Abstract
3, 3'-Diindolylmethane (DIM) has been studied for its putative anti-cancer properties, especially against prostate cancer; however, its exact mechanism of action remains unclear. We recently provided preliminary data suggesting down-regulation of uPA during B-DIM (a clinically active DIM)-induced inhibition of invasion and angiogenesis in prostate cancer cells. Since the expression and activation of uPA plays important role in tumorigenicity, and high endogenous levels of uPA and uPAR are found in advanced metastatic cancers, we investigated their role in B-DIM-mediated inhibition of prostate cancer cell growth and motility. Using PC3 cells, we found that B-DIM treatment as well as the silencing of uPA and uPAR by siRNAs led to the inhibition of cell growth and motility. Conversely, over-expression of uPA/uPAR in LNCaP and C4-2B cells resulted in increased cell growth and motility, which was effectively inhibited by BDIM. Moreover, we found that uPA as well as uPAR induced the production of VEGF and MMP-9, and that the down-regulation of uPA/uPAR by siRNAs or B-DIM treatment resulted in the inhibition of VEGF and MMP-9 secretion which could be responsible for the observed inhibition of cell migration. Interestingly, silencing of uPA/uPAR led to decreased sensitivity to B-DIM indicating important role of uPA/uPAR in B-DIM-mediated regulation of prostate cancer cell growth and migration. Our data suggests that chemo-preventive and/or therapeutic activity of B-DIM is in part due to down-regulation of uPA-uPAR leading to reduced production of VEGF/MMP-9 which ultimately leads to the inhibition of cell growth and migration of aggressive prostate cancer cells.
Keywords: 3, 3'-Diindolylmethane; Prostate cancer; PC3; Migration; VEGF; MMP-9; uPA
Introduction
With an estimated 186,320 new cases and 28,660 deaths in the year 2008 in the United States alone (Jemal et al., 2008), prostate cancer continues to be one of the deadliest cancers and currently ranks the second most frequent cause of cancer-related death in males in the United States. The slow and unsatisfactory progress for prostate cancer treatment warrants novel preventive and/or therapeutic approaches. One such approach could be the use of naturally occurring dietary substances that are classically known to be non-toxic to normal cells but effective in the killing of cancer cells. Epidemiological studies have shown beneficial effect of high dietary intake of fruits and vegetables against carcinogenesis (Heber and Bowerman, 2001). The dietary indoles present in a variety of cruciferous vegetables have shown a lot of promise against several cancers (Steinmetz and Potter, 1991). In particular, Indole-3-Carbinol (I3C) has exhibited anti-tumor activity by inducing apoptotic cell death in prostate cancer cells (Chinni et al., 2001; Nachshon-Kedmi et al., 2004b). I3C is chemically unstable in the acidic environment of the stomach and is rapidly converted into its dimeric form, 3, 3'-Diindolylmethane (DIM). DIM has been shown to exert anti-carcinogenic effects in experimental animals (Hong et al., 2002); however, the molecular mechanism(s) by which DIM exerts its biological effects is still under investigation.
Previous studies from our laboratory have shown a protective role of DIM against prostate cancer (Li et al., 2003; Li et al., 2005). These results as well as reports from other laboratories (Nachshon-Kedmi et al., 2004a; Savino, III et al., 2006) suggested the potential benefits of DIM in the prevention and/or treatment of prostate cancer. In an attempt to explain the mechanism of action, we have shown earlier that B-DIM, a formulated DIM with higher bioavailability, induces growth inhibition and apoptotic cell death in prostate cancer cells by down-regulation of androgen receptor and Akt (Bhuiyan et al., 2006) and by inactivation of FOX03a/β-catenin/GSK-3β signaling pathways (Li et al., 2007). In addition, we have shown that B-DIM inhibits angiogenesis and invasion of human prostate cancer cells (Kong et al., 2007). These results suggested, for the first time, a potential role of uPA in B-DIM-induced killing of prostate cancer cells. The uPA, or urokinase-type plasminogen activator, is a member of the urokinase plasminogen activator system, a serine protease family comprising of uPA, plasminogen activator inhibitors (PAI's), tissue-type plasminogen activator (tPA) and the uPA receptor (uPAR). The urokinase plasminogen system is particularly associated with the processes of metastasis (Rao, 2003) and is believed to play a very important role in tissue degradation, cell migration, angiogenesis, cancer cell invasion and metastasis (Binder et al., 2007; Ge and Elghetany, 2003; Blasi and Carmeliet, 2002; Sheng, 2001). Involvement of uPA family members in the progression of human cancers (Dass et al., 2008), especially prostate cancer (Li and Cozzi, 2007), is gaining significant interest; however, mechanistic studies to ascertain the role of uPA/uPAR in mediating the biological effects of chemopreventive agents are lacking except our previously published preliminary results (Kong et al., 2007).
Association of uPA with its receptor, uPAR provides an inducible, transient and localized cell surface proteolytic activity (Wang, 2001; Pillay et al., 2007), which is increasingly being recognized as a major factor causatively involved in tumor invasion and metastasis, and thus represents a very potent therapeutic target. Based on our recent preliminary results (Kong et al., 2007) and the emerging role of uPA system in carcinogenesis (Dass et al., 2008), we believe that uPA may play an important role in mediating the biological activity of B-DIM in prostate cancer cells. Therefore, we undertook the current study using PC3, LNCaP and C4-2B prostate cancer cell lines and investigated the role of uPA as well as its receptor, uPAR, in cell growth and migration of prostate cancer cells. For our studies, we used a specially formulated DIM (marketed by BioResponse, CO) and referred to as B-DIM in this study. This preparation of DIM has a highly improved bioavailability (Anderton et al., 2004) and is also currently available for human use. Our results showed that silencing of uPA as well as uPAR led to reduced cell growth and migration of highly aggressive PC3 cells. The treatment with B-DIM also had similar effects but the silencing of uPA/uPAR significantly attenuated the ability of B-DIM to inhibit cell growth and migration of PC3 cells, suggesting the essential role of uPA-uPAR system in mediating the biological activity of B-DIM against prostate cancer cells. We further confirmed our results in LNCaP and C4-2B, cells with low endogenous levels of uPA/uPAR, and found that over-expression of uPA and uPAR led to the induction of cell growth and migration; interestingly, B-DIM was found to inhibit such processes. Our results suggest the potential mechanistic role of B-DIM in the inhibition of cell growth and tumor progression by inhibiting the uPA system in prostate cancer, and thus we believe that B-DIM could be useful for the prevention of tumor progression and/or treatment.
Materials and Methods
Cell lines and Reagents
Prostate cancer cell line, PC3 [highly aggressive, androgen receptor negative (AR-) and uPA/uPAR expressing] was maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin. Two other prostate cell lines, LNCaP (AR+ and responsive to androgen) and C4-2B (AR+ derived from LNCaP cells but unresponsive to androgen), both of which express low levels of endogenous uPA/uPAR, were also maintained in RPMI 1640 with supplements as shown above with an additional 10 μmol/L HEPES. All cells were cultured in a 5% CO2-humidified atmosphere at 37°C. B-DIM, a formulated DIM with higher bioavailability, was kindly provided by Dr. Michael Zeligs (BioResponse, Boulder, CO) and was dissolved in DMSO to make 25 mM stock solutions and stored at -20°C in multiple aliquots. Antibodies against human VEGF, uPA and uPAR were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-MMP-9 monoclonal antibody was obtained from R&D Systems (Minneapolis, MN). The monoclonal antibody to β-actin was purchased from Sigma-Aldrich (St. Louis, MO). The VEGF quantikine ELISA kits and MMP-9 fluorescent assay kits were purchased from R&D Systems.
Plasmids, siRNAs and transfection conditions
For over-expression studies, uPA/uPAR-cDNA containing vectors were purchased from Origene Technologies (Rockville, MD). LNCaP and C4-2B cells were transiently transfected with uPA or uPAR cDNA or the vector control, using Lipofectamine 2000 following the transfection protocol described earlier (Ahmad et al., 2008). For silencing studies, uPA/uPAR small interfering RNAs (siRNAs) were obtained from Santa Cruz, CA, and were transfected into PC3 cells using DharmaFECT3 siRNA transfection reagent (Dharmacon, Inc., Lafayette, CO). Cells were allowed to propagate for 18-24 hours post-transfection and then treated with varying concentrations of B-DIM as described under individual experiments.
Cell Growth Inhibition Studies by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
PC3 cells were seeded at a density of 2 × 103 cells per well while LNCaP and C4-2B cells were seeded at a density of 4 × 103 cells per well in 96-well microtiter culture plates. After overnight incubation, cells were transfected with cDNA or siRNA for uPA/uPAR, as mentioned for individual experiments. 24 h post-transfection, medium was removed and replaced with a fresh medium containing DMSO (vehicle control) or different concentrations of B-DIM diluted from a 25mM stock. After 48 h of incubation, 25μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5mg/ml in phosphate-buffered saline, PBS) was added to each well and incubated further for 2 h at 37°C. Upon termination, the supernatant was aspirated and the MTT formazan, formed by metabolically viable cells, was dissolved in isopropanol (100μl). The plates were mixed for 30 min on a gyratory shaker, and the absorbance was measured at 595 nm on Ultra Multifunctional Microplate Reader (TECAN, Durham, NC). Each treatment had eight replicate wells and the amount of DMSO in reaction mixture never exceeded 0.1%. Moreover, each experiment was repeated at least thrice.
Soft Agar Colonization Assays
PC3, LNCaP and C4-2B cells were first transfected with appropriate siRNA/cDNA in 6-well plates, allowed to grow for 24 h and then collected by trypsinization. 3 × 104 cells were then plated in 0.5 ml of culture medium containing 0.3% (w/v) top agar layered over a basal layer of 0.7% (w/v) agar (with culture medium and the supplements) in 24-well plates. At the time of seeding, the culture was supplemented with the amounts of B-DIM or the DMSO. After 21 days of culture, colonies (>50 cells) were counted. Experiments were carried out in quadruplicate and results are representative of three independent observations.
Histone/DNA ELISA for Detection of Apoptosis
The Cell Death Detection ELISA Kit (Roche, Palo Alto, CA) was used to detect apoptosis in prostate cancer cells treated with B-DIM, according to the manufacturer's protocol as described earlier (Ahmad et al., 2008). Briefly, cells were treated with B-DIM or DMSO control for 48 h. In uPA/uPAR-silencing/over-expression studies, cells were transfected with siRNA/cDNA for 24 hours prior to B-DIM treatment. After treatment, the cytoplasmic histone/DNA fragments from cells were extracted and incubated in the microtiter plate modules coated with anti-histone antibody. Subsequently, peroxidase-conjugated anti-DNA antibody was used for the detection of immobilized histone/DNA fragments followed by color development with ABTS substrate for peroxidase. The spectrophotometric absorbance of the samples was determined by using Ultra Multifunctional Microplate Reader (TECAN) at 405 nm.
Homogeneous Caspase-3/7 Assay for Apoptosis
Caspase-3/7 homogeneous assay was performed using a kit from Promega (Madison, WI) and the protocol provided by the manufacturer. Cells were treated with B-DIM or DMSO control for 48 h. In uPA/uPAR-silencing/over-expression studies, cells were transfected with siRNA/cDNA for 24 hours prior to B-DIM treatment. After treatment, 100μl Apo-ONE® caspase-3/7 reagent was added and plates were shaken for 2 minutes, followed by incubation at room temperature for 3h. The fluorescence was then evaluated using ULTRA Multifunctional Microplate Reader (TECAN) at excitation/emission wavelengths of 485/530 nm.
Western blot assay
Cell lysates were obtained from cells using cold RIPA buffer as described previously (Ahmad et al., 2008). Protein concentration was measured by BCA Protein Assay (Pierce, Rockford, IL). After the resolution on 12% polyacrylamide gels under denaturing conditions, proteins were transferred to nitrocellulose membranes, and appropriate primary antibodies were added. This was followed by incubation with horseradish peroxidase-conjugated secondary antibody. Proteins were visualized using the chemiluminescence detection system (Pierce, Rockford, IL). For re-probing the blots, membranes were incubated for 30 minutes at 50°C in buffer containing 2% SDS, 62.5mM Tris-Hcl (pH 6.7), and 100mM β-mercaptoethanol, washed and incubated with desired primary followed by secondary antibodies and the signals were detected as described above.
ELISA assay for VEGF and MMP-9
To investigate the effect of uPA/uPAR on the production of VEGF/MMP-9, over-expression or silencing of uPA/uPAR was performed in six-well plates. After 24 h of seeding, cells were either transfected with uPA/uPAR or vector (control) cDNA or with non-specific (control) or uPA/uPAR siRNAs. Cells were then treated with DMSO control or B-DIM in serum-free media and incubated for 48 h. The culture media were collected, centrifuged to remove cellular debris, and stored at -70°C until used for the assay. The cells in the plate were trypsinized, and the total number of cells was determined by cell counting. The assay was done using a commercially available VEGF and MMP-9 ELISA kit (R&D System) according to the manufacturer's instruction. Results were normalized to the cell number.
Cell migration assay
Cell migration was assessed using 24-well inserts (BD Biosciences) with 8-μm pores according to the manufacturer's protocol. Briefly, transiently transfected cells (5 × 104) with serum-free medium were seeded into the upper chamber of the system. Bottom wells in the system were filled with complete medium. After 24 h of incubation, the cells in the upper chamber were removed, and the cells that had migrated through Matrigel matrix membrane were stained with 4 μg/ml calcein AM in Hanks buffered saline at 37°C for 1 h. The fluorescence of the invaded cells was read in ULTRA Multifunctional Microplate Reader (TECAN) at excitation/emission wavelengths of 530/590 nm.
Data analysis
The experimental results presented in the figures are representative of three or more independent observations. The data are presented as the mean values ± SE. Comparisons between groups were evaluated by Student's t test. Values of P < 0.05 were considered to be statistically significant.
Results
Silencing of uPA and uPAR in highly metastatic PC3 cells and the effect of B-DIM
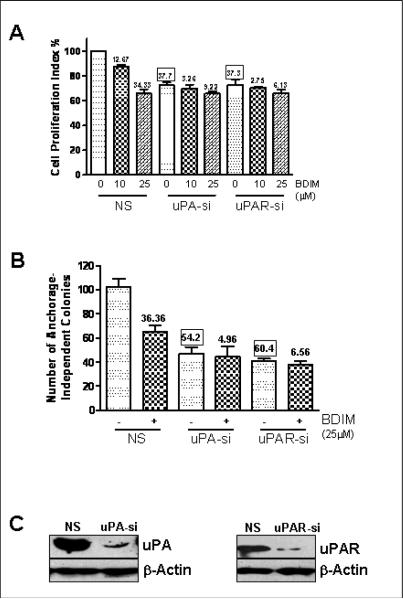
The expression and activation of uPA and its receptor, uPAR, is known to play an important role in survival and progression of human tumors (Dass et al., 2008). Moreover, our earlier study provided preliminary data in support of the involvement of uPA in prostate cancer cells (Kong et al., 2007) where we have shown that the anti-cancer effects of B-DIM against prostate cancer cells involve down-regulation of uPA. Therefore, in the present study we further investigated the role of uPA as well its receptor in aggressiveness of human prostate cancer cell lines, particularly in relation to the observed anti-cancer properties of B-DIM. We started our experiments with PC3 prostate cancer cell line which is an aggressive prostate cancer cell line with higher endogenous levels of uPA/uPAR expression. We first evaluated the effect of B-DIM treatment on PC3 cell growth. The study was performed simultaneously with silencing of uPA/uPAR. As a control, we used non-specific siRNA (NS) which was found to have no effect on the growth of PC3 cells. Figure 1A illustrates the effect of B-DIM treatment on PC3 cell growth as determined by MTT assay. In the presence of non-specific siRNA (control), exposure to 10μM B-DIM resulted in 12.67% inhibition of cell growth while exposure to 25μM B-DIM resulted in the inhibition of 34.33% cell growth after 48 hours. Silencing of uPA, by the use of uPA-specific siRNA caused 37.7% inhibition (0μM B-DIM-NS vs. 0μM B-DIM-uPA) while the silencing of uPAR resulted in a similar degree (37.3%) of growth inhibition (0μM B-DIM-NS vs. 0μM B-DIM-uPAR). The effect of silencing of uPA as well as uPAR on the growth of PC3 cells was found to be highly significant (p<0.001) compared to non-specific control. These results suggest that uPA as well its receptor, uPAR, play a significant role in the proliferation of highly metastatic PC3 prostate cancer cells. An interesting observation was made when we treated PC3 cells with B-DIM in conjunction with silencing of uPA/uPAR. When uPA siRNA was used, 10μM B-DIM inhibited the growth of PC3 cells by only 3.26% while 25μM B-DIM inhibited the same by 9.22% (compared to 0μM B-DIM-uPA siRNA). In the case of uPAR siRNA, 10μM B-DIM inhibited the growth of PC3 cells by only 2.75% while 25μM B-DIM inhibited the growth by 6.13% (compared to 0μM B-DIM-uPAR siRNA). These results suggest that the inhibition of PC3 cell growth by B-DIM occurs via down-regulation of uPA/uPAR system and that the cell growth inhibitory effects of B-DIM could be attenuated in prostate cancer cells lacking the expression of uPA/uPAR.
Figure 1.
Evaluation of (A) cell growth and (B) anchorage-independent growth in B-DIM-treated PC3 cells by MTT and soft agar assay, respectively. Cells were either vehicle-treated (DMSO-control) or treated with B-DIM, as indicated, for 48 h and then analyzed, as described under Materials and Methods. Silencing of uPA/uPAR was achieved by the use of specific siRNAs. Non-specific siRNA (NS) was used as silencing control. The amount of DMSO (solvent) never exceeded 0.1% during the treatment. The number of cells counted /O.D. value in DMSO (control)-treatment was considered 100% and the number of cells in B-DIM-treated cells was calculated relative to this control as the percentage surviving cells. Each data point represents Mean ± S.E. of 8 replicates from, at least, three independent experiments. The values (in squares) over some bars represent % decrease relative to the NS-0μM B-DIM, a measure for the base-line effect of uPA/uPAR silencing while the other values (without squares) mentioned over some bars represent % decrease corresponding to the respective 0μM B-DIM treatment, a measure for the effect of B-DIM treatment. (C) Western Blot analysis confirming the down-regulation of uPA and uPAR by the use of specific siRNAs. β-Actin protein was used as protein loading control.
Next, we studied the effect of B-DIM treatment and uPA/uPAR silencing on the potential of PC3 cells to form viable colonies in soft agar (anchorage-independent growth assay). PC3 cells treated with 25μM B-DIM were found to form 36.36% less colonies in soft agar compared to DMSO-treated control cells in the presence of non-specific siRNA (Figure 1B). However, when uPA siRNA was used, treatment with 25μM B-DIM could result in only 4.96% less colonies while the same treatment in the presence of uPAR siRNA resulted in only 6.56% less colonies, compared to respective controls. Silencing of uPA inhibited anchorage-independent growth of PC3 cells by more than 54% while silencing of uPAR inhibited it by more than 60% (p<0.001 for both, compared to NS control). These results correlate with those reported in Figure 1A and, taken together, these results demonstrate that a significant fraction of inhibitory effect of B-DIM on the growth and anchorage-independent colony formation of PC3 prostate cancer cells is due to down-regulation of uPA system. Figure 1C shows Western blot analysis confirming the down-regulation of uPA and uPAR by specific siRNAs.
uPA/uPAR transfection in prostate cells with low endogenous expression and effect of BDIM treatment
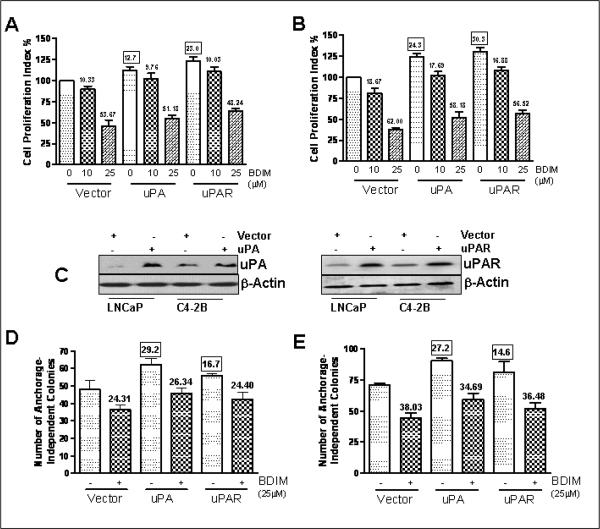
Next we tested the effects of uPA and uPAR re-expression in prostate cancer cell lines lacking the endogenous expression of uPA and uPAR. Prostate cancer cell lines, LNCaP and C4-2B, have considerably low invasive potential compared with PC3 cells. Also, LNCaP have very low endogenous levels of uPA as opposed to PC3 cells (Ustach and Kim, 2005), which made us to hypothesize that uPA and uPAR might play a role in the aggressiveness of prostate cancer cell lines. To test this hypothesis, we over-expressed uPA as well as uPAR in LNCaP and C4-2B cells. Transfection of uPA cDNA resulted in 12.7% increase in LNCaP and 24.3% increase in C4-2B cell growth (Figures 2A-B) whereas uPAR transfection resulted in 23.0% and 30.3% increase in cell proliferation, respectively, compared to vector-transfected controls (p<0.01 in all cases). We also treated the vector (control)-transfected cells as well as uPA/uPAR-transfected cells with B-DIM to test the effect of transfections on the sensitivity of these cells to B-DIM treatment. B-DIM treatment resulted in a dose-dependent inhibition of cell proliferation in both the cells lines. It is worth mentioning that, in an earlier study (Kong et al., 2007), we observed dose-dependent down-regulation of uPA by B-DIM in both LNCaP and C4-2B cells. Transfections with uPA/uPAR did not significantly alter the sensitivity of these cell lines to BDIM treatment and the percent inhibition by B-DIM using both the doses was similar in control vs. uPA/uPAR-transfected cells. The efficacy of transfection was assessed by Western Blot analysis and it was confirmed that uPA as well as uPAR transfection resulted in higher expression (Figure 2C). Subsequently, we tested the effect of uPA/uPAR transfection on B-DIM treatment by soft agar assay. As can be seen in Figures 2D-E, 25μM B-DIM treatment resulted in a significant loss in colony formation after 3 weeks of growth under anchorage-independent conditions. Transfections with both uPA and uPAR resulted in increased anchorage-independent growth in both the cell lines (as depicted by bars with 0μM B-DIM treatment). In LNCaP cells, uPA transfection increased the anchorage-independent colonies by 29.2% and uPAR transfection increased the colonies by 16.7% (p<0.01) while in C4-2B cells, the increases were 27.2% and 14.6%, respectively (p<0.01). These increases represent the basal-level induction of anchorage-independent colonies by uPA/uPAR-transfections. Upon treatment with 25μM B-DIM, it was observed that, once again, transfections with uPA/uPAR did not alter sensitivity to B-DIM treatment, which essentially confirms the results of MTT assay. We also observed (Figures 2DE) that while B-DIM treatment resulted in a similar percentage inhibition in all cases, the base-line anchorage-independent growth was raised in the uPA as well as uPAR transfected cells. This can be inferred by looking at the bars shown in the figures (uPA/uPAR-transfected cells with 25μM B-DIM vs. vector-transfected cells with 25μM B-DIM). B-DIM treatment (25μM) in all the cases caused similar inhibition (24.31% in control; 26.34% in uPA-transfected and 24.40% in uPAR-transfected LNCaP cells); however, the number of colonies was visibly increased in uPA/uPAR-transfected cells. This increase reflects the effect of uPA/uPAR-over-expression under artificial promoter, suggesting that the effect of B-DIM on uPA/uPAR requires its natural promoter for regulation. While B-DIM can effectively down-regulate endogenous uPA/uPAR leading to decreased cell growth, as seen in PC3 cells, the over-expression of uPA/uPAR achieved by cDNA transfection cannot be attenuated by B-DIM treatment. These results might also explain the results obtained with MTT assay in LNCaP and C4-2B cells wherein the basal level cell proliferation was increased by over-expression of uPA/uPAR, and that the inhibition by B-DIM was almost similar, irrespective of transfections.
Figure 2.
Evaluation of cell proliferation in (A) LNCaP and (B) C4-2B cells by MTT assay. Cells were transiently transfected with vector or uPA/uPAR-containing plasmid and were either vehicle-treated (DMSO-control) or treated with increasing concentrations of B-DIM, as indicated, for 48 h and then analyzed, as described under Materials and Methods. The number of cells counted /O.D. value in DMSO (control)-treatment was considered 100% and the number of cells in B-DIM-treated cells was calculated relative to this control as the percentage surviving cells. (C) Western Blot analysis confirming the increased expression of uPA and uPAR after transfection with specific cDNAs. β-Actin protein was used as protein loading control. Determination of anchorage-independent growth in (D) LNCaP and (E) C4-2B cells by soft agar assay. Cells were transfected with either vector alone or with the vector containing cDNA for uPA/uPAR, as described under Materials and Methods, and then treated with either vehicle (DMSO-control) or 25μM B-DIM.. The amount of DMSO during B-DIM treatment never exceeded 0.1%. Each data point represents Mean ± S.E. of 8 replicates from, at least, two independent experiments. The values (in squares) over some bars represent % increase relative to the vector-0μM B-DIM, a measure for the base-line effect of uPA/uPAR over-expression while the other values (without squares) mentioned over some bars represent % decrease corresponding to the respective 0μM B-DIM treatment, a measure for the effect of B-DIM treatment.
Role of uPA/uPAR in the induction of apoptosis by B-DIM
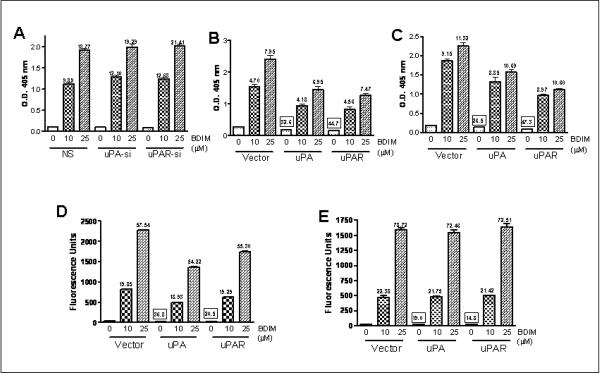
Earlier studies from our laboratory have shown that B-DIM treatment could result in the induction of apoptosis in prostate cancer cell lines (Bhuiyan et al., 2006). Therefore, we evaluated the role of uPA/uPAR system during B-DIM induced apoptosis. We found that the treatment of cells with B-DIM induced apoptosis in a dose-dependent manner in all the cell lines tested [PC3 (Figure 3A), LNCaP (Figure 3B) and C4-2B (Figure 3C)], as determined by Histone/DNA ELISA method. The values over the individual bars (without squares) represent `fold-increase' compared to respective 0μM B-DIM controls. Silencing of uPA and uPAR in PC3 cells resulted in a marginal increase in apoptosis-induction (non-significant). Over-expression of uPA and uPAR, on the other hand, in LNCaP as well as C4-2B cells, resulted in a significant reduction in apoptosis in control cells (0μM B-DIM; vector-transfected vs. uPA/uPAR-transfected cells). The exact `%-decreases' are given as values (with squares) over the bars. In LNCaP cells, uPA transfection resulted in 32.6% reduction in apoptosis-induction while uPAR transfection resulted in 44.7% reduction (Figure 3B). In C4-2B cells, the values were 26.5% and 47.3%, respectively (Figure 3C). However, once the cells were treated with increasing doses of B-DIM, the protective effect of uPA/uPAR-transfection was found to be diminished. In LNCaP and C4-2B cells, B-DIM treatment induced similar rate of apoptosis in vector as well as uPA/uPAR-transfected cells (Figures 3B-C).
Figure 3.
Detection of apoptosis was assessed by Histone/DNA ELISA method in prostate cancer cells, (A) PC3, (B) LNCaP and (C) C4-2B; and by Homogeneous Caspase-3/7 Assay in (D) LNCaP and (E) C4-2B cells. Cells were either vehicle-treated (DMSO-control) or treated with indicated concentrations of B-DIM for 48 h and assayed for apoptosis as described under Materials and Methods. Transfections with siRNA or cDNA, as appropriate, were performed prior to B-DIM treatment. The amount of DMSO added never exceeded 0.1% and the values represent a Mean ± S.E. of 3 samples from, at least, two independent experiments. The values (in squares) over some bars represent % decrease relative to the vector-0μM B-DIM, a measure for the base-line effect of uPA/uPAR over-expression while the other values (without squares) mentioned over some bars represent fold-change corresponding to the respective 0μM B-DIM treatment, a measure for the effect of B-DIM treatment.
Our results with apoptosis-induction in LNCaP and C4-2B cells (Figures 3B-C) revealed that uPA/uPAR transfections resulted in a significant reduction in apoptosis (% decreases provided over the bars, as values with squares, p<0.05). We performed additional experiments for confirming these results using another assay for apoptosis, the caspase-3/7 homogeneous assay. This assay determines the levels of active caspase-3 and/or caspase-7 by fluorescence as a measure of apoptosis. As shown in Figure 3D, uPA transfection in LNCaP cells resulted in 36.8% decrease in caspase-3/7 activation while uPAR transfection resulted in 20.5% decrease (p<0.01). The corresponding decrease in C4-2B cells was found to be 19.0% and 14.8%, respectively (p<0.05) (Figure 3E). As expected, B-DIM treatment was found to induce dose-dependent increase in caspase-3/7 activation in both the cell lines (depicted in the figure as fold-increase). The transfections with uPA/uPAR did not reduce the B-DIM-induced activation of caspase-3/7, thus confirming the results obtained with Histone/DNA ELISA method.
Effect of B-DIM treatment and uPA/uPAR silencing on VEGF and MMP-9 secretion
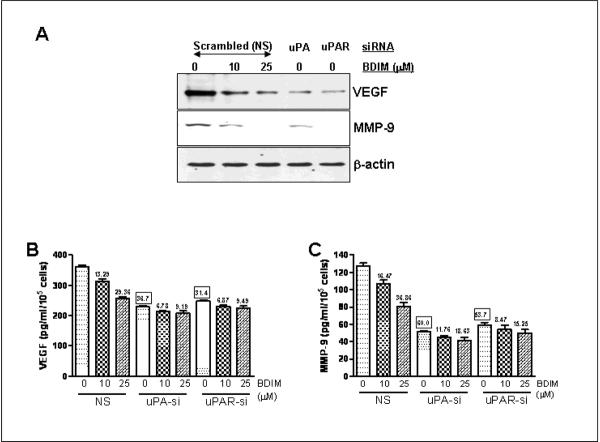
VEGF and MMP-9 are reliable markers of tumor aggressiveness and metastasis. The mechanistic details for inhibitory effect of B-DIM in prostate cancer cells are not clearly understood. Moreover, uPA/uPAR system is known to be involved in the activation of MMP-9, which causes the degradation of extracellular matrix, resulting in increased invasion (Dass et al., 2008). We, therefore, tested the relevance of uPA-uPAR system in our model system by studying the effect of uPA/uPAR silencing on the production of VEGF and MMP-9 using PC3 cells. As seen in Figure 4A, B-DIM treatment resulted in a dose-dependent inhibition of the secreted VEGF. VEGF was also found to be reduced by the silencing of uPA as well as uPAR. We also assessed the expression of MMP-9 and found similar results. These results confirmed that uPA and its receptor play a role in the regulation of VEGF and MMP-9 and, as such, could play a significant role in the processes of prostate cancer metastasis. Subsequently, we tested the effect of uPA/uPAR silencing on the B-DIM-induced down-regulation of VEGF and MMP-9 by ELISA. Conditioned media was collected from test samples and subjected to ELISA for the quantitation of VEGF and MMP-9. As seen in Figure 4, B-DIM treatment resulted in a dose-dependent down-regulation of VEGF (Figure 4B) as well as MMP-9 (Figure 4C). Silencing of uPA as well as uPAR significantly reduced the B-DIM-induced down-regulation of VEGF as well as MMP-9. B-DIM was still able to down-regulate VEGF and MMP-9 but the degree of down-regulation was only a fraction of that observed in cells transfected with non-specific siRNAs.
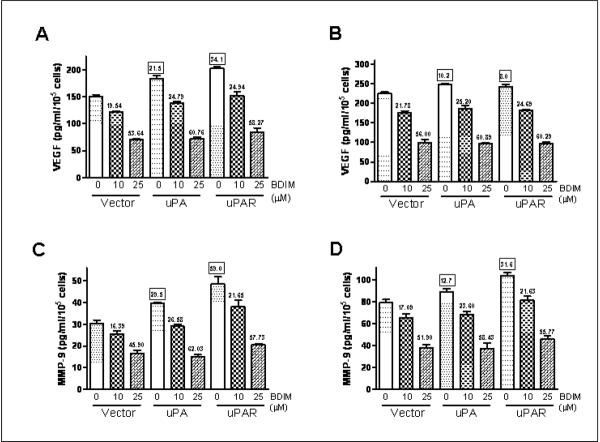
Figure 4.
Effect of B-DIM treatment and uPA/uPAR silencing on VEGF and MMP-9 production in PC3 cells. (A) Representative Western Blots for analysis of secreted VEGF and MMP-9 in the conditioned media obtained from B-DIM/siRNA transfected PC3 cells. Cell extracts were prepared using RIPA buffer, as described under Materials and Methods and β-Actin protein was used as protein loading control as shown for each blot. Effect of B-DIM on the secretion (B) VEGF and (C) MMP-9 in PC3 cells treated with indicated doses of B-DIM and/or silenced for uPA/uPAR. The culture media was collected and VEGF/MMP-9 was assayed using ELISA kits, as described in Materials and Methods. Results were normalized to the cell number and expressed as pg/mL/105 cells. The values (in squares) over some bars represent % decrease relative to the NS-0μM B-DIM, a measure for the base-line effect of uPA/uPAR silencing while the other values (without squares) mentioned over some bars represent % decrease corresponding to the respective 0μM B-DIM treatment, a measure for the effect of B-DIM treatment.
We extended these studies to LNCaP and C4-2B cells and the results are shown as Figure 5. As evident from the figure, B-DIM treatment resulted in a dose-dependent down-regulation of VEGF and MMP-9 in these two cell lines as well. In support of the putative role of uPA and uPAR in metastasis and invasion of prostate cells, we observed that transfection with uPA and uPAR caused an increase in the secretion of VEGF and MMP-9 activity. In LNCaP cells, uPA transfection resulted in 21.5% increase in VEGF and 29.5% increase in MMP-9 production while transfection with uPAR caused 34.1% increase in VEGF and 59.0% increase in MMP-9 production (Figures 5A and 5C, see the values in squares over the bars). A similar trend was observed in C4-2B cells where we found that uPA transfection resulted in 10.2% increase in VEGF and 12.7% increase in MMP-9 production, and the transfection with uPAR caused 8.0% increase in VEGF and 31.6% increase in MMP-9 production (Figures 5B and 5D). All the values were found to be significant compared to vector-transfected control cells (p<0.05).
Figure 5.
Effect of B-DIM treatment and uPA/uPAR over-expression on VEGF secretion in (A) LNCaP and (B) C4-2B cells; and MMP-9 production in (C) LNCaP and (D) C4-2B cells. Culture media was collected and VEGF/MMP-9 was assayed using ELISA kits, as described in Materials and Methods. Results were normalized to the cell number and expressed as pg/mL/105 cells. The values (in squares) over some bars represent % increase relative to the vector-0μM B-DIM, a measure for the base-line effect of uPA/uPAR over-expression while the other values (without squares) mentioned over some bars represent % decrease corresponding to the respective 0μM B-DIM treatment, a measure for the effect of B-DIM treatment.
Role of uPA/uPAR in the migration of prostate cells
The results described above, Figures 4-5, provided indirect evidence in support of the involvement of uPA and uPAR in invasion and metastasis of prostate cancer cells. To investigate the direct effect of uPA and uPAR on the migration of prostate cancer cells, we performed migration assay. As seen in Figure 6, transfection of uPA and uPAR in less aggressive cell lines, LNCaP and C4-2B cells, resulted in a significant increase in the migration of these cells. Silencing of uPA and uPAR was found to inhibit the migration of highly aggressive PC3 cells significantly. In LNCaP cells, uPA transfection caused 39.7% increase in the number of migratory cells while uPAR transfection caused 34.5% increase (Figure 6A). In C4-2B cells, the corresponding increases were found to be 26.9% and 27.7%, respectively (Figure 6B). Exposure to 25μM B-DIM resulted in a significant loss of migration in both the cell lines but the transfection with uPA or uPAR was not found to be sufficient to reverse the inhibitory effect of B-DIM treatment. In PC3 cells, exposure to 25μM B-DIM as well uPA/uPAR siRNA resulted in a significant inhibition of migration (Figure 6C). B-DIM treatment resulted in 15.56% less migration, uPA-silencing resulted in 22.3% less migration while uPAR-silencing resulted in 24.6% less migration of PC3 cells. Compared to 0μM B-DIM, 25 μM B-DIM caused only 3.63% further reduction in migration in uPA-transfected cells and 3.52% reduction in uPAR-transfected cells. Comparing these numbers with 15.56% (as observed in non-specific silencing) suggests that knock-down of uPA/uPAR significantly diminished the inhibitory effect of B-DIM treatment. Also, in LNCaP and C4-2B cells, the basal-level migration was elevated by uPA and uPAR transfections under all experimental conditions (see 0μM B-DIM; vector vs. uPA/uPAR-transfected cells and also 25 μM B-DIM; vector vs. uPA/uPAR-transfected cells). Once again, these results underscore the relevance of uPA and uPAR towards migration and aggressiveness of prostate cancer cells.
Figure 6.
Effect of B-DIM treatment and over-expression of uPA/uPAR on migration of (A) LNCaP and (B) C4-2B cells; and effect of B-DIM treatment and silencing of uPA/uPAR on migration of (C) PC3 cells. Cells were seeded into the upper chamber of the system. They were first transfected with cDNA/siRNA for 24 hours and then serum-free media containing 10 and 25 μM B-DIM or DMSO was added. Bottom wells were filled with complete media. After 24 h incubation, the cells that migrated through the membrane were stained with 4 μg/mL Calcein AM. The fluorescently labeled cells were detached from the membrane by trypsinization and the fluorescence of migrated cells was read at excitation/emission wavelengths of 530/590 nm. The values (in squares) over some bars represent % change relative to the vector/NS-0μM B-DIM, a measure for the base-line effect of uPA/uPAR over-expression/silencing while the other values (without squares) mentioned over some bars represent % decrease corresponding to the respective 0μM B-DIM treatment, a measure for the effect of 25μM B-DIM.
Discussion
Carcinoma of prostate is the most frequently diagnosed malignancy in men over 40 years of age; a trend likely to continue with the increasing aging population in the USA. Micronutrient supplements and phytochemicals have generated considerable interest in recent years because of their potential role in cancer prevention and treatment. It has been estimated that up to 60-80% of cancer patients take micronutrients, phytochemicals or herbs as dietary supplements (Nam et al., 1999; Richardson et al., 2000). Consumption of fruits and vegetables, which provides several classes of compounds including indole compounds (indole-3-carbinol, I3C, or its in vivo dimeric product 3-3'-diindolylmethane, DIM), is believed to have a protective effect against the development of prostate cancer in humans. In view of such a beneficial role of dietary components against prostate cancer, numerous studies are underway to fully understand the role and the exact mechanism of action of 3-3'-diindolylmethane (DIM) in cancer therapeutics.
We focused our present work on elucidating the mechanistic role of uPA/uPAR in mediating the growth inhibitory effect of DIM (B-DIM) against prostate cancer cells, primarily based on our recent preliminary findings (Kong et al., 2007). Our results clearly suggest that B-DIM inhibits cell growth and migration of androgen-sensitive as well as androgen-insensitive prostate cancer cells. We also show that B-DIM induces apoptosis in prostate cancer cells in a dose-dependent manner. These results are in complete agreement with our earlier published findings (Bhuiyan et al., 2006). As a mechanism in support of the observed effects of B-DIM, we found that down-regulation of uPA/uPAR leads to the reduced production of VEGF and MMP-9, which could be responsible for reduced migration and aggressiveness of prostate cancer cells. Our results also revealed that although B-DIM treatment was able to inhibit the growth of PC3 as well as LNCaP and C4-2B cells, the degree of inhibition was different. For example, 25μM B-DIM inhibited the growth of PC3 cells by only 34.33% (Figure 1A) while the inhibition in LNCaP and C4-2B cells, using the same B-DIM concentration, was found to be 53.67% and 62.00% respectively (Figures 2A-B). This inhibition by B-DIM correlates inversely with the endogenous expression of uPA and uPAR and therefore we suspected that uPA and uPAR might play an important role in prostate cancer cell growth and proliferation. As such, the inhibition of uPA and uPAR by B-DIM is of relevance in view of the suggested anti-cancer properties of B-DIM. B-DIM, by virtue of being a pleiotropic agent, is able to inhibit proliferation of prostate cancer cells by acting on various pathways (Li et al., 2005; Bhuiyan et al., 2006). This might explain its ability to inhibit growth of androgen-sensitive (LNCaP) as well as androgen-insensitive (C4-2B and PC3) cells.
It is interesting to note that PC3 cells, the cells with higher metastatic potential, have high endogenous expression of uPA as well as its receptor, uPAR (Figure 1C). We found that B-DIM could significantly inhibit cell growth and colony formation of PC3 cells, and this led us to question what would have happen if uPA or uPAR is down-regulated and then the cells are further exposed to B-DIM. An increased inhibition of cell growth and anchorage-independent growth would have indicated additive effect. However, instead, we observed that silencing of uPA and uPAR resulted in decreased sensitivity of PC3 cells to the effects of B-DIM treatment. These results clearly showed that a major part of B-DIM-induced anti-proliferative action against PC3 cells is dependent on its ability to modulate uPA-uPAR system. Once we had artificially down-regulated uPA-uPAR system, using specific siRNAs, the effect of B-DIM against cell proliferation and anchorage-independent growth of PC3 cells was considerably less profound. These results prompted us to expand our studies to other prostate cancer cell lines and we carefully selected two additional cells lines, AR positive (sensitive to androgen)- LNCaP cells, and C4-2B cells (derived from LNCaP cells containing AR but insensitive to androgen). Both these cell lines have lower invasive potential compared to the PC3 cells and we found that these cells have very low endogenous expression of uPA and uPAR (Figure 2C), which could be associated with their lower invasive capacity, and further suggests that uPA and uPAR may play important role in invasion and metastasis of prostate cancer cells.
We over-expressed uPA and uPAR in both these cell lines and compared the growth potential of these cells. We found that both uPA as well as uPAR transfection resulted in a significant increase in cell growth and anchorage-independent growth of LNCaP and C4-2B cells. Treatment with B-DIM, as expected, led to an inhibition of cell growth and anchorage-independent growth (Figure 2); however over-expression of uPA or uPAR did not show any altered sensitivity of these cells to B-DIM-induced cell growth inhibition. These results provide strong evidence in support of the role of uPA and uPAR in cell growth. Our study also demonstrates that in highly metastatic prostate cancer cells such as PC cells, wherein uPA and uPAR are over-expressed, B-DIM might be able to exert its anti-cancer properties by down-regulation of these proteins. In prostate cancer cells such as LNCaP and C4-2B, wherein uPA and uPAR are relatively less abundant, B-DIM is still able to inhibit the proliferation via regulation of alternative pathways and these findings provide evidence for the pleiotropic nature of B-DIM.
Our results, as described here, suggest that the down-regulation of uPA and its receptor, uPAR, contributes to anti-cancer properties of B-DIM against prostate cancer cell lines. The results clearly show that B-DIM can down-regulate uPA as well as uPAR. This is in direct agreement with our earlier published results (Kong et al., 2007) where we have shown down-regulation of uPA by B-DIM as a mechanism for its observed anti-cancer properties. Since the effect of B-DIM on uPAR was not investigated earlier, our present results provide a clue that B-DIM might have very similar effects on uPAR as well. Under our experimental conditions, B-DIM has similar modulatory effects on uPAR as it has on uPA. It might be tempting to suggest that down-regulation of uPA as well as uPAR by B-DIM can explain its observed inhibitory effects on proliferation and anchorage-independent growth of prostate cancer cells; however, given the intricate relationship between uPA and uPAR that governs their physiological functions, more elaborate studies need to be carried out to answer the question as to the mechanistic role of uPA and uPAR in prostate cancer aggressiveness and the role of B-DIM in the inhibition of tumor aggressiveness of prostate cancer. As reviewed earlier (Dass et al., 2008), much of the biological activities associated with uPA are believed to be dependent on its binding to uPAR. There does exist evidence in the literature to suggest that uPA and uPAR can influence biological processes independent of each other (Carmeliet et al., 1998; Koopman et al., 1998; Jo et al., 2003; Nagai et al., 2008); however, it should be noted that the biological activity of uPA increases several hundred folds by binding to uPAR (Koopman et al., 1998). It is generally believed that the activities of uPA and uPAR exclusive of each other are only a fraction of their overall physiological activities (Dass et al., 2008). In such a scenario it is possible that B-DIM down-regulates uPA as well as uPAR leading to its robust biological effects. It is also possible that B-DIM preferentially down-regulates one component (either uPA or uPAR) which might lead to the observed effects based on the inter-dependence of uPA and uPAR.
uPA, directly or via plasmin formation, leads to the release or activation of angiogenic factors such as basic FGF (bFGF) and VEGF. Urokinase plasminogen activator system is involved in proteolysis of plasmin and matrix metalloproteinase (MMP) leading to migration of vascular smooth muscle cells (Carmeliet et al., 1998), and mitogenic effects on melanoma cells (Koopman et al., 1998). Therefore, we were also interested in evaluating the role of VEGF and MMP-9 in B-DIM-induced inactivation of uPA-uPAR system. In PC3 cells, we found a significant down-regulation of secreted VEGF and MMP-9 in response to both B-DIM treatment as well as siRNA-mediated silencing of uPA/uPAR (Figure 4). VEGF and MMP-9 down-regulation, achieved by the silencing of uPA/uPAR, was found to be similar to that observed by B-DIM treatment although the combined effect was not additive or synergistic. VEGF and MMP-9 assays in LNCaP and C4-2B cells, transfected with uPA/uPAR, showed increased production of VEGF/MMP-9 in response to over-expression and that B-DIM treatment inhibited the secretion to a similar extent (Figure 5). The increased production of VEGF and MMP-9, in cells over-expressing uPA/uPAR, should result in increased motility and metastasis. To confirm this notion, we performed migration assays and found that, indeed, over-expression of uPA as well as uPAR led to an increased motility of LNCaP and C4-2B cells across the membrane barrier (Figure 6). These observations provided molecular evidence (increased production of VEGF and MMP-9 in response to increased uPA/uPAR) in support of the positive correlation of uPA/uPAR with tumor aggressiveness. Further support came from silencing of uPA/uPAR in highly metastatic PC3 cells, showing significant inhibition in cell migration comparable to B-DIM treatment.
Although the involvement of uPA system in the progression of human cancers is increasingly being recognized (Dass et al., 2008), one of the earliest indications that uPA-uPAR system might be involved in the process of chemoprevention of prostate cancer came few years back when we treated PC3 cells with genistein and microarray analysis was performed showing alterations in the expression of several genes (Li and Sarkar, 2002). This study identified uPA as well as uPAR, among many other angiogenesis-related genes, that were down-regulated by genistein (a known chemopreventive agent with similar biological activity as DIM) in prostate cancer cells. In support of the role of uPA-uPAR system in prostate cancer metastasis, it has been shown that blockage of uPAR by antisense oligonucleotides results in highly significant reduction in bone metastases in an experimental prostate cancer bone metastasis model (Margheri et al., 2005). More recently it has been reported that silencing of uPA in bone xenograft model results in a significant inhibition of bone tumor growth and turnover (Dong et al., 2008). It has also been shown that the down-regulation of uPA and uPAR by RNA interference inhibits invasion, survival and tumorigenicity of prostate cancer cells (Pulukuri et al., 2005) and specific targeting of uPA promoter by siRNA induces epigenetic transcriptional repression leading to the inhibition of prostate tumor growth and metastasis (Pulukuri and Rao, 2007). Thus, the urokinase plasminogen activator system represents a highly potential candidate for designing comprehensive studies involving the use of dietary compounds as chemopreventive and/or chemo-sensitizing agents as part of a combinational therapy. The universal role of uPA system in different cancers (Dass et al., 2008) further underscores the importance of this system as a leading target for designing novel therapies towards the cure of patients diagnosed with cancer.
Taken together, we believe that targeting uPA/uPAR by B-DIM could be one of many novel approaches for the prevention and/or treatment of highly metastatic and aggressive prostate cancer. Moreover, the down-regulation of uPA/uPAR by B-DIM could also be a useful strategy for chemo-sensitization of metastatic prostate cancer cells to conventional therapeutics in the future for designing optimal therapy for hormone-refractory and metastatic prostate cancer.
Acknowledgments
This work was partly supported by grant from the National Cancer Institute, NIH (5R01CA108535). We also thank Dr. Michael Zeligs (BioResponse, Boulder, CO), for providing B-DIM, a formulated DIM with higher bioavailability.
References
- Ahmad A, Banerjee S, Wang Z, Kong D, Sarkar FH. Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J. Cell Biochem. 2008;105:1461–1471. doi: 10.1002/jcb.21966. [DOI] [PubMed] [Google Scholar]
- Anderton MJ, Manson MM, Verschoyle R, Gescher A, Steward WP, Williams ML, Mager DE. Physiological modeling of formulated and crystalline 3,3'-diindolylmethane pharmacokinetics following oral administration in mice. Drug Metab Dispos. 2004;32:632–638. doi: 10.1124/dmd.32.6.632. [DOI] [PubMed] [Google Scholar]
- Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, Sarkar FH. Down-regulation of androgen receptor by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb. Haemost. 2007;97:336–342. [PubMed] [Google Scholar]
- Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat. Rev. Mol. Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Dewerchin M, Rosenberg S, Herbert JM, Lupu F, Collen D. Receptor-independent role of urokinase-type plasminogen activator in pericellular plasmin and matrix metalloproteinase proteolysis during vascular wound healing in mice. J. Cell Biol. 1998;140:233–245. doi: 10.1083/jcb.140.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- Dass K, Ahmad A, Azmi AS, Sarkar SH, Sarkar FH. Evolving role of uPA/uPAR system in human cancers. Cancer Treat. Rev. 2008;34:122–136. doi: 10.1016/j.ctrv.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Dong Z, Saliganan AD, Meng H, Nabha SM, Sabbota AL, Sheng S, Bonfil RD, Cher ML. Prostate cancer cell-derived urokinase-type plasminogen activator contributes to intraosseous tumor growth and bone turnover. Neoplasia. 2008;10:439–449. doi: 10.1593/neo.08106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Elghetany MT. Urokinase plasminogen activator receptor (CD87): something old, something new. Lab Hematol. 2003;9:67–71. [PubMed] [Google Scholar]
- Heber D, Bowerman S. Applying science to changing dietary patterns. J. Nutr. 2001;131:3078S–3081S. doi: 10.1093/jn/131.11.3078S. [DOI] [PubMed] [Google Scholar]
- Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis. 2002;23:1297–1305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jo M, Thomas KS, Wu L, Gonias SL. Soluble urokinase-type plasminogen activator receptor inhibits cancer cell growth and invasion by direct urokinase-independent effects on cell signaling. J. Biol. Chem. 2003;278:46692–46698. doi: 10.1074/jbc.M308808200. [DOI] [PubMed] [Google Scholar]
- Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- Koopman JL, Slomp J, de Bart AC, Quax PH, Verheijen JH. Mitogenic effects of urokinase on melanoma cells are independent of high affinity binding to the urokinase receptor. J. Biol. Chem. 1998;273:33267–33272. doi: 10.1074/jbc.273.50.33267. [DOI] [PubMed] [Google Scholar]
- Li Y, Chinni SR, Sarkar FH. Selective growth regulatory and pro-apoptotic effects of DIM is mediated by AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci. 2005;10:236–243. doi: 10.2741/1523. [DOI] [PubMed] [Google Scholar]
- Li Y, Cozzi PJ. Targeting uPA/uPAR in prostate cancer. Cancer Treat. Rev. 2007;33:521–527. doi: 10.1016/j.ctrv.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Sarkar FH. Gene expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J. Nutr. 2003;133:1011–1019. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- Li Y, Sarkar FH. Down-regulation of invasion and angiogenesis-related genes identified by cDNA microarray analysis of PC3 prostate cancer cells treated with genistein. Cancer Lett. 2002;186:157–164. doi: 10.1016/s0304-3835(02)00349-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J. Biol. Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- Margheri F, D'Alessio S, Serrati S, Pucci M, Annunziato F, Cosmi L, Liotta F, Angeli R, Angelucci A, Gravina GL, Rucci N, Bologna M, Teti A, Monia B, Fibbi G, Del RM. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther. 2005;12:702–714. doi: 10.1038/sj.gt.3302456. [DOI] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Fares FA, Yannai S. Therapeutic activity of 3,3'-diindolylmethane on prostate cancer in an in vivo model. Prostate. 2004a;61:153–160. doi: 10.1002/pros.20092. [DOI] [PubMed] [Google Scholar]
- Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3'-diindolylmethane through the mitochondrial pathway. Br. J. Cancer. 2004b;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai N, Okada K, Kawao N, Ishida C, Ueshima S, Collen D, Matsuo O. Urokinase-type plasminogen activator receptor (uPAR) augments brain damage in a murine model of ischemic stroke. Neurosci. Lett. 2008;432:46–49. doi: 10.1016/j.neulet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Nam RK, Fleshner N, Rakovitch E, Klotz L, Trachtenberg J, Choo R, Morton G, Danjoux C. Prevalence and patterns of the use of complementary therapies among prostate cancer patients: an epidemiological analysis. J Urol. 1999;161:1521–1524. [PubMed] [Google Scholar]
- Pillay V, Dass CR, Choong PF. The urokinase plasminogen activator receptor as a gene therapy target for cancer. Trends Biotechnol. 2007;25:33–39. doi: 10.1016/j.tibtech.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Pulukuri SM, Gondi CS, Lakka SS, Jutla A, Estes N, Gujrati M, Rao JS. RNA interference-directed knockdown of urokinase plasminogen activator and urokinase plasminogen activator receptor inhibits prostate cancer cell invasion, survival, and tumorigenicity in vivo. J. Biol. Chem. 2005;280:36529–36540. doi: 10.1074/jbc.M503111200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pulukuri SM, Rao JS. Small interfering RNA directed reversal of urokinase plasminogen activator demethylation inhibits prostate tumor growth and metastasis. Cancer Res. 2007;67:6637–6646. doi: 10.1158/0008-5472.CAN-07-0751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat. Rev. Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin. Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- Savino JA, III, Evans JF, Rabinowitz D, Auborn KJ, Carter TH. Multiple, disparate roles for calcium signaling in apoptosis of human prostate and cervical cancer cells exposed to diindolylmethane. Mol. Cancer Ther. 2006;5:556–563. doi: 10.1158/1535-7163.MCT-05-0355. [DOI] [PubMed] [Google Scholar]
- Sheng S. The urokinase-type plasminogen activator system in prostate cancer metastasis. Cancer Metastasis Rev. 2001;20:287–296. doi: 10.1023/a:1015539612576. [DOI] [PubMed] [Google Scholar]
- Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- Ustach CV, Kim HR. Platelet-derived growth factor D is activated by urokinase plasminogen activator in prostate carcinoma cells. Mol. Cell Biol. 2005;25:6279–6288. doi: 10.1128/MCB.25.14.6279-6288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis. Med. Res. Rev. 2001;21:146–170. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]