Abstract
Malignant gliomas are extremely lethal cancers that display striking cellular heterogeneity. A highly tumorigenic glioma tumor subpopulation, termed cancer stem cells or tumor-initiating cells, promotes therapeutic resistance and tumor angiogenesis. Therefore, targeting cancer stem cells may improve patient survival. We interrogated the role of a neuronal cell adhesion molecule, L1CAM, in glioma stem cells as L1CAM regulates brain development and is expressed in gliomas. L1CAM+ and CD133+ cells co-segregated in gliomas, and levels of L1CAM were higher in CD133+ glioma cells than normal neural progenitors. Targeting L1CAM using lentiviral-mediated short hairpin RNA (shRNA) interference in CD133+ glioma cells potently disrupted neurosphere formation, induced apoptosis, and inhibited growth specifically in glioma stem cells. We identified a novel mechanism for L1CAM regulation of cell survival as L1CAM knockdown decreased expression of the basic helix-loop-helix transcription factor Olig2 and up-regulated the p21WAF1/CIP1 tumor suppressor in CD133+ glioma cells. To determine if targeting L1CAM was sufficient to reduce glioma stem cell tumor growth in vivo, we targeted L1CAM in glioma cells prior to injection into immunocompromised mice or directly in established tumors. In each glioma xenograft model, shRNA targeting of L1CAM expression in vivo suppressed tumor growth and increased the survival of tumor-bearing animals. Together, these data demonstrate that L1CAM is required for maintaining the growth and survival of CD133+ glioma cells both in vitro and in vivo, and L1CAM may represent a cancer stem cell specific therapeutic target for improving the treatment of malignant gliomas and other brain tumors.
INTRODUCTION
The cancer stem cell hypothesis posits that brain tumors contain a subset of neoplastic cells that propagate and maintain tumors through sustained self-renewal and potent tumorigenecity (1-6). Cancer stem cells can be enriched from many human brain tumor biopsy specimens using the cell surface marker CD133 (Prominin 1) although there are tumors that may contain CD133− tumor initiating cells (6, 7), albeit often with lower tumor potency. We previously demonstrated CD133+ glioma cells derived from human tumors are radioresistant (5), promote angiogenesis (6), and display greater tumorigenic potential in immunocompromised mice relative to CD133− glioma cells (5). Thus, targeting CD133+ glioma cell survival and pro-tumorigenic behaviors by identifying novel molecular targets specific in cancer stem cells may improve patient outcome.
Molecular targets which are secreted or located on the cell surface are particularly enticing as antibody and small-molecule inhibitor therapies that do not have to cross the cell membrane can be developed for clinical use. To select a potential target, we consulted the literature to select a cell surface protein that has been reported to be expressed in malignant gliomas and contribute to tumor malignancy. Based on these criteria, the neural cell adhesion molecule, L1CAM (L1, CD171) has been identified as a potential therapeutic target in Neuro-oncology. L1CAM regulates neural cell growth, survival, migration, and axonal outgrowth and neurite extension during central nervous system development (8). Although the role of L1CAM in the normal adult nervous system is not well defined, L1CAM is overexpressed in gliomas and other solid cancers (9-15) including colorectal cancer where L1CAM is a prognostic indicator (15). Although L1CAM has many potential biological effects which can contribute to tumor formation and maintenance, recent studies suggest that overexpression of L1CAM in cancer can protect cells from apoptosis induced by nutrient deprivation (16) or chemotherapeutics (17). As glioma stem cells display a chemoresistant phenotype, we sought to determine if L1CAM mediates survival and tumorigenic potential of CD133+ glioma cell subpopulation.
MATERIALS AND METHODS
Cell Isolation and Culture
Matched glioma cell populations enriched or depleted in cancer stem cells were isolated and cultured as previously described (5, 6) from human glioma surgical specimens (designated Txxx, Supplementary Table 1) or from the D456MG pediatric glioblastoma xenograft. Briefly, tumors were immediately dissected with removal of gross necrosis, washed in Earle's balanced salt solution, subjected to a Papain digestion followed by tituration, filtering, and lysis of red blood cells in phosphate buffered saline:water (1:3) solution, and then cultured overnight (∼12 hours) in neurobasal media (with B27 and epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) at 20 ng/ml) for recovery of cellular surface antigens before cell sorting. Primary glioma tumor samples were obtained from patients undergoing resection in accordance with a protocol approved by the Duke University Medical Center Institutional Review Board. Normal human fetal neural progenitor cells were purchased from Lonza. To control for differences in media conditions, all cells were cultured in stem cell media for experiments.
Fluorescent-activated Cell Sorting (FACS) Analysis
Since Papain digestion may cause loss of some cellular surface antigens such as CD133, it is critical to allow the isolated total tumor cells to re-express their surface antigens in the neurobasal media overnight (12 hours) before sorting for CD133+ glioma cells. Tumor cell cultures were subjected to FACS analysis and cell sorting after the antigen recovery period. Human specific anti-CD133 (293C3) conjugated to allophycocyanin (APC) or anti-CD133 (293C3) conjugated to phycoerythrin (PE) (Milyteni) was used for cell sorting and FACS analysis as previously described (5, 6). Anti-L1CAM-PE used for the FACS was generated using the Lightning-Link PE kit (Innova Biosciences) in combination with a GeneTex monoclonal antibody against human L1CAM (UJ127 clone).
shRNA L1CAM Targeting
Knockdown of L1CAM was achieved through the use of lentiviral vector-mediated short hairpin RNA (shRNA) interference using Mission RNAi system clones (Sigma-Aldrich). L1CAM-targeting lentivirus and the non-targeting control lentivirus were produced in HEK293FT cells with the ViraPower Lentiviral Expression System (Invitrogen). Five different shRNA clones were characterized in terms of knockdown efficiency using L1CAM immunoblotting of glioma stem cells infected with lentiviruses encoding L1CAM shRNA or a non-targeting control shRNA. The most efficient clone was utilized for all further experiments. Produced lentiviruses were concentrated by using Centricon Plus-20 centrifugal filter device (Millipore). To ensure the same number of L1CAM-targeting and the control lentiviruses were used in the same experiment, produced lentiviral stock was titered and stored according to the manufacturer's instruction (Invitrogen). For in vitro infection of glioma stem cells with the lentivirus, cultured neurospheres were disaggregated prior to infection in order to increase the infection efficiency and unifromity.
Antibodies and Western blotting
Specific monoclonal antibodies for human L1CAM (UJ127) were purchased from GeneTex and NeoMarker. L1CAM-PE used for FACS analysis was generated using the Lightning-Link PE kit (Innova Biosciences). Anti-Olig2 goat IgG was obtained from R & D Systems. Anti-OCT4 monoclonal antibody (MAB4401) was purchased from Millipore. Anti-p21 and anti-p27 antibodies were obtained from Cell Signaling. Immunoblotting was performed as previously described (5).
In vivo Tumor Formation Assays
Intracranial or subcutaneous transplantation of glioma stem cells into nude mice was performed as described (5, 6). Glioma stem cells were infected with lentivirus expressing non-targeting shRNA or L1CAM-targeting shRNA for 24 hours, and then 105 cells/mouse of viable brain tumor stem cells transduced with control lentivirus or the L1CAM-targeting lentivirus were transplanted into athymic BalbC nu/nu mice through intracranial or subcutaneous injection. To establish glioma xenografts for lentiviral-mediated L1CAM shRNA treatment, glioma stem cells were injected into the athymic nude mice (105 cells/mouse, 10 mice/group) first, and the same number of non-tageting lentivirus or L1CAM-targeting lentivirus were delivered to tumor sites through intratumoral injection once every two days.
Neurosphere Formation Efficiency
To determine the effect of knockdown of L1CAM on the ability of glioma stem cells to form neurospheres, disaggregated cells were infected with non-targeting or L1CAM-targeting lentivirus for 48 hours and then plated into 96 well plates at a density of one cell per well via a flow cytometry cell sorter (FACSAria, BD Bioscience) or through serial dilution. The percentage of wells with neurospheres was determined on day 10 as shown.
Immunofluorescent Staining
CD133+ and CD133− glioma cells were fixed with 4% paraformaldehyde, washed with Tris-buffered saline, incubated with anti-L1CAM monoclonal antibody (UJ127, GeneTex) and FITC-conjugated donkey anti-mouse IgG secondary antibody. Cells were co-stained with DAPI and mounted with the anti-fade medium. Stained cells were examined under a fluorescent microscope (Zeiss Axiovert 200).
Annexin V-FITC Staining
Annexin V-FITC staining for detecting apoptosis in CD133+ and CD133− glioma cells and normal neural progenitor cells before and after L1CAM targeting for 48 hours was performed with the Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen) according to the manufacturer's instructions. The cells stained with Annexin V-FITC were analyzed by FACS, or fixed and co-stained with DAPI, and then examined under a fluorescent microscope (Zeiss Axiovert 200).
Real Time PCR
To examine the transcriptional regulation of L1CAM on Olig2 and p21WAF1/CIP1 in CD133+ cells, CD133+ glioma cells were treated with non-targeting lentivirus or L1CAM-targeting lentivirus for 48 hours, and RNA samples were isolated these cells using RNeasy Kit (Qiagen), and then used for Real time PCR analysis with pairs of specific primers for Olig2, p21WAF1/CIP1, L1CAM and GAPDH (internal PCR control).
Statistical Analyses
Descriptive statistics and significance were determined as described (5, 6).
RESULTS AND DISCUSSION
L1CAM is differentially overexpressed in CD133+ glioma cells
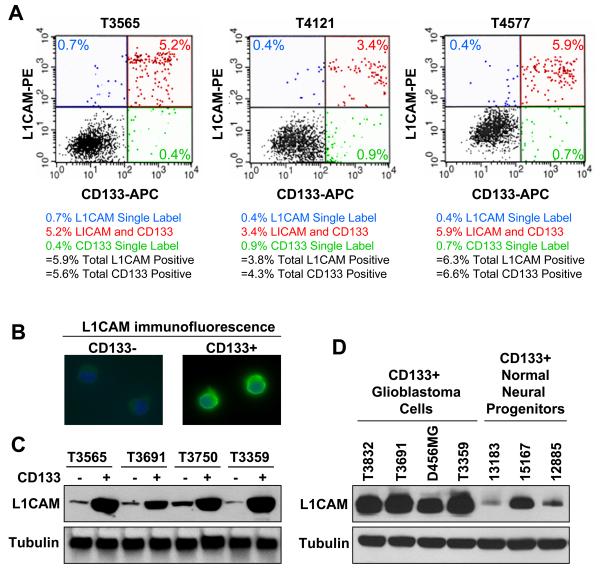
We examined L1CAM expression in CD133+ glioma cells derived from 12 human primary tumors (Supplementary Table 1) and one xenograft through complementary techniques. Using flow cytometry we determined that the majority of CD133+ cells were doubly positive for L1CAM+ when isolated directly from human patient specimens or passaged in short term cell culture (Fig. 1A; Supplementary Fig. S1A). In striking contrast, the vast majority of CD133− cells were L1CAM negative (>99% in T3565, T4121, and T4577; Fig. 1A; Supplementary Fig. S1B). In addition, the total percentage of CD133+ and L1CAM+ cells in patient glioma specimens were very similar (Fig. 1A; Supplementary Fig. S1B). L1CAM expression on the surface of CD133+ cells, but not CD133− cells, was further verified using immunofluorescence (Fig. 1B), and Western analysis confirmed that L1CAM protein was expressed at levels 18-42 fold higher in CD133+ cells from glioma patient specimens relative to matched CD133− tumor cells (Fig. 1C). In addition, CD133+ glioblastoma cells expressed L1CAM at significantly higher levels than normal neural progenitor cells derived from human fetal tissue that were enriched for CD133+ cells (Fig. 1D). Together, these data suggest that L1CAM is a surface glycoprotein specifically expressed by glioma stem cells.
Figure 1.
L1CAM is highly expressed on the surface of CD133+ glioma cells. A, Flow cytometry demonstrated most CD133+ glioma cells isolated from surgical biopsy specimens were also L1CAM+. Total population fractions of CD133+ cells and L1CAM+ cells were very similar. Unsorted tumor cells from primary brain tumor samples were labeled with anti-CD133-APC and anti-L1CAM-PE, and then subjected to FACS analysis to determine CD133+ and L1CAM+ subpopulations. The total fractions of CD133+ cells and L1CAM+ cells in unsorted tumor cells were very similar in each case and there was significant overlap between the populations. B, Immunofluorescent staining with L1CAM antibody demonstrated that L1CAM was overexpressed on the cell surface of CD133+ glioma stem cells in comparison to matched CD133− cells. C, CD133+ cells from glioma surgical biopsy specimens including the anaplastic astrocytoma T3565 and the glioblastomas T3691, T3750, and T3359 expressed L1CAM protein at higher levels than matched CD133− cells by Western blot analysis. D, CD133+ glioma cells from the glioblastoma patient tumor specimens T3832, T3691, and T3359 as well as the pediatric glioblastoma xenograft D456MG expressed L1CAM protein at higher levels than CD133+ normal neural progenitor cells (designated by lot number).
Targeting L1CAM decreases growth and survival of CD133+ glioma cells
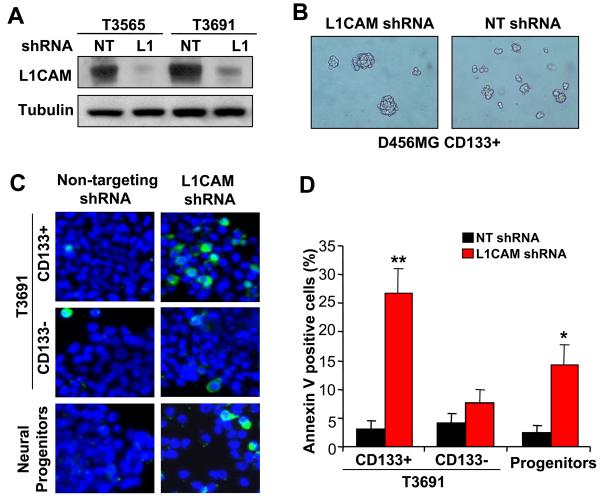
To assess the functional significance of the relative overexpression of L1CAM in CD133+ glioma cells as compared to CD133− glioma cells and normal neural progenitors, we targeted L1CAM expression using lentivirus expressing shRNA directed against L1CAM causing a 90% reduction of L1CAM expression relative to non-targeting control shRNA (Fig. 2A). As neurosphere formation is a key behavior of neural stem cells and brain tumor stem cells (1-6, 18) and is used as a measure of stem cell capacity for self-renewal, we determined neurosphere formation competence in cells with knockdown of L1CAM. Targeting L1CAM expression markedly decreased the ability of CD133+ glioblastoma cells to form neurospheres as indicated by the reduction in neurosphere formation efficiency (Fig. 2B, Supplementary Fig. S2A, B) and the size of the neurospheres formed (Fig. 2B). In addition, L1CAM knockdown resulted in significant growth inhibition in CD133+ glioblastoma cells, only modest attenuation in normal neural progenitors, and no effect on CD133− glioma cells (Supplementary Fig. S3). To determine if the reduction in neurosphere formation and growth of CD133+ glioma cells with L1CAM knockdown was due to decreased cell survival, we determined the percentage of apoptotic cells using Annexin V staining. Similar to the effects of L1CAM directed shRNA on cell growth, L1CAM shRNA significantly increased the percentage of Annexin V positive cells in CD133+ glioblastoma cells when compared to the effects of a non-targeting shRNA control (Fig. 2C, Supplementary Fig. S2C). In contrast, L1CAM knockdown had essentially no significant effect on apoptosis in CD133− glioblastoma cells (Fig. 2C, Supplementary Fig. S2C) and a moderate induction of apoptosis in normal neural progenitor cells (Fig. 2C, D) which was significantly less than that induced in CD133+ glioma cells. These data support a significant role for L1CAM in maintaining the growth of CD133+ glioblastoma cells and suggest that targeting L1CAM decreases CD133+ glioma self-renewal due to decreased survival.
Figure 2.
Knockdown of L1CAM in CD133+ glioma cells reduces self-renewal due to increased apoptosis. A, Knockdown of L1CAM expression in CD133+ cells isolated from the primary anaplastic astrocytoma T3565 and glioblastoma T3691 patient specimens was performed using L1CAM lentiviral shRNA (L1) without effects by non-targeting control shRNA (NT) as determined by Western blotting. B, Targeting L1CAM expression disrupted neurosphere formation of CD133+ brain tumor cells derived from a T3691 glioblastoma patient specimen or a D456MG pediatric glioblastoma xenograft. CD133+ cells infected with lentivirus expressing non-targeting shRNA (NT) formed neurospheres, whereas infection with lentivirus expressing L1CAM shRNA attenuated neurosphere formation. C, Representative images of CD133+ and CD133− brain tumor cells isolated from the primary glioblastoma T3691 and normal neural progenitors stained with Annexin V-FITC (green) and DAPI (blue) after treatment with lentivirus expressing non-targeting shRNA or L1CAM shRNA are shown. D, FACS analysis of apoptosis with Annexin V-FITC staining in CD133+ and CD133− brain tumor cells isolated from the primary glioblastoma T3691 and normal neural stem cells confirmed that CD133+ brain tumor cells have a greater dependence on L1CAM for cell survival. *, p < 0.01; **, p < 0.001 with comparison to non-targeting shRNA.
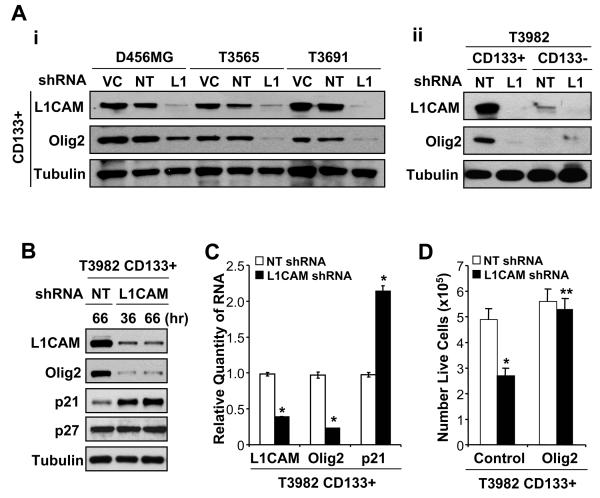
Reduction of L1CAM protein induces down-regulation of Olig2 and up-regulation of p21WAF1/CIP1
To delineate the molecular mechanisms through which L1CAM regulates CD133+ glioma cell survival, we interrogated intracellular signaling pathways in L1CAM knockdown cells with a focus on regulators of neural stem cell maintenance. As the neural stem cell transcription factor Olig2 was recently found to regulate neurosphere formation in vitro and glioma formation in vivo (19), we determined Olig2 expression in CD133+ glioma cells infected with lentivirus expressing no shRNA as a vector control, non-targeting control shRNA, or L1CAM shRNA. L1CAM knockdown reduced Olig2 protein expression in CD133+ glioma cells derived from primary glioma surgical specimens and a glioblastoma xenograft (Fig. 3A-i, B). The suppression of Olig2 induced by L1CAM knockdown is expected to have specific effects on CD133+ glioblastoma cells, as mimimal Olig2 was detected in matched CD133− glioma cells (Fig. 3A-ii). In contrast, L1CAM reduction did not alter the protein levels of other stem cell transcription factors such as Oct4 in CD133+ glioma cells (data not shown). As Olig2 may mediate its growth effects by suppressing expression of the cyclin-dependent kinase inhibitor p21WAF1/CIP1 (19), we further determined the effect of L1CAM shRNA on p21WAF1/CIP1 expression. Knockdown of L1CAM in CD133+ glioblastoma cells led to a specific up-regulation of p21WAF1/CIP1, but not p27KIP1, protein (Fig. 3B). Real time PCR further confirmed that L1CAM knockdown was associated with Olig2 down-regulation and p21WAF1/CIP1 up-regulation at the RNA level (Fig. 3C). These data suggest that the poor growth and survival of CD133+ glioma cells with L1CAM knockdown results, at least in part, from the loss of Olig2 expression and resulting increase in p21WAF1/CIP1. This model was further supported by data demonstrating that Olig2 overexpression rescued CD133+ glioblastoma cells from L1CAM shRNA induced reductions in cell growth (Fig. 3D). Thus, our data provide the first evidence that L1CAM can mediate cancer stem cell self-renewal and survival by regulating Olig2 expression with associated changes in the downstream effector, p21WAF1/CIP1.
Figure 3.
L1CAM regulates Olig2 and p21WAF1/CIP1 expression to maintain CD133+ glioma cell growth and survival. A, Reduction of L1CAM down-regulated expression of Olig2 transcription factor specific in glioma stem cells (CD133+). i, L1CAM knockdown with lentiviral mediated shRNA reduced Olig2 expression in CD133+ glioma cells isolated from the anaplastic astrocytoma T3565 and glioblastoma T3691 surgical specimens as well as the pediatric glioblastoma xenograft D456MG. Whole cell lysates from CD133+ cells infected with lentivirus expressing no shRNA (VC), non-targeting shRNA (NT) or L1CAM-targeting shRNA (L1) for 48 hours were resolved by SDS-PAGE and immunoblotted for L1CAM, Olig2, and tubulin as a loading control. ii, Knockdown of L1CAM dramatically and specifically down-regulated Olig2 protein in CD133+ glioma cells isolated from the glioblastoma patient specimen T3982. Olig2 was not detectable in CD133− cells before or after treatment with L1CAM-targeting lentivirus (L1) in comparison to non-targeting shRNA (NT) for 48 hours. B, L1CAM knockdown in CD133+ cells isolated from the glioblastoma patient specimen T3982 up-regulated p21WAF1/CIP1 but not p27KIP1 protein in comparison to non-targeting control shRNA (NT). Cells were infected with the non-targeting or L1CAM-targeting shRNA lentivirus for the indicated time. C, Knockdown of L1CAM decreased Olig2 and increased p21WAF1/CIP1 mRNA expression. RNA was purified from CD133+ cells isolated from the glioblastoma patient specimen T3982 infected with lentivirus encoding either non-targeting shRNA or L1CAM shRNA for 48 hours, and then reverse transcribed to cDNA used for Real Time PCR with sequences specific for L1CAM, Olig2, and p21 and results normalized to GAPDH levels. *, p < 0.01. D, Olig2 overexpression rescued CD133+ cells from L1CAM knockdown induced cell death. CD133+ cells isolated from the primary glioblastoma patient specimen T3982 were transfected with vector control or vector expressing Olig2 for 24 hours, and then infected with lentivirus expressing either non-targeting shRNA (NT) or L1CAM shRNA for 48 hours. L1CAM knockdown significantly reduced numbers of live cells when CD133+ cells were transfected with control vector (*, p < 0.01), but this effect was prevented by Olig2 overexpression (** , p < 0.001 with comparison to vector control with L1CAM shRNA).
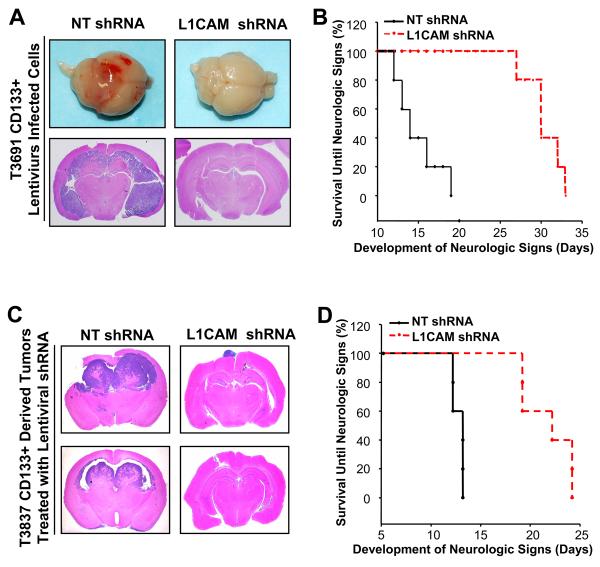
Targeting L1CAM in CD133+ glioma cells suppresses glioma growth in vivo and increases survival of mice bearing glioma xenografts
As L1CAM shRNA-mediated decreases in CD133+ glioma cell growth were associated with reduced Olig2 expression and Olig2 is critical for the growth of gliomas in vivo (19), we next sought to determine if targeting L1CAM expression could attenuate the tumorigenic potential of CD133+ glioma cells. To accomplish this goal, L1CAM expression was modulated in CD133+ glioma cells prior to implantation into immunocompromised mice (Fig. 4A, B, Supplementary Fig. S4). Knockdown of L1CAM expression in CD133+ cells derived from glioma surgical biopsy specimens prior to intracranial injection reduced tumor volumes (Fig. 4A; Supplementary Fig. S4A), and significantly increased survival until the development of neurologic signs (Fig. 4B, Supplementary Fig. S4B) relative to CD133+ glioma cells expressing non-targeting control shRNA. Furthermore, the tumorigenic capacity of CD133+ glioma cells was decreased by targeting L1CAM expression prior to intracranial injection as demonstrated in the limiting dilution assay (Supplementary Figs. S4C, D). While these in vivo data demonstrate a requirement for L1CAM in CD133+ glioma cell mediated tumorigenesis, they do not address the effects of targeting L1CAM in established tumors. To determine if targeting L1CAM could represent a potential therapeutic paradigm targeting CD133+ glioma cells, we intracranially implanted CD133+ glioma cells into immunocompromised mice and allowed tumors to establish for five days. Tumor-bearing mice were then intracranially injected with lentivirus expressing non-targeting shRNA as a control or L1CAM shRNA as a novel therapy. Brains of mice treated with non-targeting shRNA displayed aggressive high grade gliomas (Fig. 4C) whereas the brains of mice receiving intracranial injections of lentivirus expressing L1CAM shRNA showed significantly reduced tumors with small extraparenchymal tumor in most cases (Fig. 4C). Most importantly, receiving lentivirus expressing L1CAM shRNA nearly doubled the lifespan of tumor bearing mice relative to mice receiving the non-targeting shRNA expressing lentivirus (Fig. 4D). A similar decrease in tumor growth was observed in subcutaneous tumors injected with lentivirus expressing L1CAM shRNA (Supplementary Fig. S5A, B) in which L1CAM expression was decreased in comparison to mice receiving non-targeting shRNA expressing lentivirus (Supplementary Fig. S5C). FACS analysis further confirmed that the CD133+ glioblastoma cell population was reduced in tumors treated with lentivirus expressing L1CAM shRNA (Supplementary Fig. S5D). Together, these data demonstrate that molecular targeting of L1CAM in CD133+ glioma cells reduces tumor growth and increases survival in immunocompromised mice in vivo and may be a novel therapeutic paradigm with important clinical implications.
Figure 4.
Lentiviral shRNA targeting of L1CAM suppresses tumor growth and increased survival of mice bearing intracranial brain tumor xenografts. A, Representative images of brains of immunocompromised mice implanted with CD133+ cells isolated from the glioblastoma specimen T3691 and infected with non-targeting shRNA (NT) or L1CAM shRNA prior to intracranial implantation are shown. 48 hours after infection, identical numbers of viable cells (105 cells/mouse) were implanted and brains examined on day 17 after injection. Gross images and coronal sections from representative brains bearing glioma xenografts are displayed. B, Knockdown of L1CAM in CD133+ cells isolated from the glioblastoma patient specimen T3691 prior to intracranial implantation increased survival. * p < 0.002 with comparison to non-targeting control. CD133+ cells isolated from brain tumor patient specimens and infected with non-targeting shRNA (NT) or L1CAM shRNA prior to injection were implanted into the brains of immunocompromised mice as in (A). C, Representative images of coronal sections of brains of mice implanted with CD133+ brain tumor cells isolated from the T3837 glioblastoma patient specimen and then infected with lentivirus expressing non-targeting shRNA (NT) or L1CAM shRNA are shown. Identical numbers of CD133+ brain tumor cells (105 cells/mouse) were implanted into mice brains to establish intracranial tumors. After 5 days to allow tumor engraftment, lentivirus expressing non-targeting shRNA (NT) or shRNA directed against L1CAM were delivered to the tumor site through direct injection (once every other day for 12 days). D, Targeting L1CAM in vivo through lentiviral mediated shRNA suppressed intracranial tumor growth of the established brain tumor xenograft and significantly increased the survival of mice bearing intracranial glioma xenografts (*, p < 0.004).
The recent ability to prospectively identify cell subpopulations that are highly resistant to cancer therapies, drive tumor angiogenesis, and promote tumor spread (1-6, 20) makes it possible to identify molecular targets specific to these highly tumorigenic subpopulations for novel therapeutic targets. We have discovered a novel molecular target that had not been linked to CD133+ glioma cells, the cell adhesion molecule L1CAM. The dramatic overexpression of L1CAM in CD133+ brain tumor cells, the increased survival of mice bearing gliomas in which L1CAM has been targeted, and the decreased percentage of CD133+ cells in tumors treated with lentivirus expressing L1CAM shRNA all suggest that targeting L1CAM may be useful as a cancer stem cell directed therapy. As there is a very small percentage of (<1%) L1CAM+ cells which are CD133−, it remains possible that the CD133− cells reported in the literature to be tumorigenic (7) may be L1CAM+. Future studies will be needed to determine if L1CAM itself may be useful as a cancer stem cell marker in ex vivo and immunohistochemical studies. Regardless of whether L1CAM can be used to prospectively identify cancer stem cells, our studies provide evidence that L1CAM should be considered for further exploitation in therapeutic development and biological investigation.
Supplementary Material
Acknowledgments
Financial support was provided by the Goldhirsh Foundation, Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure, Alexander and Margaret Stewart Trust, Brain Tumor Society, Duke Comprehensive Cancer Center Stem Cell Initiative Grant (J.R.), NIH grants NS047409, NS054276, CA116659 and CA129958 (J.R.). J.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation and a former Sidney Kimmel Foundation for Cancer Research Scholar. We thank Y. H. Sun, S. Keir, D. Satterfield, L. Ehinger and J. Faison for technical assistance; M. Cook, B. Harvat and T. R. Dissanayake for assistance with flow cytometry; and Z. Lu for assistance with fluorescent microscopy. We are also grateful to R. Wechsler-Reya and D. Bigner for helpful discussions.
REFERENCES
- 1.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–83. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 3.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 4.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 6.Bao S, Wu Q, Sathornsumetee S, et al. Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66:7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 7.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–5. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 8.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 9.Izumoto S, Ohnishi T, Arita N, Hiraga S, Taki T, Hayakawa T. Gene expression of neural cell adhesion molecule L1 in malignant gliomas and biological significance of L1 in glioma invasion. Cancer Res. 1996;56:1440–4. [PubMed] [Google Scholar]
- 10.Suzuki T, Izumoto S, Fujimoto Y, Maruno M, Ito Y, Yoshimine T. Clinicopathological study of cellular proliferation and invasion in gliomatosis cerebri: important role of neural cell adhesion molecule L1 in tumour invasion. J Clin Pathol. 2005;58:166–71. doi: 10.1136/jcp.2004.020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figarella-Branger DF, Durbec PL, Rougon GN. Differential spectrum of expression of neural cell adhesion molecule isoforms and L1 adhesion molecules on human neuroectodermal tumors. Cancer Res. 1990;50:6364–70. [PubMed] [Google Scholar]
- 12.Thies A, Schachner M, Moll I, et al. Overexpression of the cell adhesion molecule L1 is associated with metastasis in cutaneous malignant melanoma. Eur J Cancer. 2002;38:1708–16. doi: 10.1016/s0959-8049(02)00105-3. [DOI] [PubMed] [Google Scholar]
- 13.Allory Y, Matsuoka Y, Bazille C, Christensen EI, Ronco P, Debiec H. The L1 cell adhesion molecule is induced in renal cancer cells and correlates with metastasis in clear cell carcinomas. Clin Cancer Res. 2005;11:1190–7. [PubMed] [Google Scholar]
- 14.Arlt MJ, Novak-Hofer I, Gast D, et al. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res. 2006;66:936–43. doi: 10.1158/0008-5472.CAN-05-1818. [DOI] [PubMed] [Google Scholar]
- 15.Boo YJ, Park JM, Kim J, et al. L1 Expression as a Marker for Poor Prognosis, Tumor Progression, and Short Survival in Patients with Colorectal Cancer. Ann Surg Oncol. 2007;14:1703–11. doi: 10.1245/s10434-006-9281-8. [DOI] [PubMed] [Google Scholar]
- 16.Stoeck A, Gast D, Sanderson MP, Issa Y, Gutwein P, Altevogt P. L1CAM in a membrane-bound or soluble form augments protection from apoptosis in ovarian carcinoma cells. Gynecol Oncol. 2007;104:461–9. doi: 10.1016/j.ygyno.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 17.Muerkoster SS, Werbing V, Sipos B, et al. Drug-induced expression of the cellular adhesion molecule L1CAM confers anti-apoptotic protection and chemoresistance in pancreatic ductal adenocarcinoma cells. Oncogene. 2007;26:2759–68. doi: 10.1038/sj.onc.1210076. [DOI] [PubMed] [Google Scholar]
- 18.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 19.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–17. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermann PC, Huber SL, Herrler T, et al. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.