Abstract
The dystroglycans (α-DG and β-DG), which play important roles in the formation of basement membranes, have been well studied in skeletal muscle and nerve, but their expression and localization in intestinal epithelial cells has not been previously investigated. Here, we demonstrated that the DG complex, composed of α-DG, β-DG, and utrophin, is specifically expressed in the basolateral membrane of the Caco-2-BBE monolayer. The DG complex coprecipitated with β1-integrin, suggesting a possible interaction among these proteins. In addition, we observed that activation of DG receptors by laminin-1 enhanced the interaction between β1-integrin and laminin-1, whereas activation of DG receptors by laminin-2 reduced the interaction between β1-integrin and laminin-2. Finally, we demonstrated that the intracellular COOH-terminal tail of β-DG and its binding to the DG binding domain of utrophin are crucial for the interactions between laminin-1/-2 and β1-integrin. Collectively, these novel results indicate that dystroglycans play important roles in the regulation of interactions between intestinal epithelial cells and the extracellular matrix.
Keywords: laminin, β1-integrin, Caco-2-BBE, electric cell-substrate impedance sensing
Dystroglycans (DGs) were first identified in skeletal muscle as a component of the dystrophin-glycoprotein complex (DGC) (15). DGs are composed of α- and β-subunits derived from a posttranslational modification of a protein encoded by a single gene (25, 26). In skeletal muscle, α-DG is an extracellular peripheral membrane glycoprotein that binds to laminin-2 in the extracellular matrix (ECM) (47), whereas β-DG is a transmembrane glycoprotein that anchors α-DG to the membrane (56) and binds intracellularly to dystrophin/utrophin and the associated cytoskeletal F-actin (29, 48). Thus the DGs span the sarcolemma and provide a link between the ECM and the cytoskeleton (14). Mutations in genes encoding a number of DGC components have been shown to disrupt this DG-mediated linkage, leading to various forms of muscular dystrophies in humans and mice (7). These biochemical and genetic data suggest that the connection between the ECM and the cytoskeleton provided by the DG complex serves to stabilize the sarcolemma during contraction-induced stress. However, to date, no form of muscular dystrophy has been associated to the DG gene itself. Unlike some of the other DGC components, DG is expressed in a broad array of adult tissues (25, 26). In addition, several dystrophin and utrophin isoforms are ubiquitously expressed (6, 44). Of these, DG is the most widely expressed component; it is found at high levels in many cell types including epithelial cells, where it is particularly prominent on the basal sides that face basement membranes (11, 25, 52). Studies have shown that α-DG binds to other molecules such as laminin-1, agrin, and perlecan (18, 19, 43, 49), whereas β-DG binds to dystrophin isoforms Dp71, Dp116, and Dp260 (29), and has been shown to be associated with utrophin (28). Thus different DG complexes may form in different tissues, implying that DG may have important roles outside of skeletal muscle. Indeed, DG has been implicated as a laminin/agrin receptor involved in epithelial cell development, basement membrane formation, and synaptogenesis (11, 22, 52).
Full-length dystrophin (Dp427) is mainly expressed in muscle (3) and in the brain (23). Other NH2-terminally truncated isoforms have been described (Dp260, Dp140, Dp116, and Dp71); of these, Dp71 appears to be ubiquitously expressed (6, 17). Utrophin (Up395) is a dystrophin homolog that shows some conserved expression of alternative transcripts (Up140, Up113, and Up71) (2, 53, 57). The large utrophin protein is widely expressed, and is most abundant in several nonskeletal muscle tissues (16, 40), including small intestine epithelial cells (36, 44). It is not known whether the dystrophin-associated protein complex is assembled with utrophin at these sites; in general, little is known about the function of utrophin, dystrophin, and the DGC in nonmuscle tissues such as intestinal epithelial cells.
In addition to DGs, other cell surface receptors for laminins are the integrins (24), which are a large family of cell surface receptors involved in cell adhesion to the ECM. In epithelial cells, the main integrins belong to the β1 and β4 classes and bind to basement membrane molecules such as laminins and type IV collagens. Previous studies have shown that integrins and DGs can play critical roles in cell morphogenesis and growth control of epithelial cells (38). Although previous studies have examined the integrin complex in intestinal epithelial cells (1), no study has investigated nonintegrin laminin receptors in intestinal epithelial cells. Here, we examined the expression and role(s) of DG in the cell/ECM interactions of intestinal epithelial cells.
MATERIALS AND METHODS
Antibodies
The mouse anti-β-DG monoclonal antibody (MAb), 43DAG, was obtained from Novocastra (Burlingame, CA) and the goat polyclonal anti-β-DG was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-α-DG MAbs, VIA41 and IIH6, were obtained from Upstate Biotechnologies (Lake Placid, NY). In the function-blocking experiments, the IIH6 antibody was used as a function-blocking antibody, and VIA41 was used as a control at a minimum concentration of 10 μg/ml (4, 11, 13). The mouse anti-utrophin MAb, MANCHO7 (39), was kindly donated by Professor G. E. Morris (N. E. Wales Institute). The mouse anti-β1-integrin MAb, MAB1959, was obtained from Chemicon International (Temecula, CA) and was also used for the function-blocking studies at 10 μg/ml. The mouse anti-β3 integrin MAb, MAB1957, was obtained from Chemicon International and used as the control in the function-blocking experiments at 10 μg/ml. The anti-V5 MAb was obtained from Invitrogen (Carlsbad, CA). For irrelevant antibodies, the mouse anti-IgG1 and IgG2 isotypes MAb (R&D Systems, Minneapolis, MN), were used as negative controls for immunoprecipitations and confocal experiments. For the secondary antibodies, horseradish peroxidase-linked anti-mouse antibodies were purchased from Amersham Bioscience (Buckinghamshire, UK) and fluorescein isothiocyanate-conjugated/ -goat anti-mouse immunoglobulin IgG was purchased from Santa Cruz Biotechnology.
Cell culture
Caco-2-BBE (5, 37) cells (passages 30–50) were grown in high glucose Dulbecco’s Vogt modified Eagle’s media (Invitrogen) supplemented with 14 mM NaHCO3, 10% (vol/vol) heat-inactivated fetal bovine serum (Invitrogen), and 1.5 μg/ml Plasmocin (Invitrogen). Transfected cells were maintained in the same medium containing 1.2 mg/ml G418 (Life Technologies, Rockville, MD). Cells were kept at 37°C in 5% CO2 and 90% humidity, and the medium was changed every day. Monolayer was subcultured every 7 days by trypsinization with 0.25% trypsin (Gibco, Invitrogen). Biotinylation and confocal microscopy experiments were performed with confluent monolayer plated on permeable supports (area = 1 cm2; pore size = 0.4 μm; Transwell-Clear polyester membranes from Costar VWR, Suwannee, GA) and examined 15 days postplating. For protein, membrane, or RNA extractions, Caco-2-BBE cells were plated in six-well plates (Costar VWR) at a density of 104 cells/cm2 and examined 15 days postplating. For experiments using the electric cell-substrate impedance sensing (ECIS) system, 8W1E arrays were used (Applied BioPhysics, Troy, NY). Cells were trypsinized, counted, and plated at 0.2 × 106 cells/well. In experiments studying cell adhesion to protein-coated surfaces, we flooded the electrode with a 10 μg/ml solution of laminin-1 (Sigma, St. Louis, MO) or laminin-2 (Chemicon International) in phosphate-buffered saline (PBS) for 30–40 min at room temperature. Human laminin-2 protein was affinity purified with the anti-laminin-2 specific MAb 5H2 (MAB1922) and was thus immunologically and biologically similar to human laminin-2 (Chemicon International).
Reverse transcription polymerase chain reaction (RT-PCR)
The expressions of DG and utrophin in Caco-2-BBE cells were determined by RT-PCR using oligonucleotide primers specific for DG and utrophin. Total RNA was isolated from Caco-2-BBE cells using a Qiagen kit (Qiagen, Valencia, CA). RT was carried out using random oligonucleotide primers to generate first-strand cDNA, and PCR conditions were determined according to the primer characteristics. The following specific primers were designed and ordered from Invitrogen: single DG gene, sense 5′-AAC CCA ACC AGC GCC CAG AGC-3′, antisense 5′-CGG GTG ATA TTC TGC AGG GTG ATG G-3′; utrophin isoform Up71, sense 5′-TGG GGA AGA TGT ACG AGA CT-3′, antisense 5′-TGA TGA GTG TGA TAG GAG TCT-3′; utrophin isoform Up113, sense 5′-CCT GAA TAA TGT ACG TTT TTC TGC-3′, antisense 5′-GAG ACA CAT ATC AAC ACA GAG T-3′; utrophin isoform Up140, sense 5′-AAA GGT GGT GCT AGT ATC ATC TGC G-3′, antisense 5′-CTG AGG ATG GCG CTG TTC TAA G-3′; utrophin isoform Up395, sense 5′-AGA GCA GTG TGG GCA GCG TC-3′, antisense 5′-CAT CAT CCA GGG GGC AAG TTT CCA-3′; β-actin (internal control), sense 5′-TCA CCC ACA CTG TGC CCA TCT-3′, antisense 5′-ACG GAG TAC TTG CGC TCA GG-3′. PCR products were electrophoresed on ethidium bromide-stained 1% agarose gels in Tris-acetate-EDTA.
Plasmid construction and transfections
The full-length DG cDNA was PCR cloned using specific primers (sense 5′-GGG ATG AGG ATG TCT GTG GGC CTC TCG CTG CTG-3′, antisense 5′-AGG TGG GAC ATA GGG AGG AGG-3′). To generate the COOH-terminally truncated DG, we used a different antisense primer (5′-CTT CTC CTC CTG CAG AAT GAG TGG CA-3′). The DG binding domain of the utrophin (residues between 3057 and 3107) was amplified using the following utrophin-specific primers: sense 5′-CGA GTG GCA GCA GCG GAG ACT-3′, antisense 5′-GTG ACC TTT TGC TGT TCG ACC CG-3′. This region of 51 amino acids includes the three cysteine residues C3070, C3073, and C3076 that were shown to be the most critical residues for the binding between utrophin and β-DG (27). After gel extraction using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), the PCR products representing DG, truncated DG, and the DG binding domain of utrophin were ligated into the pcDNA 3.1/V5-His TOPO TA expression vector (Invitrogen). Plasmids were purified using the Qiaprep Miniprep kit (Qiagen) and sequenced (Lark Technologies, Houston, TX). The V5 epitope is present in the COOH-terminal side of the inserted protein (Fig. 3A). Subconfluent Caco-2-BBE cells were stably transfected with the various constructs using Lipofectine (Invitrogen) according to the manufacturer’s instructions. Transfectants were selected in medium containing 1.2 mg/ml G418 (Life Technologies).
Fig. 3.
Basolateral membrane expression of V5-tagged β-DG in Caco-2-BBE monolayers. We generated three types of constructs. One expressing the whole human DG gene (A1), a construct expressing a COOH-terminal truncated mutant of the DG lacking amino acids 816–895 (A2), and a construct expressing the utrophin amino acids 3057–3107 corresponding to its binding domain to DG (A3). Western blot analysis was performed on whole cell lysates from Caco-2-BBE cells transfected with DG (B1, lane 1), empty vector (B1, lane 2), truncated DG (B1, lane 3), and DG binding domain of utrophin (B2). Whole cell proteins (50 μg/lane) were subjected to 4–20% SDS-polyacrylamide-gel electrophoresis, and blots were immunostained with a mouse anti-V5 antibody (B1 and B2). Caco-2-BBE cells transfected with the DG binding domain of utrophin and with the empty vector were immunoprecipitated with anti-β-DG antibody, and the immunoprecipitates were immunoblotted with anti-V5 antibody (B3). Confocal microscopy was used to localize actin (red) and V5-tagged DG (green) in Caco-2-BBE monolayers transfected with the V5-tagged DG (C1). Horizontal sections (xy) were taken near the basolateral domain of the monolayer. C2 is a negative control showing the actin localization on the monolayer without the anti-V5 antibody. C3 is stained with an irrelevant IgG isotype antibody (green) as negative control together with actin (red).
Western blot analysis
For total protein extraction, cells were lysed for 30 min at 4°C in ristocetin-induced platelet aggregation (RIPA) lysis buffer [150 mM NaCl, 0.5% sodium deoxycholate, 50 mM Tris · HCl (pH 8.0), 0.1% SDS, 0.1% NP-40] supplemented with protease inhibitors (Roche Diagnostics, Mannheim, Germany). The homogenates were centrifuged at 13,000 g for 30 min at 4°C, and the supernatants were collected for Western blot analysis. Protein concentrations were determined using the Lowry method (DC protein assay; Bio-Rad, Hercules, CA). Protein extracts were mixed in tricine sample buffer (Bio-Rad), boiled for 5 min, separated on a 4–20% gradient Tris-glycine-SDS polyacrylamide gel (Costar VWR), and then transferred to nitrocellulose membranes. Membranes were blocked overnight at 4°C or for 1 h at room temperature with 5% nonfat milk in blocking buffer and then incubated 1 h at room temperature with the specific primary antibody using the manufacturer’s recommended dilutions. After three 10-min washes in blocking buffer, membranes were incubated for 1 h at room temperature with the appropriate horseradish peroxidase-conjugated secondary antibody (diluted 1:1,000). Membranes were washed three times, for 10 min each time, in blocking buffer and immunoreactive proteins were detected on films using an enhanced chemiluminescence substrate according to the manufacturer’s instructions (Amersham Biosciences, Uppsala, Sweden).
Cell surface biotinylation
Cells were grown on filters and rinsed twice with PBS supplemented with 0.1 mM CaCl2 and 1 mM MgCl2. The apical or basolateral sides of the monolayer were incubated with freshly prepared sulfosuccinimidobiotin (EZ-link sulfo-NHS-biotin; Pierce, Rockford, IL) in PBS supplemented with 0.1 mM CaCl2 and 1 mM MgCl2 (1 mg/ml) for 30 min at 4°C. The reaction was quenched with 50 mM NH4Cl (5 min at room temperature). Cells were then scraped off and lysed for 30 min at 4°C in (RIPA) lysis buffer supplemented with protease inhibitors (Roche Diagnostics). After 30 min centrifugation (13,000 g at 4°C), protein concentrations were determined using the Bradford protein assay (Bio-Rad), and the supernatants were incubated with immobilized NeutrAvidin (Pierce) overnight at 4°C to bind biotinylated proteins. After centrifugation, the beads were washed twice in PBS (20 min at 4°C), once in a buffer of 500 mM NaCl, 20 mM Tris (pH 8.0), 0.5% Triton X-100, and 0.2% BSA, and rinsed in PBS. The beads were then boiled for 5 min in tricine sample buffer (Bio-Rad), and Western blot analysis was performed as described above.
Membrane preparations
For membrane preparations, cells were washed twice in PBS and then scraped into PBS. After centrifugation (400 g for 10 min), each cell pellet was resuspended and carefully homogenized with a Dounce homogenizer in HEPES (5 mM) containing protease inhibitors. After centrifugation at 13,000 g for 30 min at 4°C, the resulting pellet was suspended in PBS by repeated passage through an 18-gauge needle. The protein concentrations in the membrane suspension and total extracts were quantified using the Bradford assay.
Confocal immunofluorescence
Caco-2-BBE cells grown on filters were washed twice with PBS (pH 7.4) (Invitrogen) supplemented with 0.1 mM CaCl2 and 1 mM MgCl2 (PBS-Ca/Mg), and fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Washington, PA) in PBS-Ca/Mg for 15 min at room temperature. After three washes with PBS-Ca/Mg, cells were preincubated at room temperature for 30 min in immunostaining buffer (PBS-Ca/Mg with 3% BSA and 0.1% Triton X-100) and incubated for 40 min at room temperature with Alexa Fluo 568-conjugated phalloidin (1 U/filter; Molecular Probes, Eugene, OR) diluted in immunostaining buffer. Cells were then washed twice in PBS and exposed to mouse anti-utrophin MAb or anti-V5 or irrelevant anti-IgG isotype for 1 h at room temperature. After washing twice in PBS, cells were incubated with a goat anti-mouse antibody conjugated to Alexa Fluo 488 (1:200, Molecular Probes), for 1 h at room temperature. After washing, coverslips were mounted with the Slowfade medium (Molecular Probes) and microscopy was performed using a Zeiss epifluorescence microscope equipped with a Bio-Rad MRC600 confocal unit, computer, and laser-scanning microscope image analysis software (Carl Zeiss, Jena, Germany) (5).
Cell attachment/spreading assays
Cell attachment studies were carried out using the ECIS technology (Applied BioPhysics) (20, 21, 31). The ECIS technology has been proved to be one of the most precise and sensitive research methods to monitor real-time cell spreading and attachment. Although ECIS does not directly detect cell attachment, it does detect cell spreading, and this requires cell attachment. ECIS measures cell spreading as a reduction in the electrode capacitance or an increase in electrode resistance and impedance. The ECIS device is based on alternating current (AC) impedance measurements using weak and noninvasive AC signals, as previously described (51). Attachment and spreading of cells on the electrode surface changes the impedance, allowing morphological interpretations of the attached cells. The measurement system consisted of an eight-well culture dish (ECIS 8W1E plate) kept at 37°C in 5% CO2 and 90% humidity. Each well contained a small active electrode (area = 5 × 10−4 cm2) and a large counter electrode (area = 0.15 cm2) at the bottom of each well. A lock-in amplifier (model SR830; Stanford Research Systems, Sunnyvale, CA) with an internal oscillator relay was used to switch among the different wells, and a computer was used to control measurements and store data. For the capacitance measurements of Caco-2-BBE cells, we used a frequency of 40 kHz and a voltage of 1 V. The capacitance data at 40 kHz has been shown to be a measure of the amount of open electrode area. Under these operating conditions, the plasma membranes of attached cells acted as insulating particles, blocking current flow and allowing detection. The measurements essentially report only the fraction of the electrode covered with cells and therefore mimic the data obtained with normal microscopy. Details on the operation, equivalent resistance-capacitance circuit, and modification of the ECIS system have been described elsewhere (55). Capacitance measurements were collected from each well every 30 s. In experiments studying cell adhesion to a protein-coated surface, we flooded the electrode with a solution of the relevant protein in saline for 60 min. For dose-response experiments, electrodes were separately coated with laminin-1 or laminin-2 at concentrations of 0.1, 1, 10, and 20 μg/ml. Cell attachment inhibition assays were performed based on the cell adhesion assays. Cell suspensions [4 × 105 cells/ml in serum-free DMEM containing 10 mM HEPES (pH 7.4) and 0.5% BSA] were incubated with 10 μg/ml of function-blocking MAb or control antibody for 20 min at room temperature. The preincubated cells were added to the wells that had been coated with different proteins. We determined the capacitance shift (s = −ΔC/Δt) between C = 4 nF and C = 2 nF by means of linear regression, as described elsewhere (51); “s” corresponds to the rate of cell spreading. We chose this range of capacitance data for analysis, as it more or less symmetrically embraced the capacitance values at t1/2. We calculated the t1/2 for each result.
Statistics
Results are expressed as means ± SE. Statistical significance was determined using the ANOVA test.
Immunoprecipitation
Cells were washed with ice-cold PBS and then lysed on ice in 1 ml of lysis buffer [50 mM Tris · HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40] containing 1 tablet of protease inhibitors. The lysates were centrifuged at 12,000 g for 30 min at 4°C, and the resulting supernatants were subjected to immunoprecipitation and immunoblot analysis. For immunoprecipitation, supernatants were transferred to fresh tubes, the appropriate amount of specific antibody (1/50 dilution) was added, and samples were gently rocked for 3 h at 4°C. Subsequently, 50 μl of protein G suspension was added to the mixture and incubated overnight at 4°C. Beads alone and irrelevant anti-IgG isotype MAb were used as negative controls. The complexes were collected by centrifugation at 12,000 g for 20 s by microcentrifuge. The beads were washed for 20 min each twice with buffer 1 [50 mM Tris · HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P40], twice with buffer 2 [50 mM Tris · HCl (pH 7.5), 500 mM NaCl, 1 mM EDTA, 0.1% Nonidet P-40] and once with buffer 3 [10 mM Tris · HCl (pH 7.5), 0.1% Nonidet P-40]. The agarose pellets were overlain with 50 μl gel Tricine loading buffer (Bio-Rad), boiled 5 min at 100°C, and subjected to Western blot analysis as described above.
TNT expression
We used the TNT T7 quick coupled transcription/translation system (Promega) to verify our constructs. We mixed 40 μl of TNT T7 quick master mix, 2 μl of [35S]methionine, 1 μg of pcDNA 3.1/V5-His TOPO TA expression vector, and H2O for a total volume of 50 μl. The mixture was incubated at 30°C for 90 min. After addition of 20 μl of Tricine sample buffer, the product was denatured at 65°C for 10 min and resolved by 4–20% SDS-PAGE. The resulting gel was dried with a model 583 gel dryer (Bio-Rad) and exposed to film (Pharmacia) overnight at room temperature.
RESULTS
Caco-2-BBE cells express DG and utrophin
The expression of DG and utrophin genes in Caco-2-BBE cells was analyzed by RT-PCR. We observed the expression of the single DG mRNA and two utrophin isoforms, apparently Up140 (exons 46–47) and Up395 (exons 10–11) (results not shown). DG and utrophin expression was further tested at the protein level. Immunoblot experiments performed on Caco-2-BBE whole cell, membrane, and cytosol extracts showed that both α-DG and β-DG were present predominantly in the membrane fraction (Fig. 1, A1 and A2). A single broad band was detected for α-DG with a molecular mass of ~120 kDa (Fig. 1A1), whereas β-DG was identified as a ~43-kDa band accompanied by a ~31-kDa band (Fig. 1A2), presumably originating from proteolytic fragmentation and/or changes in glycosylation (14, 25, 26). For β-DG, an intermediate band was also observed (Fig. 1A2); consistent with what was previously described in other cell lines (35). Immunoblotting for utrophin unexpectedly revealed a ~200-kDa band in addition to the ~395-kDa full-length band (Fig. 1A3). We also noticed two other faint bands of ~120 and ~130 kDa (Fig. 1A3). These utrophin isoforms were mainly expressed in the cytosol fraction, whereas the full-length protein was strongly detected in the whole cell lysate fraction and was present at a relatively low level in the membrane fraction (Fig. 1A3).
Fig. 1.
α-Dystroglycan (α-DG), β-DG, and utrophin are expressed in Caco-2-BBE monolayers and coprecipitate with β1-integrin. Cytosol (C), membrane (M), and whole cell (WC) lysate proteins (50 μg/lane) from Caco-2-BBE were subjected to a 4–20% SDS-polyacrylamide-gel electrophoresis, and blots were immunostained with a VIA41 anti-α-DG MAb (A1), 43DAG anti-β-DG MAb (A2), and MANCHO7 anti-utrophin MAb (A3). Caco-2-BBE cell lysates were immunoprecipitated with anti-α-DG (B1 and B6), anti-β-DG (B2 and B4), anti-utrophin (B3), and anti-β1-integrin (B5). Immunoprecipitates were immunoblotted with anti-β-DG (B1 and B3), anti-α-DG (B2 and B5), anti-utrophin (B4), and anti-β1-integrin (B6). Beads alone and irrelevant IgG isotype antibodies were used as negative controls for each experiment. No bands were detected for the entire negative control experiments.
DGs and utrophin proteins form a common complex in Caco-2-BBE cells
As shown in Fig. 1, B1 and B2, when α-DG immunoprecipitates were probed with an anti-β-DG antibody, we observed the expected immunoreactive band at ~43 kDa (Fig. 1B1), and when β-DG immunoprecipitates were probed with an anti-α-DG antibody, we observed the expected immunoreactive band at ~120 kDa (Fig. 1B2). These results demonstrate that α-DG and β-DG co-precipitate. We then tested whether DG associates with utrophin in Caco-2-BBE cells. When utrophin immunoprecipitates were probed with an anti-β-DG antibody, a specific immunoreactive band was observed at ~43 kDa associated with a light ~31-kDa band (Fig. 1B3). In addition, when β-DG immunoprecipitates were probed with an anti-utrophin antibody, we observed immunoreactive bands at ~120, ~130, ~200, and ~395 kDa, representing the major forms of utrophin in Caco-2-BBE cells (Fig. 1B4). For all experiments, no bands were detected using beads only as a negative control or when using irrelevant isotype MAb. Together, these results indicate that α-DG, β-DG, and utrophin form a common complex in Caco-2-BBE epithelial cells.
α-DG associates with β1-integrin in Caco-2-BBE cells
We tested whether α-DG was associated with β1-integrin in Caco-2-BBE cells. When β1-integrin immunoprecipitates were probed with an anti-α-DG antibody, we observed the expected immunoreactive band at ~120 kDa (Fig. 1B5). When α-DG immunoprecipitates were probed with an anti-β1-integrin antibody, we observed an immunoreactive band at ~110 kDa, representing β1-integrin (Fig. 1B6). No apparent band was visualized in the beads used only as a negative controls or when using irrelevant IgG isotype MAb. Together, these results suggest that α-DG and β1-integrin associate in Caco-2-BBE cells.
DGs and utrophin are basolaterally polarized in Caco-2-BBE cells
The membrane localizations of the human DGs and utrophin were assessed in confluent Caco-2-BBE monolayer. We examined the plasma membrane expression of DGs and utrophin by surface biotinylation and confocal immunofluorescence microscopy. Plasma membrane domain-specific proteins were separately labeled by biotinylating each of them (apical and basolateral) (Fig. 2A). Under nonreducing conditions, Western blots probed with anti-α-DG showed a band at ~120 kDa (Fig. 2A1, lane 2), and the anti-β-DG displayed one immunoreactive band at ~43 kDa and one lighter band at ~31 kDa, all exclusively on the basolateral membrane (Fig. 2A2, lane 2). As a control experiment, we showed that Na-K-ATPase (a basolateral marker) reacted with the appropriate bands at ~112 and ~150 kDa under nonreducing conditions (Fig. 2A3, lane 2), all exclusively on the basolateral membrane, whereas hPepT1 (an apical marker) reacted with the appropriate band at ~75 kDa under nonreducing conditions (Fig. 2A4, lane 1). Confocal microscopy revealed that utrophin was strongly present in the basolateral membrane (Fig. 2B) but showed only slight/questionable positive expression in the apical plasma membrane. No positive expression was observed using irrelevant IgG isotype MAb. Together, these results demonstrated that the DGs and utrophin are basolaterally polarized in Caco-2-BBE cells.
Fig. 2.
DGs and utrophin are basolaterally polarized in Caco-2-BBE monolayers. Filter-grown Caco-2-BBE monolayers were subjected to domain-specific biotinylation (ap, apical domain; bl, basolateral domain) for 30 min followed by Western blot analysis of total cell lysates. Biotinylated proteins were separated on a 4–20% SDS-polyacrylamide-gel and transferred to a nitrocellulose membrane. Blots were immunostained with anti-α-DG (A1), anti-β-DG (A2), anti-Na-K-ATPase (A3), or anti-hPepT1 antibodies (A4). Confocal microscopy was used to localize actin (red) and utrophin (green) in Caco-2-BBE monolayers. Horizontal sections (xy) were taken near the basolateral domain in Caco-2-BBE monolayers (B1). Irrelevant IgG isotype antibody (green) was used as negative control (B2) together with actin (red).
Overexpression of DG, truncated DG, and DG binding domain of utrophin in Caco-2-BBE cells
Caco-2-BBE cells expressing V5-DG (Fig. 3A1), truncated V5-DG (Fig. 3A2), and V5-DG binding domain of utrophin (Fig. 3A3) were isolated in selective media (see MATERIALS AND METHODS). All constructs were verified by the TNT T7 quick coupled transcription/translation system (Promega) prior to transfections (results not shown). V5-DG, truncated V5-DG, and V5-DG binding domain of utrophin proteins expressed in Caco-2-BBE cells were detected by Western blot analysis using an anti-V5 antibody. Caco-2-BBE cells transfected with V5-DG displayed one immunoreactive band at ~43 kDa (Fig. 3B1, lane 1), representing the expected size of β-DG fused with the V5 epitope. Under these conditions, α-DG was not detected because the DG gene product was postranslationally cleaved into an NH2-terminal α-DG and a COOH-terminal β-DG (26). Caco-2-BBE cells transfected with mutated V5-DG showed one immunoreactive band at ~35 kDa, representing the expected size of V5-DG without the COOH-terminal amino acid sequence (Fig. 3B1, lane 3). Caco-2-BBE cells transfected with an empty vector (negative control) did not react with the anti-V5 antibody (Fig. 3B1, lane 2). Finally, the V5-DG binding domain of utrophin-transfected Caco-2-BBE cells showed one immunoreactive band at ~12 kDa (Fig. 3B2), representing the expected size of the V5-DG binding domain of the utrophin construct. Furthermore, to confirm that the DG binding domain of the utrophin (residues between 3057 and 3107) binds to the β-DG, we immunoprecipitated the whole cell lysate of Caco-2-BBE transfected with DG binding domain of utrophin using the anti-β-DG antibody. The immunoprecipitate was analyzed using an anti-V5 antibody (Fig. 3B3). We obtained two clear bands at ~42 and ~55 kDa. The ~55-kDa band suggests a binding of the DG binding domain of the utrophin (residues between 3057 and 3107, ~12 kDa) with the β-DG (~43 kDa). The ~42-kDa band can be explained as a result of the association between DG binding domain of the utrophin and the ~31-kDa band of β-DG (Fig. 1A2). The binding domain of the β-DG to utrophin is conserved in its 31-kDa form (35). The association between the 31-kDa form of β-DG and the ~12 kDa of the transfected V5-DG binding domain of the utrophin fragment should be the same as the binding between the full-length β-DG and the full utrophin (27). The stability of this association under denaturing conditions suggests a strong binding. As negative control, we used the vector alone transfected cells as initial lysate and no immunoreactive band was detected.
Confocal microscopy revealed that V5-DG was mostly localized at the basolateral membrane (Fig. 3, C1 and C2). Similar membrane localizations were found for truncated V5-DG and the V5-DG binding domain of utrophin (results not shown).
Kinetics of Caco-2-BBE cell attachment and spreading on different extracellular matrixes
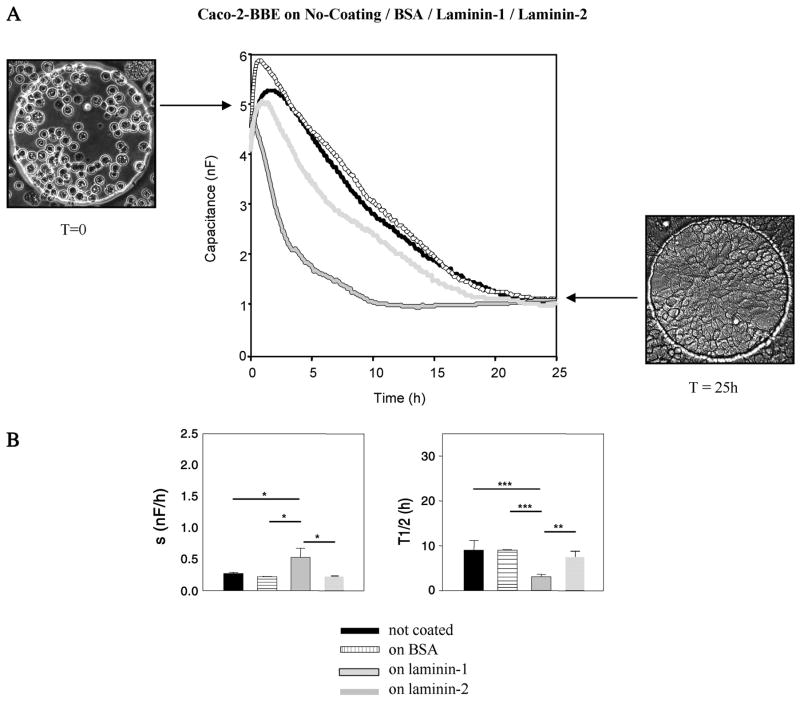
We demonstrated that the time course capacitance measured at 40 kHz of Caco-2-BBE cells spread differently on electrodes coated with laminin-1 and laminin-2 compared with electrodes coated with BSA or not coated (Fig. 4). We examined the capacitance shift (s), the time (t1/2) necessary for Caco-2-BBE cells to spread out on half the available electrode, and the rate of Caco-2-BBE cell spreading (Fig. 4B). Cells on electrodes coated with laminin-1 attach and spread significantly faster (Fig. 4, green: t1/2 = 3.12 ± 0.51 h, s = 0.5 ± 0.14 nF/h, n = 4) than cells plated on noncoated electrodes (Fig. 4, black: t1/2 = 9.0 ± 2.17 h, s = 0.27 ± 0.02 nF/h, n = 3). Similarly, cell attachment and spreading on electrodes coated with laminin-2 (Fig. 4, blue: t1/2 = 7.50 ± 1.29 h, s = 0.23 ± 0.01 nF/h, n = 3) are significantly faster than cells on noncoated electrodes (Fig. 4, black: t1/2 = 9.0 ± 2.17 h, s = 0.27 ± 0.02 nF/h, n = 3). We also noticed a significant difference in attachment and spreading between cells plated on laminin-1 and laminin-2 matrixes. We coated electrodes with BSA (Fig. 4, red: t1/2 = 9.0 ± 0.14 h, s = 0.22 ± 0.00 nF/h, n = 3) as a control protein and showed that cell attachment and spreading are very similar to cell attachment and spreading on noncoated electrodes (Fig. 4, black: t1/2 = 9.0 ± 2.17 h, s = 0.27 ± 0.02 nF/h, n = 3).
Fig. 4.
Time-course capacitance measured at 40 kHz when Caco-2-BBE cells spread into the electric cell-substrate impedance sensing (ECIS) array whose electrodes were not coated (black: t1/2 = 9.0 ± 2.17 h, s = 0.27 ± 0.02 nF/h, n = 3), coated with BSA (segmented: t1/2 = 9.0 ± 0.14 h, s = 0.22 ± 0.00 nF/h, n = 3), coated with laminin-1 (grew with border: t1/2 = 3.12 ± 0.51 h, s = 0.5 ± 0.14 nF/h, n = 4), and coated with laminin-2 (light gray: t1/2 = 7.50 ± 1.29 h, s = 0.23 ± 0.01 nF/h, n = 3). Half times (t1/2) and apparent spreading “s” rates were determined for each lane. We coated the electrodes with 10 μg/ml BSA, laminin-1 and laminin-2 were used for coating the electrodes. *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001. Pictures of the electrodes were taken after plating the cells on the wells (T = 0) and in the end of the experiment when cells are at confluence (T = 25).
Kinetics of Caco-2-BBE cell attachment and spreading on laminin-1 and laminin-2
To examine the role of laminin-1 and laminin-2 in cellular adhesion, we studied the time course of attachment and spreading of Caco-2-BBE cells on various concentrations of laminin-1- and laminin-2-coated electrodes. We measured capacitance at 40 kHz when Caco-2-BBE cells suspended in medium were seeded into measuring arrays with electrodes precoated with laminin-1 (Fig. 5A) or laminin-2 (Fig. 5B) at final concentrations of 0.1, 1.0, 10, or 20 μg/ml. As shown in Fig. 5, A1 and A2, the attachment and spreading of Caco-2-BBE cells on the laminin-1-precoated electrode was directly related to the laminin-1 concentration (0.1 μg/ml laminin-1: t1/2 = 4.90 ± 0.58 h, s = 0.40 ± 0.06 nF/h, n = 4; 1.0 μg/ml laminin-1: t1/2 = 3.70 ± 0.28 h, s = 0.58 ± 0.04 nF/h, n = 4; 10 μg/ml laminin-1: t1/2 = 1.40 ± 0.08 h, s = 1.60 ± 0.06 nF/h, n = 4; 20 μg/ml laminin-1: t1/2 = 1.10 ± 0.08 h, s = 2.22 ± 0.10 nF/h, n = 3). Electrodes precoated with laminin-2 showed a barely significant laminin-2 concentration dependence effect compared with laminin-1 (Fig. 5B). Interestingly, Caco-2-BBE cells attached and spread faster on electrodes precoated with 10 μg/ml laminin-1 vs. those precoated with 10 μg/ml laminin-2 [10 μg/ml laminin-1: t1/2 = 1.40 ± 0.08 h, s = 1.60 ± 0.06 nF/h, n = 4; vs. 10 μg/ml laminin-2: t1/2 = 39.0 ± 0.25 h (P = 5.3e−9), s = 0.06 ± 0.00 nF/h, n = 2 (P = 1.0e−4)]. Together these results demonstrate that Caco-2-BBE cells attached and spread faster on laminin-1 than on laminin-2. For all the following experiments, electrodes were precoated with 10 μg/ml laminin-1 or laminin-2.
Fig. 5.
Caco-2-BBE cells attach and spread faster on laminin-1 than on laminin-2. The time course of capacitance measured at 40 kHz during attachment and spreading of Caco-2-BBE cells on electrodes precoated with laminin-1 (A1) at 0.1 μg/ml (black: t1/2 = 4.90 ± 0.58 h, s = 0.40 ± 0.06 nF/h, n = 4), 1 μg/ml (segmented: t1/2 = 3.70 ± 0.28 h, s = 0.58 ± 0.04 nF/h, n = 4), 10 μg/ml (gray with border: t1/2= 1.40 ± 0.08 h, s = 1.60 ± 0.06 nF/h, n = 4), or 20 μg/ml (light gray: t1/2 = 1.10 ± 0.08 h, s = 2.22 ± 0.10 nF/h, n = 3) or laminin-2 (B1) at 0.1 (black: t1/2 = 33.0 ± 0.88 h, s = 0.08 ± 0.00 nF/h, n = 3), 1 (segmented: t1/2 = 37.0 ± 8.0 h, s = 0.10 ± 0.02 nF/h, n = 2), 10 (grew with border: t1/2 = 39.0 ± 0.25 h, s = 0.06 ± 0.00 nF/h, n = 2) or 20 μg/ml (light gray: t1/2 = 39.0 ± 0.33 h, s = 0.07 ± 0.00 nF/h, n = 3). Each trace and bar represents an average of n wells. Half times (t1/2) and apparent spreading rates (s) were determined for each lane on laminin-1 (A2) and on laminin-2 (B2). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001 vs. 0.1 μg/ml.
DG receptor plays a role in Caco-2-BBE cell attachment and spreading
To study whether DG plays a role in cell attachment and spreading, we compared the attachment and spreading of DG-overexpressing Caco-2-BBE cells vs. vector-transfected controls on electrodes precoated with 10 μg/ml laminin-1 or laminin-2. Attachment and spreading of the two Caco-2-BBE transfectants were similar on electrodes precoated with laminin-1 (DG-overexpressing Caco-2-BBE cells: t1/2 = 2.75 ± 0.60 h, s = 0.64 ± 0.13 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells: t1/2 = 3.00 ± 0.34 h, s = 0.60 ± 0.10 nF/h, n = 4) (Fig. 6A). It is likely that the overexpression of DG does not affect Caco-2-BBE attachment and spreading that is already maximal on laminin-1. However, when the electrodes were precoated with laminin-2, the DG-overexpressing Caco-2-BBE cells attached and spread significantly slower than the vector-transfected Caco-2-BBE cells (DG-overexpressing Caco-2-BBE cells: t1/2 = 31.25 ± 3.5 h, s = 0.10 ± 0.01 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells: t1/2 = 16.5 ± 0.8 h, s = 0.16 ± 0.01 nF/h, n = 4) (Fig. 6B). These results show that DG receptor expressed in Caco-2-BBE cells significantly affected cell attachment and spreading when the ECM is laminin-2.
Fig. 6.
DGs play a role in Caco-2-BBE cell attachment and spreading on laminin-1 and laminin-2. The time courses of capacitance were measured at 40 kHz during attachment and spreading of Caco-2-BBE cells on electrodes precoated with 10 μg/ml laminin-1 (A1): cells transfected with empty vector (black: t1/2 = 3.00 ± 0.34 h, s = 0.60 ± 0.10 nF/h, n = 4), cells overexpressing DG (gray: t1/2 = 2.75 ± 0.60 h, s = 0.64 ± 0.13 nF/h, n = 4); electrodes precoated with 10 μg/ml laminin-2 (B1): cells transfected with empty vector (black: t1/2 = 16.5 ± 0.8 h, s = 0.16 ± 0.01 nF/h, n = 4), cells overexpressing DG (gray: t1/2 = 31.2 ± 3.5 h, s = 0.10 ± 0.01 nF/h, n = 4). Each trace and bar represents an average of n wells. Half times (t1/2) and apparent spreading rates (s) were determined on laminin-1 (A2) and on laminin-2 (B2). *P ≤ 0.05, **P ≤ 0.005 vs. vector-transfected Caco-2-BBE cells.
β1-Integrin receptor has higher affinity to laminin-1 than to laminin-2 in Caco-2-BBE cells
We found that the addition of an anti-β1-integrin function-blocking antibody completely abrogated the relatively fast spreading and attachment of empty vector-transfected or DG-overexpressing Caco-2-BBE cells on laminin-1 (Fig. 7A). When electrodes were precoated with laminin-2, empty vector-transfected Caco-2-BBE cells had a slight blocking of attachment and spreading properties in the presence or absence of the β1-integrin-blocking antibody (vector-transfected Caco-2-BBE cells in the presence of β1-integrin-blocking antibody: t1/2 = 34.0 ± 1.00 h, s = 0.07 ± 0.01 nF/h, n = 3; vs. vector-transfected Caco-2-BBE cells in the absence of β1-integrin-blocking antibody: t1/2 = 27.0 ± 1.89 h, s = 0.12 ± 0.02 nF/h, n = 4), whereas the attachment and spreading of Caco-2-BBE cells overexpressing DG on laminin-2 was significantly reduced in the presence of the β1-integrin-blocking antibody (DG-transfected Caco-2-BBE cells in the presence of β1-integrin-blocking antibody: t1/2 = 39.0 ± 8.22 h, s = 0.05 ± 0.00 nF/h, n = 4; vs. DG-transfected Caco-2-BBE cells in the absence of β1-integrin-blocking antibody: t1/2 = 36.5 ± 1.02 h, s = 0.11 ± 0.00 nF/h, n = 4) (Fig. 7B). These results collectively demonstrate that the β1-integrin is crucial for attachment and spreading of Caco-2-BBE cells on laminin-1 and that DG may play a specific role on β1-integrin in the cell attachment and spreading on laminin-2.
Fig. 7.
β1-Integrin has a higher affinity to laminin-1 than to laminin-2 in Caco-2-BBE cells. The time courses of capacitance were measured at 40 kHz during attachment and spreading of Caco-2-BBE cells transfected with empty vector (solid black), overexpressing DG (solid gray), DG in the presence of β1-blocking antibody (dashed gray), or a nonfunctional β1-blocking antibody (dashed black) on electrodes precoated with laminin-1 (A1) or laminin-2 (B1). Each trace and bar represents an average of a minimum of three wells. Half times (t1/2) and apparent spreading rates (s) were determined on laminin-1 (A2) and on laminin-2 (B2). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001 vs. vector transfected Caco-2-BBE cells. Horizontal bars compare P values between cells treated with blocking MAbs and nonblocking MAbs.
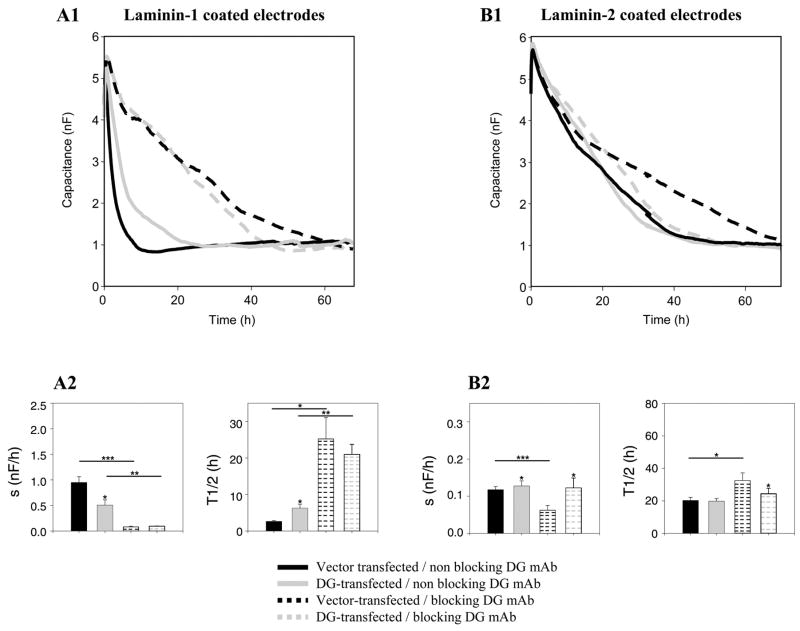
Activation of DG receptor increases laminin-1/β1-integrin interactions and decreases laminin-2/β1-integrin interactions in Caco-2-BBE cells
As shown in Fig. 8A, Caco-2-BBE cells transfected with empty vector and treated with an anti-DG function-blocking antibody showed reduced attachment and spreading on electrodes precoated with laminin-1 (vector-transfected Caco-2-BBE cells in the presence of anti-DG function-blocking antibody: t1/2 = 25.0 ± 5.70 h, s = 0.07 ± 0.00 nF/h, n = 3; vs. vector-transfected Caco-2-BBE cells in the absence of anti-DG function-blocking antibody: t1/2 = 2.60 ± 0.21 h, s = 1.00 ± 0.11 nF/h, n = 4). Interestingly, when Caco-2-BBE cells overexpressing DG were treated with the anti-DG function-blocking antibody, we observed a significant reduction of attachment and spreading on laminin-1 precoated electrodes as compared as the DG-transfected Caco-2-BBE cells in the absence of anti-DG function-blocking antibody (DG-transfected Caco-2-BBE cells in the presence of anti-DG function-blocking antibody: t1/2 = 22.0 ± 2.70 h, s = 0.09 ± 0.00 nF/h, n = 3; vs. DG-transfected Caco-2-BBE cells in the absence of anti-DG function-blocking antibody: t1/2 = 6 ± 1.0 h, s = 0.66 ± 0.10 nF/h, n = 3). On laminin-2 (Fig. 8B), only vector transfected Caco-2-BBE cell attachment and spreading were significantly blocked in the presence of DG-blocking MAb compared with the vector-transfected Caco-2-BBE cells in the absence of DG function-blocking MAb (vector-transfected Caco-2-BBE cells in the presence of anti-DG function-blocking antibody: t1/2 = 32.0 ± 4.8 h, s = 0.06 ± 0.01 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells in the absence of anti-DG blocking MAb: t1/2 = 20.0 ± 1.8 h, s = 0.11 ± 0.00 nF/h, n = 4). Interestingly, when Caco-2-BBE cells overexpressing DG were treated with the anti-DG function-blocking antibody, we observed a significant reduction of attachment and spreading on laminin-2-precoated electrodes compared with empty vector-transfected Caco-2-BBE cells under the same conditions (Fig. 8, B1 and B2) (DG-transfected Caco-2-BBE cells in the presence of anti-DG function-blocking antibody: t1/2 = 24.0 ± 3.4 h, s = 0.11 ± 0.01 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells in the presence of anti-DG blocking antibody: t1/2 = 32.0 ± 4.8 h, s = 0.06 ± 0.01 nF/h, n = 4). Taken together, these results demonstrate that the DG receptor is involved in Caco-2-BBE attachment and spreading on laminin-2. In contrast, overexpression of DG receptor in Caco-2-BBE cells did not affect Caco-2-BBE attachment and spreading on laminin 1.
Fig. 8.
DG receptor activation plays a role in the interactions of laminin-1 and -2 with β1-integrin. The time courses of capacitance were measured at 40 kHz during attachment and spreading of Caco-2-BBE cells transfected with empty vector (solid black), overexpressing DG (solid gray), DG in the presence of a α-DG-blocking antibody (dashed gray), or a nonfunctional α-DG blocking antibody (dashed black) on electrodes precoated with laminin-1 (A1) or laminin-2 (B1). Each trace and bar represents an average of a minimum of three wells. Half times (t1/2) and apparent spreading rates (s) were determined on laminin-1 (A2) and on laminin-2 (B2). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001 vs. vector-transfected Caco-2-BBE cells. Horizontal bars compare P values between cells treated with blocking MAbs and nonblocking MAbs.
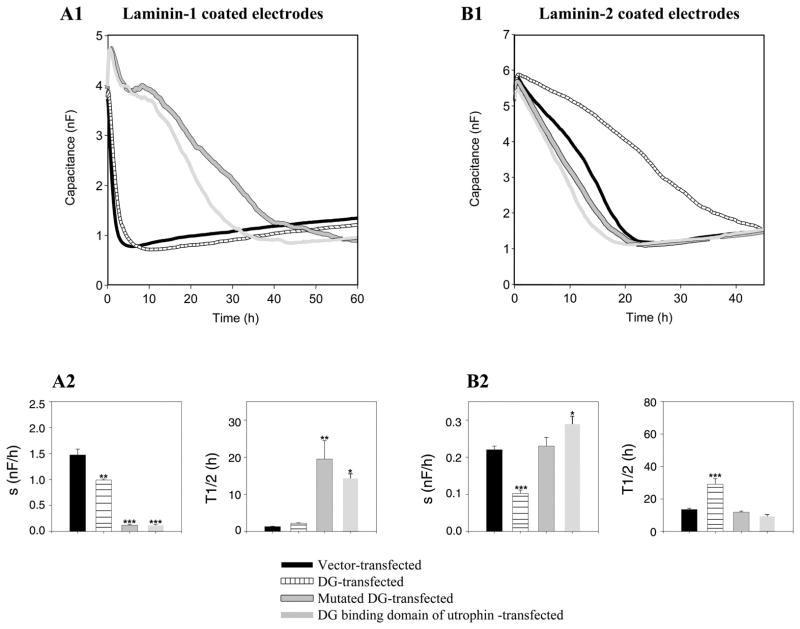
The intracellular COOH termini of β-DG and utrophin appear necessary and sufficient to induce the increase of spreading of Caco-2-BBE cells on laminin-1 and its decrease on laminin-2
To investigate whether the cytoplasmic tail of β-DG plays a role in attachment and spreading, Caco-2-BBE cells were transfected with DG lacking the COOH-terminal cytoplasmic tail. As shown in Fig. 9A, Caco-2-BBE cells overexpressing the truncated DG showed decreased attachment and spreading on laminin-1-precoated electrodes, compared with Caco-2-BBE cells transfected with empty vector or full-length DG (mutated DG-transfected Caco-2-BBE cells: t1/2 = 20.0 ± 4.9 h, s = 0.11 ± 0.02 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells: t1/2 = 1.30 ± 0.10 h, s = 1.50 ± 0.12 nF/h, n = 4, or DG-transfected Caco-2-BBE cells: t1/2 = 2.20 ± 0.12 h, s = 1.00 ± 0.02 nF/h, n = 4). When we overexpressed the DG binding domain of utrophin in Caco-2-BBE cells, we observed similar results under these conditions (DG binding domain of utrophin-transfected Caco-2-BBE cells: t1/2 = 15.0 ± 1.10 h, s = 0.12 ± 0.01 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells: t1/2 = 1.30 ± 0.10 h, s = 1.50 ± 0.12 nF/h, n = 4, or DG-transfected Caco-2-BBE cells: t1/2 = 2.2 ± 0.12 h, s = 1.0 ± 0.02 nF/h, n = 4) (Fig. 9A). Similar experiments on laminin-2-precoated electrodes revealed that Caco-2-BBE cells expressing truncated DG showed increased attachment and spreading, compared with Caco-2-BBE cells transfected with full-length DG or empty vector (mutated DG-transfected Caco-2-BBE cells: t1/2 = 11.0 ± 0.8 h, s = 0.23 ± 0.02 nF/h, n = 4; vs. vector-transfected Caco-2-BBE cells: t1/2 = 13.0 ± 0.8 h, s = 0.22 ± 0.01 nF/h, n = 4, or DG-transfected Caco-2-BBE cells: t1/2 = 29.0 ± 3.5 h, s = 0.10 ± 0.01 nF/h, n = 4) (Fig. 9B). Overexpression of the DG binding domain of utrophin in Caco-2-BBE cells yielded similar results under the same conditions (DG binding domain of utrophin-transfected Caco-2-BBE cells: t1/2 = 8.20 ± 1.00 h, s = 0.29 ± 0.02 nF/h, n = 3; vs. vector-transfected Caco-2-BBE cells: t1/2 = 13.0 ± 0.8 h, s = 0.22 ± 0.01 nF/h, n = 4, or DG-transfected Caco-2-BBE cells: t1/2 = 29.0 ± 3.5 h, s = 0.10 ± 0.01 nF/h, n = 4) (Fig. 9B). These results collectively suggest that the intracellular COOH-terminal of DG and the DG binding domain of utrophin play a role in the interactions between Caco-2-BBE cells and both laminin-1 and laminin-2 matrixes.
Fig. 9.
The intracellular COOH terminus of β-DG and the DG binding domain of utrophin are crucial for the observed changes in laminin-1 and -2 interactions with β1-integrin in Caco-2-BBE cells. The time courses of capacitance were measured at 40 kHz during attachment and spreading of Caco-2-BBE cells transfected with empty vector (black), DG (segmented), truncated DG (gray with border), or DG binding domain of utrophin (light gray) on electrodes precoated with laminin-1 (A1) or laminin-2 (B1). Each trace and bar represents an average of four wells. Half times (t1/2) and apparent spreading rates (s) were determined on laminin-1 (A2) and on laminin-2 (B2). *P ≤ 0.05, **P ≤ 0.005, ***P ≤ 0.001 vs. vector transfected Caco-2-BBE cells.
DISCUSSION
Polarized epithelial cells contain apical and basolateral membrane domains with unique protein and lipid compositions. In normal intestinal epithelium, the basal surface of epithelial cells faces the ECM, and the apical surface faces the intestinal lumen. The apical surface of intestinal epithelial cells contains microvilli and specific proteins (i.e., chloride channels) that are quite unlike those on the basolateral surface. Receptors that interact with components of the basal lamina, such as integrins, are located on the basal surface where their intracellular portions can interact with the cytoskeleton and their extracellular portion can interact with the ECM. For example, when integrins bind to ECM ligands, they aggregate in the plane of the plasma membrane and interact on the cytoplasmic side with elements of the cytoskeleton and various signaling molecules. The basement membrane provides a substrate for adhesion of the epithelium to its underlying ECM. For a long time, integrins were considered to be the main receptors linking epithelial cells to basement membranes (33, 45). In our study, we used Caco-2-BBE cells. Although this cell line derives from a human colonic adenocarcinoma, it is remarkably well differentiated (8), expressing a luxuriant microvillar brush border with a full complement of intestinal hydrolases, tight junctions, polar sorting of endogenous membrane proteins, and an apically directed section (9, 12, 46). Morphologically resembling adult small intestinal mucosa, the Caco-2-BBE cell has frequently been used as a model for normal human intestinal mucosa (12, 34). Here, we demonstrated that α-DG, β-DG, and utrophin are present in Caco-2-BBE cells and co-localize in the basolateral aspect of the monolayer. As suggested by others (30), the latter observation suggests that DG, a functional nonintegrin laminin receptor, may play a direct or indirect role in epithelium/ECM interactions in intestinal epithelia.
Laminins are some of the most abundant glycoproteins in the basement membranes of the intestine (50). Functionally, the various laminins promote intestinal cell adhesion, growth, polarization, and differentiation (10). Laminin-integrin interactions have emerged as a major cell-adhesion system in the intestinal epithelium. Both laminins and integrins are differentially expressed in the various compartments of the cryptvillus axis in developing and adult intestines. Alterations in the expression of laminins occurring in colon cancers and intestinal pathologies such as Crohn disease and ulcerative colitis have also been observed. In the present study, we demonstrated that Caco-2-BBE cells attach and spread faster on laminin-1 than on laminin-2. We found similar results using nontransformed epithelial cells such as the IEC6 rat small intestinal cell line and Madin-Darby canine kidney (MDCK) cells (data not shown). The latter observation confirms that Caco-2 BBE cells represent an appropriate cell model to study cell attachment and spreading on laminin-1 and laminin-2. Two laminin cell receptors of the integrin family (β1- and β4-integrins) appear to be expressed in the intestinal cells (1). Although the β1- and β4-integrins generally bind laminins (1), it is likely that Caco-2-BBE cells interact with laminin-1 and -2 via α2β1-integrin, which is highly expressed in these cells (54). Thus the greater Caco-2-BBE cell attachment and spreading on laminin-1 may indicate that α2β1-integrin has a higher binding capacity for laminin-1 than for laminin-2 (1).
We further demonstrated that DG receptors bind to laminin-1 and laminin-2; Caco-2-BBE cells overexpressing DG showed different cell attachment and spreading properties on laminin-1 and -2 vs. wild-type Caco-2-BBE cells. These results are in agreement with previous studies showing that α-DG binds to laminin-1, laminin-2, and laminin-10, but not to laminin-5 (14, 18, 32, 42). The interaction between the laminin-2 and DG could be minimal at the resting level (wild-type Caco-2-BBE) (Figs. 4 and 5). However, the overexpression of DG in Caco-2-BBE cells reduced significantly the cell attachment and spreading when cultured on laminin-2 (Fig. 6), suggesting a negative signal of laminin-2 on the DG receptor, controlling the cell spreading and attachment. In addition, we observed that DG and β1-integrin coprecipitated in the Caco-2-BBE cell membrane fraction, suggesting that these are located in close proximity and that they may physically associate. Thus activation of DG receptors by laminin-1 or -2 appears to play a role in regulating the affinity between β1-integrin and laminin-1 and -2, which directs Caco-2-BBE cell attachment and spreading on laminin-1 and -2. Based on our antibody perturbation experiments, we propose the model shown on Fig. 10. Activation of DG receptors by laminin-1 increases the interaction between β1-integrin and laminin-1, leading to increased Caco-2-BBE cell attachment and spreading on laminin-1 (Fig. 10A). Activation of DG receptors by laminin-2 reduced the interaction between β1-integrin and laminin-2, leading to decreased Caco-2-BBE cell attachment and spreading on laminin-2 (Fig. 10B). In addition, we showed that the DG-blocking antibody reduced the interaction between β1-integrin/laminin-1 and increased the interaction between β1-integrin/laminin-2, demonstrating that the β1-integrin/laminin-1 and β1-integrin/laminin-2 interactions are likely regulated by laminin-1 or laminin-2-induced activation of the DG receptor (Fig. 10, A and B). Together, these results suggest that the DG receptor is essential for signaling events mediated through the utrophin/DG complex. Like most other biological phenomena, cell adhesion is likely to be governed by both positive and negative signals.
Fig. 10.
Model of DG receptor involved in integrin activation in intestinal epithelia. Our results indicate that activation of DG receptors by laminin-1 enhances the interaction between β1-integrin and laminin-1 (A1), whereas activation of DG receptors by laminin-2 reduces the interaction between β1-integrin and laminin-2 (B1). Furthermore, we propose that the intracellular COOH-terminal tail of α-DG and its binding to the DG binding domain of utrophin is crucial for delivering intracellular signals responsible for modulating the interactions between laminin-1/-2 and β1-integrin.
The unstructured proline-rich cytoplasmic tail of β-DG is known to contain multiple sites for signaling molecule interactions; studies in rat myotubes reveal an association between DG and α5β1-integrin and other focal adhesion proteins such as focal adhesion kinase (FAK) (41). Here, we demonstrated that Caco-2-BBE cells overexpressing a COOH-terminally truncated DG protein showed less attachment and spreading on laminin-1 and better attachment and spreading on laminin-2. Similar results were found with Caco-2-BBE cells overexpressing the DG binding domain of utrophin. This may indicate that under these conditions, DG receptor and β1-integrin are functionally uncoupled, leading to loss of the intracellular signals regulating the β1-integrin/laminin interactions.
In sum, we herein demonstrated that the DGC (α-DG, β-DG, and utrophin) is specifically expressed in the basolateral membrane of Caco-2-BBE monolayers. The co-localization of the DG complex with β1-integrin suggests a possible interaction among these proteins. In addition, we showed that laminin-1-induced activation of DG receptor was able to enhance the interaction between β1-integrin and laminin-1. In contrast, laminin-2-induced activation of DG receptor reduced the interaction between β1-integrin and laminin-2. Finally, we showed that the intracellular COOH-terminal tail of β-DG and its binding to the DG binding domain of utrophin is crucial for modulation of the interaction between laminin-1/-2 and β1-integrin. These findings have implications for our understanding of the role of integrin-associated proteins such as DGs in the intestinal epithelia. We conclude that DGs may not only transmit signals from the exterior of the cell to its interior, but are likely to affect the affinity, specificity, and distribution of integrins making DGs transmitters of signals in both directions through the plasma membrane.
Acknowledgments
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-061941-02 (to D. Merlin), DK-064711-01 (to S. Sitaraman), DK-55850, and a Center Grant (R24-DK-064399). L. Charrier and Y. Yan are supported by a Research Fellowship Award from the Crohn’s and Colitis Foundation of America and Elvin and Janet Price.
References
- 1.Beaulieu JF. Integrins and human intestinal cell functions. Front Biosci. 1999;4:D310–D321. doi: 10.2741/beaulieu. [DOI] [PubMed] [Google Scholar]
- 2.Blake DJ, Schofield JN, Zuellig RA, Gorecki DC, Phelps SR, Barnard EA, Edwards YH, Davies KE. G-utrophin, the autosomal homologue of dystrophin Dp116, is expressed in sensory ganglia and brain. Proc Natl Acad Sci USA. 1995;92:3697–3701. doi: 10.1073/pnas.92.9.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown RH, Jr, Hoffman EP. Molecular biology of Duchenne muscular dystrophy. Trends Neurosci. 1988;11:480–483. doi: 10.1016/0166-2236(88)90006-9. [DOI] [PubMed] [Google Scholar]
- 4.Brown SC, Fassati A, Popplewell L, Page AM, Henry MD, Campbell KP, Dickson G. Dystrophic phenotype induced in vitro by antibody blockade of muscle alpha-dystroglycan-laminin interaction. J Cell Sci. 1999;112:209–216. doi: 10.1242/jcs.112.2.209. [DOI] [PubMed] [Google Scholar]
- 5.Buyse M, Sitaraman SV, Liu X, Bado A, Merlin D. Luminal leptin enhances CD147/MCT-1-mediated uptake of butyrate in the human intestinal cell line Caco-2-BBE. J Biol Chem. 2002;277:28182–28190. doi: 10.1074/jbc.M203281200. [DOI] [PubMed] [Google Scholar]
- 6.Byers T, Lidov HG, Kunkel LM. An alternative dystrophin transcript specific to peripheral nerve. Nat Genet. 1993;4:87–93. doi: 10.1038/ng0593-77. [DOI] [PubMed] [Google Scholar]
- 7.Campbell KP. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995;80:675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- 8.Chantret I, Barbat A, Dussaulx E, Brattain MG, Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988;48:1936–1942. [PubMed] [Google Scholar]
- 9.Chantret I, Trugnan G, Dussaulx E, Zweibaum A, Rousset M. Monensin inhibits the expression of sucrase-isomaltase in Caco-2 cells at the mRNA level. FEBS Lett. 1988;235:125–128. doi: 10.1016/0014-5793(88)81246-8. [DOI] [PubMed] [Google Scholar]
- 10.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–234. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 11.Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle alpha-dystroglycan is involved in epithelial development. J Cell Biol. 1995;130:79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eilers U, Klumperman J, Hauri HP. Nocodazole, a microtubule-active drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2) J Cell Biol. 1989;108:13–22. doi: 10.1083/jcb.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 14.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ervasti JM, Ohlendeick K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 16.Fabbrizio E, Latouche J, Rivier F, Hugon G, Mornet D. Reevaluation of the distributions of dystrophin and utrophin in sciatic nerve. Biochem J. 1995;312:309–314. doi: 10.1042/bj3120309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feener CA, Koenig M, Kunkel LM. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989;338:509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- 18.Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem. 1993;268:14972–14980. [PubMed] [Google Scholar]
- 19.Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273:600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- 20.Giaever I, Keese CR. A morphological biosensor for mammalian cells. Nature. 1993;366:591–592. doi: 10.1038/366591a0. [DOI] [PubMed] [Google Scholar]
- 21.Giaever I, Keese CR. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc Natl Acad Sci USA. 1984;81:3761–3764. doi: 10.1073/pnas.81.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman EP, Hudecki MS, Rosenberg PA, Pollina CM, Kunkel LM. Cell and fiber-type distribution of dystrophin. Neuron. 1988;1:411–420. doi: 10.1016/0896-6273(88)90191-2. [DOI] [PubMed] [Google Scholar]
- 24.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 25.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 26.Ibraghimov-Beskrovnaya O, Milatovich A, Ozcelik T, Yang B, Koepknik K, Franke U, Campbell KP. Human dystroglycan: skeletal muscle cDNA, genomic structure, origin of tissue specific isoforms and chromosomal localization. Hum Mol Genet. 1993;2:1651–1657. doi: 10.1093/hmg/2.10.1651. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa-Sakurai M, Yoshida M, Imamura M, Davies KE, Ozawa E. ZZ domain is essentially required for the physiological binding of dystrophin and utrophin to beta-dystroglycan. Hum Mol Genet. 2004;13:693–702. doi: 10.1093/hmg/ddh087. [DOI] [PubMed] [Google Scholar]
- 28.James M, Nguyen TM, Wise CJ, Jones GE, Morris GE. Utrophin-dystroglycan complex in membranes of adherent cultured cells. Cell Motil Cytoskeleton. 1996;33:163–174. doi: 10.1002/(SICI)1097-0169(1996)33:3<163::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- 30.Kachinsky AM, Froehner SC, Milgram SL. A PDZ-containing scaffold related to the dystrophin complex at the basolateral membrane of epithelial cells. J Cell Biol. 1999;145:391–402. doi: 10.1083/jcb.145.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keese CR, Wegener JR, Walker S, Giaever I. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci USA. 2004;101:1554–1559. doi: 10.1073/pnas.0307588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kikkawa Y, Yu H, Genersch E, Sanzen N, Sekiguchi K, Fassler R, Campbell KP, Talts JF, Ekblom P. Laminin isoforms differentially regulate adhesion, spreading, proliferation, and ERK activation of beta1 integrin-null cells. Exp Cell Res. 2004;300:94–108. doi: 10.1016/j.yexcr.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Korhonen M, Ylanne J, Laitinen L, Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990;111:1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Bivic A, Quaroni A, Nichols B, Rodriguez-Boulan E. Biogenetic pathways of plasma membrane proteins in Caco-2, a human intestinal epithelial cell line. J Cell Biol. 1990;111:1351–1361. doi: 10.1083/jcb.111.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losasso C, Di Tommaso F, Sgambato A, Ardito R, Cittadini A, Giardina B, Petrucci TC, Brancaccio A. Anomalous dystroglycan in carcinoma cell lines. FEBS Lett. 2000;484:194–198. doi: 10.1016/s0014-5793(00)02157-8. [DOI] [PubMed] [Google Scholar]
- 36.Love DR, Hill DF, Dickson G, Spurr NK, Byth BC, Marsden RF, Walsh FS, Edwards YH, Davies KE. An autosomal transcript in skeletal muscle with homology to dystrophin. Nature. 1989;339:55–58. doi: 10.1038/339055a0. [DOI] [PubMed] [Google Scholar]
- 37.Mooseker MS. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- 38.Muschler J, Levy D, Boudreau R, Henry M, Campbell K, Bissell MJ. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 2002;62:7102–7109. [PubMed] [Google Scholar]
- 39.Nguyen TM, Ellis JM, Love DR, Davies KE, Gatter KC, Dickson G, Morris GE. Localization of the DMDL gene-encoded dystrophin-related protein using a panel of nineteen monoclonal antibodies: presence at neuromuscular junctions, in the sarcolemma of dystrophic skeletal muscle, in vascular and other smooth muscles, and in proliferating brain cell lines. J Cell Biol. 1991;115:1695–700. doi: 10.1083/jcb.115.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen TM, Helliwell TR, Simmons C, Winder SJ, Kendrick-Jones J, Davies KE, Morris GE. Full-length and short forms of utrophin, the dystrophin-related protein. FEBS Lett. 1995;358:262–266. doi: 10.1016/0014-5793(94)01441-3. [DOI] [PubMed] [Google Scholar]
- 41.Osses N, Brandan E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol. 2002;282:C383–C394. doi: 10.1152/ajpcell.00322.2001. [DOI] [PubMed] [Google Scholar]
- 42.Pall EA, Bolton KM, Ervasti JM. Differential heparin inhibition of skeletal muscle alpha-dystroglycan binding to laminins. J Biol Chem. 1996;271:3817–3821. doi: 10.1074/jbc.271.7.3817. [DOI] [PubMed] [Google Scholar]
- 43.Peng HB, Ali AA, Daggett DF, Rauvala H, Hassel JR, Smalheiser NR. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun. 1998;5:475–489. doi: 10.3109/15419069809005605. [DOI] [PubMed] [Google Scholar]
- 44.Schofield JN, Blake DJ, Simmons C, Morris GE, Tinsley JM, Davies KE, Edwards YH. Apo-dystrophin-1 and apo-dystrophin-2, products of the Duchenne muscular dystrophy locus: expression during mouse embryogenesis and in cultured cell lines. Hum Mol Genet. 1994;8:1309–1316. doi: 10.1093/hmg/3.8.1309. [DOI] [PubMed] [Google Scholar]
- 45.Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stieger B, Matter K, Baur B, Bucher K, Hochli M, Hauri HP. Dissection of the asynchronous transport of intestinal microvillar hydrolases to the cell surface. J Cell Biol. 1988;106:1853–1861. doi: 10.1083/jcb.106.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- 48.Suzuki A, Yoshida M, Hayashi K, Mizuno Y, Hagiwara Y, Ozawa E. Molecular organization at the glycoprotein-complex-binding site of dystrophin. Three dystrophin-associated proteins bind directly to the carboxy-terminal portion of dystrophin. Eur J Biochem. 1994;220:283–292. doi: 10.1111/j.1432-1033.1994.tb18624.x. [DOI] [PubMed] [Google Scholar]
- 49.Talts JF, Zeynep A, Goring W, Brancaccio A, Timpl R. Binding of the G domains of laminin α1 and α2 chains and perlecan to heparin, sulfatides, α-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teller IC, Beaulieu JF. Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev Mol Med. 2001;2001:1–18. doi: 10.1017/S1462399401003623. [DOI] [PubMed] [Google Scholar]
- 51.Wegener J, Keese CR, Giaever I. Electric cell-substrate impedance sensing (ECIS) as a noninvasive means to monitor the kinetics of cell spreading to artificial surfaces. Exp Cell Res. 2000;259:158–166. doi: 10.1006/excr.2000.4919. [DOI] [PubMed] [Google Scholar]
- 52.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 53.Wilson J, Putt W, Jimenez C, Edwards YH. Up71 and up140, two novel transcripts of utrophin that are homologues of short forms of dystrophin. Hum Mol Genet. 1999;8:1271–1278. doi: 10.1093/hmg/8.7.1271. [DOI] [PubMed] [Google Scholar]
- 54.Winder SJ. The complexities of dystroglycan. Trends Biochem Sci. 2001;26:118–124. doi: 10.1016/s0968-0004(00)01731-x. [DOI] [PubMed] [Google Scholar]
- 55.Xiao C, Lachance B, Sunahara G, Luong JH. An in-depth analysis of electric cell-substrate impedance sensing to study the attachment and spreading of mammalian cells. Anal Chem. 2002;74:1333–1339. doi: 10.1021/ac011104a. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida M, Suzuki A, Yammamoto H, Noguchi S, Mizuno Y, Ozawa E. Dissociation of the complex of dystrophin and its associated proteins into several unique groups by n-octyl beta-D-glucoside. Eur J Biochem. 1994;222:1055–1061. doi: 10.1111/j.1432-1033.1994.tb18958.x. [DOI] [PubMed] [Google Scholar]
- 57.Zuellig RA, Bornhauser BC, Knuesel I, Heller F, Fritschy JM, Schaub MC. Identification and characterization of transcript and protein of a new short N-terminal utrophin isoform. J Cell Biochem. 2000;77:418–431. doi: 10.1002/(sici)1097-4644(20000601)77:3<418::aid-jcb7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]