Abstract
Although first-degree relatives of colorectal cancer (CRC) patients diagnosed at an early age are at increased risk for CRC, their compliance with colorectal cancer screening (CRCS) is not high. Relatively little is known about why these intermediate-risk family members do not comply with CRCS. Study aims were to identify subgroups of siblings of individuals diagnosed with CRC prior to age 61 who were not compliant with CRCS using cluster analysis and to identify demographical, medical and attitudinal correlates of cluster membership. A total of 421 siblings completed measures of pros, cons, processes of change, CRCS knowledge, physician and family CRCS support, CRC risk, severity, preventability, curability, closeness with the affected sibling, distress about the sibling's cancer and screening intentions. Three clusters characterized as ‘Positive about Screening’, ‘Uncertain about Screening’ and ‘Negative about Screening’ were identified. External validation revealed that those in the Positive about Screening cluster reported significantly stronger CRCS intentions than those who are Uncertain about Screening and Negative about Screening clusters. Results provide an empirical typology for understanding motivations for CRCS among at-risk family members and may lead to the development of more effective interventions to improve screening uptake.
Introduction
Colorectal cancer (CRC) is the fourth most commonly diagnosed cancer and the second leading cause of cancer deaths in the United States [1]. Although most CRC deaths could be prevented through colorectal cancer screening (CRCS) [2], participation in CRCS is relatively low [3]. CRCS is particularly important for those individuals at increased risk for CRC because of a diagnosis of CRC in a first-degree relative (FDR) [4]. Risk for CRC is particularly high if the FDR was diagnosed with CRC before age 60. These family members have an estimated lifetime risk of CRC that is between two and four times the average risk [5, 6]. This greater risk has led to the recommendation that ‘intermediate-risk’ FDRs (defined as individuals with a FDR diagnosed with CRC before the age of 61 years) begin CRCS at the age of 40 years, a decade earlier than average-risk people [7] and that they undergo more aggressive screening than average-risk individuals [8].
Although FDRs of CRC patients diagnosed at an early age are at higher risk, their compliance with screening is not high. Compliance rates range from 21% [9] to 79% [10]. Little is known about why individuals at increased risk for CRC do not choose to have appropriate CRCS even after a sibling is diagnosed with this disease. Previous research indicates that at-risk family members who do not engage in CRCS report fewer perceived pros of screening, greater perceived cons to screening, lower perceived risk for CRC, less commitment to CRCS, greater avoidance of the health care system and lower levels of closeness with the sibling diagnosed with CRC [10, 11].
One approach that has commonly been used in studies of health behaviors has been to identify distinct subgroups of individuals who share similar characteristics. The logic behind using a clustering approach is that specifying the shared and distinct characteristics of subgroups of individuals will lead to the development of more effective treatment approaches. These subgroups can subsequently be used to characterize typologies that may facilitate the conceptualization of problems. For example, typologies have been developed for individuals at risk for initiating smoking [12] or at increased mortality risk after cardiac transplant [13]. Cluster analysis can facilitate more effective treatments by allowing subgroup-specific intervention materials to be developed and facilitating the identification of groups at high risk for poor outcomes. For example, Litt et al. [14] identified three typologies of dental fear and then subsequently evaluated the cluster typologies as a moderator for treatments for reducing dental fear. A similar approach was adopted by Hodges and Wotring [15] to classify levels of psychosocial impairment among adolescents. Overall, cluster analysis may advance our understanding of factors associated with CRCS beyond what we can learn using multivariate regression approaches by characterizing groups of individuals who share beliefs about screening, so that we can identify high-risk groups and develop interventions to target each group.
The present study had three aims. Our primary aim was to identify meaningful subgroups of individuals at intermediate risk for CRC who share similar attitudes and knowledge about CRCS using cluster analysis. Theoretical models (TTMs) are helpful for guiding variable selection for such cluster analyses. Variables included in the clustering were guided by the TTM [16, 17] (pros, cons and processes of change). We clustered based on TTM constructs because our prior work has shown that these constructs are strong predictors of CRCS uptake in this population [11]. We also included knowledge about CRCS procedures in the clustering as this variable is independent conceptually from the TTM constructs but likely associated with cluster membership. Once clusters were identified, our second aim was to determine whether there were participant sociodemographic and attitudinal characteristics that might aid in understanding the cluster subgroups. Our selection of variables was guided by the Health Belief Model [18, 19] (risk, severity, preventability and curability) and the Theory of Planned Behavior [20, 21] (physician and family support for screening, distress about the affected sibling's cancer and closeness with the affected sibling). Our third aim was to determine the validity of the cluster types by evaluating their association with CRCS intentions.
Materials and methods
Participants
Data were collected as the baseline survey for a larger longitudinal study testing the efficacy of three behavioral interventions to increase CRCS (S. Manne, unpublished data). Participants were siblings of patients recruited from medical practices or clinics at 27 participating medical centers across the United States. Prospective patients were identified from lists of CRC patients from Tumor Registry and/or medical records of surgical and/or outpatient follow-up visits. Institutional Review Board approval was received at each site. Physicians providing patient names gave permission for their patients to be contacted. Sample recruitment began in December 2003 and ended in July 2007.
Eligibility criteria for patients whose siblings were the focus of this study included (i) diagnosed with colon or rectal cancer since 1997, (ii) currently living, (iii) age <61 years at diagnosis, (iv) no history of hereditary cancer syndrome, (v) no history of inflammatory bowel disease and (vi) able to comprehend English. Patients were mailed a letter and then contacted by telephone. At this time, eligible patients gave permission to contact all siblings and for medical information to be obtained. Next, identified siblings were mailed a letter describing the study. They were contacted by telephone and eligibility was determined. After informed consent and the Health Insurance Portability and Accountability Act of 1996 (after April 2003) were received, a telephone interview was conducted. During the telephone interview, data for the present study were obtained. Eligibility criteria for siblings were (i) age ≥35 or 10 years younger than the age at which the patient was diagnosed (if the proband's diagnosis was made at an age <50 years); (ii) full biological sibling; (iii) not on schedule with regard to CRCS defined as not had a colonoscopy in the past 10 years or not had a flexible sigmoidoscopy in the past 5 years and a fecal occult blood test (FOBT) in the past year [22], sibling's age at screening was appropriate; (iv) no cancer history with the exception of non-melanoma skin cancer or childhood cancer diagnosed under the age of 2 years; (v) no family history of hereditary cancer syndromes; (vi) no history of inflammatory bowel disease; (vii) able to provide meaningful informed consent and (viii) able to read and speak English.
A total of 5242 patients were identified, 1258 (24.0%) of whom were ineligible and 590 could not be located (11.3%). Of the 3394 remaining patients, 2244 (66.1%) refused participation and 1150 patients gave permission to contact their siblings (33.9%). These 1150 patients provided 2620 sibling names (M = 2.3 siblings per patient), 1262 (48.2%) of whom were found to be ineligible. Of the 1358 eligible siblings, 129 siblings could not be located. Among the remaining 1229 siblings, 808 siblings refused participation (65.7%) and 421 siblings consented and participated (participation rate = 34.3%).
A comparison between siblings who refused with participants on demographical information indicated that refusers were more likely to be male than participants [χ2 (1, 1190) = 33.9, P < 0.001; percent maleparticipant sample = 40%; percent malerefuser sample = 57%]. Participants were also significantly younger than refusers [t (1188) = 2.79, P < 0.05; Mparticipants = 47.93, standard deviation (SD) = 9.06, Mrefusers = 49.92, SD = 9.31].
Clustering variable measures
Participants completed measures of Perceived Pros of CRCS (10 items, sample item, ‘Colon cancer tests are safe’, α = 0.85), Perceived Cons of CRCS (18 items, sample item, ‘Too many things can lead to a wrong result on a colon cancer exam’, α = 0.79), Commitment to Screening (nine items, sample item, ‘I know I feel better about myself if I have a colorectal cancer test’, α = 0.81), Information Sharing and Communication (seven items, sample item, ‘I can talk with at least one other person about colorectal cancer screening tests’, α = 0.72), Thinking Beyond Oneself (eight items, sample item, ‘I think I could come up with some ideas that could get doctors to recommend cancer tests more often’, α = 0.71), Avoiding Contact with Health Care (four items, sample item, ‘If I feel well, I do not go to the doctor for a regular check-up’, α = 0.73) and CRCS Procedural Knowledge (35 items, sample item, ‘As part of a colonoscopy, they take X-rays of the colon’). All items were used in our previous work [11]. Scale scores were computed as sums of the items on each scale.
Predictor variable measures
Predictors included marital status, ethnicity, employment, age, income, education, medical insurance, the number of siblings in the family, the gender of the affected sibling, the cancer stage at diagnosis, the affected sibling's age at the time of diagnosis and time elapsed since diagnosis. Additional measures were self-reported physician support for CRCS (three items, α = 0.95), self-reported family support for CRCS (two items, α = 0.66), perceived CRC risk (four items, α = 0.81), perceived CRC severity (five items, α = 0.83), perceived CRC preventability (two items, analyzed separately), perceived CRC curability (two items, analyzed separately), closeness with the affected sibling (five items, α = 0.93) and distress about the affected sibling's CRC (one item). Scale scores were computed as sums of the items on each scale.
Validation measure: screening intentions
Four questions asked about intentions to have a CRCS test of any kind, intention to ask one's doctor about CRCS tests, intention to try to make a CRCS test appointment and intention to follow the doctor's recommendation if the doctor recommended a CRCS test. Items were rated on a seven-point Likert scale (1= not at all likely, 7 = extremely likely) (α = 0.90).
Results
Preliminary analyses
All the continuous variables were normally distributed except the CRCS Pros scale on which there was a single outlier with a score 4 SDs from the mean. Since outliers can exert undue influence in cluster analyses, the data for this person were removed from further analyses.
The study sample was 60% female, reported an average age of 47.9 years (SD = 9.1) and was primarily White (91%), employed (68.6%) and reported having medical insurance (88.6%). Patients contributing siblings to the study were on average 2 years since diagnosis and had an average age of 49 years.
Cluster analyses
The sample was randomly split into two equal subsamples and a k-means clustering algorithm was conducted on these subsamples. The cluster solution identified in each randomly selected subsample was then cross-validated. Since there are no standardized procedures for determining the optimum number of clusters, the computer program was instructed to extract a different number of clusters on each run. Solutions ranging between three and six clusters were evaluated using the following criteria: (i) the algorithm converged successfully, (ii) the clusters were interpretable, (iii) the same clusters could be identified in each subsample and (iv) there were sufficient individuals in each cluster, so that the resulting clusters could be used as a grouping variable predicting CRCS intention.
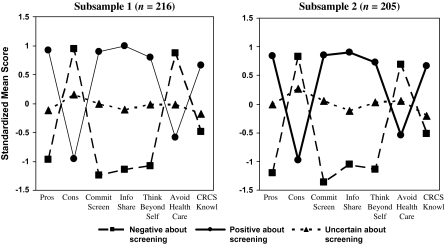
A three-cluster solution was the only solution that met all the four criteria listed above. Figure 1 illustrates the three-cluster solution in each subsample. There were different but corresponding typologies in each subsample. As shown in Fig. 1, the profile of Cluster 1 in both samples can be characterized as people who are Positive about Screening (nearly 1 SD above the mean on the Pros, Commitment to CRCS, Information Sharing and Thinking Beyond Oneself and one-half a SD above the mean on CRCS Knowledge; nearly 1 SD below the mean on the Cons and about one-half a SD below the mean on Avoids the Health Care System). The profile of Cluster 2 in both samples suggested individuals who were Negative about Screening. Their profile was almost the reverse of those who were Positive about Screening: nearly 1 SD below the mean on the Pros, >1 SD below the mean on Commitment to Screening, Information Sharing and Thinking Beyond Oneself and one-half a SD below the mean on CRCS Knowledge; nearly 1 SD above the mean on the Cons and Avoids Health Care System. The profile of Cluster 3 was essentially flat on most scales. However, people in this group were slightly above the mean on the Cons scale and below the mean on CRCS Knowledge. Because of the relatively limited variability around the mean on each of the scales, we named this clustering Uncertain about Screening.
Fig. 1.
Cluster pattern profiles from cluster analyses of the two subsamples.
To determine the extent to which the cluster solutions were replicable, the procedure described by McIntyre and Blashfield [23] was used. In the cross-validation of Subsample 1 replicating Subsample 2, average agreement between the cluster analysis and the nearest-centroid classification was 93.19%. The kappa statistic was 0.89 (z = 17.86; P < 0.0001) indicating a high degree of agreement. Similar results were found for the cross-validation of Subsample 2 replicating Subsample 1. Average agreement was 91.62% and the kappa statistic was 0.80 (z = 15.65; P < 0.0001). These results suggest that the three-cluster solution is replicable.
Table I summarizes the means and SDs for each of the seven scales in the two subsamples. Table II summarizes the standardized means and SDs for each of the seven scales in the two subsamples.
Table I.
Descriptive information on measures included in cluster analyses (n = 421)
| Measure | Positive about screening, M (SD) | Negative about screening, M (SD) | Uncertain about screening, M (SD) |
| Sample 1 | n = 63 | n = 47 | n = 106 |
| Perceived Pros | 45.8 (3.1) | 36.9 (4.0) | 40.9 (3.4) |
| Perceived Cons | 31.7 (7.0) | 46.6 (6.0) | 40.4 (4.6) |
| Commitment to Screening | 35.7 (4.4) | 23.8 (3.1) | 30.7 (3.4) |
| Information Sharing | 26.6 (2.1) | 20.2 (2.4) | 23.4 (1.6) |
| Thinking Beyond Oneself | 30.1 (3.5) | 22.9 (2.9) | 26.9 (2.4) |
| Avoids Health Care System | 9.3 (3.5) | 14.6 (3.0) | 11.4 (2.8) |
| CRCS Knowledge | 24.0 (5.7) | 16.3 (6.5) | 18.4 (6.0) |
| Sample 2 | n = 64 | n = 44 | n = 97 |
| Perceived Pros | 45.8 (2.8) | 36.0 (4.3) | 41.8 (3.1) |
| Perceived Cons | 31.1 (5.7) | 45.6 (6.5) | 41.1 (5.6) |
| Commitment to Screening | 35.4 (3.9) | 22.8 (4.1) | 30.8 (3.1) |
| Information Sharing | 26.3 (2.4) | 20.5 (2.6) | 23.3 (2.8) |
| Thinking Beyond Oneself | 30.1 (3.5) | 22.8 (2.6) | 27.4 (2.8) |
| Avoids Health Care System | 9.7 (3.6) | 14.1 (3.0) | 11.8 (3.1) |
| CRCS Knowledge | 24.4 (5.5) | 18.5 (6.1) | 16.3 (7.4) |
Table II.
Standardized means and SDs for measures in the three clusters
| Measure | Positive about Screening, M (SD) | Negative about Screening, M (SD) | Uncertain about Screening, M (SD) |
| Sample 1 | n = 63 | n = 47 | n = 106 |
| Perceived Pros | 0.92 (0.67) | −0.97 (0.84) | −0.12 (0.73) |
| Perceived Cons | −0.96 (0.89) | 0.95 (0.77) | 0.16 (0.58) |
| Commitment to Screening | 0.90 (0.79) | −1.24 (0.56) | −0.00 (0.61) |
| Information Sharing | 0.99 (0.70) | −1.14 (0.80) | −0.10 (0.55) |
| Thinking Beyond Oneself | 0.80 (0.91) | −1.08 (0.77) | −0.02 (0.63) |
| Avoids Health Care System | −0.59 (0.98) | 0.87 (0.83) | −0.02 (0.80) |
| CRCS Knowledge | 0.66 (0.85) | −0.49 (0.97) | −0.18 (0.90) |
| Sample 2 | n = 64 | n = 44 | n = 97 |
| Perceived Pros | 0.84 (0.59) | −1.20 (0.88) | −0.01 (0.65) |
| Perceived Cons | −0.98 (0.71) | 0.83 (0.81) | 0.27 (0.69) |
| Commitment to Screening | 0.85 (0.68) | −1.36 (0.72) | 0.05 (0.54) |
| Information Sharing | 0.90 (0.81) | −1.05 (0.88) | −0.12 (0.55) |
| Thinking Beyond Oneself | 0.73 (0.89) | −1.14 (0.66) | 0.03 (0.70) |
| Avoids Health Care System | −0.54 (1.01) | 0.69 (0.83) | 0.05 (0.86) |
| CRCS Knowledge | 0.66 (0.80) | −0.52 (1.05) | −0.20 (0.87) |
All variables were standardized with a mean of 0 and a SD of 1.
Correlates of cluster membership
We determined the characteristics predicting each cluster using a generalized estimating equation (GEE) approach (using the SAS procedure GENMOD) to accommodate the non-independence of observations. The GEE models were tested using a logit link function along with an exchangeable working correlation matrix to handle both the binary outcome (cluster membership) and the non-independence of observations, respectively. In order to maximize statistical power, a three-cluster solution was requested using the entire sample rather than the two subsamples when assessing the predictors of cluster membership. For each of the three comparisons, an initial model was run with all predictors in the model. All non-significant variables were subsequently removed and a final model was run with only significant predictors.
Because of the known role that medical insurance plays in CRCS, this variable was entered first in each of the models described below. In the next step, all other predictors were entered and only significant parameters will be reported.
Comparison of those who were Positive About Screening to those who were Uncertain about Screening
Participants lacking medical insurance were significantly [z = 2.05; 95% confidence interval (CI) −1.92 to −0.04; P = 0.04] more likely to be Uncertain about Screening than those who were Positive about Screening. Table III summarizes the results for the remaining predictors. Results showed that men were more likely to be in the Uncertain about Screening cluster, as were individuals reporting lower income, lower levels of family support for screening or individuals who were less likely to believe that CRC can be cured. In the context of these predictors, having medical insurance was no longer significant. None of the other variables was significant predictors.
Table III.
Comparisons of correlates of cluster group membership
| Predictors | Positive versus Uncertain about Screening |
Uncertain versus Negative about Screening |
Positive versus Negative about Screening |
|||||||||
| Parameter estimate | 95% CI | z | P | Parameter estimate | 95% CI | z | P | Parameter estimate | 95% CI | z | P | |
| Gender (male) | 0.6624 | 0.60 to 1.50 | 2.34 | 0.0195 | — | — | — | — | — | — | — | — |
| Income | −0.3573 | −0.54 to −0.18 | −3.92 | <0.0001 | −0.312 | −0.64 to 0.01 | 1.89 | 0.0592 | — | — | — | — |
| Education | — | — | — | — | 0.5901 | 1.35 to 2.32 | 2.80 | 0.0050 | −0.5688 | −0.95 to −0.19 | −2.95 | 0.0032 |
| Physician support | — | — | — | — | 0.1677 | 0.07 to 0.27 | 3.33 | 0.0009 | −0.2556 | −0.37 to −0.14 | −4.23 | <0.0001 |
| Family support | −0.3873 | −0.70 to −0.08 | −2.43 | 0.0152 | 0.2349 | −0.02 to 0.49 | 1.80 | 0.0717 | — | — | — | |
| Perceived risk | — | — | — | — | 0.1774 | 0.06 to 0.29 | 2.99 | 0.0028 | −0.1994 | −0.34 to −0.06 | −2.73 | 0.0063 |
| Perceived severity | — | — | — | — | 0.0782 | 0.02 to 0.14 | 2.51 | 0.0120 | −0.1018 | −0.19 to −0.01 | −2.21 | 0.0274 |
| Perceived curability | −0.2887 | −0.57 to −0.003 | −1.98 | 0.0474 | — | — | — | — | — | — | — | — |
| Perceived curability if caught early | — | — | — | — | — | — | — | — | −0.9929 | −1.54 to −0.45 | −3.59 | 0.0003 |
| Closeness with sibling | — | — | — | — | 0.1563 | 0.04 to 0.30 | 2.72 | 0.0064 | −0.2424 | −0.36 to −0.12 | −3.81 | 0.0010 |
—, indicates non-significant comparisons. Variables not represented (marital status, ethnicity, current employment, age, medical insurance status, number of siblings in the family, Prevent 1, Prevent 2, distress about sibling's cancer, proband gender, proband stage at diagnosis, proband age at diagnosis and time since proband's diagnosis) were not significant predictors for any of the comparisons.
Comparison of those Uncertain about Screening to those who were Negative about Screening
Having medical insurance was not significant in differentiating those who were Uncertain about Screening compared with those who were Negative about Screening. Table III summarizes the role of the remaining explanatory variables. Compared with respondents who were Negative about Screening, those who were Uncertain about Screening were significantly more likely to be better educated, report stronger physician support for screening, believe that they have a greater risk of developing CRC, believe that CRC will be more severe or be closer to the affected sibling. None of the other variables were significant predictors.
Comparison of those who were Positive about Screening to those who were Negative about Screening
Individuals who do not have health insurance were significantly (z = −2.94; 95% CI −2.52 to −0.50; P = 0.003) more likely to be Negative about Screening compared with those who were Positive about Screening. Results for the remaining predictors are shown in Table III. Compared with respondents who are Positive about Screening, those who are Negative about Screening reported less education, less physician support for screening, believed that they were at lower risk of developing CRC, believed that CRC will be less severe, were less likely to think that CRC can be cured if caught early or were less close to the proband with CRC. In the context of these predictors, medical insurance no longer differentiated between these two groups.
External validation of the three-cluster solution
External validation of the three-cluster solution was based on the solution's ability to predict CRCS intention. To increase power, the full sample was used and three clusters were requested. Since intention to screen is a continuous variable, a mixed linear models’ analysis of covariance approach with nesting was used. In the first step, medical insurance was examined. In the second step, potential screening covariates were evaluated. These included the same set of variables used in the analysis of cluster membership reported earlier. In the third step, the categorical cluster variable was entered as a main effect along with the covariates. To meet one of the assumptions of the analysis of covariance [24], in the final step, the cluster variable was crossed with each of the covariates.
In the first step, medical insurance was a significant [t (93) = 3.66; P < 0.001] predictor of CRCS intention. Those having medical insurance were more likely to say they intended to have CRCS (M = 6.10) compared with those without insurance (M = 4.97). In the second step, the covariates were added to the model. Medical insurance remained a significant predictor [t (84) = 2.24; P = 0.03]. Higher education [t (84) = 4.25; P < 0.0001], greater physician support for CRCS [t (84) = 4.94; P < 0.0001], greater family support for CRCS [t (84) = 3.26; P = 0.001], higher CRC risk [t (84) = 2.80; P = 0.01] or greater closeness with affected sibling CRC [t (84) = 2.17; P = 0.03] were associated with greater CRCS intentions. No other variables were predictive.
The main effect for cluster was added to the previous model and was a highly significant [F (2,82) = 34.02; P < 0.0001] predictor of CRCS intention. Tukey's adjusted post hoc tests indicated significant differences among all three clusters, with those in the Positive about Screening cluster having the highest and those in the Negative about Screening cluster the lowest CRCS intention scores (MPositive about Screening cluster = 6.41; MUncertain about Screening cluster = 5.98 and MNegative about Screening cluster = 4.43). Having medical insurance remained a significant [t (82) = 2.08; P = 0.0405] predictor of stronger CRCS intention as did physician support for CRCS [t (82) = 3.87; P < 0.001], family support for CRCS [t (82) = 2.86; P = 0.005] and higher education [t (82) = 2.92; P = 0.004]. Neither perceived risk nor sibling closeness remained significant.
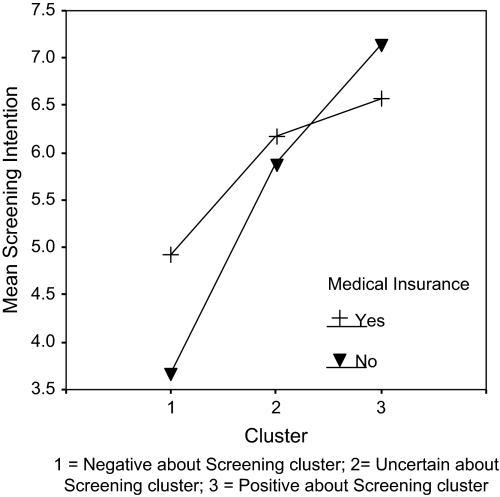
In the final step, the cluster variable was crossed with each of the covariates in the Step 2 model and non-significant effects were removed. The results of the final model are shown in Table IV. Results indicated that there was a significant interaction between cluster membership and having medical insurance. Figure 2 illustrates the interaction. Among participants who were Negative about Screening, having medical insurance was associated with a stronger CRCS intention compared with those without insurance, although the Tukey's adjusted mean difference was only marginally significant [t (81) = −2.11; P = 0.09]. By contrast, among participants who were Positive about Screening, those without medical insurance reported a significantly [t (81) = 3.80; p = 0.004] stronger Tukey's adjusted mean CRCS intention compared with those with medical insurance. Among participants in the Uncertain about Screening cluster, having medical insurance or not had no significant effect on CRCS intention (the sample size for each cell is as follows: NNo insurance, Negative about Screening = 15, NNo insurance, Uncertain about Screening = 27, NNo insurance, Positive about Screening = 6; NInsurance, Negative about Screening = 64, NInsurance, Uncertain about Screening = 190, NInsurance, Positive about Screening = 120).
Table IV.
Correlates of CRCS intentions
| Effect | Numerator, df | Denominator, df | F | P |
| Cluster membership | 2 | 81 | 46.48 | <0.0001 |
| Medical insurance | 1 | 81 | 2.17 | 0.1442 |
| Education | 1 | 81 | 7.59 | 0.0073 |
| Family support | 1 | 81 | 8.69 | 0.0042 |
| Physician support | 1 | 81 | 15.76 | 0.0002 |
| Cluster × medical insurance | 2 | 81 | 7.75 | 0.0008 |
Fig. 2.
Interaction between cluster membership and medical insurance predicting CRCS intentions.
Discussion
We created an empirical typology of motivation to have CRCS among individuals at intermediate risk for CRC who were not ‘on schedule with regard to’ CRCS. Three groups were identified through cluster analyses: Positive about Screening, Uncertain about Screening and Negative about Screening. These clusters were interpretable and replicated through internal validation analyses. External validation analyses showed significant group differences with regard to CRCS intentions among the three clusters. As anticipated, we were also able to identify demographical and psychosocial correlates of cluster membership. Overall, the CRCS typology we identified supports the hypothesis that intermediate-risk, non-CRCS compliant siblings of CRC patients (as defined by the guidelines published in Winawer et al. [22]) can be categorized into distinct groups which are related to CRCS intentions. In the discussion that follows, we characterize the clusters in more detail and discuss clinical and research applications of these findings.
Of the three clusters, individuals in the Positive about Screening cluster had the strongest intention to have CRCS. They were more likely to have medical insurance, be female, to have a higher income, to have higher levels of family support for screening and perceive CRC as more curable than individuals who are Uncertain about Screening. This cluster describes a person who has not had screening but has a proclivity to do so and has both the external support and access to the test. We would predict that these individuals would be most responsive to efforts to increase screening uptake and may not require as intensive an intervention to motivate behavioral change.
Those who are Negative about Screening displayed an opposite pattern. This cluster describes a person who has not had screening, has little motivation to do so, has little external support for CRCS and is less likely to be in the medical system and/or have access to CRCS. These individuals may be very hard to motivate, be least responsive to interventions to increase CRCS and may require behavioral interventions specifically developed for and targeted toward them. In particular, it may be important to focus on reducing these individuals’ general avoidance of the health care system as well as emphasizing the benefits of having screening done for their family. The lack of medical insurance points to the need for policy changes to make free or low-cost CRCS available.
Individuals in the Uncertain about Screening cluster fell ‘in the middle’ of the three-cluster groups in terms of their CRCS attitudes and knowledge. Although they reported above-average perceived pros and below-average CRCS knowledge, this group did not hold strong beliefs about CRCS. Their motivation to have CRCS was not as low as the Negative about Screening group but not as high as the Positive about Screening group. They were more likely to be male, reported lower incomes, had low levels of family support and perceived CRC to be less curable than those in the Positive about Screening group. They were more educated, had greater physician support for CRCS, perceived themselves to be at greater CRC risk, perceived CRC to be a more severe disease and reported higher levels of closeness to the sibling with CRC than those who are Negative about Screening. The psychological profile of this group is the most interesting as their attitudes are neither definitively negative nor positive about screening. Although this could indicate disinterest or ambivalence, it may also indicate a group of individuals who are undecided about CRCS and thus may be amenable to change. Future research to further understand the reasons underlying this ambivalence may provide a more comprehensive assessment of this group.
Overall, the factors which defined the typologies—perceived pros [25, 26], perceived cons [27], commitment to screening [28], thinking of the importance of screening for others [28], sharing information with others [28], avoidance of the health care system [28] and CRCS knowledge [28]—have previously been linked with CRCS intentions.
It is of note that most psychosocial variables were significant correlates of cluster membership. Also, the only factor that moderated the associations between cluster membership and CRCS intentions was medical insurance, which played a stronger role in intentions among participants who were Positive about Screening and Negative about Screening than among members of the Uncertain about Screening group. However, the associations were not entirely consistent. In the Negative about Screening cluster, having medical insurance was associated with a stronger screening intention. In the Positive about Screening cluster, the findings were opposite and harder to interpret: Lacking medical insurance was associated with higher screening intention than having medical insurance. Insurance status was not related to screening intentions in the Uncertain about Screening group. It is possible that, with a more favorable attitude toward CRCS, people are more willing to use their own resources to pay for the test or undergo a less costly test such as FOBT. These individuals may also see the financial benefit of identifying CRC early. Attitudes have not been explored as a predictor of willingness to pay or cost share for medical procedures, although recent studies suggest that a greater ‘need’ for a medication positively influences patients’ willingness to make co-payments for that medication [29].
The variables that predict membership in each cluster may provide guidance for more precise targeting of interventions to improve CRCS. Adopting this approach, interventions targeted toward motivating members of the Uncertain about Screening group might uniquely benefit from emphasizing ways of garnering family support for screening and increasing the understanding that CRC can be a curable disease in addition to focusing on pros, cons, commitment to screening, willingness to discuss screening with others and gather information about screening and increasing knowledge about the screening tests. Interventions targeted toward motivating the Negative about Screening group might benefit from devising methods to increase perceptions of perceived CRC risk and perceived CRC severity along with pros, cons, commitment to screening, willingness to discuss screening with others and gather information about screening and increasing knowledge about screening. Because these individuals may have less access to, or avoid, the medical system, utilizing the recommendation of a health care provider to promote screening may be challenging for individuals in this cluster. This group may be at particular risk for not having screening done and may benefit from messages that emphasize greater engagement with the health care system and the value of screening for maintaining one's health and benefit for one's family.
These findings lead to a number of ideas for future studies. First, the inclusion of other factors such as self-efficacy in the clustering procedure (e.g. [26]) may provide a more comprehensive characterization of cluster groups. Second, it is particularly important to further examine persons in the Uncertain about Screening cluster because they held both positive and negative views about CRCS. Third, it would be valuable to evaluate whether interventions designed to increase CRCS uptake also have an impact on cluster membership.
This study has several limitations. First, CRCS intention was the dependent variable rather than CRCS behavior. Validating cluster types by predicting CRCS behavior would provide a stronger test of the external validity of the clusters. Second, the fact that sibling refusers were more likely to be male and older suggests that our results may be subject to bias. For example, males are more likely to be in the Uncertain about Screening cluster and thus the number of males in the Uncertain about Screening group may be an underestimate. Third, we were not able to collect longitudinal observational data as all participants were assigned to a behavioral intervention designed to increase screening. Therefore, neither the natural stability of cluster membership nor longitudinal predictors of cluster membership over time could be studied. More importantly, our validation measure was screening intention rather than behavior. Fourth, the rate of refusal for both probands (66%) and siblings (65.7%) was relatively high. Finally, the sample was comprised primarily White, married, employed, relatively educated family members. The ability to generalize our findings to low income or minority family members may be limited.
In conclusion, we provide an empirical typology for understanding motivations for CRCS among at-risk family members. This information enhances our understanding of reasons why these family members do not present for CRCS when they have a sibling diagnosed with CRC by identifying subgroups at risk for not having CRCS who may require specialized intervention approaches. These results may thereby facilitate effective interventions to improve CRCS.
Funding
National Institutes of Health (RO1CA75795) to S.L.M.; CCSG grant to Fox Chase Cancer Center (P30CA006927).
Conflict of interest statement
None declared.
Acknowledgments
We would like to thank Jackie Allen, Christine Armetta, Hela Bakal, Pat Barry, Trudy Bennett, Cathy Betz, Angela Calnon, Lauren DeEcheandia, Terry Del Rio, Gail DeMaio, Rebekah Dunn, Tim Estrella, Millie Fleetwood, Diane Foglia, Lugenia Ford, Dot Freeman, Jolene Garney, Linda Gray, Leigh Hamilton, Laura Hammond, Danielle Hawthorne, Dr Tim Hoops, Kim Kiefer, Betsy Kopp, Nancy Koppelman, Dr Ben Krevsky, Stefanie Lappe, Denise LaRue, Debra Marra, Michele Marshall, Katie Masters, Tracy Max, Stacy McConnell, Kristine Miranda, Cheryl Mongillo, Eileen Morris, Dr Peter O'Dwyer, Melanie Pirollo, Nancy Rohowyj, Kevin Schumann, Maggie Sibert, Marianna Silverman, Wendy Stanton, Richard Throm, Amanda Tow, Gail Upshaw, Katherine Waite and Pat Weiser for assistance with data collection. Maryann Krayger provided technical assistance in preparation of this article. We would like to thank the physicians and nurses at all study sites, as well as the staff of the Cancer Community Oncology Program for their assistance in identifying participants for this study.
References
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2006. [Google Scholar]
- 2.Hewitson P, Glasziou P, Irwig L. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev. 2007;24 doi: 10.1002/14651858.CD001216.pub2. CD001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson NB, Murf HJ, Ness RM. Colorectal cancer screening among men and women in the United States. J Womens Health (Larchmt) 2007;16:57–65. doi: 10.1089/jwh.2006.0131. [DOI] [PubMed] [Google Scholar]
- 4.Bishop D, Lynch H, Rozen P. Risk and surveillance of individuals with heritable factors for colorectal cancer. WHO Collaborating Centre for the Prevention of Colorectal Cancer. Bull World Health Organ. 1990;68:655–65. [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CS, Giovannucci E, Colditz G. A prospective study of family history and risk of colorectal cancer. N Engl J Med. 1994;331:1669–74. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 6.St. John DJB, McDermott FT, Hopper LJ. Cancer risk in relatives of patients with common colorectal cancer. Ann Int Med. 1993;118:785–90. doi: 10.7326/0003-4819-118-10-199305150-00005. [DOI] [PubMed] [Google Scholar]
- 7.Levin B, Lieberman A, McFarland B. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer and the American College of Radiology. CA Cancer J Clin. 2008;58:130–60. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network (NCCN) Colorectal Cancer Screening. Clinical Practice Guidelines in Oncology. Jenkintown, PA: NCCN; 2006. Available at: http://www.nccn.org. Accessed: 6 February 2008. [DOI] [PubMed] [Google Scholar]
- 9.Rawl SM, Menon U, Champion VL. Do benefits and barriers differ by stage of adoption for colorectal cancer screening? Health Educ Res. 2005;20:137–48. doi: 10.1093/her/cyg110. [DOI] [PubMed] [Google Scholar]
- 10.Caffarey S, Broughton C, Marks C. Faecal occult blood screening for colorectal neoplasia in a targeted high-risk population. Br J Surg. 1993;80:1399–400. doi: 10.1002/bjs.1800801114. [DOI] [PubMed] [Google Scholar]
- 11.Manne S, Markowitz A, Winawer S, et al. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychol. 2002;21:3–15. [PubMed] [Google Scholar]
- 12.Velicer W, Redding C, Anatchkova M, et al. Identifying cluster subtypes for the prevention of adolescent smoking acquisition. Addict Behav. 2007;32:228–47. doi: 10.1016/j.addbeh.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Brandwin M, Trask P, Schwartz SM, et al. Personality predictors of mortality in cardiac transplant candidates and recipients. J Psychosom Res. 2000;49:141–7. doi: 10.1016/s0022-3999(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 14.Litt MD, Kalinowski L, Shafer D. A dental fears typology of oral surgery patients: matching patients to anxiety interventions. Health Psychol. 1999;18:614–24. doi: 10.1037//0278-6133.18.6.614. [DOI] [PubMed] [Google Scholar]
- 15.Hodges K, Wotring J. Client typology based on functioning across domains using the CAFAS. J Behav Health Serv Res. 2000;27:257–70. doi: 10.1007/BF02291738. [DOI] [PubMed] [Google Scholar]
- 16.Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychother Theory Res Pract. 1982;20:161–73. [Google Scholar]
- 17.Prochaska JO, Butterworth S, Redding CA, et al. Initial efficacy of MI, TTM tailoring and HRI's with multiple behaviors for employee health promotion. Prev Med. 2008;46:226–31. doi: 10.1016/j.ypmed.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janz NK, Becker MH. The Health Belief Model: a decade later. Health Educ Q. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- 19.Rosenstock IM. The health belief model. Explaining health behavior through expectancies. In: Glanz K, Lewis FM, Rimer B, editors. Health Behavior and Health Education. San Francisco, CA: Jossey Bass; 1990. pp. 39–62. [Google Scholar]
- 20.Azjen I, Fishbein M. Understanding Attitudes and Predicting Social Behavior. Englewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- 21.Montano DE, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer B, Viswanath K, editors. Health Behavior and Health Education: Theory, Research, and Practice. San Francisco, CA: Jossey-Bass; 2008. pp. 67–92. [Google Scholar]
- 22.Winawer S, Fletcher R, Rex D. Colorectal cancer screening and surveillance: Clinical guidelines and rationale—Update based on new evidence. Gastroenterology. 2003;124:544–60. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre RM, Blashfield RK. A nearest-centroid technique for evaluating the minimum-variance clustering procedure. Multivariate Behav Res. 1980;15:2225–38. [Google Scholar]
- 24.Edwards A. Experimental Design in Psychological Research. New York: Holt, Rinehart, and Winston; 1972. [Google Scholar]
- 25.Madlensky L, Esplen MJ, Goel V. Reasons given by relatives of colorectal cancer patients for not undergoing screening. Prev Med. 2004;39:643–8. doi: 10.1016/j.ypmed.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Menon U, Champion VL, Larkin GN, et al. Beliefs associated with fecal occult blood test and colonoscopy use at a worksite colon cancer screening program. J Occup Environ Med. 2003;45:891–8. doi: 10.1097/01.jom.0000083038.56116.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costanza M, Luckmann R, Stoddard A, et al. Applying a stage model of behavior change to colon cancer screening. Prev Med. 2005;41:707–19. doi: 10.1016/j.ypmed.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Manne S, Markowitz A, Winawer S, et al. Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: a test of a mediational model. Prev Med. 2003;36:71–84. doi: 10.1006/pmed.2002.1122. [DOI] [PubMed] [Google Scholar]
- 29.Yu EI, Glassman PA, Asch SM, et al. Cost-sharing for prescriptions of sildenafil and finasteride: a case study in veteran patients. Am J Manag Care. 2001;7:345–53. [PubMed] [Google Scholar]