Abstract
Plasmodium falciparum and Schistosoma haematobium are co-endemic parasitic diseases with worldwide distribution. Evidence suggests interactions occur between helminthic and malaria infections, although it is unclear whether this effect is beneficial or harmful to the host. Malian children 4–14 years of age with asymptomatic S. haematobium infection (SP) (n = 338) were prospectively matched by age, sex, and residence to children without schistosomiasis (SN) (n = 338) who were cleared of occult intestinal parasites, and followed-up for one malaria transmission season (25 weeks). The time to the first clinical malaria infection, incidence of malaria episodes, and parasitemia were recorded. Age associated protection from malaria in children with schistosomiasis was observed. SP children (4–8 years of age) compared with SN children demonstrated delayed time to first clinical malaria infection (74 versus 59 days; P = 0.04), fewer numbers of malaria episodes (1.55 versus 1.81 infections; P = 0.03) and lower geometric mean parasite densities (6,359 versus 9,874 asexual forms/mm3; P = 0.07) at first infection. No association between schistosomiasis and P. falciparum malaria was observed in children 9–14 years of age. We conclude that underlying schistosomiasis is associated with protection against clinical falciparum malaria in an age-dependent manner.
INTRODUCTION
Co-infection with multiple parasites is common in malaria-endemic areas. Although much is known about the epidemiology and immunology of specific parasitic illnesses, little is known about the interaction of concurrent infections. Mounting evidence suggests an interaction occurs between helminthic and malaria infections, although it is unclear as to whether this effect harms or protects the host. Plasmodium falciparum and Schistosoma spp. are co-endemic parasitic diseases with worldwide distribution. Revised estimates suggest that P. falciparum malaria causes 515 million infections annually1 while schistosomiasis affects nearly 200 million people in 75 countries. Both parasitic diseases predominate in sub-Saharan Africa, but the host impact of dual infection is unknown.
Helminth infection has been reported to have both beneficial and deleterious effects on subsequent protozoal infections. Rats co-infected with Strongyloides ratti and Trypansoma brucei have increased survival over those with T. brucei infection alone.2 Similarly, protection from cerebral malaria is conferred upon mice with pre-existing Brugia pahangi infection.3 However, mice with ova-producing S. mansoni infections have increased parasitemias with P. chabaudi infection4 and delayed parasite clearance after chloroquine treatment of P. bergehi5 compared with those with Plasmodia spp. infection alone. Limited studies of clinical interaction in human infection have also been reported. Pre-existing helminth infection is associated with protection from cerebral malaria in Thai adults6 and reduced malaria-related renal and liver abnormalities.7 Conversely, a negative interaction has been noted with increased incidence of uncomplicated P. falciparum malaria in individuals with intestinal helminth infection.8–10 Small sample sizes, disparate enrollment ages, and retrospective analyses limit interpretation of these results.
To determine whether pre-existing helminth infection affects the acquisition, incidence, or clinical course of P. falciparum malaria, we conducted a matched cohort study. Schistosoma haematobium-infected Malian children were matched by age, residence, and sex to children without schistosomiasis and monitored during a malaria transmission season. Further understanding of co-parasitic interaction is needed to guide public health measures in endemic areas.
MATERIALS AND METHODS
Study site
Bandiagara (population 13,600) is located in east central Mali, and has intense seasonal transmission (July–December) of P. falciparum malaria. During peak transmission, the number of infected bites per person per month ranges from 20 to 6011 with P. falciparum comprising 95% and P. malariae 5% of infections. Bandiagara is divided into eight sectors and is bisected by a tributary of the Niger River. The dominant ethnic group is Dogon (81%).
Most individuals have daily water exposure and S. haematobium and S. mansoni are endemic to the area.12,13 Pilot studies demonstrated that 91 (25.4%) of 381 local children 4–20 years old excrete S. haematobium eggs in urine and 5% excrete S. mansoni in stool (K. Lyke, O. Doumbo, unpublished data). Prior to two sequential malaria transmission seasons (2002–2003), parents of children 4–14 years of age were invited to bring their children for screening of S. haematobium and intestinal helminth infections.
Screening and enrollment
Baseline demographic data, urine samples, and stool samples were collected at screening. Morning urine samples were obtained and 10 mL were strained through filters (Nucleopore, Acton, MA) with What-man (Brentford, United Kingdom) filter paper (22 μm). Filter papers were stained with 5% ninhydrin and examined for the presence of S. haematobium eggs when dry. Individuals excreting S. haematobium eggs were quantified as low (1–49 eggs/filter) or high (> 50 eggs/filter) excreters. Each individual submitted 2–3 sequential urine samples to improve sensitivity. Stool samples were collected and processed using the Kato-Katz method for parasite detection. Slides were read and intestinal parasites were quantified.
Based on screening results, asymptomatic schistosomiasis-positive (SP) children and schistosomiasis-negative (SN) controls between the ages of 4 and 14 years were invited to participate in a study examining malaria incidence. Children less than 10 years of age were preferentially enrolled over older children. The SP subjects were matched by age, sex, and residence to a child without S. haematobium. Age categories were defined as 4, 5, 6, 7–8, 9–10, 11–12, and 13–14 years. Residence was defined as one of eight distinct sectors of Bandiagara. Pregnancy tests were performed on all females of child-bearing age. Exclusion criteria were presence of S. mansoni eggs in stool, evidence of acute or chronic illness, and pregnancy. All children displaying symptomatic effects related to underlying S. haematobium infection (gross hematuria, renal insufficiency, peripheral edema) were offered treatment with praziquantel and excluded. Participants who were enrolled in year one were ineligible for participation in year two.
At enrollment, clinical information was obtained, a physical examination was conducted, and a study identification number was assigned. Baseline hemoglobin levels (g/dL) and thin and thick blood smears identifying Plasmodia parasites were obtained. Each child, regardless of presence of intestinal helminths, was given a single dose of albendazole 400 mg orally to eradicate occult intestinal parasites.
Longitudinal monitoring
Each child was followed weekly for 25 weeks. Study personnel were available at a clinic 24 hours a day, 7 days a week for passive follow-up. Participants were evaluated for symptoms of acute malaria infection and asked to come to the clinic any time they became ill. Blood smears and hemoglobin analysis were performed monthly and at the onset of symptoms consistent with malaria. The SP children were monitored for evidence of active symptoms related to schistosomiasis infection. All individuals displaying gross hematuria or symptoms of genito-urinary pathology were treated with praziquantel therapy and discharged from the study. Children missing more than two sequential appointments were excluded from further participation. Children who did not experience an episode of clinical malaria during the 25-week period of follow-up were assigned a days to first malaria value of 175 days.
A clinical episode of malaria was defined as P. falciparum parasitemia and an axillary temperature ≥ 37.5°C on active surveillance, or parasitemia and symptoms leading to treatment-seeking behavior in the absence of other clear cause on passive surveillance. Symptomatic infections were treated with a three-day course of chloroquine, according to the National Malaria Control Program policy at that time. Treatment failures are less than 10% in Bandiagara.14 Each child was examined on days 0, 1, 2, 7, and 14 after the diagnosis of malaria with serial hemoglobin levels obtained and peripheral smears prepared. Recrudescence of symptoms within the 14-day follow-up period was counted as a treatment failure because of probable chloroquine resistance and sulfadoxine-pyrimethamine was administered as second-line therapy.
Children presented for one dry season follow-up (eight weeks after standing water pools dried to ensure maturation of underlying schistosomula in individuals). Hemoglobin analysis, peripheral smears, and urine and stool studies were repeated and all children in the SP group as well as SN individuals who had acquired schistosomiasis were treated with praziquantel (40 mg/kg).
Study protocols were reviewed and approved by the University of Bamako Institutional Review Board (IRB) and the University of Maryland IRB. Village permission to conduct research was obtained from village chiefs, government officials, and traditional healers prior to study initiation following established procedures.15 Individual informed consent was obtained from the parent or legal guardian of each child prior to screening and enrollment.
Ethical considerations
Infection with S. haematobium is a chronic disease in the absence of therapy. Genito-urinary inflammation secondary to migrating eggs is usually sustained over decades, although most individuals never experience clinical morbidity. After consultation with experts and IRBs, a nine-month interval between detection and treatment of asymptomatic infections was believed to be safe and reasonable. Schistosomiasis transmission is seasonal in Mali. The efficiency of transmission increases in the dry season when standing water evaporates and cercariae are concentrated. Immature schistosomula are resistant to praziquantel and require eight weeks for larval maturation. Premature treatment of infections and treatment during ongoing transmission ensures a high degree of treatment failure and/or re-infection.16 The consequences of treatment in disease-endemic populations with ongoing, frequent parasitic exposure are unclear. Acquired partial resistance to infection occurs with down-regulation of inflammatory response to parasites.17,18 Treatment may interrupt this partial resistance and trigger a surge of immunologically mediated disease upon re-exposure. We took a measured approach ensuring the best clinical cure and least harm to our study subjects. Although a small risk of acute inflammation due to egg migration existed, all subjects were monitored for evidence of renal abnormality and symptomatic individuals were treated immediately.
Statistical analysis
A sample size of 300 matched pairs was calculated by assuming a 20% difference in time to acquisition of the first case of malaria between the SP and SN groups with 90% power. Paired analyses between matched clinical groups were made using the Wilcoxon signed rank test for variables that were not normally distributed (SigmaStat version 3.1; SPSS Inc., Chicago, IL). Pooled analyses between clinical groups were made using the two-sided Student’s t-test for continuous variables with normal distribution and equal variance and the chi-square test for categorical variables using SPSS version 10.0 (SPSS Inc., Chicago, IL) and Epi-Info (Centers for Disease Control and Prevention, Atlanta, GA) with Fisher’s Exact testing for a cell quantity less than five.
RESULTS
Patients
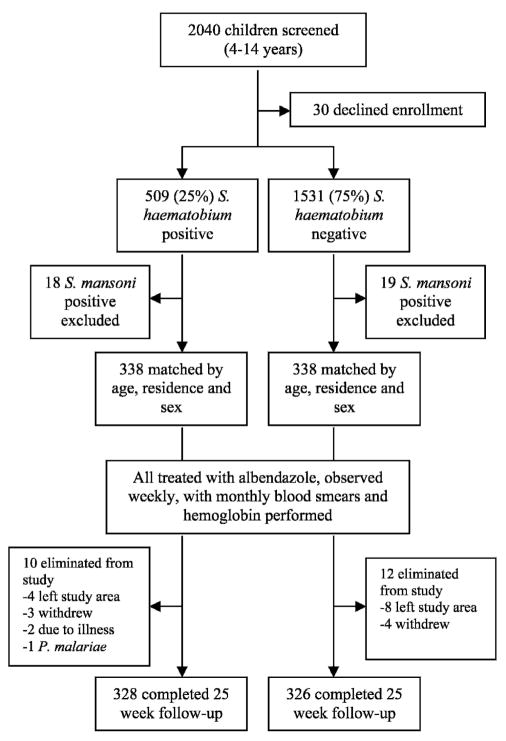
Over two transmission seasons, 2,040 children were screened (1,177 in year one and 863 in year two) (Figure 1). Schistosoma haematobium egg excretion was detected in 509 (25%) of 2,040. Sixty-seven individuals were excluded from enrollment. Of the 1,973 asymptomatic children eligible for enrollment, 676 children (338 matched pairs) were enrolled over two seasons. All SP children less than 10 years of age were preferentially enrolled with older children randomly chosen between the eight residential sectors of Bandiagara until the target sample size was attained. Eligible matching SN children were pooled with the child residing closest to the index SP volunteer chosen for enrollment. Twenty-one cases (3.1%) exited the study because of a lack of follow-up due to development of clinical symptoms (subsequently judged not to be schistosomiasis related, n = 2), or movement from the study area over the monitoring period (n = 19). One case was eliminated from analysis because of identification of P. malariae as the causative organism of the first malaria episode.
Figure 1.
Trial profile for the matched cohort trial. S. = Schistosoma.
Analysis was performed on 328 SP children and 326 SN children. The mean age was 8.8 years. The groups had similar distributions of hemoglobin subtypes, rates of concomitant intestinal parasites prior to albendazole therapy, and exposure to protective measures against malaria. Females accounted for 51% of the study subjects (Table 1).
Table 1.
Demographic characteristics at enrollment of Schistosoma haematobium–positive and age-, sex-, and residence-matched S. haematobium-negative participants
| S. haematobium negative (n = 326) | S. haematobium positive (n = 328) | |
|---|---|---|
| Age in years | ||
| 4–6 | 73 | 74 |
| 7–8 | 88 | 91 |
| 9–10 | 64 | 63 |
| 11–12 | 61 | 63 |
| 13–14 | 40 | 37 |
| Mean age | 8.80 | 8.75 |
| Female (%) | 165 (50.6) | 167 (50.9) |
| Hemoglobin (g/dL) | 12.2 | 12.3 |
| Hemoglobin subtypes (%) | ||
| AA | 250 (76.7) | 246 (75) |
| AC | 42 (13) | 47 (14.3) |
| CC | 4 (1.2) | 2 (0.6) |
| CS | 0 (0) | 1 (0.3) |
| AS | 15 (4.6) | 21 (6.4) |
| Missing | 15 (4.6) | 11 (3.4) |
| Residential sector (%) | ||
| I | 30 (9.1) | 29 (8.9) |
| II | 30 (9.1) | 26 (8) |
| III | 134 (40.9) | 129 (39.6) |
| IV | 45 (13.7) | 47 (14.4) |
| V | 18 (5.5) | 12 (3.7) |
| VI | 10 (3) | 14 (4.3) |
| VII | 44 (13.4) | 48 (14.7) |
| VIII | 17 (5.2) | 21 (6.4) |
| Intestinal infection (%)* | 26 (8) | 31 (9.5) |
| Protective measures (%)† | ||
| None | 38 (11.6) | 41 (12.5) |
| Sporadic | 81 (24.8) | 95 (29) |
| Daily | 209 (64.1) | 192 (58.5) |
Intestinal infection refers to presence of Necator americanus, Ascaris lumbricoides, Hy-menolepsis nana, and Enterobius vermicularis at enrollment.
Protective measures against malaria include reported use of bed nets, coils, or spray.
Malaria incidence
Longitudinal analysis determined that SP children had increased time to first clinical infection compared with SN children (74.6 versus 64.8 days; P = 0.05 (Table 2). No difference was observed in overall number of malaria episodes (range = 0–5) over the follow-up period in SP versus SN children (1.49 versus 1.60; P = 0.19) nor was a difference observed in geometric mean parasite density (henceforth termed parasite density) at first clinical malaria infection (5,521 versus 6,761 asexual parasites/mm3; P = 0.19). Sixty-three (19.3%) SN participants remained malaria-free throughout the follow-up period versus 77 (23.4%) of the SP participants (relative risk [RR] = 0.82, 95% confidence interval [CI] = 0.61–1.11, P = 0.2) No difference was noted between the SP and SN groups in the number of participants with more than one clinical malaria episode during the study period (146 [45%] of 328 versus 156 [48%] of 326; RR = 0.87, 95% CI = 0.64–1.2, P = 0.39) or in the parasite densities of these subsequent malaria episodes (8,071 versus 7,800 asexual parasites/mm3; P = 0.90). No difference was noted in the prevalence of asymptomatic parasitemia between groups.
Table 2.
Number of malaria episodes, time to first clinical Plasmodium falciparum infection, and geometric mean parasite density in Schistosoma haematobium–negative and –positive groups matched by age, residence, and sex and followed for 175 days
| Study group* | Age group, years | n | Malaria episodes | P† | Time (days) to first malaria infection | P | First clinical parasitemia‡ (range) | P |
|---|---|---|---|---|---|---|---|---|
| SN | All: 4–14 | 326 | 1.60 | – | 64.8 | – | 6,761 (100–568,125) | – |
| SP | 328 | 1.49 | 0.19 | 74.6 | 0.05 | 5,521 (50–512,025) | 0.19 | |
| SN | 4–8 | 161 | 1.81 | – | 59.4 | – | 9,875 (100–568,125) | – |
| SP | 166 | 1.55 | 0.03 | 73.9 | 0.04 | 6,359 (125–512,025) | 0.07 | |
| SN | 9–14 | 165 | 1.39 | – | 70.2 | – | 4,531 (100–107,575) | – |
| SP | 162 | 1.41 | 0.59 | 75.3 | 0.53 | 4,754 (50–245,150) | 0.95 |
SN = Schistosoma haematobium negative; SP = S. haematobium positive.
Wilcoxon signed rank test comparing age-stratified SN vs. SP groups. Bold text indicates significant P value with level of significance set at P ≤ 0.05.
Parasitemia reported in geometric mean density of asexual parasites per mm3.
After group stratification, analysis by age category showed similar malaria incidence patterns among SP children 4, 5, 6, and 7–8 years of age (therefore examined in a combined age grouping of 4–8 years). Analysis showed differences in disease acquisition and disease character in children 4–8 years of age with peak differences noted between the ages of 6–8 years. The SP children were compared with their matched SN counterparts and had increased time to first clinical malaria infection (74 versus 59 days; P = 0.04), fewer malaria episodes over the follow-up period (1.55 versus 1.81; P = 0.03), and lower parasite densities at first malaria infection (6,359 versus 9,875 asexual parasites/mm3; P = 0.07). The number of SP children who remained free of malaria through a single transmission season (36 [22%] of 165) was greater than SN children (22 [13.7%] of 161; RR = 0.63, 95% CI = 0.39–1.02, P = 0.05). These differences were more pronounced between SP versus SN children 6–8 years of age (time to first clinical malaria infection = 79 versus 58 days, P = 0.004; malaria incidence = 1.5 versus 1.84 episodes; P = 0.003, and parasite density = 4,647 versus 9,741 asexual parasites/mm3; P = 0.014). The number of SP children in this age group who remained free of malaria over the course of a single transmission season (28 [24%] of 117) was greater than the number of SN children (16 [13.8%] of 116; RR = 0.58, 95% CI = 0.33–1.01, P = 0.048). Children 9–14 years of age had no difference in malaria disease characteristics.
Dose-dependent effect of egg production
The SP children were categorized as low (1–49 eggs/10 mL of urine) or high (> 50) egg excreters with inter-group analysis and comparisons to SN children assessed. No age-associated difference in egg excretion was seen. An inverse relationship between egg excretion and protection from malaria was noted with decreased malaria episodes, longer time to first clinical malaria episode, and reduced parasitemia in low excreters compared with high excreters, but statistical significance was noted only in time to first malaria infection in SP low excreters versus SN children (77 versus 64.8 days; P = 0.02).
Age-stratified analysis showed that low egg-excreting children 4–8 years of age had reduced incidence of malaria, increased time to first malaria presentation, and decreased parasite densities than high excreters, although statistical significance was noted only in time until first malaria episode (76.6 versus 65.1 days; P = 0.015). No difference in malaria parameters was noted in children 9–14 years of age.
Hematologic parameters
Hemoglobin at enrollment, first malaria episode, nadir, and study termination was compared between the SN and SP groups. Results were analyzed between the groups as a whole and between age-stratified subgroups. Enrollment hemoglobin was similar between the groups (Table 1). Likewise, nadir hemoglobin, mean hemoglobin of all clinical malaria episodes, and at study termination was similar between the SN and SP groups and age-stratified subgroups.
Seasonal acquisition of helminth infection
Study participants were evaluated at a single dry season follow-up appointment. Thirteen children were unavailable for follow-up (SP = 6 and SN = 7). Interim analysis showed that two children acquired S. mansoni infection, and 29 acquired other intestinal helminths. Fifty-four of 326 SN children (16.6%) acquired S. haematobium. After eliminating all SN children who acquired schistosomiasis after enrollment, analysis was repeated. An increased time to first clinical malaria infection (74 versus 62 days; P = 0.05) was noted in SP children compared with SN children (4–8 years of age) with a trend in reduced malaria episodes (1.55 versus 1.82 episodes; P = 0.08) and lower parasite densities (6,359 versus 9,533 asexual parasites/mm3; P = 0.09). These differences remained pronounced among SP children versus SN children 6–8 years of age (time to first clinical malaria infection = 79 versus 60 days; P = 0.02, malaria incidence = 1.47 versus 1.86 episodes; P = 0.02, and parasite density = 4,647 versus 9,135 asexual parasites/mm3; P = 0.035.
DISCUSSION
This study suggests that underlying infection with S. haematobium offers a modest degree of clinical protection against P. falciparum malaria in an age-specific fashion to children exposed to both parasitic infections. Through a matched cohort study we have followed children over a malaria transmission season and demonstrated that children infected with schistosomiasis have less malaria, longer disease-free intervals until the first clinical episode, and reduced parasitemias during that episode. This difference is manifest between the ages of four and eight years. Differences disappear after nine years of age. Similarly, differences are less pronounced after the first clinical malaria episode. Little difference in hematologic parameters was noted at any time point between groups.
Although the study was not powered to detect difference according to schistosoma ova production, there appears to be decreased malaria protection with increased egg secretion. We hypothesized that increased egg production might result in enhanced protection from clinical malaria (possibly by increased immune reactivity to egg production) but our results contradict this prediction. An age-specific correlation between malaria and schistosomiasis egg production was found in children 4–8 years of age excreting low quantities of eggs. These children had an increased time to first malaria infection. An association between low egg excretion and reduced malaria parasitemias has been described.19 It is possible that children who produce fewer eggs are better able to control the schistosoma infection through an enhanced immune response, which might also help to protect from falciparum malaria. Conversely, participants secreting large amounts of eggs may experience immune tolerance to high antigenic stimulation, which results in blunting the immune protective responses and eliminates the interactive effect noted with secretion of low amounts of eggs.
The mechanisms behind helminth-associated protection from malaria are likely multifactorial. We hypothesize that it is immunologically mediated. Clinical malaria is characterized by a bimodal immune response requiring Th1 cytokine production for control of the initial parasitemia that converts to Th2-mediated cytokine production for parasite clearance.20,21 Helminth infections, which are known to be potent inducers of host Th2 cytokine production,22–24 can down-regulate the effects of a secondary Th1-dependent parasitic challenge. This is illustrated in mice with ova-producing S. mansoni infections in which impaired Th1-dependent lesional healing is noted in Leishmania major infections,25 while increased anemia and death is noted in P. chabaudi infection (normally non-lethal to these mice) along with measurably suppressed Th2 responses.4 Recently, mice co-infected with filariasis and P. chabaudi were noted to have more severe malaria along with increased interferon-γ responsiveness, which suggests an imbalance in malaria-induced immunopathology.26
In humans, blunted peripheral blood mononuclear cell (PBMC)–derived Th1 cytokine production has been demonstrated in subjects with underlying intestinal helminth infections after stimulation with tetanus toxoid,27 oral cholera toxin B vaccine,28 and schistosomal antigens.22,29,30 Helminth infections have also been associated with cellular anergy17,31 and decreased proliferative response to hepatitis C virus antigen.32 We are evaluating the immunologic response to malaria and schistosoma using sera and PBMCs collected from these volunteers at the time of clinical infection to validate our theory.
An immunologic explanation for schistosomiasis-mediated resistance to malaria might explain the age-associated differences noted in this study. Studies at this site with seasonal malaria transmission have found that children up to 10 years of age remain highly susceptible to clinical malaria, with disease burden decreasing significantly in children more than 10 years of age.11,14 Previous prevalence studies at our site have found in children 1–4 years of age an incidence of S. haematobium of 5.6% (5 of 89) (K. Lyke, O. Doumbo, unpublished data). Younger children are unlikely to acquire schistosomiasis in part because of limited independence and water exposure, whereas acquisition increases after the age of six, peaks near the age of nine, and diminishes after the age of 14 with the development of immunity.33 It is possible that enhanced parasitic interaction occurs between the ages of four and eight years when disease susceptibility overlaps. Given the difficulties of collecting urine in children 1–3 years of age and the low prevalence rate of schistosomiasis, we elected to enroll only children 4–14 years of age. We cannot rule out the fact that schistosomiasis may be exerting an even greater protective effect against malaria acquisition in children younger than four years of age.
An intriguing alternate possibility is that iron-scavenging parasites provide protection against malaria. Iron deficiency and subsequent anemia has been shown to be protective against the acquisition of malaria.34 Conversely, iron supplementation has been shown to increase susceptibility to P. vivax.35 We did not find reduced hemoglobin levels in children infected with S. haematobium despite monthly surveillance, but we cannot rule out the possibility of sub-clinical iron deficiency as a causative mechanism for protection against malaria.
Seventeen percent of the SN children acquired S. haematobium upon examination after the study period. Schistosomiasis transmission peaks in early dry season when cercariae are concentrated in standing water pools. Therefore, most of the transmission likely occurred after study monitoring ceased and not during the rainy season when water sources were rapidly flowing. When analysis was repeated after eliminating individuals who had subsequently acquired schistosomiasis, power was lost as the sample size decreased below that calculated as necessary for statistical significance (300 matched pairs). Nevertheless, the robust differences noted in the 6–8-year-old age group remained, as did trends in children 4–8 years of age.
Efforts were made to reduce confounding variables. The effect of other concomitant infections is unknown. Previous studies performed in 120 children from Bandiagara showed no detectable filarial infections (i.e., Loa loa, Onchocerca vol-vulus, or Wuchereria bancrofti). The prevalence of HIV is less than 2% among adults in Mali. Study children were not tested individually for these infections, but children with clinically apparent diseases were excluded from the study. No difference was reported in the use of bed nets or other malaria-preventative techniques. Close attention was paid to matching individuals by residence sector (and by extension, water sources), although local environmental differences in exposure to mosquitoes could not be controlled. In the interest of safety, we treated all children for schistosomiasis after one season, negating the ability to determine ongoing clinical interaction of dual infection.
This study reports a modest protective effect of S. haematobium against P. falciparum malaria. We found reduced clinical malaria, reduced parasitemias, and increased time to first clinical infection in those with S. haematobium. We hypothesize that an imbalance of Th1/Th2-mediated cytokines might account for this finding. Immunologic studies to evaluate this possibility are ongoing. The protective effects disappear after the first infection of the malaria season although the course of these infections over multiple transmission seasons is unclear. The implications of these findings could be far-reaching in that they may extend to other helminth co-infections and could affect vaccine trial outcomes, parasite treatment programs, and preventive health care maintenance policy worldwide.
Acknowledgments
We acknowledge the population of Bandiagara, Mali who continue to provide support to this and other research endeavors.
Financial support: This work was supported by a contract (N01-AI-85346) as well as a career development award (K23-AI-49203) from the National Institutes of Allergy and Infectious Diseases.
Contributor Information
KIRSTEN E. LYKE, Email: klyke@medicine.umaryland.edu.
MARCELO B. SZTEIN, Email: msztein@medicine.umaryland.edu.
CHRISTOPHER V. PLOWE, Email: cplowe@medicine.umaryland.edu.
OGOBARA K. DOUMBO, Email: OKD@mrtcbko.org.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onah DN, Onyenwe IW, Ihedioha JI, Onwumere OS. Enhanced survival of rats concurrently infected with Trypanosoma brucei and Strongyloides ratti. Vet Parasitol. 2004;119:165– 176. doi: 10.1016/j.vetpar.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Yan Y, Inuo G, Akao N, Tsukidate S, Fujita K. Down-regulation of murine susceptibility to cerebral malaria by inoculation with third-stage larvae of the filarial nematode Brugia pahangi. Parasitology. 1997;114:333–338. doi: 10.1017/s0031182096008566. [DOI] [PubMed] [Google Scholar]
- 4.Helmby H, Kullberg M, Troye-Blomberg M. Altered immune responses in mice with concomitant Schistosoma mansoni and Plasmodium chabaudi infections. Infect Immun. 1998;66:5167–5174. doi: 10.1128/iai.66.11.5167-5174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Legesse M, Erko B, Balcha F. Increased parasitaemia and delayed parasite clearance in Schistosoma mansoni and Plasmodium berghei co-infected mice. Acta Trop. 2004;91:161–166. doi: 10.1016/j.actatropica.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Nacher M, Gay F, Singhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, Vouldoukis I, Looareesuwan S. Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22:107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Nacher M, Singhasivanon P, Silachamroon U, Treeprasertsuk S, Vannaphan S, Traore B, Gay F, Looareesuwan S. Helminth infections are associated with protection from malaria-related acute renal failure and jaundice in Thailand. Am J Trop Med Hyg. 2001;65:834–836. doi: 10.4269/ajtmh.2001.65.834. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel A, Tall A, Raphenon G, Trape JF, Druilhe P. Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2003;97:198–199. doi: 10.1016/s0035-9203(03)90117-9. [DOI] [PubMed] [Google Scholar]
- 9.Nacher M, Singhasivanon P, Yimsamran S, Manibunyong W, Thanyavanich N, Wuthisen R, Looareesuwan S. Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol. 2002;88:55 –58. doi: 10.1645/0022-3395(2002)088[0055:IHIAAW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulibaly D, Diallo DA, Thera MA, Dicko A, Guindo AB, Kone AK, Cissoko Y, Coulibaly S, Djimde A, Lyke K, Doumbo OK, Plowe CV. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg. 2002;67:604–610. doi: 10.4269/ajtmh.2002.67.604. [DOI] [PubMed] [Google Scholar]
- 12.de Clercq D, Rollinson D, Diarra A, Sacko M, Coulibaly G, Landoure A, Traore M, Southgate VR, Kaukas A, Vercruysse J. Schistosomiasis in Dogon country, Mali: identification and prevalence of the species responsible for infection in the local community. Trans R Soc Trop Med Hyg. 1994;88:653– 656. doi: 10.1016/0035-9203(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 13.Corachan M, Ruiz L, Valls ME, Gascon J. Schistosomiasis and the Dogon country (Mali) Am J Trop Med Hyg. 1992;47:6–9. doi: 10.4269/ajtmh.1992.47.6. [DOI] [PubMed] [Google Scholar]
- 14.Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 15.Diallo DA, Doumbo OK, Plowe CV, Wellems TE, Emanuel EJ, Hurst SA. Community permission for medical research in developing countries. Clin Infect Dis. 2005;41:255–259. doi: 10.1086/430707. [DOI] [PubMed] [Google Scholar]
- 16.Chandiwana SK, Woolhouse ME, Bradley M. Factors affecting the intensity of reinfection with Schistosoma haematobium following treatment with praziquantel. Parasitology. 1991;102:73 –83. doi: 10.1017/s0031182000060364. [DOI] [PubMed] [Google Scholar]
- 17.King CL, Medhat A, Malhotra I, Nafeh M, Helmy A, Khaudary J, Ibrahim S, El Sherbiny M, Zaky S, Stupi RJ, Brustoski K, Shehata M, Shata MT. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–4721. [PubMed] [Google Scholar]
- 18.Grogan JL, Kremsner PG, Deelder AM, Yazdanbakhsh M. Antigen-specific proliferation and interferon-gamma and in-terleukin-5 production are down-regulated during Schistosoma haematobium infection. J Infect Dis. 1998;177:1433–1437. doi: 10.1086/517832. [DOI] [PubMed] [Google Scholar]
- 19.Briand V, Watier L, Le Hesran JY, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: Protective effect of schistosomiasis on malaria in Senegalese children? Am J Trop Med Hyg. 2005;72:702–707. [PubMed] [Google Scholar]
- 20.von der WT, Honarvar N, Langhorne J. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J Immunol. 1996;156:2510–2516. [PubMed] [Google Scholar]
- 21.Stevenson MM, Tam MF. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993;92:77 –83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwingenberger K, Hohmann A, de Brito MC, Ritter M. Impaired balance of interleukin-4 and interferon-gamma production in infections with Schistosoma mansoni and intestinal nematodes. Scand J Immunol. 1991;34:243–251. doi: 10.1111/j.1365-3083.1991.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 23.Golding B, Zaitseva M, Golding H. The potential for recruiting immune responses toward type 1 or type 2 T cell help. Am J Trop Med Hyg. 1994;50:33–40. doi: 10.4269/ajtmh.1994.50.33. [DOI] [PubMed] [Google Scholar]
- 24.Cooper PJ, Chico ME, Sandoval C, Espinel I, Guevara A, Kennedy MW, Urban JF, Jr, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–1213. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 25.La Flamme AC, Scott P, Pearce EJ. Schistosomiasis delays lesion resolution during Leishmania major infection by impairing parasite killing by macrophages. Parasite Immunol. 2002;24:339–345. doi: 10.1046/j.1365-3024.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 26.Graham AL, Lamb TJ, Read AF, Allen JE. Malaria-filaria coinfection in mice makes malarial disease more severe unless filarial infection achieves patency. J Infect Dis. 2005;191:410–421. doi: 10.1086/426871. [DOI] [PubMed] [Google Scholar]
- 27.Sabin EA, Araujo MI, Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–272. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 28.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with suppression of the in-terleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viana IR, Sher A, Carvalho OS, Massara CL, Eloi-Santos SM, Pearce EJ, Colley DG, Gazzinelli G, Correa-Oliveira R. Interferon-gamma production by peripheral blood mono-nuclear cells from residents of an area endemic for Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1994;88:466–470. doi: 10.1016/0035-9203(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 30.Zwingenberger K, Irschick E, Siqueira Vergetti JG, Correia Da-cal AR, Janssen-Rosseck R, Bienzle U, Huber C, Feldmeier H. Release of interleukin 2 and gamma interferon by peripheral mononuclear cells in human Schistosoma mansoni infection normalizes after chemotherapy. Scand J Immunol. 1989;30:463–471. doi: 10.1111/j.1365-3083.1989.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 31.Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, Bentwich Z. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest. 2000;106:1053–1060. doi: 10.1172/JCI10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamal SM, Bianchi L, Al Tawil A, Koziel M, El Sayed KK, Peter T, Rasenack JW. Specific cellular immune response and cytokine patterns in patients coinfected with hepatitis C virus and Schistosoma mansoni. J Infect Dis. 2001;184:972– 982. doi: 10.1086/323352. [DOI] [PubMed] [Google Scholar]
- 33.Etard JF, Audibert M, Dabo A. Age-acquired resistance and predisposition to reinfection with Schistosoma haematobium after treatment with praziquantel in Mali. Am J Trop Med Hyg. 1995;52:549–558. doi: 10.4269/ajtmh.1995.52.549. [DOI] [PubMed] [Google Scholar]
- 34.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, Alexander ND, Back R, Kortok M, Chemtai AK, Marsh K, Williams TN. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190:439–447. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 35.Oppenheimer SJ, Gibson FD, Macfarlane SB, Moody JB, Harrison C, Spencer A, Bunari O. Iron supplementation increases prevalence and effects of malaria: report on clinical studies in Papua New Guinea. Trans R Soc Trop Med Hyg. 1986;80:603–612. doi: 10.1016/0035-9203(86)90154-9. [DOI] [PubMed] [Google Scholar]