Abstract
Songbirds respond to initial playback of a recorded conspecific song in numerous ways, from changes in gene expression in the brain to changes in overt physical activity. When the same song is presented repeatedly, responses have been observed to habituate at multiple levels: molecular, cellular and organismal. Core criteria of habituation have been established at each level, although in no case have all the formal parameters been rigorously measured. At the level of overt behavior, classical field studies showed that territorial birds respond to the song of a potential challenger with a variety of behaviors, and many (but not all) of these behaviors decline with repeated stimulus presentation. More recent laboratory studies have defined analogous responses to song presentation in the zebra finch (Taeniopygia guttata), the dominant species in current molecular and neurobiological research and one that does not use song for territorial defense. Studies in the zebra finch have also demonstrated activation followed by habituation of responses measured at both electrophysiological and molecular (gene expression and signal transduction) levels. In all cases, habituation is specific for a very particular stimulus - an individual song presented in a particular context. There are strong correlations between habituation measurements made at these different levels, but some dissociations have also been observed, implying that molecular, electrophysiological and behavioral habituations are not equivalent manifestations of a single core process.
Keywords: Zebra finch, sparrow, zenk, ERK, IEG, habituation, latency
Introduction
Habituation is one of the most fundamental forms of adaptation to experience (Harris, 1943; Thompson, 2000; Thompson and Spencer, 1966). Songbirds (oscines of Order Passeriformes) are uniquely attractive subjects for study of how organisms adapt to natural experience. There are roughly 4000 extant songbird species, spread across diverse terrestrial niches, and many species are easy to observe in their natural habitats. Thus songbirds have become great focal points for both formal behavioral ecology and casual birdwatching. A few songbird species - most notably the Australian zebra finch (Taeniopygia guttata) - have been domesticated and are well adapted to laboratory studies. Appreciation for the cognitive complexity of songbirds is growing with discoveries of complex social structures, tool making abilities and sophisticated communication systems (Clayton, in press).
Songbirds are also one of the few animal clades apart from humans to engage in robust learned vocal communication. In most songbird species, the young male must learn to reproduce the song of one or more tutors; studies of this aspect of developmental learning represent one important tradition in songbird research (Zeigler and Marler, 2004). Another strand of research has focused on the use of song by adult birds in social recognition. It is in this latter context that the concepts of habituation most resonate. Song has a role in territorial defense, reproduction, and possibly other aspects of social organization in colonial species (Marler and Hamilton, 1966; Thorpe, 1968). Birds respond to the singing of other birds, and these responses change with song repetition. The term “habituation” has been used to describe both overt behavior and the behavior of specific physiological measurements, in both field and laboratory studies of birds responding to repeated playbacks of recorded birdsong. We will review these diverse phenomena in this article, leading up to a consideration of how these observations inform an understanding of habituation more generally.
Habituation of Behavioral Responses to Song in the Field
For territorial species, a major function of song is to announce and defend the occupation of a particular breeding territory. Competition over territories can be fierce, but may lead eventually to a relatively stable social order where neighboring males in adjacent territories come to recognize and acknowledge one another through often-complex rites of singing and countersinging. If a new challenger is heard singing on the edge of a territory, the occupying male may initially respond with physical aggression (Blanchard, 1941; Kroodsma, 2005). However, if the challenger respects the boundary, the occupying male may cease responding to the song of the challenger - the aggressive response is thus said to habituate. Classical studies of sparrows showed that a male would physically attack a speaker placed at the edge of his territory when that speaker first began broadcasting a recorded song, but would eventually cease the attack - until the speaker was moved to a new position (Brooks and Falls, 1975; Falls, 1982; Falls, 1992; Falls and Brooks, 1975; Verner and Milligan, 1971).
In one study of white-crowned sparrows, male sparrows changed nearly every aspect of their observable behaviors when they first heard the playback of a novel song (Verner and Milligan, 1971). They increased vocal activities, stayed closer to the speaker, and flew short distances more frequently throughout the first song exposure session. Two to four weeks later, however, when those birds were re-exposed to the same song, their behavior was less markedly altered. On the third exposure, their behavior was not significantly altered at all. Similar phenomena were also observed in other species of birds in field (Harris and Lemon, 1974; Krebs, 1976).
These observations led Lewis Petrinovich and Thomas Patterson to formally characterize song habituation in wild white-crowned sparrows, emphasizing the use of species-meaningful stimuli presented in natural contexts (Patterson and Petrinovich, 1979; Petrinovich and Patterson, 1979; 1980; 1981; 1982). Their study also included observations of both sexes, ultimately describing 11 male and 5 female behavioral responses to song playback. They found that some but not all of these responses habituate with repeated song presentations (e.g., 160 song repeats over 80 minutes), with the most robust decrements observed for male song production, male trill, male flutter and female chinks. Considering the original Thompson and Spencer formulation of habituation (1966), they confirmed that habituation is stimulus-(song-) specific, so it is not caused by sensory adaptation or motor fatigue. They also observed a limited degree of what may be considered stimulus generalization, in that introduction of a new song after habituation of one song tended to generate an intermediate response. Consistent with dual-process theory (Groves and Thompson, 1970), they found that shorter trial intervals did not change the rate of habituation but did increase the response level, whereas habituation developed more easily when trial intervals were increased. (In dual-process theory, short interval training favors sensitization, whereas long interval training favors the slower but more lasting process of habituation.)
Petrinovich and Patterson also encountered variations in habituation that foretell some of the complexities observed in later studies (below). Somewhat surprisingly, they were unable to demonstrate spontaneous recovery even after several weeks. They also found that the absolute levels of the behaviors and their habituation rates were strongly affected by the states of the birds. One factor was the stage of the reproduction cycle (whether the female has eggs, nestlings or fledglings). A second was the general responsiveness of male. Interestingly, both factors exerted a strong influence on the patterns of all behaviors for both members of a mated pair. Thus many parameters of song response habituation fit the general Thompson/Spencer/Grove model, but there appear to be additional factors that modulate the process according to the individual, the individual’s state, and the particular response modality.
Habituating Responses to Song in the Laboratory
A new dimension was brought to habituation research with the surprising discovery that song playbacks activate rapid changes in specific messenger RNA (mRNA) levels in the auditory forebrain of songbirds (Mello and Clayton, 1994; Mello, Vicario, and Clayton, 1992), and that song repetition leads to stimulus-specific habituation of this gene response (Mello, Nottebohm, and Clayton, 1995). This was first observed using probes for the product of the gene known by the acronym zenk, representing four names given to the orthologous gene by independent investigators (zif-288, egr1, ngfi-a, krox-24). Even a single exposure to a novel song would cause an increase in zenk mRNA (Kruse, Stripling, and Clayton, 2000), although the peak mRNA level was not reached until about 30 minutes after stimulus onset before returning to baseline levels after about 2 hours (Mello and Clayton, 1994; Mello et al., 1995), and the ZENK protein only peaked at about 90 minutes (Mello and Ribeiro, 1998). After repetition of one song (e.g., for 1 hour daily for several days, or several hours on one day), subsequent presentations a day later caused no change in zenk mRNA levels, but introduction of a new song stimulus reactivated the gene response in the same brain region and apparently the same set of brain cells (reviewed in (Mello, 2004). Hence the zenk gene response to song playbacks was said to habituate (see Figure 1A). Although these experiments were initially conducted with cage-bred zebra finches and canaries, the phenomena are likely to be relevant to songbird species in the wild as song playbacks were shown to induce a zenk response in song sparrows in the field (Jarvis, Schwabl, Ribeiro, and Mello, 1997). Current research using DNA microarrays is showing that zenk is by no means the only gene that responds and habituates to song playback, but in fact is probably only one of literally hundreds of such genes (Dong, Replogle, Hasadsri, Imai, Yau, Rodriguez-Zas, Southey, Sweedler, and Clayton, submitted; Replogle, Arnold, Ball, Band, Bensch, Brenowitz, Dong, Drnevich, Ferris, George, Gong, Hasselquist, Hernandez, Kim, Lewin, Liu, Lovell, Mello, Naurin, Rodriguez-Zas, Thimmapuram, Wade, and Clayton, 2008).
Figure 1.
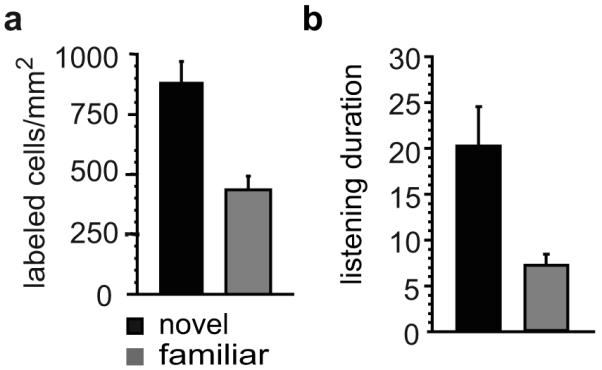
Two measures of habituation in the laboratory zebra finch. A) Playback of a novel song initially triggers expression of the zenk mRNA in cells in the auditory forebrain. After the song has been made familiar by repetition, fewer cells show this response when the song is presented again. B) Birds hearing song playbacks also adopt a distinctive listening posture upon stimulus onset. After the song has been made familiar by repetition, the duration of this “listening response” becomes shorter (scale bar: square root of seconds spent in the listening posture). Adapted from (Dong and Clayton, in press).
To investigate behavioral correlates of this changing gene response in the zebra finch, Stripling et al (Stripling, Milewski, Kruse, and Clayton, 2003) began by counting the spontaneous occurrence of various overt behaviors (e.g., sing, call, hop, fly, groom, eat, drink) prior to song playback. A single song stimulus was then repeated for 3 hours (two-second song bout every ten seconds), sufficient to achieve persistent gene habituation in prior reports. A day later the same song was repeated again, and the frequencies were compared across each period for each behavior (prior to song, hearing novel song, a day later hearing the now-familiar song). At the onset of initial song playback, the birds dramatically arrested all ongoing behaviors and sat silently with occasional head movement, apparently listening intently to the song. The latency to resume spontaneous activity was then measured. This latency was relatively long when birds heard the song for the first time, sometimes extending even more than 30 minutes. A day after the training experience, the birds showed a much shorter latency to resume activity, and this change in response was specific to the particular training song (see Figure 1B).
Thus zebra finches in the laboratory, like birds in the wild, do show a form of behavioral habituation to repeated song playbacks. Interestingly, however, their response to song playbacks is somewhat the reciprocal of what is observed in territorial species in the field: when the song is novel, instead of attacking the speaker, a zebra finch halts other activities and simply listens. The duration of listening is inferred from the lack of other overt behavioral activities, and habituation of listening is defined by the rapid resumption of these other behaviors after onset of the test song playback. Perhaps the difference in response pattern reflects the different contexts of the experience (field vs. laboratory), or it may reflect the intrinsic nature of the species: zebra finches live closely in colonies and have no documented use of song in territorial defense (Zann, 1996). In any case, the description of song response habituation complements past behavioral studies in the zebra finch which have mostly focused on demonstrations of mate preference by the female, showing for example that females respond more slowly to novel songs than to familiar songs, e.g. father’s song or the song from the same subspecies (Clayton, 1990; Miller, 1979).
“Molecular Habituation”
To what degree is it appropriate to use the term “habituation” to refer to a changing molecular response, such as zenk gene transcription in the brain, as opposed to an overtly behavioral one? This is an argument perhaps more suited for semanticists, but certainly it is valid to consider whether the criteria for classical habituation (see Introduction to this Issue) are fulfilled by the declining zenk gene response after repeated song playbacks. We argue that the core requirements are met, although only four of the 10 criteria have been directly examined. Gene response habituation is indeed a decrement that arises from repeated stimulation (criterion 1: Repeated application of a stimulus results in a progressive decrease in some parameter of a response to an asymptotic level) and it can persist for long durations after the period of stimulation (criterion 10: With some stimulus repetition protocols certain aspects of the response decrement may last hours, days or weeks). In experiments described in a doctoral thesis (Kruse, 2001), it was estimated that repetition of a song for 30-60 minutes (frequency of 2 seconds of song every 10 seconds) was the minimum needed to see a response decrement to that song 3 hours later. Other experiments have indicated a minimum of 1-3 hours of stimulus repetition for development of habituation that persists for at least 24 hours (Dong and Clayton, in press; Kruse, 2001). Consistent with criterion 2 (If the stimulus is withheld after response decrement, the response recovers at least partially over time), the habituated response does exhibit spontaneous recovery, although rigorous parametric analysis of this phenomenon has never been attempted.
Most importantly, the response decrement is clearly specific to the repeated song stimulus (criterion 6: Within the same stimulus modality the response decrement shows some stimulus specificity). Indeed, the decrement may depend not only on the acoustic information in the song but also the environmental context in which the song is heard. Thus presenting the habituated song from a different speaker position, at a lower volume, or in a new visual context will trigger a new round of gene expression (Kruse, Stripling & Clayton 2004), as will pairing the familiar song with a footshock (Jarvis, Mello, and Nottebohm, 1995). This apparent recovery of response to a repeated song stimulus has been called, erroneously, “dishabituation.” However, the true test of dishabituation (criterion 7: Presentation of a different stimulus results in an increase of the decremented response to the original stimulus) remains to be performed, where effects of a strong intervening stimulus are examined on subsequent presentations of the habituated stimulus in the same original, habituated context. The other formal criteria of habituation (3-5, 8, 9) also remain to be tested; both pragmatic and ethical considerations challenge the design of extensive parametric experiments of gene habituation, which would require terminal procedures with large numbers of songbirds.
Habituation in Other Molecular Pathways
The identification of a dynamic molecular correlate of song experience led to an active research focus on the mechanisms associated, first, with the initial gene wave of gene expression induced by novel song. Presumably the eventual habituation of the gene response involves some alteration along this activation pathway. Based on analysis of the canary zenk gene promoter and precedents from other species, (Cheng and Clayton, 2004) tested the hypothesis that the increase in zenk mRNA requires activation of the ERK (Extracellular Receptor Kinase) pathway. They found that cannula-directed infusion of an ERK inhibitor into the auditory forebrain indeed blocks the song-induced increase in zenk. Activation of ERK itself was detected using an antibody specific for the activated (phosphorylated) protein. Interestingly, ERK activation peaked within 1-2 minutes after song onset, long before zenk expression itself peaked. Song repetition also resulted in apparent habituation of the ERK response. Hence processes along the pathway leading to ERK activation may mediate both the genomic response to novelty and the eventual habituation of that response. As we discuss below, these processes could be centered within the responding cell, but they could just as easily involve external circuits that impinge on receptors feeding into the ERK pathway. Moreover, the development of response habituation may or may not require the initial wave of gene expression itself.
One other molecule has been implicated rather unexpectedly in habituation of gene responses to song: caspase-3, an intracellular protease best known as the “executioner” enzyme in apoptosis, the process of programmed cell death. Novel song exposure triggers release of activated caspase from an intracellular store apparently near postsynaptic terminals (Huesmann and Clayton, 2006). The wave of activated caspase-3 is brief, peaking ∼10 minutes after song onset. The function of caspase release is not clear - there is no evidence that it effects any change in neuronal survival or turnover. However, pharmacological inhibition of caspase-3 activity during initial song training blocks the emergence of zenk gene habituation to the training song, as measured by subsequent exposure to the song a day later.
The temporal and proposed functional relationships of these various molecular responses are summarized in Figure 2.
Figure 2.
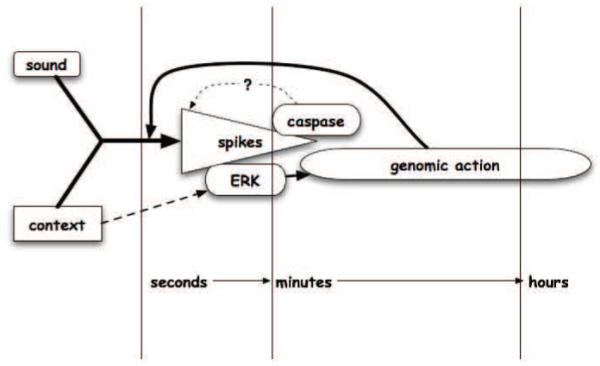
Schematic diagram illustrating distinct process and timelines in habituation of molecular and cellular responses to song playback.
Habituation of Electrophysiological Responses to Song
Following the discovery of dynamic gene responses to song in the auditory forebrain, two groups of investigators applied multiunit and single unit recording methods to characterize the rates of neuronal firing in this brain region in response to novel and repeated song stimuli (Chew, Mello, Nottebohm, Jarvis, and Vicario, 1995; Chew, Vicario, and Nottebohm, 1996; (Stripling, Kruse, and Clayton, 2001; Stripling, Volman, and Clayton, 1997). Unlike zenk response studies, electrophysiological studies require that the birds be physically restrained throughout the recording process, which alters the stimulus-response properties of the zenk response itself (Park and Clayton, 2002). The measurements in the studies listed above were collected, however, in birds that were awake and unanesthetized.
Most neurons in the auditory forebrain fire in response to a broad range of stimuli including simple tones, white noise, and birdsong. However, they are more responsive to conspecific vocalizations (including songs, male/female contact calls, and the bird’s own song) than to heterospecific songs and artificial sounds. Neurons vary greatly in their patterns of firing relative to the structure of a song. Thus, each song may generate a unique signature of activity pattern across a population of neurons. When a song stimulus is repeated, the spike response begins to decrement essentially immediately, dropping by an average of about 15% between the first and second presentation (Stripling et al., 1997). After 50 repetitions of the stimulus over 11 minutes, the spike rate decreases to about 40% to 60% of the initial response but still remains well above the spontaneous rate observed between stimulus presentations (Chew, Mello, Nottebohm, Jarvis, and Vicario, 1995; Stripling et al., 1997). Additional playbacks do not decrease the spike rate any further below this baseline response, although they may increase the duration the habituation persists.
Hence, in contrast the complete loss of the zenk gene response in auditory neurons after song habituation, these cells continue to maintain a significant though diminished electrophysiological response. Interestingly, electrophysiological habituation only occurs in response to conspecific and heterospecific songs but not to simple tones. Tones also fail to induce a zenk gene response even when they are first presented (Mello et al., 1992), suggesting a general correlation between the tendency of the spike response to habituate to a stimulus and the tendency of the stimulus to induce a zenk response. This correlation was only partially supported, however, in an analysis of song responses in the developing zebra finch (Stripling et al., 2001).
Consistent with a core criterion of habituation (#6: within the same stimulus modality the response decrement shows some stimulus specificity), the decrement in spike rate is specific to the repeated stimulus, and multiple song stimuli can be habituated independently. As observed for gene response habituation, spike response habituation can also persist for periods of up to several days before undergoing spontaneous recovery (Chew et al., 1995; Chew, Vicario, and Nottebohm, 1996). Duration of the response decrement depends on both the nature of the stimulus and the duration of initial entrainment. Under the same training pattern, habituation to conspecific song lasts longer than heterospecific song. Consistent with criterion #3 (other things being equal, more frequent stimulation results in a more rapid and/or more pronounced response decrement, and a more rapid spontaneous recovery), spontaneous recovery is dependent on the frequency of initial stimulus presentation, so that more frequent song presentations result a more rapid spontaneous recovery.
Relationships of Various Levels of Habituation
Thus in songbirds, habituation of responses to repeated song playbacks is observed at four different functional levels: the whole organism’s behavior, activity in neural circuits, intracellular signaling pathways (ERK and caspase), and nuclear gene expression. Do all of these changes reflect the same fundamental process? Does habituation at one level cause or require habituation at the other levels? Although the different experimental designs required for the four types of measurements make it difficult to compare habituation parameters across levels, there are some obvious correlations. In all four cases, response habituation is specific to the repeated song, and it develops more easily with the stimuli that are most salient to the bird - the songs of other conspecifics. Spaced training is more efficient than massed training. The same training procedure (2 seconds of song every 10 seconds, for 1-3 hours) is sufficient to develop habituation of all four responses that persists for at least a day. In the literature of learning, gene expression through new protein synthesis is often proposed as an essential step in the formation of stable, persistent memories (Gold, 2008). Might the initial activation of zenk and other genes by novel stimuli be a prerequisite for the development of persistent habituation of the response itself, and of the overt behavioral manifestation of habituation?
To test this association directly, we performed an experiment where both behavioral and zenk assays were used simultaneously to study the same individuals (Dong and Clayton, in press). During a 3-hour training period, activity of the ERK pathway necessary for zenk expression was blocked in the auditory forebrain via cannula-directed infusion of a pharmacological inhibitor (U0126) or an inactive control compound (U0124). One day later, birds were tested by exposure to the training song Behavioral responses on both days were scored as above (latency to resume activity after onset of listening), and the birds were euthanized for analysis of zenk mRNA 30 minutes after onset of the final testing period. The birds that had been trained under the influence of the inhibitor showed a full zenk response to the test song (i.e., as though it was novel), whereas birds infused with an inactive control compound showed no zenk response at final testing (i.e., habituation). This indicates that activity in the ERK/zenk pathway is indeed necessary for the development of persistent gene habituation to the training stimulus.
Unexpectedly, however, the overt behavior of the birds reported a different effect: birds infused with either the active inhibitor or the inactive control compound during training responded the next day with short latencies as though the songs were familiar, even when they were presented with novel songs. The experience of cannulation and infusion had no obvious effect on the day of training (birds showed the expected long latencies to the initial presentations), but it seemed to alter the way they responded to any song stimulus a day later. This might be interpreted as a form of stimulus generalization (the bird habituates to the procedure as a whole). In any case it appears to be another illustration of the phenomena described in sparrows by Petrinovich and Patterson (discussed above), where habituating behaviors were found to be modulated in complex ways by the animal’s individual state. The results also indicate that behavioral and molecular measures of habituation are not coupled in lock-step, and may be modulated with some independence.
The Meaning of (Song) Habituation
The formal definition of habituation refers to a loss of something - a decrement in a response. In songbirds, habituation also implies a gain of something - the selective recognition of a particular acoustic pattern and the association of that pattern with a particular context. Changing either the song’s pattern or its context is sufficient to override the habituation and trigger new overt responses (Kruse, Stripling, and Clayton, 2004). Is habituation better understood as a loss - perhaps an essentially passive process following repeated activation of a particular neural representation? Or is habituation something that is actively and purposefully maintained? Further insight into this may come soon from studies using large-scale DNA microarrays to profile the changing patterns of gene expression during the development, maintenance and eventual decline of song-specific habituation. Initial results of such studies suggest that habituation is marked not just by the loss of expression of zenk and other responsive genes, but also by the emergence of a distinctive “habituation-specific” gene expression profile (Dong et al., submitted).
For songbirds in the wild, the phenomena of habituation to a territorial challenger are central to the establishment of stable breeding territories, and hence to the social organization of many species. For these reasons, we wonder if song habituation in birds has been molded by particular evolutionary forces that may not apply to other examples of habituation. Is habituation a singular process, with lots of manifestations and applications? Or is it better understood as a broad set of distinct processes, all of which support the essential adaptation of an organism to repeated experience? Future research using songbirds may help answer these fundamental questions about biological order and design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Blanchard BD. The White-crowned Sparrows (Zonotrichia leucophrys) of the Pacific seaboard: environment and annual cycle. Univ. Calif. Publ. Zool. 1941;46:1–178. [Google Scholar]
- Brooks RJ, Falls JB. Individual recognition by song in white-throated sparrows. 1. Discrimination of songs of neighbors and strangers. Canadian Journal of Zoology. 1975;53:879–888. [Google Scholar]
- Cheng HY, Clayton DF. Activation and habituation of extracellular signal-regulated kinase phosphorylation in zebra finch auditory forebrain during song presentation. Journal of Neuroscience. 2004;24:7503–7513. doi: 10.1523/JNEUROSCI.1405-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew SJ, Vicario DS, Nottebohm F. A large-capacity memory system that recognizes calls and songs of individual birds. Proceedings of the National Academy of Sciences (USA) 1996;93:1950–1955. doi: 10.1073/pnas.93.5.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton DF. The songbird’s niche in the world of genomes (Review) Current Biology. in press. [Google Scholar]
- Clayton NS. Subspecies recognition and song learning in zebra finches. Animal Behaviour. 1990;40:1009–1017. [Google Scholar]
- Dong S, Clayton DF. Partial Dissociation of Molecular and Behavioral Measures of Song Habituation in Adult Zebra Finches. Genes, Brain and Behavior. doi: 10.1111/j.1601-183X.2008.00423.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Replogle K, Hasadsri L, Imai B, Yau P, Rodriguez-Zas S, Southey B, Sweedler J, Clayton DF. Discrete molecular states in the brain accompany changing responses to a vocal signal. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls JB. Individual recognition by sounds in birds. In: Kroodsma DE, Miller DH, Ouellet H, editors. Acoustic Communication in Birds. Academic Press; NY: 1982. pp. 237–278. [Google Scholar]
- Falls JB. In: Playback: a historical perspective. McGregor PK, editor. Plenum Press; New York: 1992. pp. 11–33. [Google Scholar]
- Falls JB, Brooks RJ. Individual Recognition by Song in White-Throated Sparrows .2. Effects of Location. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1975;53:1412–1420. [Google Scholar]
- Gold PE. Protein synthesis and memory. Introduction. Neurobiology of Learning and Memory. 2008;89:199–200. doi: 10.1016/j.nlm.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Harris J. Habituatory response decrement in the intact organism. Psychological Bulletin. 1943;40:385–422. [Google Scholar]
- Harris MA, Lemon RE. Songs of Song Sparrows: Reactions of Males to Songs of Different Localities. The Condor. 1974;76:33–44. [Google Scholar]
- Huesmann G, Clayton DF. Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song response habituation. Neuron. 2006;52:1061–1072. doi: 10.1016/j.neuron.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Mello CV, Nottebohm F. Associative learning and stimulus novelty influence the song-induced expression of an immediate early gene in the canary forebrain. Learning and Memory. 1995;2:62–80. doi: 10.1101/lm.2.2.62. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Schwabl H, Ribeiro S, Mello CV. Brain gene regulation by territorial singing behavior in freely ranging songbirds. Neuroreport. 1997;8:2073–2077. doi: 10.1097/00001756-199705260-00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JR. Habituation and song repertoires in great tit. Behavioral and Ecological Sociobiology. 1976;1:215–227. [Google Scholar]
- Kroodsma D. The Singing Life of Birds: The Art and Science of Listening to Birdsong. Houghton Mifflin; 2005. [Google Scholar]
- Kruse AA. Neuroscience. University of Illinois; Urbana, IL: 2001. Dynamic modulation of an immediate-early gene in the songbird forebrain. [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Minimal experience required for immediate-early gene induction in zebra finch neostriatum. Neurobiology of Learning and Memory. 2000;74:179–184. doi: 10.1006/nlme.2000.3968. [DOI] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiology of Learning and Memory. 2004;82:99–108. doi: 10.1016/j.nlm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Marler P, Hamilton WJI. Mechanisms of Animal Behavior. John Wiley and Sons; New York: 1966. [Google Scholar]
- Mello CV. Gene regulation by song in the auditory telencephalon of songbirds. Frontiers in Bioscience. 2004;9:63–73. doi: 10.2741/1201. [DOI] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. Journal of Neuroscience. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Nottebohm F, Clayton DF. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene’s response to that song in zebra finch telencephalon. Journal of Neuroscience. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. Zenk protein regulation by song in the brain of songbirds. Journal of Comparative Neurology. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB. Long-term recognition of father’s song by female zebra finches. Nature. 1979;280:389–391. [Google Scholar]
- Park KH, Clayton DF. Influence of restraint and acute isolation on the selectivity of the adult zebra finch zenk gene response to acoustic stimuli. Behavioural Brain Research. 2002;136:185–191. doi: 10.1016/s0166-4328(02)00129-8. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Petrinovich L. Field studies on habituation Part 2: Effect of massed stimulus presentation. Journal of Comparative and Physiological Psychology. 1979;93:351–359. [Google Scholar]
- Petrinovich L, Patterson TL. Field studies on habituation, Part 1: Effect of reproductive condition, number of traials and different delay intervals on responses of the white-crowned sparrow. Journal of Comparative and Physiological Psychology. 1979;93:337–350. [Google Scholar]
- Petrinovich L, Patterson TL. Field studies of habituation 3. Playback contingent on the response of the white-crowned sparrow Zonotrichia-leucophrys. Animal Behaviour. 1980;28:742–751. [Google Scholar]
- Petrinovich L, Patterson TL. Field studies of habituation 4. Sensitization as a function of the distribution and novelty of song playback to white-crowned sparrows Zonotrichia-leucophrys-nuttalli. Journal of Comparative and Physiological Psychology. 1981;95:805–812. [Google Scholar]
- Petrinovich L, Patterson TL. Field studies of habituation 5. Evidence for a 2-factor dual process system. Journal of Comparative and Physiological Psychology. 1982;96:284–296. [Google Scholar]
- Replogle K, Arnold AP, Ball GF, Band M, Bensch S, Brenowitz EA, Dong S, Drnevich J, Ferris M, George JM, Gong G, Hasselquist D, Hernandez AG, Kim R, Lewin HA, Liu L, Lovell PV, Mello CV, Naurin S, Rodriguez-Zas S, Thimmapuram J, Wade J, Clayton DF. The Songbird Neurogenomics (SoNG) Initiative: community-based tools and strategies for study of brain gene function and evolution. BMC Genomics. 2008;9:131. doi: 10.1186/1471-2164-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripling R, Kruse AA, Clayton DF. Development of song responses in the zebra finch caudomedial neostriatum: Role of genomic and electrophysiological activities. Journal of Neurobiology. 2001;48:163–180. doi: 10.1002/neu.1049. [DOI] [PubMed] [Google Scholar]
- Stripling R, Milewski L, Kruse AA, Clayton DF. Rapidly learned song-discrimination without behavioral reinforcement in adult male zebra finches (Taeniopygia guttata) Neurobiology of Learning and Memory. 2003;79:41–50. doi: 10.1016/s1074-7427(02)00005-9. [DOI] [PubMed] [Google Scholar]
- Stripling R, Volman SF, Clayton DF. Response modulation in the zebra finch caudal neostriatum: Relationship to nuclear gene regulation. Journal of Neuroscience. 1997;17:3883–3893. doi: 10.1523/JNEUROSCI.17-10-03883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. Encyclopedia of Psychology. Oxford University Press; 2000. Habituation. [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;173:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thorpe WH. Perceptual basis for group organization in social vertebrates, especially birds. Nature. 1968;220:124–128. doi: 10.1038/220124a0. [DOI] [PubMed] [Google Scholar]
- Verner J, Milligan MM. Responses of male white-crowned sparrows to playback of recorded songs. The Condor. 1971;73:56–64. [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. Oxford University Press; Oxford: 1996. [Google Scholar]
- Zeigler HP, Marler P. Behavioral Neurobiology Of Bird Song (Annals of the New York Academy of Sciences. Vol. 1016. New York Academy of Sciences; 2004. [DOI] [PubMed] [Google Scholar]


