Abstract
Two potential malaria virulence factors, parasite multiplication rate (PMR) and red blood cell selectivity (measured as selectivity index [SI]), were assessed in Plasmodium falciparum clinical isolates from Mali and Kenya. At both sites, PMRs were low (Kenya median = 2.2, n = 33; Mali median = 2.6, n = 61) and did not differ significantly between uncomplicated and severe malaria cases. Malian isolates from hyperparasitemic patients had significantly lower PMRs (median = 1.8, n = 19) than other Malian isolates (uncomplicated malaria median = 3.1, n = 23; severe malaria median = 2.8, n = 19; P = 0.03, by Kruskal-Wallis test). Selective invasion occurred at both sites (Kenya geometric mean SI = 1.9, n = 98; Mali geometric mean SI = 1.6, n = 104), and there was no significant association between the SI and malaria severity. Therefore, in contrast to previous results from Thailand, we found no association of PMR and SI with malaria severity in African children. This raises the possibility of differences in the mechanisms of malaria virulence between sub-Saharan Africa and Asia.
INTRODUCTION
There is a poor understanding of factors that cause severe malaria, although host genetics, environmental determinants, and parasite properties are all thought to play an important role.1 As far back as the early 20th century when malaria therapy was used to treat neurosyphilis, differences in Plasmodium falciparum strain virulence were recognized.2 In parasite evolutionary theory, differences in strain virulence can be explained by the trade-off hypothesis, which describes how parasites evolve to balance the fitness advantages of high growth and transmission with the fitness cost of host death.3 In other words, parasites that grow faster transmit more efficiently, but are also more virulent to the host. The relationship between parasite growth rate and virulence has been shown in rodent malaria models with P. chabaudi.4–6 In human malaria, P. falciparum virulence was associated with in vitro parasite multiplication rate (PMR) in a study carried out in Thailand (severe malaria isolates median PMR = 8.3, uncomplicated malaria isolates median PMR = 2.8).7 Whether this relationship between PMR and virulence is seen in other malaria-endemic countries is unknown.
Further insight into parasite multiplication in vivo can be gained by understanding whether red blood cell (RBC) invasion is a random, unrestricted process, or whether some selectivity occurs, with only a subset of RBCs being accessible for invasion. The selectivity index (SI) compares the observed number of multiple-infected RBCs (containing more than one ring-stage parasite) to the number expected from a random Poisson distribution.8 An SI of 1 indicates random, non-selective invasion of RBCs, whereas an SI of 2 indicates twofold more multiple-infected RBCs than expected by chance alone. Factors that may lead to non-random invasion (SI > 1) include parasite receptor preference, host RBC polymorphisms, or host merozoite-agglutinating antibodies.9–11 An SI < 1 could occur if invasion by one merozoite rendered an RBC refractory to further invasion.
Multiple-infected RBCs occur in all human Plasmodium infections.8 In P. vivax, the SI is high (~8), which reflects the preference of P. vivax for reticulocytes and young RBCs.8 In P. falciparum infections with parasitemias less than 2%, multiple-infected RBCs are largely the result of the high parasite load, and the SI is ~1.8 Plasmodium falciparum infections with lower parasitemias (< 2%) show a tendency towards higher RBC selectivity, with a mean SI of 2.44.8 Importantly, after accounting for parasitemia effects, RBC selectivity of P. falciparum isolates has been associated with disease severity in Thailand.8 Severe malaria isolates show random, unrestricted invasion of RBCs (geometric mean SI = 1.35), whereas uncomplicated malaria isolates show restricted, non-random invasion with a higher number of multiple-infected cells (geometric mean SI = 2.31).8
Together with the PMR results,7 the above data indicate that there may be more virulent isolates causing severe malaria in Thai patients due to rapid, unrestricted growth and subsequent high parasite burdens in the host. The influence of these factors on malaria severity in African children has not previously been studied. Therefore, we investigated PMR and RBC selectivity in relation to malaria virulence in two diverse sites in Africa.
MATERIALS AND METHODS
Parasite isolates
Parasite isolates were collected in Bandiagara, Mali and Kilifi, Kenya. Malaria transmission is seasonal at both sites, with infective bites ranging from 20 to 60 per month in Mali12 and 10 to 30 per person per year in Kenya.13 In Mali, there is a single malaria season every year (July–December), whereas in Kenya there are two malaria seasons per year (June–August and December–February). The Malian samples were collected as part of the Bandiagara Malaria Project case-control study in which uncomplicated malaria controls were age, residence, and ethnicity matched to severe malaria cases.12,14 The World Health Organization (WHO) criteria for severe malaria were applied,15 although patients with hyperparasitemia (> 500,000 parasites/μL of blood) and no other symptoms or signs of severe disease were analyzed as a separate group because previous studies indicated a good prognosis for these children.14,16 Uncomplicated malaria cases were all those recruited into the study with fever and P. falciparum parasitemia who did not have severe malaria or hyperparasitemia.14,17 In Kenya, children with cerebral malaria (unrousable coma with a Blantyre score ≤ 2), prostration (inability to sit unaided or in babies to breast feed), or respiratory distress (abnormally deep breathing) were considered to have severe malaria. The simpler clinical definition applied in Kenya identifies approximately the same group of children at risk for life-threatening malaria as those identified by the more comprehensive WHO criteria.16 In Kenya, uncomplicated cases were children with malaria who came to a hospital with no symptoms or signs of severe disease and were treated as outpatients with oral therapy. Blood samples were collected after obtaining informed consent from the children’s parents or guardians, and protocols were reviewed and approved by ethical review boards in the United Kingdom, the United States, Mali, and Kenya.
After removal of lymphocytes using Lymphoprep (Axis-Shield UK, Kimbolton, Cambridgeshire, United Kingdom), Malian isolates were washed and then frozen in glycerolyte by standard methods and shipped to Edinburgh. The isolates were thawed using a standard protocol. Briefly the isolates were diluted in a series of salt solutions (0.2 mL of 12% saline added slowly, followed by 10 mL of 1.8% saline followed by 10 mL of 0.9% saline/0.2% glucose) and washed in RPMI 1640 medium containing 2 mM glutamine, 37.5 mM HEPES, 20 mM glucose, and 25 μg/mL of gentamicin (incomplete RPMI medium). The cells were resuspended in complete RPMI medium (incomplete RPMI medium supplemented with 10% human AB serum), gassed with 3% CO2, 1% O2, 96% N2 and incubated at 37°C. Kenyan isolates were collected into heparinized tubes and put into culture within 12 hours of collection. Lymphocytes and the buffy coat were removed, the RBC were washed three times in incomplete RPMI medium, resuspended in complete RPMI medium, gassed, and incubated as above. Cultures were monitored for 18–48 hours by staining smears with Giemsa, and only those with normal morphology that matured to the schizont stage were included in the study. Thai samples were frozen and shipped to Edinburgh and then thawed and cultured in the same way as the Malian samples.
Thin blood smears for SI calculations
Thin smears were prepared from blood collected from the patient on arrival at hospital and stained with Giemsa. A subset of the slides for SI calculations are from the isolates used to collect in vitro PMR data. The remaining slides in both Kenya and Mali were collected from children recruited into other studies of factors affecting severe malaria.14,18,19
Selectivity index (SI)
Three hundred ring-infected RBC were counted per slide and the number of rings in each RBC was recorded. The SI was calculated as described in detail by Simpson and others8 Briefly, the number of observed multiple-infected RBCs (i.e., ≥ 2 rings per RBC) was divided by the number of expected multiple-infected RBCs. The latter was calculated according to a Poisson distribution with parameter λ = (− ln(1 − parasitemia). The parasitemia was estimated as the proportion of RBCs infected with at least one parasite from a count of 1,000 RBCs.
In vitro PMR assay
The assay to determine PMR in the first cycle of in vitro culture was set up as described by Choti-vanich and others.7 All parasites were cultured as described above until the mature schizont stage. The schizonts were enriched by centrifugation through 60% Percoll. The enriched schizonts were washed twice and adjusted to a 1% parasitemia with normal group A (Malian samples) or group O (Kenyan samples) RBCs, each from one single healthy Caucasian donor, respectively. They were incubated in 96-well plates in a volume of 50 μL at a 2% hematocrit with duplicate wells for each isolate. The 96-well plate was placed within a gas modulator chamber to allow culturing in 3% CO2, 1% O2, 96% N2 for 24 hours at 37°C. Thin blood films of the pre-invasion sample and the ring-invaded RBCs after 24 hours were made and stained with Giemsa to determine the parasitemia. At least 1,000 RBCs from each slide of the duplicate wells per sample were counted. The PMR is the number of ring-infected RBCs after invasion divided by the pre-invasion parasitemia. The multiplication rate can also be calculated using the total number of ring-stage parasites present in all infected RBCs after invasion.7 Analysis of the data using total number of ring-stage parasites rather than the number of ring-infected RBCs did not materially affect any of the results shown here; therefore, only data referring to the number of ring-infected RBCs are given.
Rosette formation
Rosette frequency was assessed by staining an aliquot of culture suspension with 25 μg/mL of ethidium bromide. A wet preparation of the stained culture suspension at a 2% hematocrit was viewed with a fluorescence microscope, and the percentage of 200 mature-infected erythrocytes binding ≥ 2 uninfected erythrocytes was counted (the rosette frequency).
Statistical analysis
Statistical analysis was done with Stat-View 5.0.1 software (SAS Institute, Cary, NC) and Minitab version 14.1. (Minitab Ltd., Coventry, Warwickshire, United Kingdom). The power calculation was determined using S-plus software. Non-parametric tests (Mann-Whitney U or Kruskal-Wallis) were used for most analyses because of the non-normality of the data, and correlations were assessed by Spearman rank correlation. The PMR data were square-root transformed to conform to normality and homogeneity of variance assumptions. They were then analyzed using a general linear model for the effects of disease severity, parasitemia, country of origin, and the interaction between disease severity and country of origin. Repeating the non-parametric analysis of the PMR with parametric tests using the transformed data did not materially alter the results. The SI data were normalized by log transformation. Geometric mean SI (95% confidence intervals [CIs]) for disease severity groups were calculated and compared by one-way analysis of variance (ANOVA). Correlations with SI were assessed by simple regression.
RESULTS
Clinical and laboratory details of patients in the PMR study
The laboratory data for the patients in the PMR study are shown in Table 1. Children with severe malaria tended to have higher parasitemias, higher white blood cell counts, and lower hemoglobin levels than children with uncomplicated malaria (Table 1). The Kenyan children with severe disease were younger than those with uncomplicated malaria; however, this age difference was not seen in the Malian dataset because age-matching was part of the study design (Table 1). The parasites from severe malaria cases showed higher rosette frequencies than those from uncomplicated malaria cases as described previously,18,19 although this was not statistically significant in Kenya where the sample size was small (Table 1). Of the 13 Kenyan children with severe malaria, three had cerebral malaria, five had respiratory distress and prostration, four had prostration alone, and one had respiratory distress alone. Of the 19 Malian children with severe disease, 5 had cerebral malaria (unrousable coma), 10 had other neurologic impairment (prostration, obtundation or repeated seizures), 2 had respiratory distress, 1 had severe anemia, and 1 had jaundice. One Malian child with respiratory distress died, and all the other children survived.
Table 1.
Laboratory data and parasite multiplication rates for patients included in the PMR study in Kenya and Mali*
| No. | Age (months) | Hb (g/dL) | Parasitemia† (%) | WBCs (× 109/L) | Rosette frequency‡ | Parasite multiplication rate | |
|---|---|---|---|---|---|---|---|
| Kenya | |||||||
| All patients | 33 | 33 (30) [30] | 8.7 (2.6) [27] | 6.0 (4.1) | 13.7 (12.0) [27] | 1.3 (0–5.5) [20] | 2.2 (1.1–3.3) |
| Uncomplicated | 20 | 44 (35) [17] | 9.8 (1.3) [14] | 4.0 (2.3) | 9.0 (3.6) [14] | 1.0 (0–3) [12] | 2.3 (1.2–3.6) |
| Severe§ | 13 | 20 (11) | 7.5 (3.1) | 9.1 (4.5) | 18.8 (15.6) | 3.3 (0–19.3) [8] | 1.8 (1.0–2.9) |
| P | 0.02 | 0.0125 | 0.0001 | 0.003 | 0.32 | 0.38 | |
| Mali | |||||||
| All patients | 61 | 39 (22) | 9.6 (1.8) | 8.2 (7.8) | 14.0 (7.2) [60] | 7.0 (0–17) | 2.6 (1.8–3.5) |
| Uncomplicated | 23 | 40 (22) | 9.6 (1.5) | 3.0 (1.7) | 13.2 (7.4) [22] | 2.0 (0–12) | 3.1 (2.3–4.4) |
| Severe | 19 | 34 (22) | 8.7 (2.4) | 7.3 (4.3) | 15.7 (8.3) | 14.0 (9–24) | 2.8 (2.1–3.5) |
| Hyperparasitemic | 19 | 42 (24) | 10.4 (1.1) | 15.4 (9.4) | 13.1 (5.9) | 5.0 (2–20) | 1.8 (1.2–2.7) |
| P | 0.54 | 0.04 | < 0.0001 | 0.48 | 0.003 | 0.27¶ 0.03# |
|
Values are the mean (SD) or median (interquartile range). Values in square brackets indicate no. of patients that differ due to missing data. Hb = hemoglobin; WBC = white blood cells. Rosette frequency and parasite multiplication rates were analyzed by the Mann-Whitney U test (Kenya) or the Kruskal-Wallis test (Mali). Other parameters were analyzed by analysis of variance or Student’s t-test.
Percentage of infected red blood cells in 1,000 red blood cells counted.
Percentage of infected red blood cells binding ≥ 2 uninfected red blood cells in 200 infected red blood cells counted.
Mean (SD) values for additional biochemical tests in severe case are pH 7.34 (0.15), bicarbonate 16.04 mmol/L (5.90), and lactate 5.03 mg/dL (3.56). These tests were not available for uncomplicated cases in Kenya or any samples from Mali.
Severe versus uncomplicated by Mann-Whitney U test.
Severe, uncomplicated, and hyperparasitemia groups by Kruskal-Wallis test.
Lack of an association between field isolate multiplication rates and disease severity in Kenya and Mali
We measured the PMR in the first cycle of in vitro culture for 33 Kenyan and 61 Malian field isolates. A power calculation indicated that in both Kenya and Mali we had > 99% power to detect a difference in PMR between severe and uncomplicated isolates as large as that seen in the previous study in Thailand.7 With the sample size and variance shown here for Kenya (the smallest of the two studies), we had > 90% power at a 5% error level to detect a difference in the PMR between severe and uncomplicated isolates as small as 1.8.
The multiplication rates in both Kenya and Mali were low (Kenya median = 2.2, Mali median = 2.6, Table 1). In Kenya there was no significant difference in the PMR of isolates from severe malaria patients compared with the PMR of isolates from uncomplicated malaria cases (P = 0.38 by Mann-Whitney U test, Figure 1A and Table 1). Similarly, in Mali there was no significant difference in the PMR of severe malaria isolates compared with uncomplicated malaria isolates (P = 0.27 by Mann-Whitney U test, Figure 1B and Table 1). Children with uncomplicated malaria had lower parasitemias than children with severe malaria in both Kenya and Mali (Table 1), and parasitemia was significantly negatively correlated with PMR in both Kenya and Mali (Kenya: ρ = −0.397, P = 0.025; Mali: ρ = −0.312, P = 0.02 Spearman rank correlation; Figure 2). The data on the relationship between PMR and disease severity were therefore re-analyzed after adjustment for host parasitemia, and there was still no significant difference between the PMR of uncomplicated malaria, isolates compared with severe malaria isolates (F1,29 = 0.343, P = 0.56 and F1,38 = 0.120, P = 0.73 for Kenya and Mali, respectively).
Figure 1.
Parasite multiplication rates (PMRs) of A, Kenyan and B, Malian Plasmodium falciparum isolates from children diagnosed with uncomplicated or severe malaria. Isolates from Malian children diagnosed with hyperparasitemia (Hyperpt.) are shown as a separate group. Equivalent children with hyperparasitemia and no other symptoms or signs of severe malaria occur in Kenya, but they were not recruited because of the study design. The median PMR for each group is indicated by the closed circle and line.
Figure 2.
Scatter plots indicating the negative correlation between the parasite multiplication rate and host parasitemia of Plasmodium falciparum isolates from A, Kenya (n = 33) and B, Mali (n = 61).
The PMR of isolates from cerebral malaria cases in Kenya (n = 3, median = 2.7) was not significantly different from the PMR of isolates from patients with other forms of severe disease (n = 10, median = 1.7, P = 0.74, by Mann-Whitney U test). Similarly, in Mali, the PMR of isolates from cerebral malaria cases (n = 5, median = 3.5) did not differ significantly from the PMR of isolates from other severe malaria cases (n = 14, median = 2.5, P = 0.17, by Mann-Whitney U test). The number of isolates in the sub-categories of severe disease is small, and a larger study would be required to determine conclusively whether there are any significant differences in PMR among isolates from patients with different clinical forms of severe malaria.
We also studied patients in Mali with hyperparasitemia (> 500,000 parasites/μL of blood) but no other symptoms or signs of severe malaria. These patients can be classified as severe using WHO criteria;15 however, they have a very low mortality rate and should probably be considered as an intermediate category between severe and mild uncomplicated malaria.14,16 Patients with hyperparasitemia are also found in Kenya, but were not recruited in the Kenyan study because its aim was to compare the most severely ill children to those with mild uncomplicated disease, rather than consider the intermediate category. Isolates from patients with hyperparasitemia had significantly lower PMRs (median = 1.8) than isolates from other Malian severe and uncomplicated malaria cases (P = 0.03, by Kruskal-Wallis test, Figure 1B and Table 1). The occurrence of lower PMRs in isolates from patients with higher parasite burdens was also indicated by the negative correlation between parasitemia and PMR (Figure 2).
We also examined PMR in relation to three other variables: patient age as a possible indicator of host immunity, hemoglobin as an additional measure of disease severity, and parasite rosette frequency (a known correlate of malaria virulence in African children).18,19 Age and hemoglobin levels of the children and the parasite rosette frequency were not correlated with PMR in Mali (P = 0.98, P = 0.72, and P = 0.64, respectively) or Kenya (P = 0.29, P = 0.78, and P = 0.70, respectively, by Spearman rank correlation).
The maximum PMR was 6.1 in the Kenyan isolates and 8.9 in the Malian isolates (Figure 1). This is in contrast to the previous study from Thailand where more than half of the isolates from severe malaria patients showed between 8- and 25-fold multiplication.7 We used identical methods to those described in the Thai study; however, our results are so clearly different to those reported earlier that we decided to examine the possibility of a technical problem in the PMR assay. We therefore studied PMR in a small, blinded set of Thai isolates (kindly provided by Dr. K. Chotivanich and Professor N. White, Mahidol University, Bangkok, Thailand). The PMR assay was set up using blood from the same Caucasian donor who was used for the Mali samples. Despite the small numbers studied, the Thai severe malaria isolates had higher PMRs (median = 7.7, n = 4: 1.4, 6.5, 9.0, 10.2) than the Thai uncomplicated malaria isolates (median = 2.2, n = 4: 0.4, 2.2, 2.2, 3.5), as shown previously.7 This difference in PMR between severe and uncomplicated Thai isolates was significantly greater than that found in the African samples (country × disease severity interaction: F1,77 = 5.20, P = 0.008). Thus, technical differences between studies are unlikely to explain the contrast between P. falciparum isolates from Thailand and Africa in the relationship between PMR and severe malaria.
Clinical and laboratory details of patients in the SI study
The laboratory data for the children in the SI study are shown in Table 2. The severe malaria patients had lower hemoglobin levels, higher parasitemias, and higher white blood cell counts than the children with uncomplicated malaria. The parasite isolates from the severe malaria patients had significantly higher rosette frequencies than the parasite isolates from the uncomplicated malaria and hyperparasitemic patients (Table 2). Of the 46 Kenyan children with severe malaria, 20 had cerebral malaria, 11 had respiratory distress and prostration, 11 had prostration alone, 2 had respiratory distress alone, and 2 were missing the clinical details on the sub-category of severe disease. Of the 46 Malian children with severe malaria, 13 had cerebral malaria, 23 had other neurologic impairment (prostration, obtundation, or repeated seizures), 2 had respiratory distress, 5 had severe anemia, and 3 had jaundice. Six Malian and 3 Kenyan children with severe disease died, and there were no deaths among children with uncomplicated malaria or those who were hyperparasitemic.
Table 2.
Laboratory data and selectivity index for patients included in the selectivity index analysis in Kenya and Mali*
| No. | Age (months) | Hb (g/dL) | Parasitemia† (%) | WBC (× 109/L) | Rosette frequency‡ | Selectivity index (geometric mean (95% CI)) | |
|---|---|---|---|---|---|---|---|
| Kenya | |||||||
| All patients | 98 | 33 (26) [79] | 8.1 (2.5) [74] | 6.5 (6.1) | 13.2 (13.1) [45] | 4.25 (1–13.5) [62] | 1.92 (1.65–2.19) |
| Uncomplicated | 52 | 40 (27) [37] | 9.4 (1.5) [32] | 4.2 (2.9) | 9.0 (3.9) [21] | 1.5 (0–7) [31] | 2.05 (1.55–2.55) |
| Severe§ | 46 | 27 (23) [42] | 7.0 (2.6) [42] | 9.0 (7.7) | 16.9 (16.9) [24] | 6.5 (2.5–17.5) [31] | 1.79 (1.58–2.01) |
| P | 0.022 | < 0.0001 | < 0.0001 | 0.044 | 0.009 | 0.56 | |
| Mali | |||||||
| All patients | 104 | 39 (24) | 9.0 (2.4) | 7.6 (7.0) | 13.9 (7.7) [101] | 12 (2–22) [98] | 1.61 (1.12–2.10) |
| Uncomplicated | 33 | 38 (20) | 9.4 (1.7) | 3.3 (2.5) | 12.9 (6.3) [32] | 2 (0–14.5) | 1.86 (0.60–3.11) |
| Severe | 46 | 38 (25) | 8.2 (2.9) | 6.9 (5.7) | 15.4 (9.3) [45] | 18.5 (11.5–37) [40] | 1.55 (0.86–2.25) |
| Hyperparasitemic | 25 | 44 (25) | 10.2 (1.5) | 14.8 (8.0) | 12.3 (5.5) [24] | 5 (2–19.5) | 1.44 (1.21–1.67) |
| P | 0.58 | 0.003 | < 0.0001 | 0.19 | < 0.0001 | 0.62 | |
Values are the mean (SD) or median (interquartile range) unless otherwise indicated. Values in square brackets indicate no. of patients that differ due to missing data. Hb = hemoglobin; WBC = white blood cells; CI = confidence interval. Rosette frequency was analyzed by the Mann-Whitney U test (Kenya) or the Kruskal-Wallis test (Mali). Other parameters were analyzed by analysis of variance or Student’s t-test.
Percentage of infected red blood cells in 1,000 red blood cells counted.
Percentage of infected red blood cells binding ≥ 2 uninfected red blood cells in 200 infected red blood cells counted.
Mean (SD) values for additional biochemical tests in severe case are pH 7.26 (0.15), n = 23, bicarbonate 13.97 mmol/L (5.85), n = 23, and lactate 6.91 mg/dL (4.35), n = 23. These tests were not available for uncomplicated cases in Kenya or any samples from Mali.
Negative correlation of SI of field isolates with parasitemia
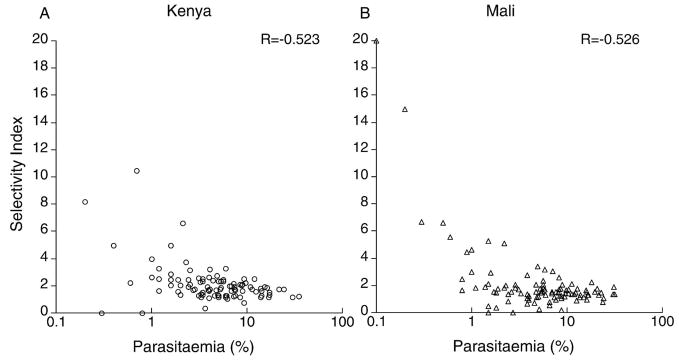
We examined thin blood smears from 98 Kenyan and 104 Malian children. The geometric mean SI was 1.92 (95% CI = 1.65–2.19) for Kenyan isolates and 1.61 (95% CI = 1.12–2.10) for Malian isolates, which indicated non-random, selective invasion at both sites. The SI in both Kenya and Mali was negatively correlated with host parasitamia (Kenya r = −0.523, Mali r = −0.526, P < 0.001 in both cases, Figure 3). To allow direct comparison with previous data,8 we grouped the SI into four categories (< 2% parasitemia, 2.1–5%, 5.1–10%, and > 10%–maximum, Figure 4). The < 2% parasitemia group had a significantly higher SI than the other parasitemia groups in both Kenya and Mali (P < 0.02, by Student’s t-test, with all clinical categories within each parasitemia group combined).
Figure 3.
Scatter plots indicating the negative correlation between the selectivity index and host parasitemia of Plasmodium falciparum isolates from A, Kenya (n = 98) and B, Mali (n = 104).
Figure 4.
Geometric mean selectivity index (SI) of Plasmodium falciparum isolates from A, Kenya and B, Mali separated by patient malaria severity (black bars = uncomplicated; white bars = severe, gray bars = hyperparasitemia) and host parasitaemia group. Errors bars show 95% confidence intervals. Four samples with an SI of 0 (i.e., no multiple-infected cells) were excluded (two Kenyan and two Malian). max = maximum.
Simple regression showed that the SI was not correlated with age (P = 0.63 and P = 0.76), hemoglobin (P = 0.49 and P = 0.68) or parasite rosette frequency (P = 0.95 and P = 0.24 for Kenya and Mali, respectively). The latter result was of particular interest because we had hypothesized that rosette formation might increase the SI if it resulted in preferential invasion into the RBCs forming the rosettes. The lack of correlation between rosette formation and SI suggests that invasion is not targeted into the uninfected erythrocytes in rosettes, a finding that agrees with previous work.20
Lack of an association between SI and disease severity
We examined whether the SI differed among isolates from children with uncomplicated or severe malaria or hyperparasitemia (Figure 4 and Table 2). One-way analysis of variance with parasitemia grouping included as a covariate showed no significant difference in the SI of isolates from the different (F1,93 = 0.14, P = 0.71 and F1,73 = 0.255, P = 0.62 disease severity groups for Kenya and Mali, respectively). There was also no significant difference between uncomplicated and severe cases if only parasitemias less than 5% were analyzed in accordance with previous data from Thailand.8 With our sample sizes and variance we had > 97% power to detect a difference between severe and uncomplicated isolates equivalent to that measured in Thailand.8
The SI of isolates from children in Kenya with cerebral malaria (geometric mean = 1.72, 95% CI = 1.29–2.15, n = 20) did not differ significantly from the SI of isolates from children with other forms of severe disease (geometric mean = 1.85, 95% CI = 1.59–2.11, n = 26, F1,44 = 0.53, P = 0.47, by ANOVA). Similarly, in Mali, the SI of isolates from children with cerebral malaria (geometric mean = 1.23, 95% CI = 0.88–1.58, n = 13) did not differ significantly from the SI of isolates from children with other forms of severe disease (Mali geometric mean = 1.70, 95% CI = 0.74–2.67, n = 33, P = 0.18, F1,43 = 1.894, P = 0.18 by ANOVA).
Multiplication rate in relation to SI
We also examined the relationship between SI and PMR. There was a negative correlation between SI and PMR in Kenya (n = 29, ρ = −0.410, P = 0.03, by Spearman rank correlation, Figure 5A). However, in Mali there was no significant correlation between the SI and PMR (n = 60, ρ = 0.015, P = 0.91, by Spearman rank correlation, Figure 5B). It might be expected that isolates with high selectivity would have a low PMR, resulting in a negative correlation between SI and PMR as seen in Kenya (Figure 5A). It is unclear why this relationship was not seen in the Malian isolates (Figure 5B).
Figure 5.
Scatter plots indicating the relationship between the parasite multiplication rate (PMR) and selectivity index (SI) of Plasmodium falciparum isolates from A, Kenya (n = 29) and B, Mali (n = 60). These samples are the subset from the SI data shown in Figure 3 for which there is corresponding PMR data.
DISCUSSION
In this study, we assessed two indicators of P. falciparum invasiveness (PMR and SI) and determined the relationship of these factors to malaria severity. The PMR measures parasite multiplication under standard conditions in the first cycle of in vitro culture, whereas the SI is determined from blood smears taken directly from patients. There was no significant difference in the PMR and SI of isolates from severe malaria patients compared with isolates from uncomplicated malaria patients in two diverse sites in Africa. This lack of association with severe malaria is in contrast to previous studies on PMR and SI from Thailand.7,8 We were able to exclude technical differences as the source of this discrepancy in results because a small, blinded study of Thai isolates in our laboratory confirmed the previously published data.7 The PMR of the African isolates were mostly in the range of 1–6 fold (Figure 1), and are comparable to those of uncomplicated malaria isolates from Thailand.7 However, isolates with high PMR in the 10–22-fold range characteristic of severe malaria cases in Thailand were absent from Africa. It seems extraordinary that many isolates in both Africa and Thailand should have such low PMR when every schizont produces approximately 24 merozoites. We only used healthy parasites that matured well for one cycle in culture for the PMR assays; however, we do not know how well the in vitro assay reflects PMR in vivo. The high PMR seen in Thai severe malaria isolates are in agreement with estimates of PMR in vivo from recent studies with the P. falciparum laboratory-adapted clone 3D7 infecting European volunteers21–23 and from retrospective analysis of data collected during neurosyphilis treatment programs.24,25 To our knowledge, there are no comparable data available for in vivo PMR using fresh clinical isolates, or from any parasite clones in semi-immune individuals.
Why should there be a difference between Asia and Africa in the association of PMR and SI with parasite virulence? In Thailand (and most of southeast Asia), malaria transmission levels tend to be low, there is little acquired immunity in the population, and severe malaria affects all age groups.26 In contrast, in sub-Saharan Africa, malaria transmission tends to be moderate to high, older children and adults show partial immunity to malaria, and severe disease mainly occurs in children less than five years of age. It is possible that the different levels of immunity to malaria in the two regions are responsible for the diverse results highlighted here. Partial immunity in African patients could delay the course of infection and lead them to present at hospitals later than Asian patients, by which time differences between isolates in PMR and SI may no longer be apparent. If such a scenario were true, one might expect African children experiencing their first malaria infections after maternally acquired immunity has waned to show higher PMR more typical of those seen in Thailand. However, there was no correlation between age and PMR in our study, and isolates from children between 6 and 12 months of age who are unlikely to have had many previous malaria infections did not have higher PMR than isolates from older children (Kenya: infants, n = 5, median PMR = 1.5; older children, n = 25, median PMR = 2.3, P = 0.52, by Mann-Whitney U test; Mali: infants, n = 4, median PMR = 2.7; older children, n = 57, median PMR = 2.6, P = 0.79, by Mann-Whitney U test). There was also no significant correlation between age and SI, and isolates from children in the 6–12-month age group did not show significantly higher SI than isolates from older children (Kenya: infants, n = 13, geometric mean SI = 1.77, 95% CI = 1.38–2.16; older children, n = 66, geometric mean SI = 1.86, 95% CI = 1.55–2.17, F1,75 = 0.15, P = 0.70, by ANOVA; Mali: infants, n = 8, geometric mean SI = 1.70, 95% CI = 0.06–3.34; older children, n = 96, geometric mean SI = 1.60, 95% CI = 1.08–2.12, F1,100 = 0.05, P = 0.82, by ANOVA). The small number of children in the 6–12-month age group at both sites reflects the relative rarity of severe malaria in children less than one year of age in these areas. A prospective study with active surveillance would be required to determine conclusively whether parasites from African children experiencing their first malaria infection show higher PMR and lower SI than parasites from older children.
An alternative explanation of our results is that parasites adapted to grow in semi-immune populations in sub-Saharan Africa have evolved different mechanisms of virulence compared with those adapted to grow in non-immune populations in Asia. The growth of parasites within the host during the asexual blood stage depends upon the multiplication potential of the parasite and ability of the host to remove infected RBCs. It is plausible that in Asia, in the absence of significant levels of immunity, virulent parasites reach high parasite burdens within the host by having high PMRs. In Africa, parasites with high PMRs may not be effective in reaching high parasite burdens because of the presence of invasion-blocking antibodies in many hosts. In this case, parasite properties contributing to avoidance of the host’s immune system clearance mechanisms may be more important in helping a parasite to achieve high parasite burdens, produce high levels of sexual stages, and maximize its transmission and fitness.
Could the relationship between PMR and in vivo parasitemia explain the differences between African and Thai isolates? The interaction between parasitemia and PMR was not commented upon in the Thai study, and the parasitemias of the patients were not shown. However, high parasite burdens equivalent to those seen in Africa are not uncommon in southeast Asia; therefore, we believe it is unlikely that differences in parasitemia levels explain the diverse results from the two areas. In the current study we have carried out additional analysis on the subset of samples with parasitemias less than 5%, and on all data after adjustment to take into account host parasitemia effects. In both cases, there was no significant association between disease severity and PMR and SI in African children.
Whatever the explanation for the discrepancies between sub-Saharan Africa and Asia indicated by this study, our results raise the possibility that different pathogenic mechanisms of severe malaria may operate in parts of the world with different malaria transmission levels. This possibility is supported by studies of another potential virulence factor, rosette formation, which is associated with severe disease in multiple sites in Africa,19,27,28 but not in Asia.29,30 The hypothesis that Asian and African parasites differ in virulence properties could be tested in the absence of confounding effects of host immunity and host genetics by studying isolates infecting non-immune travelers.
One striking finding from our study was that isolates from Malian patients with hyperparasitemia had significantly lower multiplication rates than other Malian isolates (Figure 1 and Table 1). Additionally, there was a negative correlation between in vivo parasitemia and PMR across all isolates from both Kenya and Mali (Figure 2). These results seem counterintuitive because one might expect that isolates reaching high parasitemias in vivo would have the highest multiplication rates rather than the lowest. Density-dependent mechanisms are thought to occur in vivo to prevent excessive expansion of parasite populations, and can be determined by the availability of host RBCs, the presence of host immunity, or down-regulation of parasite invasiveness.31–34 Our data are consistent with P. falciparum having the ability to down-regulate its multiplication rate to avoid overwhelming the host. We are not aware of any other experimental evidence to support such an idea; however, it is plausible that switching of invasion pathways35–39 could potentially underlie such a process. It would be of great interest to examine the relationship between particular invasion pathways and parasite PMR and SI, and to determine whether the PMR and SI are stable properties of each isolate or can vary in response to external stimuli. An initial study found no association between invasion pathways and SI in Gambian field isolates.40 An alternative interpretation of the finding that isolates from hyperparasitemic patients have very low PMRs is that slowly multiplying parasites may do less damage to their hosts and therefore are able to reach high parasitemias without causing the type of life-threatening disease manifestations seen in the severe malaria group.
One similarity between Thai and African isolates is that RBC selectivity is highest at low parasitemias, with isolates from patients with parasitemias less than 2% having significantly higher SI than other groups (Figures 3 and 4). Parasites with restricted invasion (high SIs) may be unable to reach high parasitemias. A parallel to this occurs in P. yoelii, where virulent strains that invade normocytes reach higher parasitemias than avirulent strains that have a strong reticulocyte preference.41,42 An alternative explanation for the high SI at low parasitemia is that the presence of host antibodies to merozoites could lead to partial blocking of invasion and keep the parasitemia low, while also agglutinating merozoites and increasing the number of multiple invasions.9
In this study we have found no evidence to support a role for high parasite multiplication or lack of RBC selectivity as virulence factors in African children, a finding that contrasts sharply with previous work from southeast Asia.7,8 Other virulence factors such as rosette formation19,27,28 and platelet-mediated clumping43 may play a major role in parasite virulence in sub-Saharan Africa, whereas PMR and RBC selectivity are more important in Asia. If distinct pathogenic mechanisms underlie severe malaria in different parts of the world, this could have implications for the development of new strategies to treat and prevent severe disease. The differences in pathogenic mechanisms of severe malaria in regions of varying malaria transmission in both Africa and Asia are therefore worthy of further study.
Acknowledgments
We are grateful to the Bandiagara Malaria Project team in Mali and the clinical, nursing, and laboratory staff at the Kenya Medical Research Unit in Kilifi for their assistance with this study, and to the patients and their parents at both study sites. We are also grateful to Andrew Read, David Conway, and Susana Nery for discussions and comments on the manuscript. This work is published with the permission of the director of the Kenya Medical Research Institute.
Financial support: This work was supported by the Wellcome Trust (PhD studentship to Anne-Marie Deans and a Senior Research Fellowship to J. Alexandra Rowe, grant no. 067431) and the National Institutes of Health (contract no. N01-AI-85346).
Footnotes
Disclosure: None of the authors have commercial or other associations that might pose a conflict of interest.
References
- 1.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 2.James SP, Nicol WD, Shute PG. A study of induced malignant tertian malaria. Proc R Soc Med. 1932;25:1153–1181. [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 4.Mackinnon MJ, Read AF. Genetic relationship between parasite virulence and transmission in the rodent malaria Plasmodium chabaudi. Evolution. 1999;53:689–703. doi: 10.1111/j.1558-5646.1999.tb05364.x. [DOI] [PubMed] [Google Scholar]
- 5.Mackinnon MJ, Read AF. The effects of host immunity on virulence-transmissibility relationships in the rodent malaria parasite Plasmodium chabaudi. Parasitology. 2003;126:103–112. doi: 10.1017/s003118200200272x. [DOI] [PubMed] [Google Scholar]
- 6.Mackinnon MJ, Read AF. Virulence in malaria: an evolutionary viewpoint. Philos Trans R Soc Lond B Biol Sci. 2004;359:965–986. doi: 10.1098/rstb.2003.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chotivanich K, Udomsangpetch R, Simpson JA, Newton P, Pukrittayakamee S, Looareesuwan S, White NJ. Parasite multiplication potential and the severity of falciparum malaria. J Infect Dis. 2000;181:1206–1209. doi: 10.1086/315353. [DOI] [PubMed] [Google Scholar]
- 8.Simpson JA, Silamut K, Chotivanich K, Pukrittayakamee S, White NJ. Red cell selectivity in malaria: a study of multiple-infected erythrocytes. Trans R Soc Trop Med Hyg. 1999;93:165–168. doi: 10.1016/s0035-9203(99)90295-x. [DOI] [PubMed] [Google Scholar]
- 9.Miller LH, David PH, Hudson DE, Hadley TJ, Richards RL, Aikawa M. Monoclonal antibodies to a 140,000-m.w. protein on Plasmodium knowlesi merozoites inhibit their invasion of rhesus erythrocytes. J Immunol. 1984;132:438–442. [PubMed] [Google Scholar]
- 10.Ramasamy R, Ramasamy M, Yasawardena S. Antibodies and Plasmodium falciparum merozoites. Trends Parasitol. 2001;17:194–197. doi: 10.1016/s1471-4922(00)01946-2. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Yasawardena S, Kanagaratnam R, Buratti E, Baralle FE, Ramasamy MS. Antibodies to a merozoite surface protein promote multiple invasion of red blood cells by malaria parasites. Parasite Immunol. 1999;21:397–407. doi: 10.1046/j.1365-3024.1999.00239.x. [DOI] [PubMed] [Google Scholar]
- 12.Lyke KE, Dicko A, Kone A, Coulibaly D, Guindo A, Cissoko Y, Traore K, Plowe CV, Doumbo OK. Incidence of severe Plasmodium falciparum malaria as a primary endpoint for vaccine efficacy trials in Bandiagara, Mali. Vaccine. 2004;22:3169–3174. doi: 10.1016/j.vaccine.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 13.Kinyanjui SM, Mwangi T, Bull PC, Newbold CI, Marsh K. Protection against clinical malaria by heterologous immuno-globulin G antibodies against malaria-infected erythrocyte variant surface antigens requires interaction with asymptomatic infections. J Infect Dis. 2004;190:1527–1533. doi: 10.1086/424675. [DOI] [PubMed] [Google Scholar]
- 14.Lyke KE, Diallo DA, Dicko A, Kone A, Coulibaly D, Guindo A, Cissoko Y, Sangare L, Coulibaly S, Dakouo B, Taylor TE, Doumbo OK, Plowe CV. Association of intraleukocytic Plasmodium falciparum malaria pigment with disease severity, clinical manifestations, and prognosis in severe malaria. Am J Trop Med Hyg. 2003;69:253–259. [PubMed] [Google Scholar]
- 15.World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 16.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G, Snow R. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 17.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe JA, Obiero J, Marsh K, Raza A. Correlation between rosetting and parasitaemia in Plasmodium falciparum clinical isolates. Am J Trop Med Hyg. 2002;66:458–460. doi: 10.4269/ajtmh.2002.66.458. [DOI] [PubMed] [Google Scholar]
- 19.Rowe A, Obeiro J, Newbold CI, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clough B, Atilola FA, Pasvol G. The role of rosetting in the multiplication of Plasmodium falciparum: rosette formation neither enhances nor targets parasite invasion into uninfected red cells. Br J Haematol. 1998;100:99–104. doi: 10.1046/j.1365-2141.1998.00534.x. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence G, Cheng QQ, Reed C, Taylor D, Stowers A, Cloonan N, Rzepczyk C, Smillie A, Anderson K, Pombo D, Allworth A, Eisen D, Anders R, Saul A. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine. 2000;18:1925–1931. doi: 10.1016/s0264-410x(99)00444-2. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, Carter R, Saul A. Measurement of Plasmodium falciparum growth rates in vivo: a test of malaria vaccines. Am J Trop Med Hyg. 1997;57:495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 23.Bejon P, Andrews L, Andersen RF, Dunachie S, Webster D, Walther M, Gilbert SC, Peto T, Hill AV. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J Infect Dis. 2005;191:619–626. doi: 10.1086/427243. [DOI] [PubMed] [Google Scholar]
- 24.Simpson JA, Aarons L, Collins WE, Jeffery GM, White NJ. Population dynamics of untreated Plasmodium falciparum malaria within the adult human host during the expansion phase of the infection. Parasitology. 2002;124:247–263. doi: 10.1017/s0031182001001202. [DOI] [PubMed] [Google Scholar]
- 25.Gravenor MB, McLean AR, Kwiatkowski D. The regulation of malaria parasitaemia: parameter estimates for a population model. Parasitology. 1995;110:115–122. doi: 10.1017/s0031182000063861. [DOI] [PubMed] [Google Scholar]
- 26.Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg. 1997;91:256–262. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 27.Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 28.Kun JF, Schmidt-Ott RJ, Lehman LG, Lell B, Luckner D, Greve B, Matousek P, Kremsner PG. Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambarene, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–114. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 29.Ho M, Davis TM, Silamut K, Bunnag D, White NJ. Rosette formation of Plasmodium falciparum-infected erythrocytes from patients with acute malaria. Infect Immun. 1991;59:2135–2139. doi: 10.1128/iai.59.6.2135-2139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angkasekwinai P, Looareesuwan S, Chaiyaroj SC. Lack of significant association between rosette formation and parasitized erythrocyte adherence to purified CD36. Southeast Asian J Trop Med Public Health. 1998;29:41–45. [PubMed] [Google Scholar]
- 31.Tyler KM, Higgs PG, Matthews KR, Gull K. Limitation of Trypanosoma brucei parasitaemia results from density-dependent parasite differentiation and parasite killing by the host immune response. Proc R Soc Lond B Biol Sci. 2001;268:2235–2243. doi: 10.1098/rspb.2001.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reuner B, Vassella E, Yutzy B, Boshart M. Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Mol Biochem Parasitol. 1997;90:269–280. doi: 10.1016/s0166-6851(97)00160-6. [DOI] [PubMed] [Google Scholar]
- 33.Bruce MC, Donnelly CA, Alpers MP, Galinski MR, Barnwell JW, Walliker D, Day KP. Cross-species interactions between malaria parasites in humans. Science. 2000;287:845–848. doi: 10.1126/science.287.5454.845. [DOI] [PubMed] [Google Scholar]
- 34.Bruce MC, Day KP. Cross-species regulation of Plasmodium parasitemia in semi-immune children from Papua New Guinea. Trends Parasitol. 2003;19:271–277. doi: 10.1016/s1471-4922(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 35.Dolan SA, Miller LH, Wellems TE. Evidence for a switching mechanism in the invasion of erythrocytes by Plasmodium falciparum. J Clin Invest. 1990;86:618–624. doi: 10.1172/JCI114753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soubes SC, Wellems TE, Miller LH. Plasmodium falciparum: a high proportion of parasites from a population of the Dd2 strain are able to invade erythrocytes by an alternative pathway. Exp Parasitol. 1997;86:79–83. doi: 10.1006/expr.1997.4153. [DOI] [PubMed] [Google Scholar]
- 37.Reed MB, Caruana SR, Batchelor AH, Thompson JK, Crabb BS, Cowman AF. Targeted disruption of an erythrocyte binding antigen in Plasmodium falciparum is associated with a switch toward a sialic acid-independent pathway of invasion. Proc Natl Acad Sci USA. 2000;97:7509–7514. doi: 10.1073/pnas.97.13.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowman AF, Baldi DL, Healer J, Mills KE, O’Donnell RA, Reed MB, Triglia T, Wickham ME, Crabb BS. Functional analysis of proteins involved in Plasmodium falciparum merozoite invasion of red blood cells. FEBS Lett. 2000;476:84–88. doi: 10.1016/s0014-5793(00)01703-8. [DOI] [PubMed] [Google Scholar]
- 39.Gaur D, Mayer DC, Miller LH. Parasite ligand-host receptor interactions during invasion of erythrocytes by Plasmodium merozoites. Int J Parasitol. 2004;34:1413–1429. doi: 10.1016/j.ijpara.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Baum J, Pinder M, Conway DJ. Erythrocyte invasion phenotypes of Plasmodium falciparum in The Gambia. Infect Immun. 2003;71:1856–1863. doi: 10.1128/IAI.71.4.1856-1863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walliker D, Sanderson A, Yoeli M, Hargreaves BJ. A genetic investigation of virulence in a rodent malaria parasite. Parasitology. 1976;72:183–194. doi: 10.1017/s0031182000048484. [DOI] [PubMed] [Google Scholar]
- 42.Swardson-Olver CJ, Dawson TC, Burnett RC, Peiper SC, Maeda N, Avery AC. Plasmodium yoelii uses the murine Duffy antigen receptor for chemokines as a receptor for normocyte invasion and an alternative receptor for reticulocyte invasion. Blood. 2002;99:2677–2684. doi: 10.1182/blood.v99.8.2677. [DOI] [PubMed] [Google Scholar]
- 43.Pain A, Ferguson DJ, Kai O, Urban BC, Lowe B, Marsh K, Roberts DJ. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci USA. 2001;98:1805–1810. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]