Abstract
An important factor in determining the adverse consequences of a stress experience is the degree to which an individual can exert control over the stressor. Stressor controllability is known to influence brain norepinephrine levels, but its impact on activity in noradrenergic cell bodies is unknown. In the present study we investigated whether noradrenergic neurons within the locus coeruleus (LC), the major source of forebrain norepinephrine, are sensitive to stressor controllability. We exposed adult male Sprague-Dawley rats to escapable or yoked inescapable tailshock and assessed LC activity by measuring changes in the immediate early gene c-fos and the enzyme tyrosine hydroxylase (TH). We used in situ hybridization to measure levels of c-fos mRNA, TH mRNA, and TH primary transcript in the LC. In all three cases stress exposure increased expression relative to an unstressed homecage control group, but expression did not differ between controllable and uncontrollable stress. To further examine whether stressor controllability influences the number of stress-responsive LC neurons we performed double-label immunohistochemistry for TH and Fos protein. Again we detected an overall effect of stress, which did not differ between controllable and uncontrollable stress. We conclude that exposure to stress robustly increases expression of TH and c-fos in the LC, but this effect is not influenced by stressor controllability. To the extent that the expression of these genes reflects degree of neuronal activation, our results suggest that stress-induced activity of noradrenergic cell bodies in the LC is not sensitive to stressor controllability.
Keywords: Norepinephrine, tyrosine hydroxylase, c-fos, in situ hybridization, learned helplessness, stress
1. Introduction
Uncontrollable stress can be a critical factor in the development and expression of psychiatric illnesses such as posttraumatic stress disorder and depression [32,57]. In particular, feelings of helplessness can exacerbate the emotional consequences of stress, whereas holding the belief of being generally in control of one’s life is a resilience factor associated with reduced risk for stress-related psychiatric disorders [9,10]. Additionally, having control over a discrete, acute stressor can mitigate its emotional and physiological consequences. This phenomenon is studied experimentally by manipulating stressor controllability: while one test subject can control the intensity or duration of an aversive stimulus, a “yoked” subject passively receives the same aversive stimulus without having any control. Thus while physical parameters of the stressor (intensity and duration) are identical for both subjects, their experiences differ in that one subject has control over the stressor while the other does not. Humans with control show reduced emotional and physiological reaction to a stress experience [14,55]. In rodents the behavioral consequences of stressor controllability are analogous to those seen in humans: having control attenuates the impact of stress on subsequent tests of anxiety [18,52], conditioned fear [8,46], and active/passive stress coping responses [39,69].
The neurotransmitter norepinephrine (NE) has been widely implicated as a biological substrate of stress, and may be dysregulated in stress-related psychiatric disorders [4,56]. In rodents, uncontrollable stress increases NE turnover, resulting in transient reductions in central NE tissue content [7,69]. This effect is influenced by stressor controllability: homogenates of whole-brain tissue from rats exposed to escapable shock contain higher concentrations of NE than brain homogenates from their yoked, inescapably shocked partners [70]. Subsequent studies using similar experimental designs have identified controllability effects on NE in a number of specific brain regions [6,60,64,69]. It is not known whether these effects are driven by differences in activity-dependent release of NE, or instead reflect differences in processes that might be independent of noradrenergic cell body activity such as NE synthesis, reuptake, metabolism, or conversion to epinephrine within adrenergic neurons. To assess these possibilities, it is important to determine whether stressor controllability influences noradrenergic activity at the level of the cell body.
The locus coeruleus (LC) is the largest nucleus of noradrenergic cell bodies in the brain [59] and is the major source of forebrain NE [30]. The LC is highly stress-reactive, as assessed by electrophysiological activity [1,36], NE efflux in terminal regions [2,45,63,71], and expression levels of tyrosine hydroxylase (TH, the rate-limiting enzyme in NE synthesis [37]) and the immediate early gene c-fos. A variety of stressors increase TH mRNA within the LC, including social isolation [5], subordination [68], restraint [53], exposure to cold [48], and electric shock [17]. c-fos mRNA and its protein product, Fos, are similarly upregulated in the LC after numerous stressors (reviewed by Kovacs [33]). However, expression of these genes has not been examined in yoked stress models to assess the impact of controllability. In the present study we tested the sensitivity of the LC to stressor controllability by measuring the effects of escapable and yoked inescapable tailshock on expression of TH and c-fos.
2. Results
2.1 Tyrosine hydroxylase mRNA
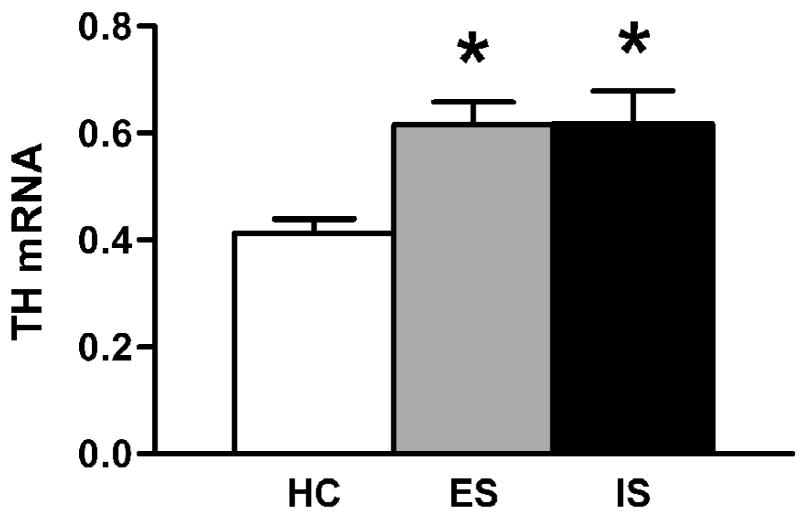
In choosing a time point for examining stressor controllability effects, we performed an initial time course experiment revealing trends for maximal increase at 24 h post-stress (Fig. 1, Table 1). We used this time point in a subsequent controllability experiment (Fig. 2) in which significant group differences were observed, F(2,14) = 5.615, p < 0.05. Post-hoc tests revealed that unstressed differed from both shock groups, p < 0.05. However, escapable and inescapable shock did not differ from each other, p > 0.9.
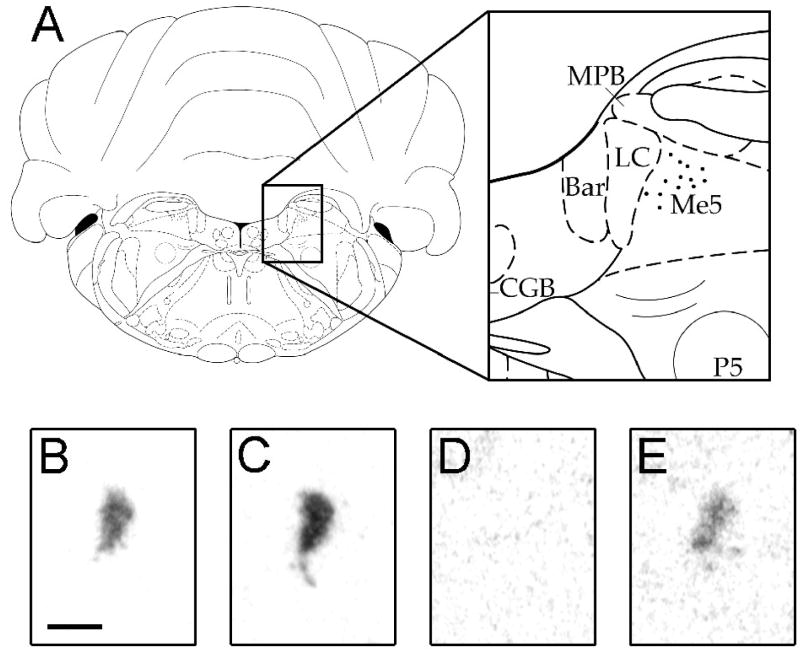
Figure 1.
Autoradiographic images of locus coeruleus tissue. A, Location of LC tissue analyzed in this study, with enlargened detail of peri-LC region. Image represents −10.04 mm bregma from the atlas of Paxinos & Watson [47]. B–E, sample autoradiographic images of TH (B, C) and c-fos (D, E). Tissue is from unstressed rats (B, D) and inescapably shocked rats (C, E). Stress time points depicted in TH and c-fos images are 24 h and 1 h, respectively. Scale bar = 500 μm.
Table 1.
Time course of gene expression in the locus coeruleus after inescapable shock
| Stressor | Time after shock session | n | TH mRNA | c-fos mRNA |
|---|---|---|---|---|
| Unstressed | 6 | 0.391 ± 0.030 | 0.0118 ± 0.0015 | |
| IS | 1 h | 4 | 0.401 ± 0.023 | 0.1113 ± 0.0215*** |
| IS | 2 h | 5 | 0.404 ± 0.072 | 0.0822 ± 0.0149** |
| IS | 4 h | 4 | 0.429 ± 0.060 | 0.0228 ± 0.0082 |
| IS | 24 h | 4 | 0.528 ± 0.066 | 0.0130 ± 0.0031 |
Values are mean ± SEM.
p < 0.01,
p < 0.001 vs. unstressed homecage control.
Figure 2.
TH mRNA in the locus coeruleus 24 h after stress. Graph shows optical density of in situ hybridization autoradiograms. Data are expressed as mean + SEM of unstressed homecage control (HC), escapable shock (ES), and inescapable shock (IS). n = 5 for HC, n = 6 for both ES and IS. * p < 0.05 vs. homecage control.
2.2 Tyrosine hydroxylase primary transcript
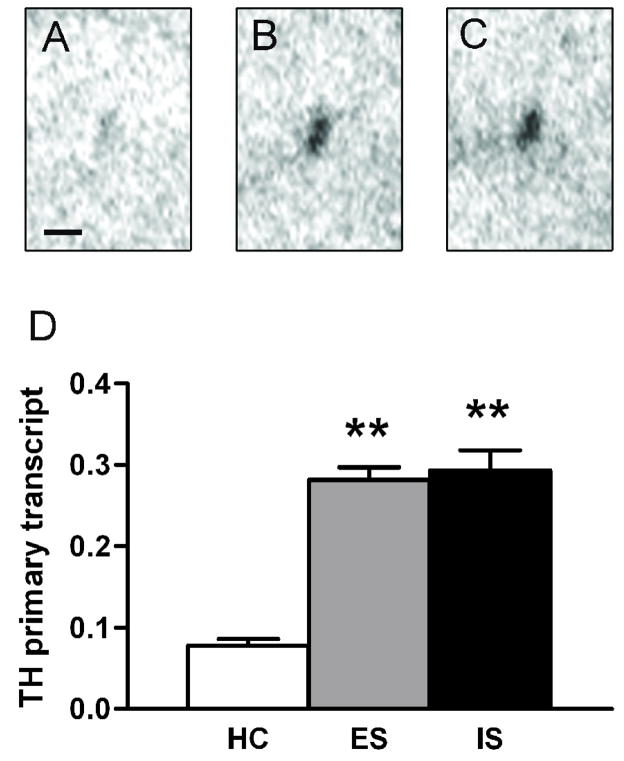
Primary transcript, also referred to as heteronuclear RNA (hnRNA), is recently synthesized RNA in which complete splicing of introns has not yet occurred. Because it is rapidly processed into mature mRNA, measuring TH primary transcript by intron-specific hybridization provides an indirect index of synthetic rate that closely matches results from the more direct technique of nuclear run-on assay [16]. Preliminary tests with an antisense oligonucleotide probe directed at intron 2 of the TH gene gave hybridization signal specifically in the LC; sense control probe assayed under identical conditions gave no detectable signal above background (data not shown). Because previous reports suggest that the effects of stress on TH primary transcript are short-lasting [17], we investigated its expression only at 1 h after stress (Fig. 3). There were significant group differences, F(2,18) = 47.334, p < 0.001, with post-hoc tests revealing that unstressed differed significantly from both shock groups, p < 0.001. However escapable and inescapable shock did not differ from each other, p = 0.885.
Figure 3.
TH primary transcript in the locus coeruleus 1 h after stress. Sample in situ hybridization autoradiograms in tissue from unstressed (A), escapably shocked (B), and inescapably shocked (C) rats. Scale bar = 500 μm. D, Optical density of autoradiograms expressed as mean + SEM of unstressed homecage control (HC), escapable shock (ES), and inescapable shock (IS). n = 7 per group. ** p < 0.001 vs. homecage control.
2.3 c-fos mRNA
c-fos displayed a rapid and transient increase in mRNA following inescapable tailshock (Fig. 1, Table 1). There was an overall effect of stress exposure, F(4,18) = 15.161, p < 0.001. Post-hoc tests revealed that these effects were significant at 1 h (p < 0.001) and 2 h (p < 0.01) after stress. In a subsequent controllability experiment, rats were sacrificed 1 h after stress and assessed for c-fos mRNA (Fig. 4). There were significant group differences F(2,18)= 50.429, p < 0.001, and post-hoc tests revealed that unstressed differed significantly from both shock groups, p < 0.001. However escapable and inescapable shock did not differ from each other, p = 0.266.
Figure 4.
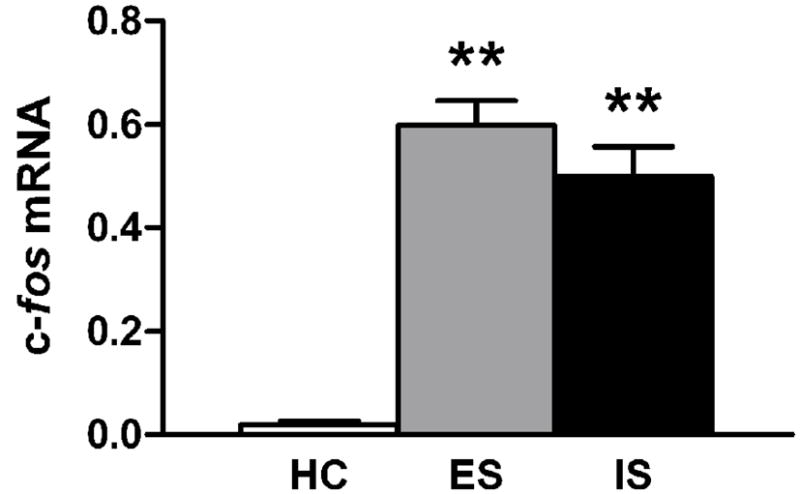
c-fos mRNA in the locus coeruleus 1 h after stress. Graph shows optical density of in situ hybridization autoradiograms. Data are expressed as mean + SEM of unstressed homecage control (HC), escapable shock (ES), and inescapable shock (IS). n = 7 per group. ** p < 0.001 vs. homecage control.
2.4 Fos immunohistochemistry
We did not detect any Fos/TH double labeling in the LC in unstressed homecage control rats in a pilot study (data not shown), and therefore omitted this group from the subsequent controllability experiment. In this experiment, both escapable and inescapable shock robustly induced Fos immunocreactivity in the LC (Fig. 5). When all Fos-positive nuclei within the LC were counted without regard to TH co-labeling (Table 2), there were no significant effects of controllability F(1,24) = 1.826, p = 0.189, or of time F(1,24) = 1.976, p = 0.173, and the interaction between controllability and time was also non-significant, F(1,24) = 0.8028, p = 0.379.
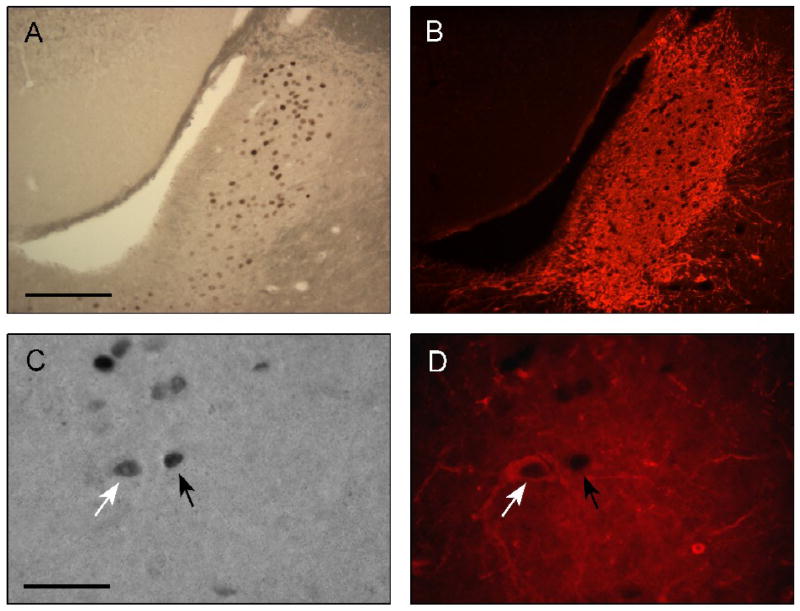
Figure 5.
Fos (A, C) and TH (B, D) immunoreactivity in the locus coeruleus 2h after exposure to inescapable shock. Top row: 20X magnification, scale bar = 250μm. Bottom row: 100X magnification, scale bar = 50 μm. White arrow denotes Fos/TH double-labeled cell, black arrow denotes Fos single-labeled cell.
Table 2.
Effects of escapable and inescapable shock on the number of Fos-positive nuclei in the locus coeruleus
| Time after shock session | Number of Fos-positive nuclei per LC region |
|
|---|---|---|
| Escapable shock | Inescapable shock | |
| 0 h | 74.06 ± 5.94 | 59.39 ± 7.20 |
| 2 h | 59.03 ± 7.87 | 56.06 ± 4.64 |
Values are mean ± SEM. n = 7 for all groups. There were no significant effects of stressor controllability or time.
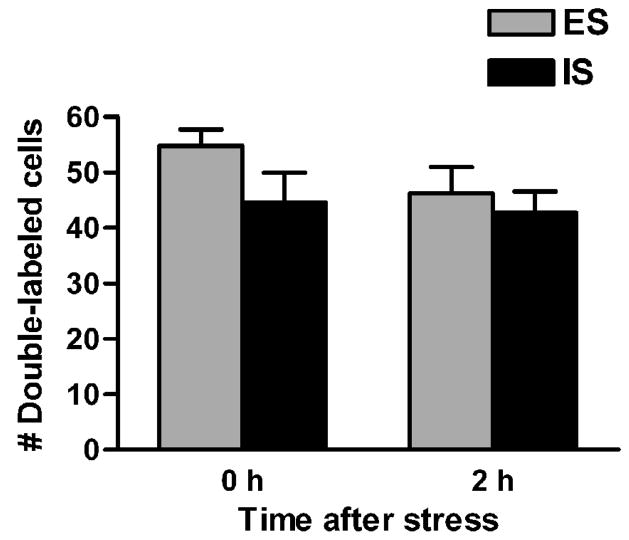
2.5 Fos/TH double-label imunohistochemistry
Stress induced Fos/TH double-labeling in the LC (Fig. 5, Fig. 6). There were no significant effects of controllability F(1,24) = 2.491, p = 0.128, or of time F(1,24) = 1.459, p = 0.239, and the interaction between controllability and time was also non-significant, F(1,24) = 0.605, p = 0.444. A fraction of the total Fos-positive nuclei throughout the LC did not show clear double-labeling for TH. Analysis of Fos-positive/TH-negative nuclei did not reveal significant effects of controllability, time, or time/controllability interaction (data not shown).
Figure 6.
Number of Fos / TH double-labeled cells per LC region following escapable shock (ES) and inescapable shock (IS). Data are expressed as mean + SEM. n = 7 / group.
3. Discussion
Here we investigated the role of stress and stressor controllability on transcriptional activation within the LC. We did so by exposing rats to escapable or yoked inescapable tailshock and examining expression of TH and c-fos at the mRNA and protein level. We conclude that while stress induces robust transcriptional activation of the locus coeruleus, this effect is not influenced by stressor controllability.
We found that c-fos mRNA increased quickly after the stress session, with greatest expression observed at the earliest time point examined. In contrast, our observed TH time course data (Table 1, Fig. 1) is suggestive of a slow increase following stress, which was confirmed in subsequent experiments showing increased TH mRNA 24 h after stress (Fig. 3). Because these effects were delayed, it might be argued that they are not a direct consequence of LC activity during the stress session, but rather an indirect effect of stress – caused, perhaps, by stress-induced changes in social interaction, sleep, or feeding over the 24 h following the stress session. However, our observation that stress induces a rapid increase in TH primary transcript (Fig. 3) suggests that at least some portion of the stress effects on TH mRNA are directly related to LC activation during the tailshock session. Differences in timing between TH primary transcript and mRNA may be due to a large, stable pool of TH mRNA at basal conditions [17]. This explanation could also account for the disparity between a large increase in TH primary transcript (Fig. 3) resulting in a relatively modest change in mature mRNA (Fig. 2). The slow TH mRNA time course that we infer from our data (Table 1, Fig. 3) corroborates other studies that have found TH mRNA to increase gradually after stress [17,50,66], but differs from that reported by Smith and colleagues [53], which may be due to differences in animal procedures or mRNA quantification.
Although both controllable and yoked uncontrollable stress increased TH mRNA relative to an unstressed control group (Fig. 2), the effects of these stressors did not differ from each other. Similar results were obtained when examining TH primary transcript (Fig. 3) and c-fos mRNA (Fig. 4), supporting our conclusion that stressor controllability does not influence the degree of transcriptional activity in the LC. To explore whether stressor controllability influences the number of active LC neurons we used immunohistochemistry to count number of Fos-positive cells at two different time points after stress exposure. We found no effect of stressor controllability on the number of Fos/TH double-labeled neurons (Fig 6) or total number of Fos-positive nuclei (Table 2). We counted a greater number of total Fos-positive nuclei than Fos/TH double-labeled neurons, suggesting a population of Fos-positive/TH-negative cells. This is probably largely an artifact of incomplete immunohistochemical detection of TH protein and our conservative criteria for determining a Fos-positive cell to be double-labeled with TH. However, this may also reflect the presence of Fos in non-noradrenergic cells. Regardless, there was no effect of stressor controllability on the number of Fos-positive, TH-negative nuclei. Because there were not effects of controllability on any metric used here, we conclude that stressor controllability does not influence the number of stress-reactive LC neurons. While it is possible that controllability may influence gene expression in a manner evident only outside the window of time used in our experiments, we consider this unlikely because our selection of time points was guided by an initial time course experiment (Table 1). We therefore conclude that stressor controllability does not affect expression of these genes in the LC.
Measuring changes in gene expression – particularly immediate early genes such as c-fos – is a widely used strategy for identifying brain regions sensitive to a given stimulus. For example, one of our laboratories previously used Fos immunoreactivity to show that activation of the dorsal raphe nucleus is sensitive to stressor controllability [23]. While the conclusions drawn from that Fos experiment are in accordance with other studies using more direct physiological measurements of neuronal activity[12,40], caution must be used when equating gene expression with neuronal activity, since genes are not uniformly regulated with regard to brain region, cell type, or stimulus. In the specific case of stress and the genes TH and c-fos, however, there are multiple lines of evidence suggesting that their upregulation in the LC is a marker of increased electrophysiological activity. First, it is well-established that stress, which increases expression of these genes, increases electrical activity within the LC [1,36] and NE efflux in its terminal regions [2,45,63,71]. Second, TH mRNA is increased within the LC by several in vivo pharmacological treatments known to increase physiological activity of LC neurons [17,19,41]. Finally, there is mechanistic evidence to suggest that acute excitation of these neurons subsequently increases TH and c-fos expression. The promoter region of the c-fos gene is sensitive to increases in intracellular Ca++ via cAMP response element binding protein (CREB) and mitogen-activated protein kinase (MAPK) signaling pathways [33], both of which are activated in the LC by stress [26,34]. Similarly, TH mRNA is upregulated in cultured catecholaminergic cells by depolarization or cAMP stimulation [54], an effect also seen in LC organotypic slice culture [49]. Thus evidence suggests that upregulation of these genes in the LC is a marker of increased neuronal activity. Furthermore, previous studies provide evidence that graded intensities of uncontrollable stress can result in graded responses of TH mRNA [5], TH primary transcript [17], c-fos mRNA [22], and number of Fos-positive nuclei [62] in the LC. Accordingly, if one assumes that expression of these genes is a reflection of degree of neuronal activity, our present experiments suggest that noradrenergic cell bodies in the LC are not sensitive to stressor controllability. Nevertheless, because gene expression is not a direct measurement of physiological activity, this conclusion would be strengthened by future experiments using in vivo measurements of electrical activity or neurotransmitter release.
The results of these experiments were surprising given the literature connecting NE and stressor controllability. There are several possible explanations that might account for an apparent discrepancy between controllability effects on tissue concentrations of NE versus LC activation. First, the relationship between NE and controllability may depend critically on stress parameters. For example, there are published accounts of uncontrollable stress resulting in hypothalamic NE concentrations that are lower [6,60], higher [64], and equal to [69] levels after an equivalent controllable stressor. These inconsistencies may be due to methodological differences across studies in shock delivery, shock intensity, duration of stress session, nature of escape response, and time of sacrifice. Underscoring the importance of stress procedures is one study which demonstrated that, when all other parameters were held constant, the duration of stress session greatly influenced NE controllability effects [64]. Thus it is possible that the specific stress procedures used in this study simply do not produce controllability-dependent differences in NE function. If this is the case, then differences in NE function need not be invoked to explain the numerous behavioral effects observed in studies using identical specific procedures to those described here for escapable and yoked inescapable tailshock [8,18,39,52].
Several other explanations could account for differences between LC activity and NE tissue content. Since NE is an intermediate in the synthesis of epinephrine [43], previously observed changes in NE tissue content might reflect changes occurring within epinephrine-containing neurons. This is unlikely, however, because epinephrine is present at much lower concentrations than NE in most brain regions and undetectable in the frontal cortex [65], a site of controllability-dependent changes in NE content [69]. Another possibility is that differences in NE tissue content may reflect changes in noradrenergic neurons from sources other than the LC, such as the lateral tegmental group or the nucleus of the solitary tract [43]. While this could account for some controllability-dependent differences in NE content, it is not sufficient to explain differences seen in frontal cortex, which receives NE innervation exclusively from the LC [38]. A last alternative explanation to reconcile our findings with previous literature is that controllability-dependent changes in NE content are not a reflection of activity at the level of the cell body, but instead reflect changes localized to NE-containing axon terminals. Noradrenergic function is regulated presynaptically by numerous heteroreceptors [51] including the serotonergic 5-HT1B receptor [42], which is expressed in LC neurons [15]. Activation of this terminally-located receptor inhibits neurotransmitter synthesis [27] and release [20,42], while increasing reuptake [21]. Thus the differential release of serotonin observed during inescapable and escapable shock [12] might affect one or more of these processes within noradrenergic terminals, creating differences in NE content that do not correlate with activity in noradrenergic cell bodies. It is difficult to interpret NE tissue content, however, as measurements of tissue content cannot distinguish between NE that was intracellular and NE that was released. To our knowledge there are no published microdialysis studies that resolve this issue.
While the dorsal raphe nucleus and locus coeruleus are similar in their reactivity to uncontrollable stress [62], the dorsal raphe is unique in showing reduced levels of activity when a stressor is controllable. The dorsal raphe receives glutamatergic projections from the medial prefrontal cortex [35] that preferentially innervate inhibitory GABAergic interneurons within the dorsal raphe [28], which in turn inhibit serotonergic neurons [25,67]. Although the medial prefrontal cortex as a whole does not appear sensitive to stressor controllability, the subpopulation of neurons within the prelimbic cortex that project to the dorsal raphe do in fact show selective activation during controllable stress (Baratta, M.V. & Maier, S.F., unpublished observations). These neurons thus silence activity of serotonergic neurons in the dorsal raphe during controllable stress, attenuating the transcriptional, physiological, and behavioral consequences of stress during conditions of behavioral control [3]. The LC, in contrast, does not appear to have the circuitry necessary for selective inhibition during controllable stress. The neurochemical identity of LC neurons is almost exclusively noradrenergic [58] and does not include GABAergic cell bodies [11], as are found in the dorsal raphe. Thus while the LC does receive glutamatergic afferents from the prefrontal cortex, these inputs are excitatory to its noradrenergic neurons [29,31]. Although it is possible that these inputs may provide additional excitatory influence selectively during controllable or uncontrollable stress, our present results suggest that these scenarios are unlikely.
A large body of literature implicates NE as an important biological mediator of acute stress and stress-related psychiatric disorders. While there is evidence that some processes in noradrenergic neurons are sensitive to stressor controllability, our present results suggest that activation of noradrenergic cell bodies in the LC is not. Future experiments may help to further characterize the influence of stressor controllability on noradrenergic function.
4. Experimental procedure
4.1 Animals
The inescapable shock time course experiment was performed at the University of Washington; procedures were approved by the University of Washington animal care committee and conducted in accordance with National Institutes of Health guidelines. Male Sprague-Dawley rats (Charles River, Wilmington, MA) were maintained in a colony room with 12-h light/12-h dark cycle (lights on at 6:00 AM) and weighed 250–300 g at the time of testing. Stressor controllability procedures were performed at the University of Colorado; procedures were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder. Male Sprague-Dawley rats (300–325 g; Harlan, Indianapolis, IN) were housed on a 12-h light/12-h dark cycle (lights on at 7:00 A.M). At both institutions rats were group housed 2–3 per cage, had access to standard lab chow and water available ad libitum, and were allowed to acclimate to colony conditions for at least one week prior to experimentation. Care was taken during all procedures to minimize animal discomfort.
4.2 Inescapable tailshock
Stress procedures took place between 9:00 AM and 11:30 AM. Subjects were placed inside Plexiglas enclosures measuring 4.625″ L x 3″ W x 5.66″ H. A rod protruded from the back of the enclosure, to which the rat’s tail was secured with athletic tape. Three rat enclosures, separated by opaque dividers, were housed together inside a large sound isolation cubicle. Each enclosure was dimly lit throughout the duration of the shock session. After rats were loaded into the enclosures, electrodes (Mansfield R&D, St. Albans, VT) were attached to the tail approximately 1 1/4″ apart. Electrode paste (D.O. Weaver & Co, Aurora, CO) was placed on each electrode to ensure consistent current delivery. A total of 100 tailshocks were delivered, each at 1.0 mA for 5 s. Shocks were controlled by a constant current shock generator (Med Associates, St. Albans, VT). Inter-shock intervals were controlled by computer software and varied randomly from 30 – 90 s, averaging 60 s. After the end of the stress session animals were returned to the vivarium, and were subsequently anesthetized with carbon dioxide inhalation at 1, 2, 4, or 24 h after the end of the tailshock session. Unstressed rats, which remained undisturbed inside their homecages in the vivarium throughout the experiment, were anesthetized at the same time of day as rats from the 2 h stress group.
4.3 Escapable tailshock
Stress procedures were carried out by experienced individuals using equipment and procedures as previously described [13]. Stress sessions took place between 8:00 AM and 3:00 PM. Each rat was placed in a Plexiglas box (14 x 11 x 17 cm) with a wheel mounted in the front and a Plexiglas rod protruding from the rear. The rat’s tail was secured to the rod with tape and affixed with copper electrodes. Rats were run in yoked pairs and each session consisted of 80 trials of tailshock (27 x 1.0 mA, 27 x 1.3 mA, and 26 x 1.6 mA) with an average 60 s inter-trial intervals. Tailshock was terminated for both rats when the escapably shocked rat met the escape requirement. Thus the duration and intensity of tailshocks were identical for each rat in the pair. The following procedure was used to ensure that the rat learned the operant response to terminate the tailshock. Initially, the shock was terminated by a one-quarter turn of the wheel. The response requirements were increased by a one-quarter turn when three consecutive trials were completed in less than 5 s. Subsequent latencies under 5 s increased the requirement by 50% up to a maximum of 4 full turns. The requirement was reduced if the trial was not completed in less than 5 s. If the requirement was not reached in less than 30 s, the shock was terminated and the requirement was reduced to one-quarter turn of the wheel. One subject failed to reach an inclusion criterion of 4 full turns by approximately 15 trials; this rat and its yoked partner were removed from subsequent analysis. Unstressed rats remained undisturbed inside their homecages in the vivarium throughout this time. At the end of the stress session, all rats were returned to the vivarium until the time of sacrifice. Animals intended for in situ hybridization were rapidly decapitated at 1 or 24 h after the end of the stress session. Animals intended for immunohistochemistry were anesthetized with sodium pentobarbital immediately or at 2 h after the end of the stress session.
4.4 In situ hybridization histochemistry
Following dissection, brains were frozen on dry ice and stored at −70°C. Brains were cut into 20 um sections on a cryostat at −20°C and mounted onto multiple sets of Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were stored at −70°C until processing. Tissue preparation and hybridization procedures were performed as previously described [61]. In brief, slides were post-fixed in cold 4% paraformaldehyde, washed in phosphate-buffered saline (PBS), treated with 0.25% acetic acid, dehydrated through a series of graded alcohols, delipidated in chloroform, partially rehydrated, and air-dried.
The TH oligonucleotide probe was a 48-base probe complementary to nucleotides 1351–1398 of rat TH mRNA [24]. The c-fos oligonucleotide probe was a 51-base probe complementary to nucleotides 270–319 of rat c-fos mRNA [44]. The TH primary transcript probe was complementary to nucleotides 3062–3105 (intron 2) of the rat TH gene, with antisense sequence CACAGGGTTCTGGCTTTAATGTCATTTGAGATTTTTTTTCTGGG. Oligonucleotides were 3′ labeled with [33P]dATP (Perkin-Elmer, Boston, MA) using terminal deoxyribonucleotidyl transferase (Invitrogen, Carlsbad, CA) and purified on a G25 spin column (Amersham, Arlington Heights, IL). Labeled probe was diluted to 2 pmol/mL in a hybridization buffer containing 50% formamide, 10% dextran sulfate, 0.3 M sodium chloride, 10 mM Tris (pH 8.0), 1 mM EDTA, 1X Denhardt’s, 0.5 mg/ml yeast tRNA, and 10mM dithiothreitol. Fifty microliters of the hybridization mixture was applied to each slide, which was then coverslipped and incubated overnight at 37°C in a humid chamber. Coverslips were removed and the slides were washed three times in 1X SSC (150 mM NaCl, 15 mM Na-citrate, pH 7.0) for 20 min at 55°C, then for 1 h in 1X SSC at room temperature. Slides were dehydrated through a graded series of alcohols containing 0.3M ammonium acetate and exposed to either Hyperfilm beta-Max (Amersham) or a high-resolution Cyclone phosphor scanner (Packard, Meriden, CT).
Optical densities were measured by an observer blind to animal treatment using MicroComputer Imaging Device (Imaging Research, Cambridge, England). Optical densities were measured from left and right LC hemispheres over 3 successive sections which were anatomically matched across animals according to coronal plates 57 and 58 in the rat brain atlas of Paxinos and Watson [47]. Tissue background was subtracted from each measurement to yield net signal. These six net signal values were averaged for each rat to yield a single representation of optical density.
4.5 Immunohistochemistry
Immediately or 2 h following the last tailshock, rats were anesthetized with sodium pentobarbital and perfused through the left cardiac ventricle with 100 ml of physiological saline followed by 250 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed and post-fixed overnight, then transferred to 30% sucrose and stored at 4°C until sectioning. Brains were sectioned on a cryostat at 20 μm and mounted onto SuperFrost Plus slides (Fisher Scientific). Slides were stored at −20°C until staining.
Immunohistochemical staining for Fos and TH were conducted sequentially in a manner modified slightly from previous reports [62]. Staining for Fos was conducted first using the avidin-biotin-horseradish peroxidase complex (ABC) method. Following a series of washes in PBS, sections were incubated for 30 min in a solution containing 0.9% hydrogen peroxide and 4% Triton X-100. Sections were incubated for 24 h with Fos primary antibody diluted 1:5,000 (Santa Cruz Biotechnology, Santa Cruz, CA) in a blocking solution containing 1% normal goat serum, 1% normal horse serum, 0.25% Triton X-100, and 0.1% sodium azide. Tissue was incubated for 2 h at room temperature in biotinylated goat anti-rabbit IgG diluted 1:200 (Jackson ImmunoResearch, West Grove, PA). Sections were incubated with avidin-biotin-horseradish peroxidase complex (Vectastain Elite ABC kit) in PBS for 1 h, then incubated in a 3,3′-diaminobenzidine tetrahydrochloride (DAB) solution for approximately 15 min. For subsequent TH immunostaining, sections were washed in PBS, incubated in blocking solution for 30 min, and then incubated in primary TH antibody diluted 1:2,000 (Pel-Freez, Rogers, AR, catalog #P40101-0) for 48 h at 4°C. After another series of PBS washes, sections were incubated with a goat anti-rabbit secondary antibody (1:200; AlexaFluor 594; Molecular Probes) for 3 h at room temperature. Sections were then washed with PBS, dried overnight in a dark environment, and coverslipped with DPX mounting media (Fluka BioChemika, Steinheim, Switzerland).
Slides were examined on a microscope by an observer blind to animal treatment on an Olympus BX-61 microscope (Olympus America, Melville, NY) and analyzed using Olympus MicroSuite software. Sections were assessed in two ways: first, counting the absolute number of Fos-positive cells irrespective of TH labeling (Table 2); second, counting the number of cells double-labeled for both Fos and TH (white arrow in Fig. 5C; Fig. 6). Fos-stained nuclei were identified by dark-brown or black ovoid particles using the brightfield setting. The double-labeled neurons were analyzed with the same microscope using a mercury lamp coupled to a TRITC filter. Neurons displaying an unambiguously distinguishable red cell body were identified as immunopositive for TH. For each animal, eight LC regions across four consecutive sections were counted and used to calculate average count per LC region. The boundary of the LC was determined using the atlas of Paxinos and Watson [47] and was aided by the TH immunofluorescence.
4.6 Statistical analysis
To determine what time point was most appropriate with our procedures to detect an increase in TH and c-fos mRNA, one-way analysis of variance (ANOVA) was performed for each gene, followed by Dunnett’s post-hoc test (when appropriate) to individually compare each stress time point to unstressed control. Experiments testing the influence of stressor controllability on TH mRNA, TH primary transcript, and c-fos mRNA were each analyzed ANOVA and Tukey post-hoc tests. Total Fos-positive labeling, Fos / TH double-labeling, and Fos-positive / TH-negative labeling were each analyzed with two-way ANOVA using stressor controllability and time as the independent variables. For all comparisons, the threshold for statistical significance was set at alpha = 0.05.
Acknowledgments
This work was supported by GM07108 (to RAM), MH 63303 (to JFN), MH 050479 (to SFM), and the Department of Veterns Affairs Research and Development Services Northwest Network Mental Illness Research, Education, and Clinical Center (to PZ). We are grateful to Simina Popa and Evelyn Vincow for critical reading of this manuscript.
Abbreviations
- LC
locus coeruleus
- NE
norepinephrine
- TH
tyrosine hydroxylase
- ABC
avidin-biotin-horseradish peroxidase complex
- PBS
phosphate buffered saline
- DAB
3,3′-diaminobenzidine tetrahydrochloride
- ANOVA
analysis of variance
- HC
homecage control
- ES
escapable shock
- IS
inescapable shock
- CREB
cAMP response binding protein
- MAPK
mitogen-activated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987;7:2837–43. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abercrombie ED, Keller RW, Jr, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- 3.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–71. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 4.Anand A, Charney DS. Norepinephrine dysfunction in depression. J Clin Psychiatry. 2000;61 Suppl 10:16–24. [PubMed] [Google Scholar]
- 5.Angulo JA, Printz D, Ledoux M, McEwen BS. Isolation stress increases tyrosine hydroxylase mRNA in the locus coeruleus and midbrain and decreases proenkephalin mRNA in the striatum and nucleus accumbens. Brain Res Mol Brain Res. 1991;11:301–8. doi: 10.1016/0169-328x(91)90039-z. [DOI] [PubMed] [Google Scholar]
- 6.Anisman H, Pizzino A, Sklar LS. Coping with stress, norepinephrine depletion and escape performance. Brain Res. 1980;191:583–8. doi: 10.1016/0006-8993(80)91311-6. [DOI] [PubMed] [Google Scholar]
- 7.Anisman H, Sklar LS. Catecholamine depletion in mice upon reexposure to stress: mediation of the escape deficits produced by inescapable shock. J Comp Physiol Psychol. 1979;93:610–25. doi: 10.1037/h0077603. [DOI] [PubMed] [Google Scholar]
- 8.Baratta MV, Christianson JP, Gomez DM, Zarza CM, Amat J, Masini CV, Watkins LR, Maier SF. Controllable versus uncontrollable stressors bi-directionally modulate conditioned but not innate fear. Neuroscience. 2007;146:1495–503. doi: 10.1016/j.neuroscience.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benassi VA, Sweeney PD, Dufour CL. Is there a relation between locus of control orientation and depression? J Abnorm Psychol. 1988;97:357–67. doi: 10.1037//0021-843x.97.3.357. [DOI] [PubMed] [Google Scholar]
- 10.Benight CC, Bandura A. Social cognitive theory of posttraumatic recovery: the role of perceived self-efficacy. Behav Res Ther. 2004;42:1129–48. doi: 10.1016/j.brat.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Berod A, Chat M, Paut L, Tappaz M. Catecholaminergic and GABAergic anatomical relationship in the rat substantia nigra, locus coeruleus, and hypothalamic median eminence: immunocytochemical visualization of biosynthetic enzymes on serial semithin plastic-embedded sections. J Histochem Cytochem. 1984;32:1331–8. doi: 10.1177/32.12.6150057. [DOI] [PubMed] [Google Scholar]
- 12.Bland ST, Hargrave D, Pepin JL, Amat J, Watkins LR, Maier SF. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–96. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- 13.Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–28. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breier A, Albus M, Pickar D, Zahn TP, Wolkowitz OM, Paul SM. Controllable and uncontrollable stress in humans: alterations in mood and neuroendocrine and psychophysiological function. Am J Psychiatry. 1987;144:1419–25. doi: 10.1176/ajp.144.11.1419. [DOI] [PubMed] [Google Scholar]
- 15.Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1D alpha, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–86. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 16.Chang MS, Hahn MK, Sved AF, Zigmond MJ, Austin MC, Sherman TG. Analysis of tyrosine hydroxylase gene transcription using an intron specific probe. J Neurosci Methods. 2000;94:177–85. doi: 10.1016/s0165-0270(99)00137-5. [DOI] [PubMed] [Google Scholar]
- 17.Chang MS, Sved AF, Zigmond MJ, Austin MC. Increased transcription of the tyrosine hydroxylase gene in individual locus coeruleus neurons following footshock stress. Neuroscience. 2000;101:131–9. doi: 10.1016/s0306-4522(00)00352-3. [DOI] [PubMed] [Google Scholar]
- 18.Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis AL, Bello NT, Connolly KR, Valentino RJ. Corticotropin-releasing factor neurones of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J Neuroendocrinol. 2002;14:667–82. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- 20.Davidson C, Stamford JA. Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br J Pharmacol. 1995;114:1107–9. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daws LC, Gould GG, Teicher SD, Gerhardt GA, Frazer A. 5-HT(1B) receptor-mediated regulation of serotonin clearance in rat hippocampus in vivo. J Neurochem. 2000;75:2113–22. doi: 10.1046/j.1471-4159.2000.0752113.x. [DOI] [PubMed] [Google Scholar]
- 22.Day HE, Masini CV, Campeau S. The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics. Brain Res. 2004;1025:139–51. doi: 10.1016/j.brainres.2004.07.079. [DOI] [PubMed] [Google Scholar]
- 23.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 24.Grima B, Lamouroux A, Blanot F, Biguet NF, Mallet J. Complete coding sequence of rat tyrosine hydroxylase mRNA. Proc Natl Acad Sci U S A. 1985;82:617–21. doi: 10.1073/pnas.82.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- 26.Hebert MA, Serova LI, Sabban EL. Single and repeated immobilization stress differentially trigger induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem. 2005;95:484–98. doi: 10.1111/j.1471-4159.2005.03386.x. [DOI] [PubMed] [Google Scholar]
- 27.Hjorth S, Suchowski CS, Galloway MP. Evidence for 5-HT autoreceptor-mediated, nerve impulse-independent, control of 5-HT synthesis in the rat brain. Synapse. 1995;19:170–6. doi: 10.1002/syn.890190304. [DOI] [PubMed] [Google Scholar]
- 28.Jankowski MP, Sesack SR. Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol. 2004;468:518–29. doi: 10.1002/cne.10976. [DOI] [PubMed] [Google Scholar]
- 29.Jodo E, Chiang C, Aston-Jones G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience. 1998;83:63–79. doi: 10.1016/s0306-4522(97)00372-2. [DOI] [PubMed] [Google Scholar]
- 30.Jones BE, Harper ST, Halaris AE. Effects of locus coeruleus lesions upon cerebral monoamine content, sleep-wakefulness states and the response to amphetamine in the cat. Brain Res. 1977;124:473–96. doi: 10.1016/0006-8993(77)90948-9. [DOI] [PubMed] [Google Scholar]
- 31.Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of noradrenaline in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur J Pharmacol. 2000;387:279–86. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- 32.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int. 1998;33:287–97. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 34.Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW. The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus coeruleus. Neuroscience. 2006;142:1281–92. doi: 10.1016/j.neuroscience.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 35.Lee HS, Kim MA, Valentino RJ, Waterhouse BD. Glutamatergic afferent projections to the dorsal raphe nucleus of the rat. Brain Res. 2003;963:57–71. doi: 10.1016/s0006-8993(02)03841-6. [DOI] [PubMed] [Google Scholar]
- 36.Levine ES, Litto WJ, Jacobs BL. Activity of cat locus coeruleus noradrenergic neurons during the defense reaction. Brain Res. 1990;531:189–95. doi: 10.1016/0006-8993(90)90773-5. [DOI] [PubMed] [Google Scholar]
- 37.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation Of The Rate-Limiting Step In Norepinephrine Biosynthesis In The Perfused Guinea-Pig Heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- 38.Levitt P, Moore RY. Noradrenaline neuron innervation of the neocortex in the rat. Brain Res. 1978;139:219–31. doi: 10.1016/0006-8993(78)90925-3. [DOI] [PubMed] [Google Scholar]
- 39.Maier SF, Albin RW, Testa TJ. Failure to learn to escape in rats previously exposed to inescapable shock depends on nature of escape response. J Comp Physiol Psychol. 1973;85:581–592. [Google Scholar]
- 40.Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–20. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- 41.Melia KR, Rasmussen K, Terwilliger RZ, Haycock JW, Nestler EJ, Duman RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase in the rat locus coeruleus: effects of chronic stress and drug treatments. J Neurochem. 1992;58:494–502. doi: 10.1111/j.1471-4159.1992.tb09748.x. [DOI] [PubMed] [Google Scholar]
- 42.Molderings GJ, Werner K, Likungu J, Gothert M. Inhibition of noradrenaline release from the sympathetic nerves of the human saphenous vein via presynaptic 5-HT receptors similar to the 5-HT 1D subtype. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:371–7. doi: 10.1007/BF00169451. [DOI] [PubMed] [Google Scholar]
- 43.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–68. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 44.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–7. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 45.Nakane H, Shimizu N, Hori T. Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am J Physiol. 1994;267:R1559–66. doi: 10.1152/ajpregu.1994.267.6.R1559. [DOI] [PubMed] [Google Scholar]
- 46.Pare WP. Enhanced retrieval of unpleasant memories influenced by shock controllability, shock sequence, and rat strain. Biol Psychiatry. 1996;39:808–13. doi: 10.1016/0006-3223(95)00220-0. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press; Sydney; Orlando: 1986. [Google Scholar]
- 48.Richard F, Faucon-Biguet N, Labatut R, Rollet D, Mallet J, Buda M. Modulation of tyrosine hydroxylase gene expression in rat brain and adrenals by exposure to cold. J Neurosci Res. 1988;20:32–7. doi: 10.1002/jnr.490200106. [DOI] [PubMed] [Google Scholar]
- 49.Rusnak M, Gainer H. Differential effects of forskolin on tyrosine hydroxylase gene transcription in identified brainstem catecholaminergic neuronal subtypes in organotypic culture. Eur J Neurosci. 2005;21:889–98. doi: 10.1111/j.1460-9568.2005.03913.x. [DOI] [PubMed] [Google Scholar]
- 50.Rusnak M, Zorad S, Buckendahl P, Sabban EL, Kvetnansky R. Tyrosine hydroxylase mRNA levels in locus ceruleus of rats during adaptation to long-term immobilization stress exposure. Mol Chem Neuropathol. 1998;33:249–58. doi: 10.1007/BF02815186. [DOI] [PubMed] [Google Scholar]
- 51.Schlicker E, Gothert M. Interactions between the presynaptic alpha2-autoreceptor and presynaptic inhibitory heteroreceptors on noradrenergic neurones. Brain Res Bull. 1998;47:129–32. doi: 10.1016/s0361-9230(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 52.Short KR, Maier SF. Stressor controllability, social interaction, and benzodiazepine systems. Pharmacol Biochem Behav. 1993;45:827–35. doi: 10.1016/0091-3057(93)90128-g. [DOI] [PubMed] [Google Scholar]
- 53.Smith MA, Brady LS, Glowa J, Gold PW, Herkenham M. Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in the locus ceruleus by in situ hybridization. Brain Res. 1991;544:26–32. doi: 10.1016/0006-8993(91)90881-u. [DOI] [PubMed] [Google Scholar]
- 54.Stachowiak MK, Goc A, Hong JS, Poisner A, Jiang HK, Stachowiak EK. Regulation of tyrosine hydroxylase gene expression in depolarized non-transformed bovine adrenal medullary cells: second messenger systems and promoter mechanisms. Brain Res Mol Brain Res. 1994;22:309–19. doi: 10.1016/0169-328x(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 55.Staub E, Tursky B, Schwartz GE. Self-control and predictability: their effects on reactions to aversive stimulation. J Pers Soc Psychol. 1971;18:157–62. doi: 10.1037/h0030851. [DOI] [PubMed] [Google Scholar]
- 56.Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–71. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- 57.Sutker PB, Uddo-Crane M, Allain AN. Clinical and research assessment of posttraumatic stress disorder: A conceptual overview. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:520–530. [Google Scholar]
- 58.Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- 59.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J Comp Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 60.Swenson RM, Vogel WH. Plasma Catecholamine and corticosterone as well as brain catecholamine changes during coping in rats exposed to stressful footshock. Pharmacol Biochem Behav. 1983;18:689–93. doi: 10.1016/0091-3057(83)90007-2. [DOI] [PubMed] [Google Scholar]
- 61.Szot P, Reigel CE, White SS, Veith RC. Alterations in mRNA expression of systems that regulate neurotransmitter synaptic content in seizure-naive genetically epilepsy-prone rat (GEPR): transporter proteins and rate-limiting synthesizing enzymes for norepinephrine, dopamine and serotonin. Brain Res Mol Brain Res. 1996;43:233–45. doi: 10.1016/s0169-328x(96)00184-2. [DOI] [PubMed] [Google Scholar]
- 62.Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, Fornal CA, Jacobs BL. Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res. 2005;162:299–306. doi: 10.1016/j.bbr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka T, Yokoo H, Mizoguchi K, Yoshida M, Tsuda A, Tanaka M. Noradrenaline release in the rat amygdala is increased by stress: studies with intracerebral microdialysis. Brain Res. 1991;544:174–6. doi: 10.1016/0006-8993(91)90902-8. [DOI] [PubMed] [Google Scholar]
- 64.Tsuda A, Tanaka M. Differential changes in noradrenaline turnover in specific regions of rat brain produced by controllable and uncontrollable shocks. Behav Neurosci. 1985;99:802–17. doi: 10.1037//0735-7044.99.5.802. [DOI] [PubMed] [Google Scholar]
- 65.Van der Gugten J, Palkovits M, Wijnen HL, Versteeg DH. Regional distribution of adrenaline in rat brain. Brain Res. 1976;107:171–5. doi: 10.1016/0006-8993(76)90107-4. [DOI] [PubMed] [Google Scholar]
- 66.Wang P, Kitayama I, Nomura J. Tyrosine hydroxylase gene expression in the locus coeruleus of depression-model rats and rats exposed to short-and long-term forced walking stress. Life Sci. 1998;62:2083–92. doi: 10.1016/s0024-3205(98)00183-0. [DOI] [PubMed] [Google Scholar]
- 67.Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29:943–8. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- 68.Watanabe Y, McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Brain Res Mol Brain Res. 1995;32:176–80. doi: 10.1016/0169-328x(95)00081-3. [DOI] [PubMed] [Google Scholar]
- 69.Weiss JM, Goodman PA, Losito BG, Corrigan S, Charry JM, Bailey WH. Behavioral depression produced by an uncontrollable stressor: relationship to norepinephrine, dopamine, and serotonin levels in various regions of rat brain. Brain Res Rev. 1981;3:167–205. [Google Scholar]
- 70.Weiss JM, Stone EA, Harrell N. Coping behavior and brain norepinephrine level in rats. J Comp Physiol Psychol. 1970;72:153–60. doi: 10.1037/h0029311. [DOI] [PubMed] [Google Scholar]
- 71.Yokoo H, Tanaka M, Yoshida M, Tsuda A, Tanaka T, Mizoguchi K. Direct evidence of conditioned fear-elicited enhancement of noradrenaline release in the rat hypothalamus assessed by intracranial microdialysis. Brain Res. 1990;536:305–8. doi: 10.1016/0006-8993(90)90039-e. [DOI] [PubMed] [Google Scholar]