Abstract
Several porphyrin and salophen complexes with Rh(III) are examined as ionophores to prepare nitrite selective polymeric membrane electrodes. All ionophores tested exhibit preferred selectivity towards nitrite anion. Enhanced potentiometric nitrite selectivity is observed in the presence of either lipophilic anionic as well as cationic sites within the membranes, suggesting that the ionophores can function via either a charged or neutral carrier response mechanism. Among a range of complexes and membrane formulations examined, optimal nitrite selectivity and reversible response down to 5 × 10−6 M is achieved using Rh(III)-tetra(t-butyl-phenylporphyrin) as the ionophore in the presence of lipophilic cationic sites in plasticized PVC membrane. Response times are substantially longer than typical membrane electrodes apparently due to slow nitrite ligation reaction however, a significant improvement in dynamic EMF response can be realized by optimizing the membrane formulation and increasing temperature. The selecitivity observed with these membranes is greater than the best nitrite selective electrodes reported to the date in the literature based on lipophilic Co(III)-corrin complexes, allowing the new nitrite electrodes to be utilized to determine the level of nitrite in meats with good correlation to the colorimetric Griess assay method.
Ion-selective polymeric membrane electrodes (ISEs) for cations and anions have become widespread analytical tools over the past three decades.1,2 They are based on a generic sensing principle in which different lipophilic ionophores can be employed within the polymer membranes to yield devices with high selectivity for a wide range of ions. Generally, it has been found that the development of ionophores capable of selective interaction with specific anions is far more challenging than for cations, owing to the highly varied sizes and shapes of anionic species.1 Among the anion-selective carriers examined to date, lipophilic metal cation-ligand complexes, such as metalloporphyrins, metallophthalocyanines, and metallosalophens have received considerable attention due to their good chemical and thermal resistance.3 Moreover, it has been demonstrated repeatedly that the anion selectivity of these complexes depends mainly on the specific nature of the central metal cation and hence fundamental knowledge about the chemical ligation properties of metal ion centers of these complexes (i.e., relative affinity towards different anions) can help predict the anion-selectivity of membranes formulated with such species.
There is a growing interest in devising simple sensors to detect nitrite due to the important role of this anion in many fields.4 Nitrite ion is commonly used as an additive in some foods and as a corrosion inhibitor.5,6,7 Moreover, it can be formed as a result of the degradation of fertilizers.8,9,10 Its determination is important for environmental reasons as well as for public health, since highly carcinogenic N-nitrosoamines can be formed by the reaction of nitrite with secondary amines and amides, which can be present in food in addition to the nitrite added as a preservative.11,12 In addition, measurements of nitrite are gaining greater interest in the biology/physiology/medicine arenas owing to its production in vivo by oxidation of nitric oxide (NO) produced by the enzyme nitric oxide synthase (NOS).13,14 Hence, levels of nitrite in physiological fluids can reflect NO production rates, and lower NOS activity can signal higher risk of thrombotic events.15
To date, only a few useful nitrite-selective ionophores have been reported. The most selective of these are those based on Co(III)-ligand complexes, including Co(III)-cyanocobyrinate,16,17,18 Co(III)-phthalocyanine,19 and Co(III)-tetraphenylporphyrin derivatives.20 In addition, membranes containing benzylbis(triphenylphosphine)palladium(II) and UO2-salophen have also been suggested as nitrite-selective sensors.21,22 However, even the best cobalt(III) based complexes reported thus far do not adequately discriminate against the most lipophilic anions such as thiocyanate and salicylate, which can interfere when determining nitrite in real samples.10
It is known that rhodium(III) exhibits similar (but not the same) ligation chemistry as cobalt(III) and therefore the idea of utilizing Rh(III)-ligand complexes as ionophores to devise more selective nitrite sensors seems plausible.23 To date, only one paper has been reported on the use of a Rh(III)-porphyrins within polymeric membrane electrodes, and the authors suggested these electrodes as thiocyanate selective sensors.24 However, no comprehensive studies were conducted on the question of nitrite-selectivity. Hence, the goal of the studies reported herein was to examine whether lipophilic complexes of the Rh(III) cation with several porphyrin and salophen ligands might be useful as nitrite-selective ionophores. Among several complexes examined, rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin chloride and rhodium(III) (S,S)-(+)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine chloride appear to offer the most promising nitrite selectivity. Results will be presented that demonstrate the influence of polymer membrane matrix (polymer and plasticizer) as well as the nature of ionic site additives on the observed potentiometric responses, selectivity, response times, functional lifetimes and detection limits for nitrite of membranes formulated with these ionophores.
EXPERIMENTAL SECTION
Materials and Reagents
The ionophores rhodium(III) 5,10,15,20-tetraphenylporphyrin chloride (Rh-TPP), rhodium(III) 2,3,7,8,12,13,17,18-octaethylporphyrin chloride (Rh-OEP), rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin chloride (Rh-tBTPP) and rhodium(III) (S,S)-(+)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine chloride (Jacobsen’s ligand) (Rh-JL) were synthesized via a metallation of the corresponding free porphyrins obtained from Frontier Scientific (Logan, UT, USA) or Jacobsen’s ligand obtained from Aldrich (Milwaukee, WI, USA). For preparation of porphyrin complexes, 0.418 g (2 mmol based on anhydrous formula weight) of RhCl3·×H2O (Aldrich) and 0.2 mmol of free porphyrin were refluxed in 30 mL of benzonitrile for 24 h under a nitrogen flow.25 The solvent was then evaporated under vacuum and the product was purified by column chromatography on silica gel using CH2Cl2 as the solvent.
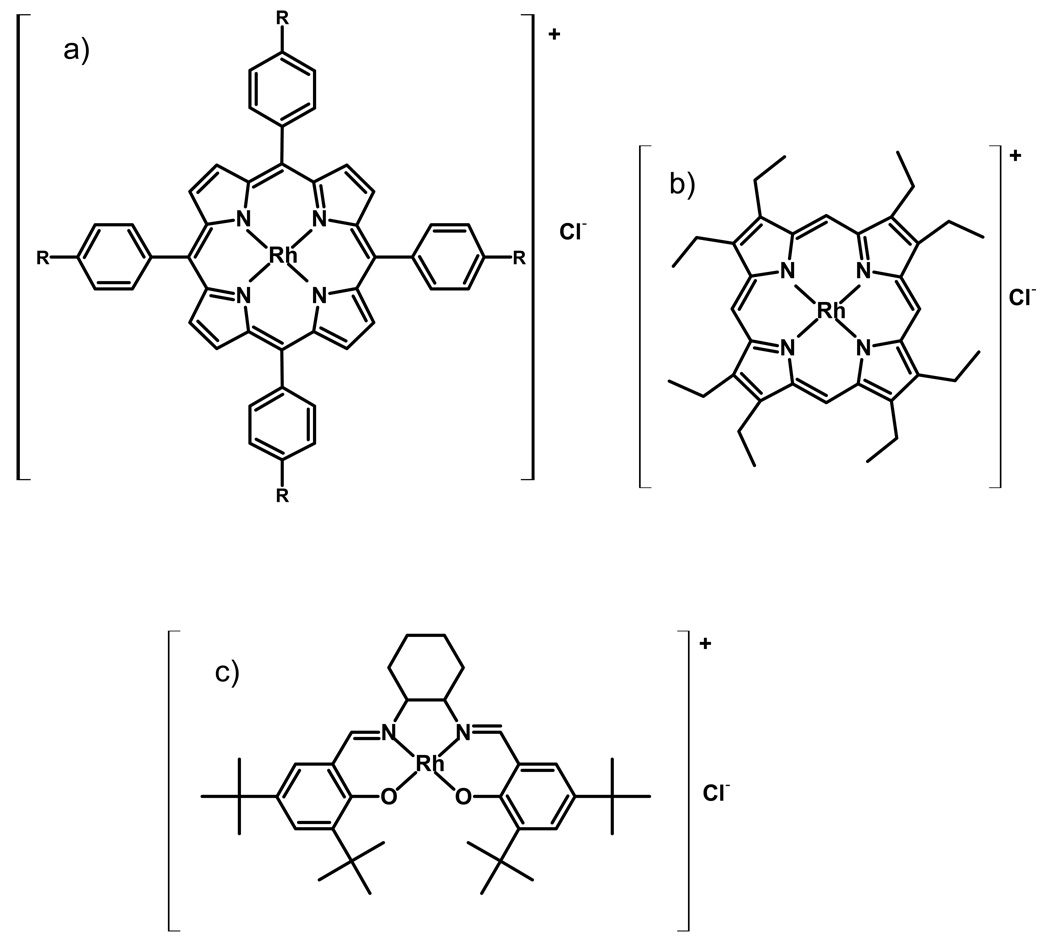
Rhodium Jacobsen’s ligand complex was obtained by dissolving 0.418 g (2 mmol based on anhydrous formula weight) of RhCl3·×H2O (Aldrich) and 0.238 g (0.4 mmol) of Jacobsen’s ligand in 50 mL of anhydrous ethanol (Aldrich).26 After 10 h of refluxing, the solvent was evaporated under vacuum and the product was purified by column chromatography on silica gel using CH2Cl2. The purity of all obtained ionophores was confirmed by thin layer chromatography, UV-Vis spectroscopy and ESI-MS spectrometry. The structures of all the Rh(III)-based ionophore compounds prepared and tested are presented in Figure 1.
Figure 1.
Structures of ionophores examined in this work: a) rhodium(III) 5,10,15,20-tetraphenylporphyrin chloride (Rh-TPP) when R=H or rhodium(III) 5,10,15,20-tetra(p-tert-butylphenyl)porphyrin chloride (Rh-tBTPP) when R=tert-butyl; b) rhodium(III) 2,3,7,8,12,13,17,18-octaethylporphyrin chloride (Rh-OEP); c) rhodium(III) (S,S)-(+)-N,N′-Bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine (Jacobsen’s ligand) (Rh-JL).
For membrane preparation, poly(vinyl chloride) (PVC), polyurethane Tecoflex SG-80A (PU), carboxylated poly(vinyl chloride) (cPVC), o-nitrophenyloctyl ether (o-NPOE), dioctyl sebacate (DOS), tributyl phosphate (TBP), potassium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (KTFPB) and tridodecylmethylammonium chloride (TDMACl) were used as received from Fluka. Tetrahydrofuran (THF) and dichloromethane (CH2Cl2) were employed to cast membrane films.
All aqueous solutions were prepared with salts of the highest purity available from Fluka. The sample solutions for potentiometric and spectrophotometric measurements consisted of sodium salts of the given anions in buffer solutions. The pH 4.5 phosphate (0.05 M) and pH 4.5 or 5.5 MES (4-Morpholineethanesulfonic acid) (0.05 M) buffers were used as background test solutions for potentiometric measurements of anion responses. For spectrophotometric measurements, a pH 5.5 MES (0.05 M) buffer was employed.
ISE Membrane Formulation and EMF Measurements
Polymer membranes employed for ISE measurements consisted of 1 wt% ionophore, PVC/plasticizer or PU/plasticizer (1:2) polymeric matrix and varying amounts of lipophilic ion additives (10 – 50 mol% relative to Rh(III) ionophore) or carboxylated PVC (10 – 50 mol% of carboxylic groups relative to ionophore). The exact compositions of the most promising membranes tested are presented in Table 1. All components were dissolved in 2 mL of THF or CH2Cl2/THF (1:3 v/v) and the mixture was then cast in 24- mm-i.d. glass ring on a glass slide. The solvent was allowed to evaporate overnight. Eight-mm diameter discs were cut out from this parent membrane and mounted in and polymer membrane electrode body (Oesch Sensor Technology, Sargans, Switzerland). Electrochemical potentials were measured with the following galvanic cell: Ag / AgCl(s), KCl (1 M) / bridge electrolyte / sample solution / ion-selective membrane / inner filing solution / AgCl(s) / Ag. The bridge electrolyte of the double-junction reference electrode was 1 M lithium acetate. A phosphate buffer solution, pH 4.5, with 10−2 M NaCl or with 10−2 M NaCl and 10−3 M NaNO2, served as the inner filling solution. Before any testing, the electrodes were first conditioned in a phosphate buffer solution, pH 4.5, containing 10−2 M NaCl and 10−3 M NaNO2 for 24 h. EMF values for the ISEs vs. reference electrode were measured at ambient temperature (ca. 24 °C) via a PC coupled to a high Z interface (VF-4, World Precision Instruments) and controlled by Labview software (version 7.0, National Instruments). Selectivity coefficients were calculated by the separate solution method (SSM).27
Table 1.
Compositions of the membranes and characteristics of the potentiometric response at ambient temperature towards nitrite for electrodes with plasticized PVC membranes containing 1 wt% of Rh-TPP, Rh-OEP, Rh-JL and Rh-tBTPP.
| ID | Ionophore | Plasticizer | Additives amount / typea [mol%] |
Slopeb [mV decade−1] |
Detection limit [M] |
|---|---|---|---|---|---|
| 1 | Rh-TPP | o-NPOE | 10 / T | −62.1 ± 0.3 | 1·10−5 |
| 2 | 25 / T | −62.5 ± 0.2 | 2·10−5 | ||
| 3 | 10 / B | −46.3 ± 0.6 | 3·10−5 | ||
| 4 | Rh-OEP | 10 / T | −60.2 ± 0.5 | 6·10−6 | |
| 5 | 25 / T | −59.4 ± 0.2 | 1·10−5 | ||
| 6 | 10 / B | −40.3 ± 0.4 | 2·10−4 | ||
| 7 | Rh-JL | 10 / T | −57.7 ± 0.3 | 8·10−6 | |
| 8 | 25 / T | −58.5 ± 0.6 | 1·10−5 | ||
| 9 | 10 / B | −42.5 ± 0.6 | 8·10−5 | ||
| 10 | Rh-tBTPP | 10 / T | −62.1 ± 0.3 | 5·10−6 | |
| 11 | 25 / T | −61.5 ± 0.6 | 8·10−6 | ||
| 12 | 10 / B | −45.6 ± 0.4 | 6·10−5 | ||
| 13 | 10 / C | −61.1 ± 0.4 | 9·10−6 | ||
| 14 | 25 / C | −60.1 ± 0.5 | 1·10−5 |
T = TDMACl; B = KTFPB, C = cPVC.
average ± s.d. of n=3 electrodes of each type
Optical Thin-Film Preparation and Spectrophotometric Measurements
UV-Vis spectroscopy data was acquired with a Lambda 35 UV-Vis spectrometer (Perkin Elmer) equipped in a quartz cell with a path length of 1 cm. Thin films of polymeric membranes of the same composition as used for potentiometric measurements were cast on glass slides (50 mm × 9 mm) according to a method described earlier.28 After placing the modified glass slide into cuvette aqueous, buffered (MES, pH 5.5) solutions containing varying concentrations of sodium nitrite were then added directly to the cuvette, allowing the polymer film to soak in the sample solution for 15 min and then the UV-Vis spectrum of the film was acquired.
Determination of Nitrite in Meats
For spectrophotometric determination of nitrite in meats, the procedure was as follows: 10 g of homogenized meats (ham or salami) were added to 100 mL of phosphate buffer (pH 4.5) and blended. This mixture was transferred into a beaker and heated to 80 °C for 30 min. Then, 5 mL of 10% ZnSO4 clarifying agent was added to the hot solution. The resulting solution was stirred and cooled at room temperature, centrifuged for 15 min and then the supernatant was filtered through a filter paper (medium texture). The solution was then diluted to 250 mL with phosphate buffer. The sulphanilamide (N-(1-naphthyl)ethylenediamine dihydrochloride (NED)) reagent was prepared as follows: 0.5 g sulphanilamide was dissolved in 150 mL of 1 M hydrochloric acid at room temperature; 6 mL of 0.20% NED solution was added and the solution diluted to 200 mL. For sample measurements 10 mL of filtrate was mixed with 5 mL of sulphanilamide – NED reagent.29,30 The absorbance was measured after 15 min at 542 nm. If the measured absorbance was too high, the sample was diluted appropriately.
For potentiometric determination of nitrite in meats with the new nitrite selective electrodes, the procedure was as follows: 10 g of homogenized meats (ham or salami) were added to 80 mL of phosphate buffer (pH 4.5) and blended. This mixture was transferred into a beaker and heated to 80 °C for 30 min. The resulting solution was stirred and cooled at room temperature and filtered through a filter paper (medium texture). The solution was then diluted to 100 mL with phosphate buffer, and the EMF of the nitrite electrode vs. double junctioned reference electrode was recorded, and this cell voltage was compared to prior calibration of nitrite standards prepared in the same phosphate buffer.
RESULTS AND DISCUSSION
To verify the usefulness of rhodium(III) complexes as ionophores for nitrite-selective electrodes, a number of different membrane formulations for each of the different ionophores were prepared (see Table 1) and examined for their response to nitrite and potential interfering anions: ClO4−, SCN−, NO3−, Br−, Cl−, F−, salicylate (Sal−) and acetate (Ace−), as well as slope of nitrite calibration curves, lower detection limit toward nitrite, and response times. Beyond the formulations listed in Table 1, a host of other compositions were tested for each ionophore, in terms of membrane plasticizer and polymer, as well as level of lipophilic ionic site additives. The listing shown in Table 1 represents the most promising formulations found, based on observed nitrite selectivity and sensitivity, from an extensive survey of different compositions.
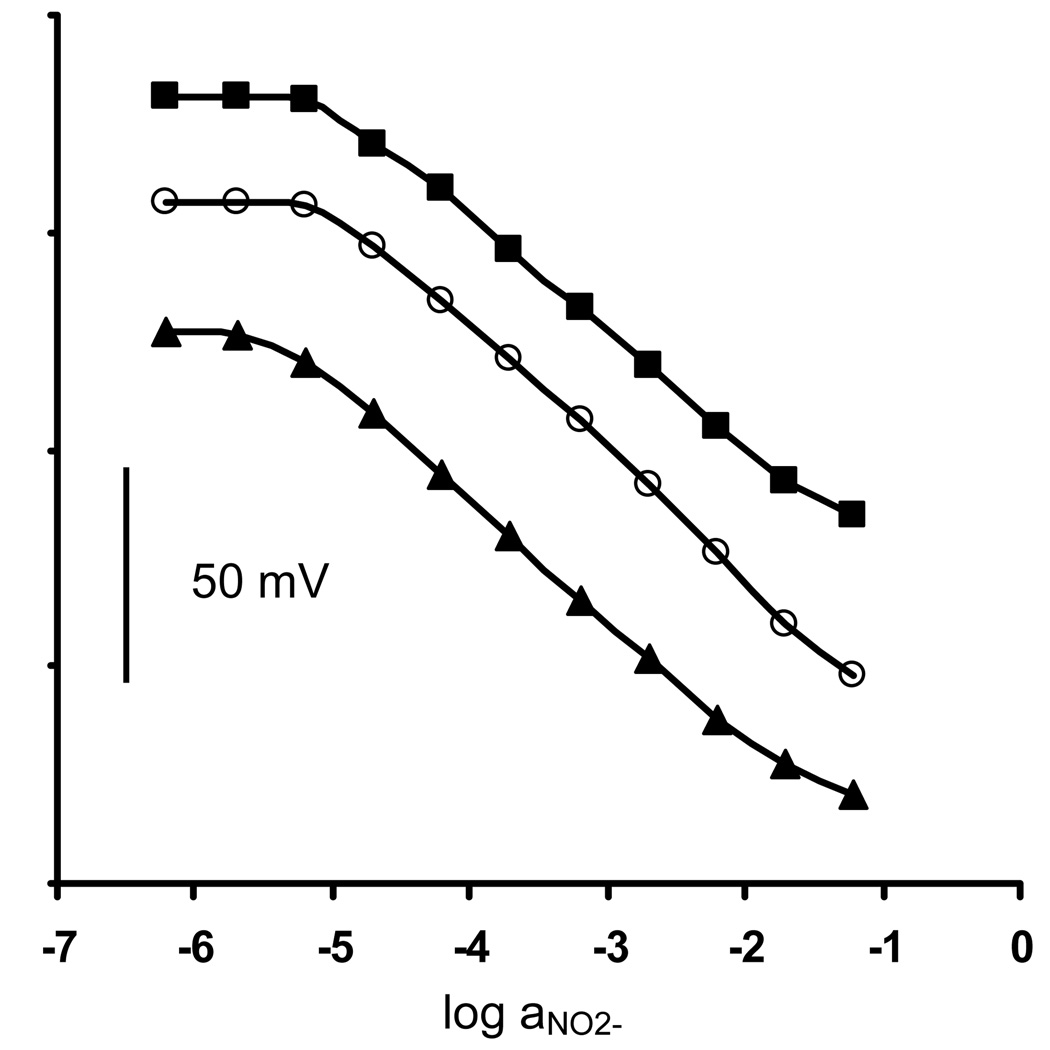
In preliminary studies, all of the membranes formulated with the Rh(III) complexes yielded significant potentiometric response to nitrite when examined in a pH 4.5 phosphate buffer background solution. Figure 2 illustrates the nitrite response in this solution for membranes 4, 7, and 10. Detection limits on the order of 5 µM were obtained for these most promising formulations and this was independent of the composition of the inner filling solution employed (with or without NO2− added), suggesting that transmembrane fluxes of nitrite when present in the internal solution do not dictate the lower limit of detection for the electrodes. Further, slopes were near nernstian values in most cases, with or without nitrite added to the internal solution. These findings suggest that the presence of 10−2 M chloride within the internal solution is adequate to poise the interfacial phase boundary potential at the inner solution/membrane interface. Because there was no dependence of potentiometric response properties on the inner filling solution composition, a phosphate buffer solution, pH 4.5, with 10−2 M NaCl added was used as the inner solution for all electrode data reported here.
Figure 2.
Potentiometric nitrite responses in phosphate buffer solution, pH 4.5, for electrodes 4 (○), 7 (Δ) and 10 (■).
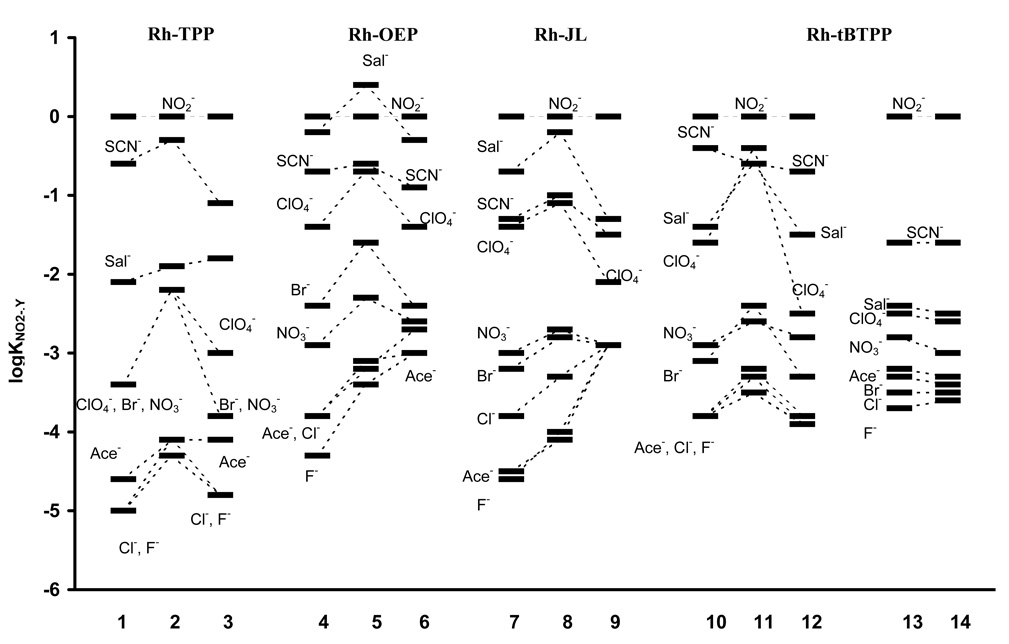
In addition to nernstian response and reasonable detection limits, preliminary studies also indicated that the selectivity coefficients observed with respect to nitrite over other anions were also quite encouraging (see Fig. 3). These promising results prompted more detailed studies regarding the effect of sample pH and membrane composition on the EMF response to nitrite.
Figure 3.
Logarithm of potentiometric selectivity coefficients of electrodes based on membranes doped with Rh-TPP, Rh-OEP, Rh-tBTPP and Rh-JL. Measurements carried out in phosphate buffer, pH 4.5. Numbers refer to membrane formulations listed in Table 1.
The Influence of pH on Electrode Nitrite Response Parameters
It is well documented that most electrodes prepared with membranes doped with ionophores that are metal ion-ligand complexes are pH-sensitive.31 Hydroxide usually exhibits high affinity towards the central metal ion of the complexes and can block the interaction between the ionophore and the other anions. Thus, the influence of sample solution pH on the nitrite response characteristics for membranes containing each of the ionophores was investigated. For this purpose, a MES/NaOH buffer solution, pH 5.5, MES/NaOH buffer solution, pH 4.5 and phosphate buffer solution, pH 4.5, were examined as the background electrolyte solutions for potentiometric nitrite measurements.
For all membrane formulations examined, changing the sample pH from 5.5 (MES buffer) to 4.5 (MES or phosphate buffer) resulted in an increase of the electrodes’ baseline EMF values. Indeed, this tenfold decrease of hydroxide concentration is reflected in a positive change of the initial baseline by approx. 50 mV, and this helps to improve the lower limit of detection toward nitrite by nearly one order of magnitude at pH 4.5 vs. pH 5.5. For example, for the electrode based on PVC/o-NPOE membranes formulated with Rh-JL as the ionophore along with 10 mol% of TDMACl (electrode 7), the LDL shifted from 6·10−5 to 8·10−6 M at pH 4.5 compared to the response in pH 5.5 solutions. In the case of the electrode prepared with the PVC/o-NPOE membrane doped with Rh-tBTPP and 10 mol% of TDMACl (electrode 10), the LDL shifted from 4·10−5 to 5·10−6 M. Moreover, there was no difference between the calibration curves for the nitrite ion obtained in MES (pH 4.5) and phosphate buffer (pH 4.5). Therefore, the phosphate buffer solution, pH 4.5, was chosen as a suitable background electrolyte for testing of all nitrite-selective membrane electrodes. A further decrease in OH− concentration to improve detection limits was avoided due to inevitable protonation of nitrite (pKaHNO2 = 3.37), which would occur when utilizing buffers with even lower pH values.
Measurements with Membranes Doped with Rh-TPP Ionophore
Rhodium(III)-tetraphenylporphyrin was suggested as a thiocyanate selective ionophore in a recent report.24 The data presented, however, indicated that this compound may have even greater affinity towards nitrite under certain conditions. Therefore, several different polymer matrices containing this species were examined for nitrite response and selectivity in detail.
Among PVC/DOS, PVC/TBP and PVC/o-NPOE membrane matrices, Rh-TPP was found to only be compatible with the latter one. Indeed, strong crystallization of this compound occurred in membranes prepared with PVC/DOS and PVC/TBP, and this crystallization occurred immediately after the solvent evaporation. It should be noted that Rh-TPP was also found not to be fully soluble in THF; therefore when preparing the membrane cocktails, independent of the matrix used, the ionophore required a CH2Cl2/THF (1:3 v/v) solvent mixture to fully dissolve the ionophore for membrane casting.
The potentiometric measurements for electrodes with PVC/o-NPOE membranes containing Rh-TPP, conducted in the presence of anionic or cationic additives in the polymeric membrane, showed relatively high selectivity towards nitrite anion over other anions compared to the selectivity order expected simply from the lipophilicity of anions: (i.e, the so-called Hofmeister selectivity pattern) ClO4−>SCN−∼I−>Sal−>Br−>NO2−∼Cl−>HCO3−>H2PO4−∼F−.32 Indeed, the observed selectivity order for membrane electrodes formulated with the Rh-TPP complex was: NO2−>SCN−>Sal−>ClO4−>Br−>NO3−>Cl−∼F−∼Ace−. As expected, thiocyanate was found to be the most interfering anion, however log selectivity coefficient values for this anion calculated relative to nitrite were still negative (see Figure 3). Potentiometric selectivity that differs significantly from the classical Hofmeister pattern was observed for electrodes prepared with membranes doped with either cationic or anionic additives, suggesting that the Rh-TPP can function via both a neutral carrier and charged carrier mechanism within the polymer membranes. Such behavior distinguishes the Rh-TPP from Co-TPP which was shown previously to function only via a neutral carrier mechanism.20 However, in contrast to the nernstian slopes for the electrodes with membranes containing cationic sites, the response slopes toward nitrite for membranes doped with anionic additives were found to be significantly lower than theoretical (−46.3 mV decade−1 for 10 mol% and −38.3 mV decade−1 for 25 mol% of KTFPB (see electrode 3 in Table 1) and decreased further with higher borate levels). In addition, the nitrite response time (defined as time required to reach 95% of equilibrium EMF value; t95) was surprisingly long (20 min when changing the concentration of nitrite from 6.3·10−5 to 2·10−4 M) and it was found to improve only with increased concentrations of NO2− (e.g., 10 min when changing the concentration of nitrite from 2·10−4 to 6.3·10−4 M and 3 min when changing the concentration of nitrite from 6·10−4 to 2·10−3 M). The sub-nernstian slopes for the membranes with KTFPB as well as the UV-Vis spectra (data not shown) of thin polymer films containing Rh-TPP and lipophilic borate additive excluded the likelihood that the slow response was due to ionophore dimer formation in the membrane phase. Moreover, the response time observed for all the other anions was much faster, suggesting that despite Rh-TPP appearing to exhibit strong interaction with nitrite, the NO2−-Rh-TPP complex formation in the membrane phase is kinetically quite slow. Indeed, when polymer membranes are formulated with only TDMACl as a general anion exchanger (to yield electrodes with Hofmeister selectivity), the response observed toward nitrite is reasonably rapid with equilibrium EMF values obtained in < 30 s. This suggests that slow ion transfer of nitrite at the sample/membrane interface is not the origin of the long response times observed when Rh-TPP is used as the ionophore, further supporting the notion that once within the organic phase, binding of nitrite to the ionophore is the rate limiting step in reaching nitrite ion equilibrium at the sample/membrane interface.
The other potential drawback of the Rh-TPP ionophore system is the relatively short electrodes lifetime. Indeed, the slope of the nitrite calibration curve decreases from −62.1 to −33.9 mV decade−1 for electrode 1 and from −46.3 to −27.8 mV decade−1 for electrode 3 after one week of use. This behavior can be attributed to strong crystallization of the ionophore within the membrane phase during this period of time.
Measurements with Membranes Doped with Rh-OEP, Rh-tBTPPand Rh-JL
Given that the results obtained for membranes containing Rh-TPP were very promising from a selectivity standpoint, three other Rh(III)-based compounds were synthesized and employed as ionophores in plasticized PVC membranes. It was hoped that changes in the structure of the ligand would influence the ionophore’s solubility in the membrane phase and/or kinetics of nitrite-ionophore interaction and overcome the drawbacks observed for the Rh-TPP complex. Rh(III)-ocatethylporphyrin (Rh-OEP), Rh(III)-tert-butyltetraphenylporphyrin (Rh-tBTPP) and Rh(III)-Jacobsen Ligand (Rh-JL) were chosen as Rh(III) complex variants (see Fig. 1). Each of these compounds was found to be fully soluble in THF, with no crystallization observed in the various membrane matrices tested. Exact compositions and the slopes of nitrite calibration curves as well the nitrite lower detection limits obtained for electrodes containing these compounds, are summarized in Table 1.
As in the case of Rh-TPP, the other rhodium(III)-based ionophores induced potentiometric anion selectivity patterns significantly different than the Hofmeister series, with pronounced nitrite selectivity (see Figure 3). Independently of the ionophore used, the best working parameters of the electrodes, such as nitrite selectivity, slopes and lower detection limit, are observed for the membranes prepared with the most polar plasticizer o-NPOE. Therefore, only results with membranes prepared with this plasticizer are described in detail here. When comparing individual ionophores the lowest detection limit was recorded for the membrane doped with Rh-tBTPP and 10 mol% of TDMACl (5·10−6 M). By increasing the cationic additives concentration (more than 25 mol%) the potentiometric parameters (nitrite-selectivity and detection limit) deteriorated. This was likely caused by increased anion-exchange properties of lipophilic cations, which promotes potentiometric response of the membrane electrode towards lipophilic anions. All the electrodes with membranes doped with anionic additives (KTFPB) showed sub-nernstian nitrite slopes; however, such membranes discriminated more against thiocyanate and salicylate, as reflected by the observed selectivity coefficients (logKNO2−,SCN−= −1.5, logKNO2−,Sal−= −1.3) for membrane 9 prepared with Rh-JL and 10 mol% of KTFPB. As with membranes containing TDMACl, the membranes with 10 mol% of KTFPB functioned the best when the most polar plasticizer (o-NPOE) was used. Experiments in which the borate concentrations were increased within the membranes resulted in even lower response slopes toward nitrite, (about −30 mV decade−1) (data not presented), and no further improvement in selectivity.
While changing ligand structure helps to avoid the crystalization process of the Rh(III)-complexes and this lead to enhanced operations lifetimes (see below), the dynamic response times of electrodes were not improved compared to the Rh-TPP-based electrodes. For example the nitrite response time for the electrode prepared with PVC/o-NPOE membrane containing 1 wt% of Rh-tBTPP and 10 mol% TDMACl (electrode 10) was 18 min, when changing the nitrite concentration from 6.3·10−5 to 2·10−4 M. Increasing the nitrite concentration lead to faster response (e.g., 3 min), when changing the nitrite concentration from 6.3·10−3 to 2·10−2 M. All of the Rh(III)-based ionophore doped membranes prepared with a conventional PVC/plasticizer matrix exhibit this slow response to nitrite when experiments are conducted at room temperature.
Methods of Improving Response Time
The main reason suggested for slow response of previously reported ISEs based on metalloporphyrin type ionophores is a dimer-monomer equilibrium of these compounds within the polymeric membrane phase.33,34 However, such chemistry does not appear to be responsible for the slow dynamic behavior of all the Rh(III)-ionophore membranes examined in this work. Indeed, UV-vis spectrophotometry measurements of thin films of the various Rh(III)-complex doped membranes showed no evidence of dimer—monomer chemistry when nitrite at high concentration was added to the bathing solution. However, it is well known that the kinetics of chemical reactions is influenced by temperature and thus if ligation reaction kinetics of the Rh(III)-nitrite interaction was limiting the response time of the sensors, a faster response time should be observed at higher temperatures. In addition membranes prepared with carboxylated PVC or polyurethanes (e.g., SG 80A) were also examined to speed response times. Such polymers assure higher water uptake within the organic membrane phase that can influence the ionophore-anion complex formation via ligation to the metal ion center (carboxyl or urethane moieties) or by catalyzing the complexation and decomplexation of nitrite by the metal ion centers of the receptor (via protons of the carboxyl moieties).
To assess the effect of temperature and increased hydrophilicity of the membrane, studies were conducted with membranes containing Rh-tBTPP and plasticized with o-NPOE, since this combination exhibited the best potentiometric selectivity and detection limit when employed in regular PVC membranes (see above). The compositions for membranes doped with cPVC, as well as the slopes and detection limits of nitrite responses for these membranes are summarized in Table 1. The highest cPVC concentration added to the normal PVC membrane was 50 mol%, but the best results were obtained for membranes doped with only 10 and 25 mol% of cPVC (electrodes 13 and 14).
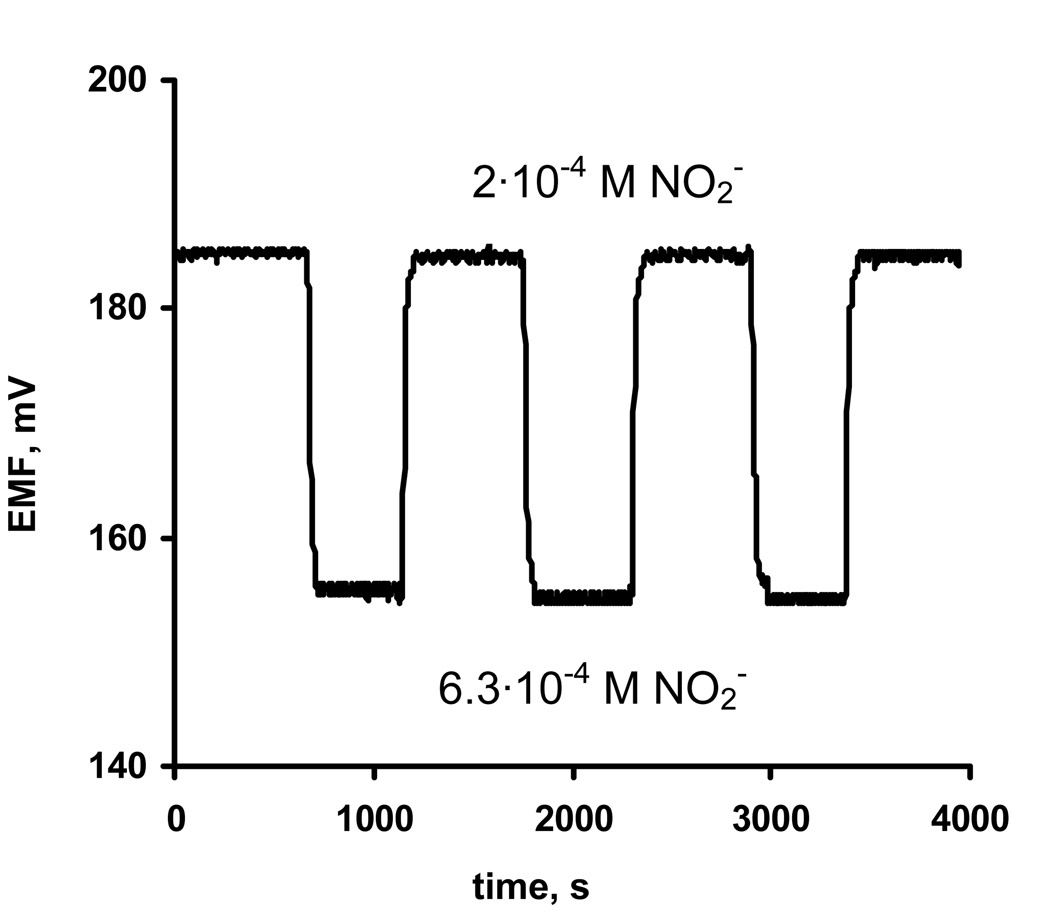
It was found that both higher temperature as well as increased hydrophilicity of membranes noticeably improves the response times of electrodes. The response time of electrode prepared with membrane 10 calibrated at 37°C when changing the nitrite concentration from 6.3·10−5 to 2·10−4 M was ca. 4 min (compare with 18 min for the same electrode at ambient temperature). Slightly better effect was obtained by using the electrode 13 (membranes doped with 10% of cPVC) at ambient temperature. For changes in nitrite concentration from 6.3·10−5 to 2·10−4 M, the response time for electrode 13 was only 3 min. Like the response time, the observed nitrite response reversibility for electrode 10 in 37°C, as well as for electrode 13 (ambient temperature) was also quite good (see Figure 4, for reversibility of electrode 13 at room temperature). The use of PU as the membrane polymer instead of PVC also helped to improve the response time, however its effect was less significant. It should be noted that increasing the measurement temperature in case of electrodes 10–12 did not alter the measured selectivity coefficients of these electrodes. The influence of changing the polymer matrix on the selectivity of electrodes is presented in Figure 3 (see data for membranes 13 and 14). The addition of cPVC (which can be considered also as anionic additive) to the PVC-based membranes improves the nitrite-selectivity (see Figure 3). Moreover, in contrast to using KTFPB, cPVC did not deteriorate the nitrite slopes or the detection limits (see Table 1). Together with improved response times, membranes doped with cPVC exhibited excellent discrimination against more lipophilic anions such as Sal−, ClO4− and even SCN− (logKNO2−,SCN−= −1.6, logKNO2−,Sal−= −2.4, logKNO2−,ClO4−= −2.5 for electrode 13) resulting in nitrite-selective electrodes with the highest degree of selectivity over these species reported to date.
Figure 4.
Reversibility of EMF nitrite response for electrode 13 when changing nitrite concentration back and forth between 2·10−4 and 6.3·10−4 M. Measurements carried out in phosphate buffer, pH 4.5, at room temperature.
Lifetime of Electrodes
The lifetime of polymeric membrane ion-selective electrodes is usually limited by leaching of the ionophore or additives from the organic membrane to the aqueous phase or by decomposition of membrane active components. In addition, ionophore crystallization will also greatly influence the operational lifetime of the electrodes. The changes in the membranes composition usually influence the electrodes properties, especially slope and selectivity.
Among all the electrodes examined, those with the best analytical response parameters (electrodes 4, 7, 10 and 13) were examined over a three month period, in terms of selectivity coefficients and slope values. As it can be seen in Table 2, the lifetime depends considerably on the ionophore structure. Among the three ionophores associated with these electrodes, the best results were obtained for membranes containing Rh-tBTPP, when PVC was employed as the membrane matrix. This porphyrin ligand structure has also been shown to be very stable in the membrane phase when used as an ionophore complex with other metal cations.35 The slopes of electrodes with membranes 10 and 13 were maintained on the useful level (over −50 mV decade−1) for over two months. Employment of PU as a membrane matrix containing Rh-tBTPP, significantly shortens the usefulness of the membrane electrodes (to two weeks). PVC membranes doped with the least lipophilic ionophore (Rh-OEP) exhibited the shortest functional lifetimes (e.g., for electrode 4, the slope reaches –50 mV decade−1 in two weeks), due to ionophore leaching. Together with slopes changes, the selectivity coefficients of each of the electrodes were also monitored over the same time periods. During periods when slopes were maintained at values > −50 mV decade−1, there were significant changes in the measured selectivity coefficients (±0.2 for logK values) (data not shown).
Table 2.
Changes in the nitrite response slopes of examined electrodes as a function of time (in mV decade−1).
| time | 1 d | 3 d | 7 d | 14 d | 28 d | 45 d | 66 d | 94 d |
|---|---|---|---|---|---|---|---|---|
| electrode | ||||||||
| 4 | −60.2 | −58.6 | −56.8 | −50.1 | −43.4 | −34.3 | −24.1 | - |
| 7 | −57.7 | −58.2 | −58.3 | −56.3 | −53.1 | −48.1 | −43.2 | −37.4 |
| 10 | −62.1 | −61.1 | −60.1 | −59.7 | −57.2 | −55.3 | −54.1 | −47.2 |
| 13 | −61.1 | −60.4 | −60.1 | −59.9 | −57.9 | −56.7 | −54.2 | −49.3 |
Application of New Nitrite Selective Electrodes
To verify the usefulness of electrodes prepared with membranes doped with Rh-tBTPP or Rh-JL, the determination of nitrite in commercial ham and salami samples was performed. Electrodes 7, 10 and 13 were used for this test and operated at room temperature. Samples were prepared via procedure similar to that described previously.30 Three of each polymeric membrane electrodes were used in this experiment and the results were compared with the results obtained using a colorimetric Griess assay method. As shown in Table 3, the results of nitrite determinations with the three types of electrodes are in excellent agreement with the results obtained using the colorimetric method. This confirms the usefulness of the Rh(III)-based ionophores to prepare nitrite-selective electrodes that have selectivity suitable for practical applications.
Table 3.
The results of nitrite determinations in commercial meats using new Rh(III)-complex based membrane electrodes compared results from classical colorimetric Griess assay method.a,b
| Sample | Electrode 7 | Electrode 10 | Electrode 13 | Griess Assay Method |
|---|---|---|---|---|
| Nitrite Content (mg of NO2−/kg of meat) | ||||
| Ham 1 | 37.0±0.3 | 37.1±0.2 | 36.9±0.5 | 36.1±0.2 |
| Salami | 40.0±0.5 | 40.2±0.3 | 39.9±0.5 | 39.5±0.2 |
| Ham 2 | 252.9±1.4 | 254.0±2.0 | 253.8±1.8 | 250.7±1.4 |
Measurements in phosphate buffer solution, pH 4.5
results reported are average ± s.d for n=3 measurements for each sample.
CONCLUSIONS
In summary, all Rh(III) species prepared and tested as ionophores in polymeric membranes electrodes induce selectivity patterns that differ significantly from the classical Hofmeister pattern, with greatly enhanced selectivity observed toward nitrite. Good nitrite response was observed in the presence of both anionic or cationic additives in the membrane, suggesting that these new ionophores can function via a dual mechanism. The best nitrite selectivity was obtained with membranes doped with carboxylated PVC. Studies on the influence of sample solution pH on the performance of the electrodes indicated that the optimal nitrite response occurs using a pH 4.5 buffered sample solution, with detection limits on the order of 5·10−6 M. It was further found that slow response times of the electrodes can be partly overcome by increasing the temperature or employing polymer matrix additives that such as polyurethanes or carboxylated PVC. The most likely origin of the relatively slow response times is the intrinsic kinetics of the Rh(III)-complex ligation reactions in the organic membrane phase. Among the examined ionophores, membranes doped with Rh-tBTPP had the longest functional lifetimes. The potentiometric nitrite selectivity over thiocyanate, perchlorate and salicylate of many of membrane electrodes tested are better than the selectivity of the best nitrite-selective ionophore described to date in the literature (cobalt(III) cobyrinate derivative with logKNO2−,SCN− = 0.3).16–18 Finally, the analytical utility of the electrodes based on Rh(III)-ionophores have been demonstrated via the accurate determination of nitrite levels in food samples.
ACKNOWLEDGEMENT
We thank the National Institutes of Health (grant # EB-000784) for supporting this work.
LITERATURE CITED
- 1.Buhlmann P, Pretsch E, Bakker E. Chem. Rev. 1998;98:1593–1687. doi: 10.1021/cr970113+. [DOI] [PubMed] [Google Scholar]
- 2.Buck RP, Lindner E. Anal. Chem. 2001;73:88A–97A. doi: 10.1021/ac012390t. [DOI] [PubMed] [Google Scholar]
- 3.Nardis S, Monti D, Di Natale C, D’Amico A, Siciliano P, Forleo A, Epifani M, Taurino A, Rella R, Paolesse R. Sens. Actuat. B. 2004;103:339–343. [Google Scholar]
- 4.Moorcroft M, Davis J, Compton R. Talanta. 2001;54:785–803. doi: 10.1016/s0039-9140(01)00323-x. [DOI] [PubMed] [Google Scholar]
- 5.Santos WJR, Lima PR, Tanaka AA, Tanaka SMCN, Kubota LT. Food Chem. 2009;113:1206–1211. [Google Scholar]
- 6.Hamano T, Mitsuhashi Y, Aoki N, Semma M, Ito Y, Oji Y. Analyst. 1998;123:1127–1129. doi: 10.1039/a707591j. [DOI] [PubMed] [Google Scholar]
- 7.Sideris KK, Savva AE. Cem. Concr. Compos. 2005;27:277–287. [Google Scholar]
- 8.Aydin A, Ercan O, Tascioglu S. Talanta. 2005;66:1181–1186. doi: 10.1016/j.talanta.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Brimblecombe P, Stedman DH. Nature. 1982;298:460–461. [Google Scholar]
- 10.Caro CA, Bedioui F, Zagal JH. Electrochim. Acta. 2002;47:1489–1494. [Google Scholar]
- 11.Pobel D, Riboli E, Cornee J, Hemon B, Guyader M. Eur. J. Epidemiol. 1995;11:67–73. doi: 10.1007/BF01719947. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch H, Ohshima H, Pignatelli B. Mutat. Res. 1988;202:307–324. doi: 10.1016/0027-5107(88)90194-7. [DOI] [PubMed] [Google Scholar]
- 13.Milsom AB, Hobbs AJ, Ahluwalia A. Nitric Oxide. 2008;19:51–52. [Google Scholar]
- 14.Oh BK, Meyerhoff ME. Biomaterials. 2004;25:283–293. doi: 10.1016/s0142-9612(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 15.Shao J, Miyata T, Yamada K, Hanafusa N, Wada T, Gordon KL, Inagi R, Kurokawa K, Fujita T, Johnson RJ, Nangaku M. J. Am. Soc. Nephrol. 2001;12:2088–2097. doi: 10.1681/ASN.V12102088. [DOI] [PubMed] [Google Scholar]
- 16.Schulthess P, Ammann D, Simon W, Caderas C, Stepanek R, Krautler B. Helv. Chim. Acta. 1984;67:1026–1032. [Google Scholar]
- 17.Schulthess P, Ammann D, Krautler B, Caderas C, Stepanek R, Simon W. Anal. Chem. 1985;57:1397–1401. [Google Scholar]
- 18.Stepanek R, Krautler B, Schulthess P, Lindemann B, Ammann D, Simon W, Caderas C. Anal. Chim. Acta. 1986;182:83–90. [Google Scholar]
- 19.Li JZ, Wu XC, Yuan R, Lin HG, Yu RQ. Analyst. 1994;119:1363–1366. [Google Scholar]
- 20.Malinowska E, Meyerhoff ME. Anal. Chim. Acta. 1995;300:33–43. [Google Scholar]
- 21.Badr IHA, Meyerhoff ME, Hassan SSM. Anal. Chem. 1995;67:2613–2618. doi: 10.1021/ac00111a019. [DOI] [PubMed] [Google Scholar]
- 22.Wroblewski W, Brzozka Z, Rudkevich DM, Reinhoudt DN. Sens. Actuat. B. 1996;37:151–155. [Google Scholar]
- 23.Cotton FA, Wilkinson G. Advanced Inorganic Chemistry. New York: Interscience; 1972. pp. 1017–1026. [Google Scholar]
- 24.Vlascici D, Spiridon Bizerea O, Fagadar-Cosma AJ. Optoel. Adv. Mater. 2006;8:883–887. [Google Scholar]
- 25.Tse A, Wu BM, Mak T, Chan KSJ. Organomet. Chem. 1998;568:257–261. [Google Scholar]
- 26.Stinziano-Eveland RA, Nguyen SBT, Liable-Sands LM, Rheingold AL. Inorg. Chem. 2000;39:2452–2455. doi: 10.1021/ic990548a. [DOI] [PubMed] [Google Scholar]
- 27.Bakker E, Pretsch E, Buhlmann P. Anal. Chem. 2000;72:1127–1133. doi: 10.1021/ac991146n. [DOI] [PubMed] [Google Scholar]
- 28.Malinowska E, Niedziolka J, Meyerhoff ME. Anal. Chim. Acta. 2001;432:67–78. [Google Scholar]
- 29.Norwitz G, Keliher PN. Analyst. 1987;112:903–907. doi: 10.1039/an9871200903. [DOI] [PubMed] [Google Scholar]
- 30.Badea M, Amine A, Benizne M, Curulli A, Moscone D, Lupu A, Volpe G, Palleschi G. Microchim. Acta. 2004;147:51–58. [Google Scholar]
- 31.Egorov VV, Rakhman'ko EM, Rat'ko AA. J. Anal.Chem. 2002;57:46–53. [Google Scholar]
- 32.Hofmeister F. Arch. Exp. Pathol. Pharmacol. 1888;24:247–260. [Google Scholar]
- 33.Steinle ED, Amemiya S, Bühlmann P, Meyerhoff ME. Anal. Chem. 2000;72:5766–5773. doi: 10.1021/ac000643x. [DOI] [PubMed] [Google Scholar]
- 34.Gorski L, Malinowska E, Parzuchowski P, Zhang W, Meyerhoff ME. Electroanal. 2003;15:1229–1235. [Google Scholar]
- 35.Pietrzak M, Meyerhoff ME, Malinowska E. Anal. Chim. Acta. 2007;596:201–209. doi: 10.1016/j.aca.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]