stage showing initial formation of the lateral elements of the synaptonemal complex stained with anti-SYCP3 antibody (green). PARP-1 (red) is an abundant protein that exhibits diffuse nucleoplasmic staining. (B) Representative micrograph of a WT oocyte showing the chromosome morphology typical of the zygotene stage as detected by SYCP3 staining (green) and prominent nucleoplasmic PARP-1 staining (red). (C) Parp-1 (−/−) oocyte at the zygotene stage, as detected by the patterns of SYCP3 staining (green), used as a negative control lacks PARP-1 staining and thus confirms antibody specificity. Scale bar=10 μM.
Supplemental Figure 2. Establishment of crossovers in Parp-1 (−/−) oocytes showing complete synapsis of homologous chromosomes
A) Comparison of the chromosomal distribution of the mismatch repair protein MLH1 (red) in wild type oocytes at the pachytene stage revealed no significant differences in the establishment of crossovers in the fraction of Parp-1 (−/−) oocytes showing complete synapsis of homologous chromosomes as determined by SYCP1 staining (green). One or two MLH1 foci (white arrows) can be detected on each bivalent. Scale bar=10 μM. B) The average number of MLH1 foci per oocyte at the pachytene stage showed no significant differences between wild type (n=36) and Parp-1 (−/−) oocytes (n=42); data are presented as the mean (±S.D.) after three independent experiments.


