Abstract
In an increasing number of cancers, tumor populations called cancer stem cells (CSCs) or tumor initiating cells have been defined in functional assays of self-renewal and tumor initiation. Moreover, recent work in several different cancers has suggested the CSC population as a source of chemo- and radiation-therapy resistance within tumors. Work in glioblastoma and breast cancers supports the idea that CSCs may possess innate resistance mechanisms against radiation- and chemotherapy-induced cancer cell death, allowing them to survive and initiate tumor recurrence. Several resistance mechanisms have been proposed, including amplified checkpoint activation and DNA damage repair as well as increased Wnt/β-Catenin and Notch signalling. Novel targeted therapies against the DNA damage checkpoint or stem cell maintenance pathways may sensitize CSCs to radiation or other therapies. Another important category of cancer therapies are anti-angiogenic and vascular targeting agents which are also becoming integrated in the treatment paradigm of an increasing number of cancers. Recent results from our laboratory and others support a role for CSCs in the angiogenic drive as well as the mechanism of anti-angiogenic agents. Identifying and targeting the molecular mechanisms responsible for CSC therapeutic resistance may improve the efficacy of current cancer therapies.
Introduction
Increasing evidence supports tumors as complex heterogeneous organ-like systems with a hierarchical cellular organization, rather than simply as collections of homogeneous tumor cells. Normal stem cells can replicate to populate an organ during normal organogenesis and tumors initiate when cells develop an unrestricted capacity for sustained proliferation. In both scenarios the initiating cell, whether a normal stem cell or a tumorigenic cell, retains the capacity to generate diverse progeny at various levels of differentiation, from uncommitted pluripotent stem cells to committed progenitor cells to fully differentiated senescent descendent cells. In this way, the tumor cell population itself is heterogeneous, adding to the heterogeneity provided by the immune, stromal and vascular cells that are also present in tumors. Some of the cells within the “aberrant” cancer organ1, the tumor, have the potential for continued proliferation, despite the frequent differentiated phenotype displayed by the majority of the tumor cells. The phylogeny of these tumor cells thus suggests the existence of a cell population that retains the ability to self-renew while also often possessing the capacity to generate progeny that differentiate. In other words, this leads us to hypothesize the existence of cancer stem cells (CSCs), alternately called tumor initiating cells and stem-like cancer cells, within tumors that are responsible for tumorigenesis as well as maintenance of the tumor bulk.
Many advanced cancers recur despite the use of chemotherapeutic and radiation modalities that initially lead to therapeutic responses. For example, irradiation of glioblastomas (GBMs) can lead to significant radiographic responses, yet these tumors invariably recur and lead to patient death. Frequently, glioblastomas recur in a nodular pattern suggesting a clonal or polyclonal source of recurrent tumor cells that are able to withstand conventional cytotoxic therapies, including radiation therapy, to cause recurrence of disease. Furthermore, recurrent tumors also demonstrate heterogeneity within the tumor cell population with regards to the presence of both CSCs and non-CSCs as well as in histological and cytogenetic differences2, suggesting that the CSCs that populated the original tumor may have withstood the treatments to repopulate the recurrent tumor even after the bulk of the tumor has been removed by resection or chemoradiation therapy3.
The idea of CSCs as being the source of post-therapeutic tumor recurrence is not a new one4. Indeed, scientists in the late nineteenth century proposed that a rare population of cells with stem-like properties may be the source of tumors5-7. As technologies improved, people began noticing that cancers contained cells that differed in their abilities to proliferate in colony formation assays8 and spleen repopulation assays9-11, suggesting that there may be sub-populations of cells with varied self-renewal capacity. The advances in technology through the 1980s and 1990s allowed for more efficient separation of cells based on cell marker phenotypes, leading to the prospective identification of normal hematopoietic stem cells in 198812. More recently, Bonnet and Dick13 validated the theoretical existence of tumorigenic stem cells in cancers with the identification of a population of primitive leukemic cells resembling hematopoietic stem cells that could give rise to acute myelogenous leukemia with multi-lineage differentiation in immunodeficient mice. Subsequently, improvements in the ability to prospectively isolate stem-like cells have generated evidence that a variety of solid tumors contain similar stem-like tumor cells. Though sometimes only present in very small numbers in human tumors, CSCs have the ability to generate tumors that recapitulate the original tumor when xenotransplanted into in animals, whereas the remaining non-CSC tumor bulk most often cannot4,14,15. The most substantiated CSC selection methods have been developed for leukemias13, central nervous system tumors including glioma16-20, and breast cancer21, but similar selection techniques appear to be applicable to other tumors, with accumulating evidence for existence of a CSC subpopulation in tumors of the colon22,23, pancreas24, prostate25, melanoma26, liver27 and head and neck28.
It is of no small concern that in a variety of tumors, CSCs seem to be particularly resistant to conventional chemo- and radiation therapies compared with the more differentiated cells in the non-CSC compartment. Furthermore, the CSCs seem to be particularly adept in stimulating angiogenesis to promote tumor growth and increase overall tumor aggressiveness both before and after therapy. In fact, there is an increasing body of evidence suggesting that radioresistance, chemotherapy resistance, and angiogenesis in these CSCs in humans could partially explain tumor recurrence in advanced or aggressive tumors treated with radiation.
Evidence for Radiation Resistance in Cancer Stem Cells
Radiation therapy remains the most effective non-surgical intervention for glioblastomas, though these tumors invariably recur after radiation therapy to result in patient death. Therefore, determination of the mechanisms of radioresistance in these tumors and others could lead to advances in the treatment of cancer. In our studies of radioresistance in glioblastomas29, we utilized short term cell cultures derived from primary human tumor specimens and xenografted tumors to investigate radiation responses in cell populations enriched for CSCs versus non-CSCs. This system allows us to bypass the many disadvantages involved in use of high-passage established cell lines, as serum-containing media induces differentiation. We showed that the population of cells enriched for glioma CSCs was dramatically increased by irradiation and that irradiated CSCs have survival advantages relative to the non-CSC population. CSCs are then able to give rise to tumors that have both CSCs and more differentiated non-CSCs. Radioresistant tumors displayed an increased percentage of CD133+ cells than the parent cell population. Furthermore, radiation had little effect on the ability of CSCs to regrow tumors.
We speculated that the CSC-enriched cell population might avoid radiation-induced cell death through activation of DNA damage repair mechanisms. Indeed, the non-CSCs had higher levels of apoptosis following irradiation relative to the CSC population. Radiation caused equal levels of damage to all cancer cells but CSCs repaired the damage more rapidly than non-stem cancer cells. Cancer cells, like all cells, respond to DNA damage through the activation of complex detection and repair mechanisms. The DNA damage and replication checkpoint includes ataxia telangiectasia mutated (ATM) and the checkpoint kinases, Chk1 and Chk2, that become activated upon genotoxic stress to initiate cell cycle arrest and attempted repair or apoptosis if the damage is too great. CSCs activate the DNA damage checkpoint more readily than matched non-stem cells. In fact, the CSCs display a basal activation of the checkpoint, indicating that they are primed to respond to genomic insults. Inhibition of the Chk1/2 kinases with a small molecule inhibitor disrupted the radioresistance of CSC-enriched cells in an in vitro colony formation assay and in in vivo tumor growth, suggesting that an intact Chk1/2 response is critical to the radioresistance of glioblastoma CSCs. Hence, this Chk1/2 response could develop into a worthwhile target in efforts to develop agents able to sensitize CSCs to radiation therapy (Figure 1a, b). Notably, the checkpoint proteins Chk1 and Chk2 and the rest of the DNA damage response cascade may contribute to tumor initiation, as activation of the DNA damage checkpoint occurs early in tumorigenesis30,31. However, it is probable that these CSCs employ more than one mechanism of cell survival after radiation, due to the multiple cellular changes caused by radiation, such as DNA damage and reactive oxygen species formation. Several studies using breast cancer cell lines have made efforts to examine other potential radioresistance mechanisms in CSC populations.
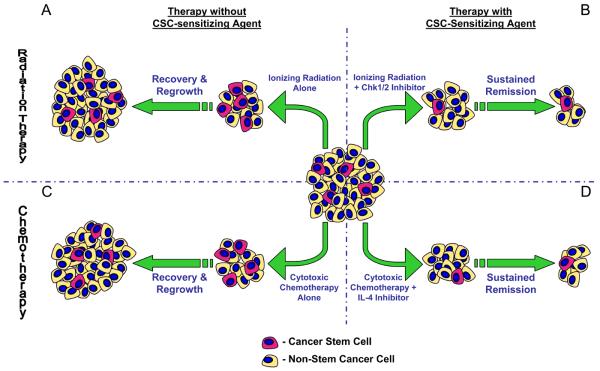
Figure 1.
CSC-sensitizing agents in radiation therapy and chemotherapy. Tumors contain both CSCs (pink) and non-stem cancer cells (yellow). CSCs may preferentially survive monotherapy with ionizing radiation (A) or cytotoxic chemotherapies (C), leading to tumor repopulation and disease recurrence. Targeting CSC-specific therapeutic resistance mechanisms like Chk1/2 activation (B) or IL-4 signalling (D) could sensitize tumors to these treatments.
The Wnt/β-catenin pathway has recently been implicated in the radiation resistance in mammary progenitor cells as well as cells expressing CSC markers in breast cancer cell lines. Woodward et al. showed in a murine mammary epithelial cell (MEC) culture that radiation treatment results in enrichment for the stem- and progenitor cell-containing side population, and particularly augments the stem cell antigen (Sca) positive compartment of the side population cells32. Wnt-induced mammary hyperplasias (from MMTV-driven Wnt-1 transgenic mice) show an increased side population relative to matched controls, and MECs from mice with a conditionally stabilized β-catenin allele showed a higher proportion of side population cells after radiation than matched controls. Interestingly, Sca+ side population cells, but not Sca- cells, had high levels of activated β-catenin by flow cytometry after irradiation. The same group also determined a role for the Wnt/β-catenin pathway in radioresistance of CSCs in an immortalized mammary gland cell line33. In this system, overexpression of β-catenin in the Sca+ cells enhanced self-renewal in a mammosphere formation assay and expression of a dominant negative β-engrailed decreased self-renewal. Intriguingly, these alterations affected the total levels of survivin, an anti-apoptotic protein that is upregulated in these cells after irradiation. No knockdown analysis of survivin was completed, so it is difficult to say that it is definitely the mediator of radioresistance in these Sca+ cells, but it is interesting as a subject for further study. These studies on Wnt/β-catenin signalling provide an insight as to another possible mechanism for CSC radioresistance, but await confirmatory animal and clinical studies.
Because radioresistance in CSCs may occur via concurrent but distinct mechanisms, these data regarding Wnt/β-catenin involvement in cell survival and self-renewal after irradiation correlate with the concept that CSCs have amplified DNA damage repair mechanisms through Chk1/2 activation, as shown by Bao et al29. Normal stem cells activate the Wnt/β-catenin signalling axis during development34, and several lines of research in non-CSC systems suggest that activation of the Wnt/β-catenin pathway promotes DNA damage tolerance. For example, Ku70 and PARP-1 compete with β-catenin for binding to the transcription T-cell factor 4 (Tcf-4), which is the downstream mediator for many of the effects caused by activation of the Wnt/β-catenin pathway35. When DNA is damaged, PARP-1 is modified to prevent its interaction with Tcf-4, thus allowing Ku70 to bind in a complex with β-catenin to activate the Wnt pathway cellular effects. Therefore, DNA damage may enhance β-catenin activity. In light of this, while possibly promoting the ability of CSCs to survive extensive DNA damage until lethal damage can be repaired, the Wnt/β-catenin pathway promotes genomic instability in colon cancer36 and may promote conversion of non-tumorigenic stem cells to glioma CSCs through the destabilization of the genome37. This signalling axis could play its role by allowing radiated cells to tolerate DNA damage, while the Chk1/2 kinases cause cell cycle arrest until lethal DNA damage can be repaired. Alternatively, these pathways could both promote genomic instability while allowing tumor cells to survive after irradiation, thus accelerating the rate of genetic change in the tumor.
Other pathways have also been implicated as playing roles in CSC radioresistance. Phillips et al.38 showed that CSC-enriched mammosphere cultures of established breast cancer cell lines showed decreased sensitivity to radiation in clonogenic assays relative to adherent cells from the same line, while the numbers of the CSCs in the culture increased in response to fractionated radiation. The levels of reactive oxygen species were reduced in the mammosphere cultures, indicating higher levels of radical scavengers in these CSC-enriched cultures. Interrogation of a possible role of the Notch signalling axis on this radioresistance revealed a modest induction of Jagged-1 expression on the surface of non-adherent CSC-enriched cells after fractionated radiation as well as increases in the levels of activated Notch-1 in the culture media of CSC-enriched cells, indicating that altered activity in the Notch pathway may partially explain the apparent radioresistance present in the CSC fraction. Though this study showed a correlation between the levels of Jagged and activated Notch-1 and radiation treatment, more in depth interrogation might reveal whether this pathway is either necessary or sufficient for CSC radioresistance. The Hedgehog-Gli1 pathway has been implicated in human glioma CSC self-renewal and tumorigenicity, so it is conceivable that this pathway could be involved in CSC-mediated tumor recurrence after radiation therapy39. In unfractionated glioma cultures, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors such as the multitargeted kinase inhibitor ZD6474 and AG1478 have been shown to block radiation and chemoradiation resistance, respectively, in the tumor bulk40,41 and a dominant negative form of EGFR can enhance radiosensitivity in glioma cell lines42. CSCs require EGF for maintenance in culture, so it is entirely possible that a pathway downstream of EGFR may contribute to CSC radioresistance. In fact, loss of the tumor suppressor PTEN, which has reduced activity in many tumors due to silencing or mutation and which functions to oppose EGFR-mediated signalling through the Akt kinase, has been shown in mouse embryonic stem cells to prevent cell cycle arrest in response to radiation by restricting Chk1 to the cytoplasm, ultimately leading to genetic instability43.
CSCs and Chemotherapy Resistance
Most cytotoxic therapies used for cancer therapy damage DNA or disrupt mitosis to induce cell death in highly proliferative tumor cells. The apparent resistance of CSCs to radiation-induced DNA damage toxicity suggests that perhaps CSCs may also play a role in mediating chemotherapy resistance in tumors. Indeed there have been several studies implicating CSCs as being chemoresistant in a variety of different cancers. Recently, it was reported that a subpopulation of pancreatic cancer cells functionally resemble stem cells and also have a strong resistance to gemcitabine both in vitro and in vivo44. Recent data suggests that preferential Akt activity may confer chemotherapy resistance to hepatocellular carcinoma CSCs45. One group found that CSCs from gliomas display marked resistance to several chemotherapeutic agents (temozolamide, carboplatin, VP16 and Taxol) relative to the non-CSC population46. Colon cancer stem cells, which were shown to have baseline resistance to cell death induced by 5-fluorouracil or oxaliplatin treatment, can be chemosensitized by an interleukin-4 blocking antibody, suggesting that autocrine stimulation of IL-4 receptors on CSCs may contribute to their chemoresistant phenotype47 and could be manipulated in efforts to sensitize CSCs to cytotoxic chemotherapies (Figure 1c, d).
Both normal stem cells and CSCs commonly express drug pumps such as ATP-binding cassette (ABC) transporters, including multidrug resistance transporter 1 (MDR1) and breast cancer resistance protein (BCRP). Leukemic side population cells, which are enriched for CSCs, have an amplified ability to pump chemotherapeutic drugs like daunorubicin and mitoxantrone out of the cell, suggesting that increased drug removal ability may contribute to the chemoresistance of cancer stem cells48, and stem-like neuroblastoma cells displayed a similar ability to pump mitoxantrone, resulting in increased cell survival49. Specifically, the ABC transporters BCRP and MDR1 have been implicated in specifically expelling chemotherapeutic agents from cells and thus may mediate chemoresistance when expressed by CSCs. MDR1 has been shown to remove vinblastine50 and paclitaxel51, while BCRP prevents accumulation of imatinib mesylate52, topotecan53 and methotrexate54.
In addition to possessing an increased capacity for drug efflux, CSCs also express molecular metabolic mediators like aldehyde dehydrogenase 1 (ALDH1) that have been shown to confer resistance to cyclophosphamide in normal stem cells55. ALDH1 activity is amplified in leukemic CSCs and thus may have implications for the resistance of these cells to chemotherapeutic agents such as cyclophosphamide56. ALDH1 is expressed by breast CSCs and is associated with a poor prognosis57 suggesting that chemotherapy resistance mechanisms expressed by CSCs may directly impact patient outcome. Furthermore, cellular sensitivity to chemotherapeutic agents relies upon cell cycle kinetics that will permit lethal cellular damage in highly proliferative cells. Normal stem cells cycle less frequently than the more differentiated transit-amplifying cells (thus, the designation of normal stem cells as label retaining). CSCs from acute and chronic myelogenous leukemias are relatively quiescent58,59, contributing to therapeutic resistance. Similar results have not been confirmed in CSCs derived from solid tumors. The rapid proliferation of solid tumor CSCs described in ex vivo assays is likely not representative of the in vivo proliferative index as CSCs are cultured with high levels of growth factors in most assays, but, it is probable that the least differentiated tumor populations mimic normal stem cells with a relatively slow rate of renewal contributing to the ability of these cells to resist chemotherapeutic agents that depend on specific cycles or on rate of cycle completion. Ultimately, the chemoresistance displayed by the CSCs in a variety of tumors as a result of increased drug efflux, metabolic alterations and cell cycle kinetics highlights the need for development of CSC radiation and chemotherapy sensitization techniques and compounds that will allow these resistant populations to be eradicated to prevent recurrence of disease.
CSCs and Angiogenesis
Clinical use of anti-angiogenic agents for neoplastic diseases has accelerated in recent years, with over 40 currently in clinical trials for various types of cancers60. Anti-angiogenic agents such as bevacizumab (Avastin) have shown promise as part of a combination therapy regimen in several advanced cancers, including colon cancer61 and glioblastoma62. Moreover, several agents that were originally developed as blocking EGFR (erlotinib, cetuximab, vandetanib) have recently been shown to have an inhibitory effect on angiogenesis by blocking the vascular endothelial growth factor (VEGF) receptor or by inhibiting pro-angiogenic protein secretion60. Thus, it seems as if the clinical success of several widely used and studied compounds may relate to inhibition of vascular growth in tumors. There are several theories regarding the clinical mechanism of anti-angiogenic drug benefit. One possibility is that anti-angiogenics simply destroy the vascular structure of the tumor, promoting profound tumor hypoxia and nutrient deprivation. Alternatively, it has been proposed that anti-angiogenics may transiently “normalize” the tumor vasculature, making it more efficient in delivering oxygen and drugs63. In addition, it appears as if some cancers may express VEGF receptors as well, raising the possibility that anti-VEGF therapies like bevacizumab actually have direct anti-tumor effects. Understanding the mechanism of anti-angiogenic agents will permit their optimal clinical use.
Interestingly, CSCs contribute to tumor angiogenesis. We have found that CSCs produce much higher levels of VEGF in both normoxic and hypoxic conditions than the non-CSC population, and this CSC-mediated VEGF production leads to amplified endothelial cell migration and tube formation in vitro64. When we supplemented these endothelial migration and tube-formation assays with the VEGF-blocking antibody bevacizumab, the in vitro endothelial cell behaviors were blocked. Moreover, in vivo administration of bevacizumab potently inhibited the growth, vascularity, and hemorrhage of xenografts derived from CSCs while no effects were seen on xenografts from non-CSCs. A VEGF-overexpression glioma model has recently provided supportive evidence for this as well by showing that glioblastoma CSCs overexpressing VEGF produce larger, more vascular and highly hemorrhagic tumors65.
It appears that while angiogenesis in tumors derives significantly from CSC-secreted VEGF, CSCs themselves depend on the presence of vascular niches. Calabrese et al.66 confirmed that CSCs generate VEGF and other factors to induce angiogenesis, but also showed that CSCs themselves are dependent on factors created by the vasculature itself (Figure 2a). In this way, CSCs mimic normal stem cells, which also seem to be dependent on vascular niches and factors secreted by the vasculature67,68. Factors like leukaemia inhibitory factor (LIF), brain derived neurotrophic factor (BDNF) and pigment epithelial derived factor (PEDF) have been implicated in normal stem cell maintenance67, so these factors may also regulate endothelium-derived CSC niche maintenance. Thus, CSCs and angiogenesis can positively feed-back on each other to promote tumor development and maintenance and represents an area of tumor biology that could be clinically manipulated to provide anti-tumor effects (Figure 2b).
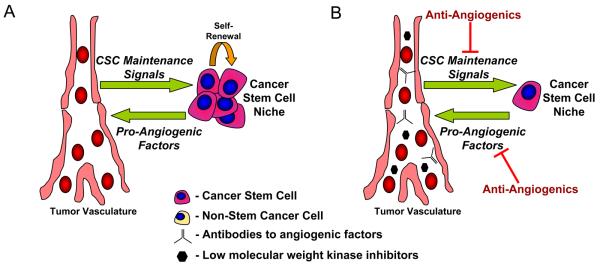
Figure 2.
Anti-angiogenic agents may target both tumor vasculature formation and CSC niche maintenance. (A) CSCs generate pro-angiogenic factors to stimulate angiogenesis while the tumor vasculature aids in maintaining CSC self-renewal and maintenance. (B) Anti-angiogenic agents like anti-VEGF therapies or low molecular weight kinase inhibitors disrupt angiogenesis and may also interrupt vascular-derived CSC maintenance cues.
The interplay between CSCs, angiogenesis and the tumor vasculature may well impact the efficacy of radiation. HIF-1 a transcription factor stabilized by hypoxic conditions, increases the production of VEGF in gliomas as well as a variety of other tumor types and has been suggested as a factor that regulates a variety of tumor radioresponses. It sensitizes tumor cells to radiation through induction of ATP metabolism, proliferation and p53 activation but it also allows endothelial cell survival69. These complex effects on radiation sensitivity have not yet been dissected, but we have noted that irradiated CSC-derived tumors are particularly vascular and hemorrhagic29, indicating that hypoxia-mediated endothelial cell survival after radiation may contribute to the angiogenesis and tumor growth noted in post-radiation tumors. Furthermore, CSCs may be enriched by hypoxic conditions70, thus stabilizing HIF in these cells. These observations suggest that HIF-mediated radioresistance in tumors may be intimately related to the often hypoxic CSCs, and that targeting the CSCs or their vascular niche may have a CSC radiosensitizing effect in addition to simply preventing the development of vascular structures that supply the tumor bulk. In fact, recent clinical studies have showed enhanced anti-tumor cell effects when anti-angiogenic therapy is combined with radiation71-73. Given the evidence for CSC dependence on tumor vasculature, combining radiation therapy with anti-angiogenic therapies has promise in possibly mediating targeted anti-CSC effects to promote prolonged recurrence-free survival.
Clinical Applications of CSC Therapeutic Resistance and Angiogenesis
Despite the recent advances in basic science research in the CSC field on the subject of chemotherapy and radiotherapy resistance, the fact remains that clinicians continue to face the challenge of recurrent or metastatic cancer despite maximal therapy. As molecular mediators of therapeutic resistance in CSCs are established, developing clinically useful inhibitors to target these pathways should be prioritized. It seems reasonable that combining radiation therapy with an agent that radiosensitizes CSCs, an agent that targets tumor angiogenesis and an agent that can debulk the mass of the tumor, would be a good approach to rationally advancing the treatment of solid tumors. For example, use of a radiosensitizing Chk1/2 inhibitor with the anti-angiogenic therapy bevacizumab and the cytotoxic drug temozolamide could amplify responses in tumors that will be irradiated. Local delivery of therapeutics to post-resection residual tumor cells through implantation of drug-eluting wafers similar to the Gliadel wafers used in glioblastoma resection cavities could be helpful in targeting the radiosensitization and cytotoxic agents to tumors for which drug delivery is a barrier, such as brain tumors. The potential for targeting CSC populations to prevent recurrence after anti-tumor therapy as part of a personalized medicine approach is also very promising, as elimination of the tumor bulk is critical during treatment and this aspect of therapy could be guided very powerfully by the molecular profile of the overall tumor.
Finally, a word should be said about developing anti-CSC therapies that have minimal or no effect on normal stem cells. Though stem cells in non-hematopoietic tissues still have poorly defined roles, they could potentially be critical for mediating tissue responses to injury. Development of targeted anti-CSC therapies should take this into account and should aim to affect molecules and pathways that are not crucial for normal stem cell maintenance. The existence of such a therapeutic window has been suggested by one recent study of normal hematopoietic stem cells and leukemic CSCs with deletions in the tumor suppressor Pten74. The authors demonstrate that while the CSCs and the resultant leukemias are effectively treated by rapamycin treatment, the proliferation of non-cancerous Pten−/− hematopoietic stem cells is maintained. This indicates the differential sensitivity of normal and cancer stem cells and suggests strongly that therapies targeting the CSCs without affecting normal stem cells is possible. Though still in its infancy, it seems likely that the field of CSC therapeutic resistance could lead to the development of unique targeted agents that may be able to sensitize these cells to chemotherapy and radiation therapy in order to improve cancer care.
Acknowledgments
Financial support was provided by the Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure, Alexander and Margaret Stewart Trust, Brain Tumor Society, Goldhirsh Foundation, Duke Comprehensive Cancer Center Stem Cell Initiative Grant (J.R.), NIH grants NS047409, NS054276, and CA116659 (J.R.). J.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation and a Sidney Kimmel Foundation for Cancer Research Scholar. C.E. receives Medical Scientist Training Program support from the National Institute of General Medical Sciences grant 2T32GM007171.
REFERENCES
- 1.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Scheck AC, Shapiro JR, Coons SW, et al. Biological and molecular analysis of a low-grade recurrence of a glioblastoma multiforme. Clin Cancer Res. 1996;2:187–199. [PubMed] [Google Scholar]
- 3.Abbott A. Cancer: the root of the problem. Nature. 2006:442. doi: 10.1038/442742a. [DOI] [PubMed] [Google Scholar]
- 4.Wicha MS, Liu S, Dontu G. Cancer stem cells: an old idea - a paradigm shift. Cancer Res. 2006;66:1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 5.Cohnheim J. Ueber entzundung und eiterung. Path Anat Physiol Klin Med. 1867:40. [Google Scholar]
- 6.Cohnheim J. Congenitales, quergestreiftes Muskelsarkon der Nireren. Virchows Arch. 1875;65:64. [Google Scholar]
- 7.Durante F. Nesso fisio-pathologico tra la struttura dei mei materni e la genesi di aleuni tumori maligni. Arch Memor Observ Chir Pract. 1874:11. [Google Scholar]
- 8.Park CH, Bergsagel DE, McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- 9.Bruce WR, Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 10.Bergsagel DE, Valeriote FA. Growth characteristics of a mouse plasma cell tumor. Cancer Res. 1968;28:2187–2196. [PubMed] [Google Scholar]
- 11.Wodinsky I, Swiniarski J, Kensler CJ. Spleen colony studies of leukemia L1210. I. Growth kinetics of lymphocytic L1210 cells in vivo as determined by spleen colony assay. Cancer Chemother Rep. 1967;51:415–421. [Google Scholar]
- 12.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 14.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 15.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells - perspectives on current status and future directions: AASCR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 16.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003:63. [PubMed] [Google Scholar]
- 17.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 18.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. P Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 20.Ignatova TN, Kukekov VG, Laywell ED, et al. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. P Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 23.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 25.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 26.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 27.Ma S, Chan KW, Hu L, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 28.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. P Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 30.Gorgoulis VG, Vassiliou L-VF, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 31.Bartkova J, Horejsi Z, Koed K, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 32.Woodward WA, Chen MS, Behbod F, et al. Wnt/b-catenin mediates radiation resistance of mouse mammary progenitor cells. P Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MS, Woodward WA, Behbod F, et al. Wnt/b-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- 34.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 35.Idogawa M, Masutani M, Shitashige M, et al. Ku70 and poly(ADP-ribose) polymerase-1 competitively regulate b-catenin and T-cell factor-4-mediated gene transactivation: possible linkage of DNA damage recognition and Wnt signaling. Cancer Res. 2007;67:911–918. doi: 10.1158/0008-5472.CAN-06-2360. [DOI] [PubMed] [Google Scholar]
- 36.Hadjihannas MV, Bruckner M, Jerchow B, et al. Aberrent Wnt/b-catenin signaling can induce chromosomal instability in colon cancer. P Natl Acad Sci USA. 2006;103:10747–10752. doi: 10.1073/pnas.0604206103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiras A, Chettiar S, Shepal V, et al. Spontaneous transformation of human adult non-tumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells. 2007;25:1478–1489. doi: 10.1634/stemcells.2006-0585. [DOI] [PubMed] [Google Scholar]
- 38.Phillips TM, McBride WH, Pajonk F. The response of CD24 -/low / CD44+ breast cancer-initiating cells to radiation. J Natl Cancer I. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 39.Clement V, Sanchez P, Tribolet Nd, et al. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007:17. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damiano V, Melisi D, Bianco C, et al. Cooperative anti-tumor effect of multitargeted kinase inhibitor ZD6474 and ionizing radiation in glioblastoma. Clin Cancer Res. 2005;11:5639–5644. doi: 10.1158/1078-0432.CCR-05-0174. [DOI] [PubMed] [Google Scholar]
- 41.Chakravarti A, Chakladar A, Delaney MA, et al. The epidermal growth factor receptor pathway mediates resistance to sequential administration of radiation and chemotherapy in primary human glioblastoma cells in a RAS-dependent manner. Cancer Res. 2002;62:4307–4315. [PubMed] [Google Scholar]
- 42.Lammering G, Hewit TH, Holmes M, et al. Inhibition of the type III epidermal growth factor receptor variant mutant receptor by dominant-negative EGFR-CD533 enhances malignant glioma cell radiosensitivity. Clin Cancer Res. 2004;10:6732–6743. doi: 10.1158/1078-0432.CCR-04-0393. [DOI] [PubMed] [Google Scholar]
- 43.Puc J, Keniry M, Li HS, et al. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell. 2005:7. doi: 10.1016/j.ccr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Hermann PC, Huber SL, Herrier T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cancer Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Ma S, Lee TK, Zheng B-J, et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2007:1–10. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 46.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006:5. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todaro M, Alea MP, DiStefano AB, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Wulf GG, Wang R-Y, Kuehnle I, et al. A leukemic stem cell with intrinsic drug efflux capacity in acute myeloid leukemia. Blood. 2001;98:1166–1173. doi: 10.1182/blood.v98.4.1166. [DOI] [PubMed] [Google Scholar]
- 49.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. P Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang FP, Wang L, Yang JS, et al. Reversal of P-glycoprotein-dependent resistance to vinblastine by newly synthesized bisbenzylisoquinoline alkaloids in mouse leukemia P388 cells. Biol Pharm Bull. 2005;28:1979–1982. doi: 10.1248/bpb.28.1979. [DOI] [PubMed] [Google Scholar]
- 51.Green H, Soderkvist P, Rosenberg P, et al. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12:854–859. doi: 10.1158/1078-0432.CCR-05-0950. [DOI] [PubMed] [Google Scholar]
- 52.Berger H, vanTol H, Boersma AW, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 53.Sparreboom A, Loos WJ, Burger H, et al. Effect of ABCG2 genotype on the oral bioavailablity of topotecan. Cancer Biol Ther. 2005;4:650–658. doi: 10.4161/cbt.4.6.1731. [DOI] [PubMed] [Google Scholar]
- 54.Chen ZS, Robey RW, Belinsky MG, et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta-estradiol 17 -(beta-D-glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res. 2003;63:4048–4054. [PubMed] [Google Scholar]
- 55.Magni M, Shammah S, Schiro R, et al. Induction of cyclophosphamide-resistance by aldehyde-dehydrogenase gene transfer. Blood. 1996;87:1097–1103. [PubMed] [Google Scholar]
- 56.Pearce DJ, Taussig D, Simpson C, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23:752–760. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 57.Ginestier C, Hur MH, Charafe-Jauffret ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holyoake T, Jiang X, Eaves C, et al. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 59.Guan Y, Hogge DE. Proliferative status of primitive hematopoietic progenitors from patients with acute myelogenous leukemia (AML) Leukemia. 2000;14:2135–2141. doi: 10.1038/sj.leu.2401975. [DOI] [PubMed] [Google Scholar]
- 60.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 61.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 62.Vredenburgh JJ, Desjardins A, Herndon JEI, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1259–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 63.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 64.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 65.Oka N, Soeda A, Inagaki A, et al. VEGF promotes tumorigenesis and angiogenesis of human glioblastoma stem cells. Biochem Bioph Res Co. 2007;360:553–559. doi: 10.1016/j.bbrc.2007.06.094. [DOI] [PubMed] [Google Scholar]
- 66.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 67.Riquelme PA, Drapeau E, Doetsch F. Brain micro-ecologies: neural stem cell niches in the adult mammalian brain. Phil Trans R Soc B. 2007:1–15. doi: 10.1098/rstb.2006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nat Rev Genet. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- 69.Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Blazer ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133− cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 71.Lee CG, Heijn M, Tomaso Ed, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 72.Hess C, Vuong V, Hegyi I, et al. Effect of VEGF receptor inhibitor PTK787/ZK222584 [correction of ZK222548] combined with ionizing radiation on endothelial cells and tumour growth. Br J Cancer. 2001;85:2010–2016. doi: 10.1054/bjoc.2001.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li J, Huang S, Armstrong EA, et al. Angiogenesis and radiation response modulation after vascular endothelial growth factor receptor-2 (VEGFR2) blockade. Int J Radiat Oncol Biol Phys. 2005;62:1477–1485. doi: 10.1016/j.ijrobp.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 74.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]