Abstract
Objective
Does psychosocial intervention for caregivers whose spouses with Alzheimer’s disease (AD) are taking donepezil delay nursing home (NH) placement or death of patients?
Design
Randomized controlled trial with 2 years of active treatment and up to 8.5 years of follow-up (M = 5.4 years, SD = 2.4) was conducted.
Setting
Outpatients of research clinics in Australia, United Kingdom (UK) and the United States (US).
Participants
155 persons with AD and their spouses.
Intervention
5 sessions of individual and family counseling (+ prn ad hoc counseling) or usual care.
Measurements
Time to institutionalization and death using Cox proportional hazards methods.
Results
Over a mean of 5.4 years (SD = 2.4), there were no differences in NH placement or mortality by intervention group but there were by country, with Australian patients admitted to NHs earlier than US and UK patients.
Conclusion
Earlier NH admission of Australian than UK and US subjects may be due to differences in health care, NH systems, availability and affordability.
Keywords: caregiver, Alzheimer’s disease, dementia, nursing home placement, mortality, intervention
Objective
Family caregivers of people with dementia experience physical, psychological, financial and/or social burden (1) and are crucial in maintaining a person with dementia in the community. Negative factors associated with caregiving have been identified as the main predictors of nursing home (NH) placement (2).
Caregiver interventions can improve caregiver outcomes such as subjective well-being, psychological morbidity, and ability/knowledge and can reduce the impact on caregivers of behavioral symptoms of care recipients and their risk of institutionalization(3–6). A structured, intensive 10-day training program conducted in Sydney, Australia decreased caregiver distress, delayed institutionalization and increased patient survival(7, 8). A family intervention conducted in Manchester, UK reduced distress and depression in caregivers of persons with dementia, (9). The New York University Caregiver Intervention (NYUCI) (10), which included individual and family counseling, support group participation, and ad hoc (on demand) counseling, generally by telephone, reduced the rate of NH placement (11, 12) and improved caregiver depression (10, 13) and management of problem behaviors (14). Delay in NH admission can benefit caregivers and patients and result in significant economic savings (15).
Cholinesterase inhibitors such as donepezil may delay NH admission (16, 17) and reduce both caregiver burden (18–20) and time spent caring (19, 20) and possibly reduce mortality in people with dementia residing both in nursing homes (21, 22) and in the community (23, 24). The present study aimed to investigate whether the addition of a psychosocial caregiver intervention to cholinesterase inhibitor treatment for patients provided further benefit to patients and spouse caregivers with respect to delay of institutionalization.
Previous studies have identified older age, greater severity of cognitive and functional decline, behavioral and psychological symptoms of dementia (BPSD) and poorer general physical health as predictors of greater mortality in persons with dementia (2, 25–33). As well as these variables, caregiver burden has been shown to be a risk factor for nursing home admission (2, 12).
The Three Country Study (3CS), conducted in Australia, the United Kingdom (UK) and the United States (US) substantially replicated the NYU intervention (10) and added donepezil treatment for patients. We previously confirmed our primary hypothesis that five counseling sessions would reduce depression two years after enrollment (34). This paper examines our secondary hypothesis, that the 3CS intervention would delay NH placement and improve patient survival after controlling for factors known to influence these outcomes. In addition we were interested in whether there were differences in these effects across the three sites.
Methods
Subjects
Subjects were a volunteer sample of 158 patient/caregiver dyads (Australia: 52; UK: 54; US: 52) recruited at one of three sites: Australia, the University of New South Wales, Academic Department for Old Age Psychiatry, Prince of Wales Hospital in Sydney; UK, the University of Manchester, Division of Psychiatry; and US, the Silberstein Aging & Dementia Research Center at the New York University School of Medicine (NYU-ADRC). All patients were required to have stable physical health for the previous year and meet the National Institute of Neurological and Communicative Diseases and Stroke - Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) (35) and the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (36) criteria for probable Alzheimer’s disease (AD), have a Global Deterioration Scale (GDS) (37) score of 4 to 5 at the time of enrollment, have no contra-indication to taking donepezil, be stable with other medications, be able to give informed consent (or not object to participating) and be community dwelling with their spouse.
Participants were required to be the spouse and primary caregiver of the patient, and give informed consent. Caregivers were excluded if they had previously received formal caregiver counseling. In addition, at least one family member other than the caregiver had to be potentially available to participate in the family counseling sessions, although on a few occasions at the UK site, family members declined to, or could not take part in the counseling sessions.
Study Design
Dyads were randomly assigned by lottery to the control (donepezil and standard services) or treatment group (donepezil, standard services and psychosocial caregiver intervention). Standard services included resource information, help in an emergency and the routine services normally provided to patients and family members at each site, but not formal structured counseling sessions.
All patients received donepezil for up to 24 months free of charge. Patients, who started taking donepezil at enrollment, began with a dose of 5 mg per day which was increased to 10 mg at first follow up. Others were maintained on the dose they were taking upon study entry. This dose was maintained throughout the duration of the study unless contraindicated by patient reaction.
Caregivers and patients were assessed by independent raters, using structured questionnaires, every three months for the first year and every six months for the second year except that there was no 9-month follow-up in the UK. Strenuous efforts were made to keep raters blind to group assignment. All follow-up assessments included the baseline measures. Caregivers were periodically contacted for up to 8.5 years (M = 5.4 years, SD: 2.4 years, range: 5 months–8.5 years) after enrollment, and patient NH placement and/or death dates were recorded if applicable.
Psychosocial Intervention
Caregivers in the treatment group received five counseling sessions within three months of enrollment. These comprised one individual session with the spouse followed by three family counseling sessions, which included the family members who were invited by the caregiver to participate, and then another individual session with the spouse. Ad hoc counseling on demand by telephone (and/or face-to-face in Australia) was available to caregivers and their family members for up to two years (whereas in the original NY study it continued indefinitely). Additional in-person counseling, as distinct from telephone counseling, was only provided at the Australian site. No consistent records were kept of the number of telephone contacts subsequent to formal counseling, but family members in the treatment group knew the counselors and could call them, while those in the control group had generally never met the counselors and were therefore unlikely to call them.
The intervention strategy was manualized and based on the NYU intervention (10), While there were four family counseling sessions in the original intervention, taking place within four months of enrollment, there were three sessions in the 3CS replication, taking place within three months of enrollment. This change was made to accommodate the need for a 3-month follow-up of patients beginning donepezil at enrollment. Previous experience in the NYUCI suggested that it was not possible to schedule four family sessions plus two individual sessions within three months. As in the original intervention, the content of the sessions was individualized and could include education about AD, information about available resources in the community family issues about helping the caregiver or care of the person with dementia and help in understanding how to manage difficult patient behavior. The importance of emotional support and assistance for the spouse caregiver was a general theme. Changes in patient status, new symptoms, other family problems and emergencies and requests for resource information often resulted in ad hoc calls from caregivers and family members. Principal investigators had regular telephone conferences during the study period, and counselors also had telephone conference discussions about specific cases. Principal investigators at each site were involved in regular meetings with counselors as well.
Measures
The assessment of the caregiver included demographic characteristics such as gender, age, race, education, and income. Caregiver depression was measured with the revised Beck Depression Inventory (BDI-II) (38), and social support with items from the Stokes Social Network List (39).
Patient assessment included the Mini-Mental State Examination (MMSE) (40), Global Deterioration Scale (GDS) (37), Alzheimer’s Disease Assessment Scale – cognitive subscale (ADAS-cog) (41), Alzheimer’s Disease Cooperative Study – Activities of Daily Living (ADCS-ADL) (42) and Revised Memory and Behavior Problems Checklist (RMBPCL) (43). Donepezil dosage, concurrent medications, including psychotropic medication; (previous and) current alcohol intake and adverse events were recorded at each visit. Caregivers rated patient physical health assessed at each visit on three items from the OARS (potential sum of scores ranges between 3–10) (44)..
Analyses
Data were analyzed using SPSS version 14. Cox Proportional Hazards models were used to assess the effect of treatment group on time to NH admission or death separately. Time in days (from first entering the study) to NH admission or death was the dependent variable. NH placement was right censored using date of death, when appropriate. Treatment group and country of residence were entered as covariates. Factors that were found to correlate with the time to NH admission or death in univariate analyses or were reported in other research were entered into the models, namely: patient age and gender, baseline ADAS-cog and ADCS-ADL scores. In addition, NH placement, which might affect survival time (26), was included as a covariate when assessing mortality. Behavioral and psychological symptoms in dementia as measured with the Revised Memory and Behavior Problem Checklist were not associated with time until NH admission or death in univariate analyses. Unlike the steady decline of cognition and function in AD, BPSD fluctuate (45, 46) and so baseline levels were not included as a covariate in our regression models. To obtain pair wise comparisons for all three countries each model was run twice changing the reference.
In the UK sample, baseline ADCS-ADL scores were missing for two patients. A sensitivity analysis excluding those two patients did not change the results of the Cox Proportional Hazards models. Their three months scores were therefore used as substitutes for the missing baseline ADCS-ADL scores. Three UK patients (2%) who withdrew prior to three months and were not included in the analysis, had all been assigned to the control group and did significantly better on the MMSE, GDS and ADAS-Cog at baseline than the remaining patients (Mann-Whitney Test, Z = −2.323, p = 0.020, Z = −2.046, p = 0.041, and Z = −2.747, p = 0.006, respectively). The results presented are for the remaining 155 patient-caregiver dyads.
Results
Baseline variables
Baseline characteristics of patients and caregivers are summarized in table 1. Briefly, the mean ages of patients and caregivers were 73.8 yrs (SD = 7.48, 52–91 yrs) and 71.3 yrs (SD = 8.20, 47–88 yrs) respectively, with patients having a mean MMSE score of 20.3 (SD = 5.61). Eighty-eight patients were rated as GDS4 on the GDS, 62 as GDS5. Five patients were inadvertently included with GDS ratings of 3 or 6 which was only discovered after randomization, so they permitted to remain in the study.
Table 1.
Baseline characteristics of patients and caregivers
| Mann-Whitney | country | Kruskal-Wallis (df=2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N=155) |
Treatment (n=79) | controls (n=76) | Z | p | AUS (n=52) | UK (n=51) | USA (n=52) | chi2 | p | ||
| Patients | age: M(SD) | 73.8 (7.48) | 73.9 (7.74) | 73.7 (7.28) | −0.858 | 0.391 | 75.0 (7.23) | 72.9 (6.37) | 73.0 (8.55) | 2.463 | 0.292 |
| gender: M:F | 87:68 | 47:32 | 40:36 | 31:21 | 22:29 | 34:18 | |||||
| MMSE: M(SD) | 20.3 (5.61) | 20.9 (5.16) | 19.8 (5.99) | −0.581 | 0.561 | 20.0 (5.46) | 20.3 (6.22) | 20.7 (5.27) | 0.453 | 0.797 | |
| ADCS-ADL: M(SD) | 55.3 (13.32) | 56.7 (11.41) | 54.0 (14.85) | −0.045 | 0.964 | 54.4 (12.91) | 53.5 (14.0) | 58.1 (13.03) | 6.559 | 0.038 | |
| ADAS-Cog: M(SD) | 25.8 (11.40) | 25.7 (11.11) | 25.9 (11.73) | −0.206 | 0.837 | 28.8 (10.45) | 21.1 (12.29) | 26.8 (10.79) | 12.876 | 0.002 | |
| GDS: M(SD) | 4.4 (0.53) | 4.4 (0.49) | 4.4 (0.58) | −0.406 | 0.685 | 4.4 (0.50) | 4.2 (0.54) | 4.5 (0.55) | 3.207 | 0.201 | |
| RMBPCL: M (SD) | 9.84 (8.43) | 10.04 (8.91) | 9.63 (7.96) | −.062 | 0.951 | 9.23 (9.27) | 9.31 (8.05) | 10.98 (7.94) | 3.437 | 0.179 | |
| Phys. health | 5.7 (1.51) | 5.5 (1.52) | 5.8 (1.50) | −1.61 | 0.107 | 5.5 (1.38) | 5.8 (1.70) | 5.8 (1.54) | 0.314 | 0.855 | |
| Caregivers | age: M (SD) | 71.3 (8.2) | 71.5 (7.98) | 71.1 (8.41) | −0.641 | 0.521 | 71.8 (8.20) | 72.6 (5.63) | 69.6 (9.46) | 1.133 | 0.567 |
| gender M:F | 69:86 | 33:46: | 36:40: | 21:31 | 29:22 | 19:33 | |||||
| BDI: M(SD) | 8.7 (6.53) | 9.2 (7.02) | 8.3 (6.05) | −0.508 | 0.611 | 9.1 (5.48) | 7.1 (6.68) | 9.8 (7.30) | 3.001 | 0.223 | |
M: mean, SD: Standard deviation, USA: United States of America, UK: United Kingdom, AUS: Australia; Significant results are printed bold
Treatment and control group subjects did not differ significantly at enrollment with respect to caregiver depression (BDI), gender or age; or patient gender, age, GDS, MMSE, RMBPCL, ADCS-ADL, ADAS-Cog or physical health (see table 1). However, comparisons by country revealed some differences as regards ADAS-Cog and ADCS-ADL scores (table 1). Post-hoc pairwise Mann-Whitney U comparisons with Bonferroni corrections revealed that there were significant differences in ADAS-Cog scores between the UK and both the US (Z = −2.55, p = 0.011) and Australia (Z = −3.45, p = 0.001), suggesting that UK patients were less cognitively impaired at baseline. Post-hoc pairwise comparisons of ADCS-ADL differences by country indicated that UK patients were also less impaired in ADLs than US patients (Mann-Whitney, Z = −2.52, p = 0.012). No differences were found between Australian and either UK or US patients. Regarding the remaining baseline parameters no differences by country were found.
Survival at home (Table 2)
Table 2.
Predictors for NH placement at up to 8.5 years.
| Wald | df | Sig. | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Treatment group | .044 | 1 | .835 | 1.057 | .627 | 1.781 |
| Patient age | 6.330 | 1 | .012 | .956 | .923 | .990 |
| Patient gender | 1.358 | 1 | .244 | 1.376 | .804 | 2.355 |
| ADCS-ADL at baseline | 13.976 | 1 | <.001 | .948 | .922 | .975 |
| ADAS-Cog at baseline | .705 | 1 | .401 | 1.013 | .983 | 1.043 |
| UK vs Australia* | 9.302 | 1 | .002 | .344 | .173 | .683 |
| USA vs Australia* | 17.408 | 1 | <.001 | .228 | .114 | .456 |
| UK vs USA*1 | .905 | 1 | .342 | 1.509 | .646 | 3.525 |
| Patient physical health at baseline | .951 | 1 | .329 | .908 | .747 | 1.103 |
| Caregiver depression at baseline | .034 | 1 | .853 | 1.004 | .967 | 1.042 |
Significant HR are printed bold,
indicates reference group,
in order to get pair wise comparisons for all three countries, Cox regression was run twice changing the reference group
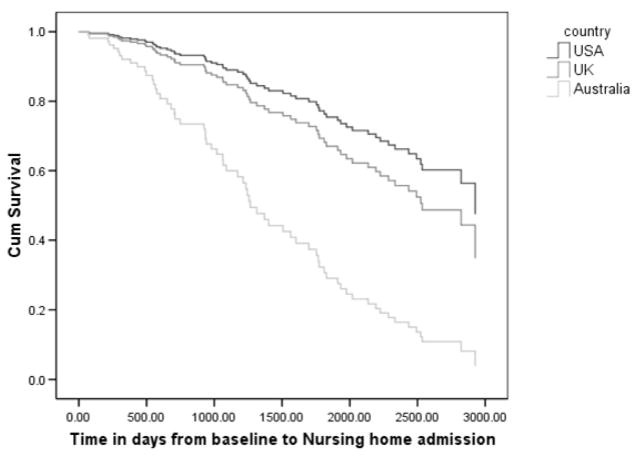
The results of the first Cox Proportional Hazards model assessing the effect of treatment and country of residence on time to NH admission are displayed in table 2. Older age and higher baseline ADCS-ADL scores (i.e. more functional independence) were found to predict increased time at home. Australians were at higher risk for NH placement than UK or US patients (table 2 & figure 1). Caregiver counseling, patient gender, baseline cognitive functioning, physical health as well as caregiver depression at baseline did not predict NH placement.
Figure 1.
Nursing home survival between Australia, the UK and the US at up to 8.5 years.
On post-hoc analyses, when data for all three countries were combined, no difference was found in times to NH placement between treatment (M = 4.1 years, SD = 2.4 years) and control (M = 4.3 years, SD = 2.4 years) patients (Log-Rank test, χ2 = 0.000, df = 1, p = 0.998). When individual country data were examined, half of the Australian patients whose spouses had had counseling (the treatment group) were still at home at date of death or censoring date compared to only 23% of control patients (Pearson χ2, df = 1, p = 0.044). This treatment group effect was not found for UK or US patients (Pearson χ2, df = 1, p = 0.322 and p = 0.749, respectively) (see table 3).
Table 3.
Nursing home status and mortality between countries and treatment groups at up to 8.5 years (M = 5.4 years).
| Treatment | Control | ||||||
|---|---|---|---|---|---|---|---|
| Australia | UK | US | Australia | UK | US | TOTAL | |
| N | 26 | 27 | 26 | 26 | 24 | 26 | |
|
| |||||||
| Not admitted to NH | 13 (50%)1 | 12 (44%) | 19 (73%) | 6 (23%)1 | 14 (58%) | 20 (77%) | 84 |
| Admitted to NH | 13 (50%) | 15 (56%) | 7 (27%) | 20 (77%) | 10 (42%) | 6 (23%) | 71 |
|
| |||||||
| Alive | 11 (42%) | 13 (48%) | 14 (54%) | 11 (42%) | 11 (46%) | 12 (46%) | 73 |
| Deceased | 15 (58%) | 14 (52%) | 12 (46%) | 15 (58%) | 13 (54%) | 14 (54%) | 85 |
Pearson χ2 significant, (df = 1, p = 0.044)
Mortality
According to the second Cox Proportional Hazards model, time to death was similar for treatment (M = 5.5 years, SD = 2.4) and control (M= 5.3 years, SD = 2.4) patients (Log-rank test not significant, χ2 = 0.258, df = 1, p = 0.612) and across the three countries (Log-rank test not significant, χ2 = 1.004, df = 1, p = 0.316). Increased survival was predicted by lower baseline ADAS-cog scores, higher baseline ADL scores and placement in a NH (not admitted to a NH: M = 4.9 years, SD = 2.5; admitted to NH: M = 5.9 years, SD = 2.2) as well as caregiver depression at baseline (Table 4). Patients’ gender, age and physical health did not predict time to death.
Table 4.
Predictors for mortality at up to 8.5 years.
| Wald | df | Sig. | HR | 95.0% CI for HR | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Treatment group | .005 | 1 | .946 | .984 | .612 | 1.581 |
| Patient age | 1.102 | 1 | .294 | 1.019 | .984 | 1.056 |
| Patient gender | .072 | 1 | .789 | .932 | .558 | 1.558 |
| ADCS-ADL at baseline | 5.005 | 1 | .025 | .971 | .947 | .996 |
| ADAS-Cog at baseline | 10.040 | 1 | .002 | 1.041 | 1.016 | 1.068 |
| NH placement | 8.253 | 1 | .004 | .449 | .260 | .775 |
| UK vs Australia* | .045 | 1 | .833 | .936 | .508 | 1.727 |
| USA vs Australia* | 3.028 | 1 | .082 | .574 | .307 | 1.073 |
| UK vs USA*1 | 1.963 | 1 | .161 | 1.630 | 0.823 | 3.230 |
| Patient physical health at baseline | .691 | 1 | .406 | .931 | .787 | 1.102 |
| Caregiver depression at baseline | 3.866 | 1 | .049 | .959 | .920 | 1.000 |
Significant HR are printed bold,
indicates reference group
in order to get pair wise comparisons for all three countries, Cox regression was run twice changing the reference group
A post-hoc analysis of deceased patients revealed that there were no national differences in mean survival times. Moreover, there was no significant interaction effect for country*NH admission on survival (MANOVA, F = 1.844, df = 2, p = 0.165).
Finally we examined whether extra sessions of in-person counseling at the Australian site influenced outcomes (M = 10.0, SD = 4.17). The total number of counseling sessions did not influence mortality outcomes (HR = 0.074, (95% CI: 0.817–1.160), p = 0.764) but was associated with slightly but significantly increased rates of NH admissions (HR = 1.188 (95% CI: 1.001–1.409), p = 0.048).
Conclusion
In this first international study of psychosocial intervention performed across three countries, counseling did not alter rates of NH admission or death though there were significant inter-country differences in rates of institutionalization. Contrary to our previous findings that comprehensive caregiver training (7, 8) and counseling and support (10, 11) significantly delayed NH placement, by more than1.5 years in the NY study (11), no difference in time to NH admission was observed between the treatment and control groups. Although in a previous study we reported that CG training increased survival until death at five years (but not at eight) (2), we did not find an effect here.
However, in our post-hoc analysis a significant difference in NH placement was observed between countries, with Australian patients being admitted to NHs significantly earlier than both UK and US patients. In particular, very few patients entered NHs in the US (25%) compared with Australia (64%), even though absolute observation times differed only slightly from one country to another (Australia 8.7 years, UK 8.4 years and USA 8.1 years). It seems likely that differences between countries outweighed the effects of CG counseling. There were several important differences between this and the original NY study: (i) participants here were much less impaired than those in the NY study and thus less likely to enter a nursing home in the US; (ii) ad hoc counseling here was available only for up to two years but continued indefinitely in the NYU study; (iii) all patients were taking donepezil; and (iv) awareness, expectations and mainstream treatment were almost certainly different in the decade since Mittelman’s intervention..
Why in Australia, but not in USA or UK, was the rate of NH admission so much lower in the counseling group? The higher rate of NH admission in Australia generally allowed more scope to demonstrate effects on institutionalization. Also, differences between countries in the health care, NH systems and in community care may mitigate admission rates. In Australia there are no long-term care insurance schemes but NHs are affordable for anyone assessed as having need, NH fees are income based and, in circumstances of hardship, can be waived altogether through government subsidies (47). The UK has a publicly funded health and social care system financed through general taxation although for long term care, persons are expected to contribute to their care costs if their income and savings is above a certain level. Means tested local authority (nursing) homes exist but most NHs are private and non-income tested. Government subsidies are available, but may not cover the fees charged by these homes (48). In the US, Medicaid will pay most NH costs for eligible low income earners, providing a Medicaid place is available. The majority of residents must initially pay privately, and some may have invested in specialized private long-term care insurance to help meet the high costs (49, 50). However, at the New York site a large proportion of patients were eligible for Medicaid, so the decision to place an ill spouse in a NH was not primarily financial, although those with means may have opted for care at home. While there may be little difference between the three countries in terms of the total number of NH places available (51), in the UK, only a small number may be government subsidized making NH placement unattainable for lower income earners in some regions.
Availability and affordability of community care, which may maintain patients at home longer, also differ across countries. In Australia, community services are well developed although they have waiting lists and some costs to pay. The UK has a publicly funded health and social care system financed through general taxation. For long term care, in England, people are expected to contribute to their care costs if their income and savings are above a certain level but the local authority will pay in full for people with low incomes. In the US, long term care insurance pays for home as well as NH care, which may be a disincentive for NH admission. Medicaid will cover custodial care at home for eligible older adults (with demonstrably low incomes); this is a disincentive to place people in nursing homes.
“Usual care” might also differ by country. In Australia, aged care is funded and administered nationally thereby minimizing regional differences in delivery of services. The system of care is similar to that in the UK with the majority of community care available at minimal cost and domiciliary assessment and consultations with dementia nurses the norm. In the UK usual care consists of outpatient clinic appointments and follow-up, usually in the person’s own home to monitor the effects of medication and solve problems with care that may emerge. GP advice is also freely available and attendance at local day centres is offered. At the US site usual care includes a family conference with a social worker after each diagnostic evaluation and availability of a social worker to answer questions, give advice or resource information when requested. Moreover, one or more caregiver support groups are available, and more recently also support groups for people in both the early and middle stages of AD. It is also suggested that all families contact the Alzheimer’s Association for additional information and resources.
We note that counselors and counseling techniques possibly differed both qualitatively and quantitatively despite each counselor providing the prescribed five sessions, using the manual developed by Mittelman et al. (52) and offering subsequent ad hoc counseling. While further support was offered by telephone in all three countries, the Australian counselor also made herself available for extra individual and/or family in-person sessions. A sensitivity analysis performed for the Australian sample found a slight but significant association between more counseling sessions and greater risk of NH placement for patients, probably indicating that those in greatest need were requesting more help. Finally, when the study was initiated, donepezil was expensive and neither subsidized in Australia nor in the UK. Consequently, some patient/caregiver dyads may have enrolled only to receive donepezil without being receptive to counseling. In contrast, many participants in the U.S. were able to obtain donepezil through health insurance and were more likely to have enrolled in order to get the benefit of the counseling.
Unexpectedly, NH placement was found to predict increased survival in Australia and the UK. Literature investigating the effect of NH placement on patients’ mortality is contradictory (53–56). Among those patients who died during the study period UK and Australian participants lived longer when they were admitted to a NH whereas the opposite occurred in the US perhaps providing more evidence suggesting that reasons for NH placement may have differed. Another possibility is that NHs may differ in their level of care.
Behavioral and psychological symptoms of dementia have been found in previous research to predict NH admission (57, 58) and death (54, 59, 60). However, in this study as BPSD at baseline were not associated with time to NH admission or death in univariate analyses and there is longitudinal variability in their levels (45, 46), BPSD were not included as a covariate in our regression models. BPSD scores more proximal to NH admission or death might be better predictors for those events.
Further research is necessary to ascertain the reasons why caregiver counseling is effective in delaying NH admission in some environments but not others. Better matching of intervention to needs is likely to result in better outcomes; for example, we did not restrict counseling to depressed caregivers. The impact of the comparative affordability and availability of resources to assist with care at home versus care in institutions on the timing of placement decisions should be examined.
We conclude that among study participants across all three countries, caregiver counseling did not delay NH admission or increase survival until death. However, there were differences between countries in time to NH placement, with Australian patients being admitted to NHs earlier than UK or US patients, possibly due to differences in national health care and NH systems, availability and affordability. Future multinational research could reveal the effects of these and other possible national differences, and thereby elucidate the reasons for nursing home placement, and perhaps lead to better interventions to assure that they occur when they are in the best interests of caregivers and persons with dementia.
Acknowledgments
We thank the patients and their caregivers, the NYU counseling staff, Sean Page and Jane Winter in Manchester, and Angi Lesmina, Karen Berman, Lynne Seifman and Annette Altendorf in Sydney. We also thank Henrietta Wolland for developing and maintaining the study database. Lee-Fay Low commented on an earlier version of this paper.
Pfizer International provided an unrestricted grant to all three centers to undertake this investigator initiated study.
Sources of support: Pfizer International
Footnotes
No disclosures to report
References
- 1.Brodaty H, Green A. In: Family caregivers for people with dementia: Carer burden and stress, in Dementia. O’Brien J, Ames D, Burns A, editors. London: Chapman & Hall; 2000. [Google Scholar]
- 2.Brodaty H, McGilchrist C, Harris L, et al. Time until institutionalization and death in patients with dementia: Role of caregiver training and risk factors. Arch Neurol. 1993;50:643–650. doi: 10.1001/archneur.1993.00540060073021. [DOI] [PubMed] [Google Scholar]
- 3.Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc. 2003;51:657–664. doi: 10.1034/j.1600-0579.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 4.Pinquart M, Sorensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18:577–595. doi: 10.1017/S1041610206003462. [DOI] [PubMed] [Google Scholar]
- 5.Selwood A, Johnston K, Katona C, et al. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord. 2007;101:75–89. doi: 10.1016/j.jad.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 7.Brodaty H, Gresham M. Effect of a training programme to reduce stress in carers of patients with dementia. BMJ. 1989;299:1375–1379. doi: 10.1136/bmj.299.6712.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodaty H, Gresham M, Luscombe G. The Prince Henry Hospital Dementia Caregivers’ Training Programme. Int J Geriatr Psychiatr. 1997;12:183–192. doi: 10.1002/(sici)1099-1166(199702)12:2<183::aid-gps584>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 9.Marriott A, Donaldson C, Tarrier N, et al. Effectiveness of cognitive-behavioural family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psych. 2000;176:557–562. doi: 10.1192/bjp.176.6.557. [DOI] [PubMed] [Google Scholar]
- 10.Mittelman MS, Ferris SH, Shulman E, et al. A comprehensive support program: Effect on depression in spouse-caregivers of AD patients. Gerontologist. 1995;35:792–802. doi: 10.1093/geront/35.6.792. [DOI] [PubMed] [Google Scholar]
- 11.Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer disease. A randomized controlled trial. JAMA. 1996;276:1725–1731. [PubMed] [Google Scholar]
- 12.Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67:1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 13.Mittelman MS, Roth DL, Coon DW, et al. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. Am J Psychiatry. 2004;161:850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- 14.Mittelman MS, Roth DL, Haley WE, et al. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: results of a randomized trial. J Gerontol B Psychol Sci Soc Sci. 2004;59:P27–P34. doi: 10.1093/geronb/59.1.p27. [DOI] [PubMed] [Google Scholar]
- 15.Access Economics: The Dementia Epidemic: Economic Impact and Positive Solutions for Australia. Canberra: Access Economics; 2003. [Google Scholar]
- 16.McRae T, Knopman D, Duttagupta S, et al. Donepezil delays time to nursing home placement in patients with Alzheimer’s disease. The 14th meeting of the American Association of Geriatric Psychiatry; San Francisco, CA. 2001. [Google Scholar]
- 17.Geldmacher DS, Provenzano G, McRae T, et al. Donepezil is associated with delayed nursing home placement in patients with Alzheimer’s disease. J Am Geriatr Soc. 2003;51:937–944. doi: 10.1046/j.1365-2389.2003.51306.x. [DOI] [PubMed] [Google Scholar]
- 18.Fillit HM, Gutterman EM, Brooks RL. Impact of donepezil on caregiving burden for patients with Alzheimer’s disease. Int Psychogeriatr. 2000;12:389–401. doi: 10.1017/s1041610200006499. [DOI] [PubMed] [Google Scholar]
- 19.Feldman H, Gauthier S, Hecker J, et al. Efficacy of donepezil on maintenance of Activities of Daily Living in patients with moderate to severe Alzheimer’s disease and the effect on caregiver burden. J Am Geriatr Soc. 2003;51:737–744. doi: 10.1046/j.1365-2389.2003.51260.x. [DOI] [PubMed] [Google Scholar]
- 20.Lingler JH, Martire LM, Schulz R. Caregiver-specific outcomes in antidementia clinical drug trials: a systematic review and meta-analysis. J Am Geriatr Soc. 2005;53:983–990. doi: 10.1111/j.1532-5415.2005.53313.x. [DOI] [PubMed] [Google Scholar]
- 21.Gasper MS, Ott BR, Lapane KL. Is Donepezil Therapy associated with reduced mortality in nursing home residents with dementia? Am J Geriatr Pharmacother. 2005;3:1–7. doi: 10.1016/j.amjopharm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Ott BR, Lapane KL. Tacrine therapy is associated with reduced mortality in nursing home residents with dementia. J Am Geriatr Soc. 2002;50:35–40. doi: 10.1046/j.1532-5415.2002.50005.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopez OL, Becker JT, Wisniewski S, et al. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatr. 2002;72:310–314. doi: 10.1136/jnnp.72.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Pousa S, Olmo JG, Franch JV, et al. Comparative analysis of mortality in patients with Alzheimer’s disease treated with donepezil or galantamine. Age Aging. 2006;35:365–371. doi: 10.1093/ageing/afj083. [DOI] [PubMed] [Google Scholar]
- 25.Aguero-Torres H, Qiu C, Winblad B, et al. Dementing disorders in the elderly: Evolution of disease severity over 7 years. Alzheimer Dis Assoc Disord. 2002;16:221–227. doi: 10.1097/00002093-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Albert SM, Sano M, Marder K, et al. Participation in clinical trials and long-term outcomes in Alzheimer’s disease. Neurology. 1997;49:38–43. doi: 10.1212/wnl.49.1.38. [DOI] [PubMed] [Google Scholar]
- 27.Boersma F, van den Brink W, Deeg DJ, et al. Survival in a population-based cohort of dementia patients: Predictors and causes of mortality. Int J Geriatr Psychiatry. 1999;14:748–753. doi: 10.1002/(sici)1099-1166(199909)14:9<748::aid-gps3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 28.Carlson MC, Brandt J, Steele C, et al. Predictor index of mortality in dementia patients upon entry into long-term care. J Gerontol A Bioll Sci Med Sci. 2001;56A:M567–M570. doi: 10.1093/gerona/56.9.m567. [DOI] [PubMed] [Google Scholar]
- 29.Claus JJ, van Gool WA, Teunisse S, et al. Predicting survival in patients with early Alzheimer’s disease. Dem Geriatr Cog Disord. 1998;9:284–293. doi: 10.1159/000017073. [DOI] [PubMed] [Google Scholar]
- 30.Freels S, Nyenhuis DL, Gorelick PB. Predictors of survival in African American patients with AD, VaD, or stroke without dementia. Neurology. 2002;59:1146–1153. doi: 10.1212/wnl.59.8.1146. [DOI] [PubMed] [Google Scholar]
- 31.Guehne U, Riedel-Heller S, Angermeyer MC. Mortality in Dementia: A Systematic Review. Neuroepidemiology. 2005;25:153–162. doi: 10.1159/000086680. [DOI] [PubMed] [Google Scholar]
- 32.Larson EB, Shadlen MF, Wang L, et al. Survival after initial diagnosis of Alzheimer disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 33.Doody R, Massman P, Dunn K. A method of estimating progression rates in Alzheimer disease. Arch Neurol. 2001;58:449–454. doi: 10.1001/archneur.58.3.449. [DOI] [PubMed] [Google Scholar]
- 34.Mittelman MS, Brodaty H, Wallen AS, et al. A three-country randomized controlled trial of a psychosocial intervention for caregivers combined with pharmacological treatment for patients with Alzheimer disease: Effects on caregiver depression. Am J Geriatr Psychiatry. 2008;16:893–904. doi: 10.1097/JGP.0b013e3181898095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 37.Reisberg B, Ferris SH, de Leon MJ, et al. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatr. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 38.Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 39.Stokes JP. Predicting satisfaction with social support from social network structure. Am J Community Psychol. 1983;11:141–152. [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatr. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 42.Galasko D, Bennett D, Sano M, et al. An inventory to assess Activities of Daily Living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study . Alz Dis Assoc Disord. 1997;11:S33–S39. [PubMed] [Google Scholar]
- 43.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: the Revised Memory and Behavior Problems Checklist. Psychol Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 44.Center for the Study of Aging and Human Development Duke University. Multidemensional Functional Assessment: the OARS Methodology. Durham, NC: Duke University Press; 1975. [Google Scholar]
- 45.Devanand DP, Jacobs DM, Tang MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatr. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 46.Marin DB, Green CR, Schmeidler J, et al. Noncognitive disturbances in Alzheimer’s disease: frequency, longitudinal course, and relationship to cognitive symptoms. J Am Geriatr Soc. 1997;45:1331–1338. doi: 10.1111/j.1532-5415.1997.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 47.Australian Government Department of Health and Ageing. 5 Steps to Entry into Residential Aged Care. Canberra: Department of Health and Ageing; 2006. [Google Scholar]
- 48.NHFA Care Fees Advise. Long Term Care Guide. Oxford: NHFA Ltd; 2002. [Google Scholar]
- 49.National Institute on Aging. Nursing Homes: Making the Right Choice. Washington DC: U.S. Department of Health and Human Services; 2007. [Google Scholar]
- 50.Centers for Medicare and Medicaid services. Guide to Choosing a Nursing Home. Washington DC: U.S. Department of Health and Human Services; 2005. [Google Scholar]
- 51.Knapp M, Comas-Herrera A, Somani A, et al. Dementia: International comparisons. London: Personal Social Services Research Unit, London School of Economics and Political Science and the Institute of Psychiatry, King’s College; 2007. [Google Scholar]
- 52.Mittelman MS, Epstein C, Pierzchala A. Counseling the Alzheimer’s caregiver, A Resource for Health Care Professionals. Chicago, IL: AMA Press; 2003. [Google Scholar]
- 53.Kopetz S, Steele CD, Brandt J, et al. Characteristics and outcomes of dementia residents in an assisted living facility. J Gerontol B Psychol Sci Soc Sci. 2000;15:586–593. doi: 10.1002/1099-1166(200007)15:7<586::aid-gps148>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.McClendon MJ, Smyth KA, Neundorfer MM. Long-term-care placement and survival of persons with Alzheimer’s disease. J Gerontol B Psychol Sci Soc Sci. 2006;61:P220–P227. doi: 10.1093/geronb/61.4.p220. [DOI] [PubMed] [Google Scholar]
- 55.Stockdale R, Dening T. Mortality in patients with dementia following their discharge from hospital. Int J Geriatr Psychiatr. 2000;15:870–871. doi: 10.1002/1099-1166(200009)15:9<870::aid-gps233>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 56.Xie J, Brayne C, Matthews FE. Survival times in people with dementia: Analysis from population based cohort study with 14 year follow-up. BMJ. 2008;336:258–262. doi: 10.1136/bmj.39433.616678.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banerjee S, Murray J, Foley B, et al. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatr. 2003;74:1315–1316. doi: 10.1136/jnnp.74.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luppa M, Luck T, Brahler E, et al. Prediction of institutionalisation in dementia. A systematic review. Dem Geriatr Cogn Disord. 2008;26:65–78. doi: 10.1159/000144027. [DOI] [PubMed] [Google Scholar]
- 59.Gilley DW. Are behavioral and psychological symptoms of dementia associated with mortality in Alzheimer’s disease? Int Psychogeriatr. 2000;12:63–66. [Google Scholar]
- 60.Walsh JS, Welch HG, Larson EB. Survival of outpatients with Alzheimer-type dementia. Ann Intern Med. 1990;113:429–434. doi: 10.7326/0003-4819-113-6-429. [DOI] [PubMed] [Google Scholar]