Abstract
Variation in hemispheric asymmetry of the planum temporale (PT) has been related to verbal ability. The degree to which genetic and environmental factors mediate PT asymmetry is not known. This study examined the heritability for planar asymmetry in 12 dizygotic (DZ) and 27 monozygotic (MZ) male twin pairs who were between 6 and 16 years of age. There was weak but positive evidence for heritability of planar asymmetry. Co-twin similarity for planar asymmetry and Sylvian fissure morphology increased when excluding twins discordant for writing hand and when excluding twins exhibiting birth weight differences >20% from the analyses. Birth weight differences were also related to twin differences in total cerebral volume, but not central sulcus asymmetry. These results suggest that exogenous perinatal factors affect the epigenesis of planar asymmetry development.
Introduction
Perisylvian cortex engages in language-related information processing (Ojemann, 1983). For this reason, neuroanatomical structures within the perisylvian region have been targets for studies attempting to explain the neurobiological underpinnings of reading and language disability. Symmetry or reversed asymmetry of the planum temporale (PT) is consistently related to language impairment (Plante et al., 1991; Gauger et al., 1997) and poor verbal ability (Rumsey et al., 1997; Eckert and Leonard, 2000; Eckert et al., 2001). Individual variability in the PT is thought to reflect the phenotypic expression of genes involved in garden variety reading impairment (low verbal ability) and language impairment (Leonard et al., 2001).
The causal factors for the development of reversed PT asymmetry have not been identified. Reversed planar asymmetry has been reported in children with a family history for reading disability (Eckert et al., 2001) and in children with congenital adrenal hyperplasia and their siblings (Plante et al., 1996). The relative impact of genetic and environmental factors on PT asymmetry is not known, however. Steinmetz and colleagues did not find significant concordance in planar asymmetry among 20 pairs of monozygotic (MZ) twins (Steinmetz et al., 1995). This finding could have been influenced by the inclusion of twins discordant for handedness. Right-handed twin pairs exhibited a trend for planar asymmetry concordance.
Studies of brain structure concordance in twins show that MZ twin brains are the same size and look similar in shape, but vary with respect to some major and most minor sulci. Brain volume is the most heritable neuroanatomical feature in twins (Bartley et al., 1997; Biondi et al., 1998; Tramo et al., 1998; Pennington et al., 2000). Genetic models account for the majority of variance in concordance for brain volume between MZ and dizygotic (DZ) twins, but fail to explain much variation in gyral/sulcal topography. Deep sulci appear to be more strongly genetically determined than superficial sulci (Biondi et al., 1998; Lohmann et al., 1999). Gyral and sulcal features are probably shaped more by experience given a 7–17% heritability estimate for gyral patterns (Bartley et al., 1997).
Fetal brains exhibit planar asymmetry by 29–31 weeks in gestation (Wada et al., 1975; Chi et al., 1977). The weak genetic findings for gyri and sulci suggest that pre- and postnatal events can affect PT asymmetry development and disrupt twin concordance in brain structure. For example, hormones have been proposed to affect planar asymmetry (Geschwind and Galaburda, 1987). The atypical planar asymmetry findings in congenital adrenal hyperplasia, in which absence of 21-hydroxylase leads to high levels of intrauterine testosterone, support this theory.
A large number of antenatal variables, including chorionic status (Machin et al., 1995; Charlemaine et al., 2000; Victoria et al., 2001) and post-zygotic genetic effects can produce phenotypic differences between MZ twin pairs (Martin et al., 1997). Chorionic status, due in large part to twin-to-twin transfusion syndrome (TTTS), is frequently associated with cognitive and birth weight differences in twins (James, 1982; O’Brien and Hay, 1987; Charlemaine et al., 2000).
This study examined PT asymmetry concordance in 27 male MZ twins and 12 DZ twins. Planar asymmetry was predicted to exhibit significant heritability based on evidence that planar symmetry is related to a family history of reading disability. Birth weight differences, as an index of TTTS, were also examined to determine if this perinatal risk factor was related to twin discordance in planum measures.
Materials and Methods
Participants
Twenty-seven MZ and 12 DZ twin pairs were recruited from the American Academy of Child and Adolescent Psychiatry, the Virginia Commonwealth University Twin Registry, Children and Adults with Attention Deficit Disorder and the National Organization of Mothers of Twins Clubs. Female twin pairs were excluded from this study to control for random X-inactivation that might lead to more dissimilarity among female MZ twins (Jorgensen et al., 1992). Zygosity was verified using 9–14 unlinked short tandem repeat loci, by BRT Laboratories Inc. (Baltimore, MD). MZ cases which did not yield a probability of twinship >99% were tested further for a total of 21 loci. Written assent from the child and consent from the parents were obtained for each participant. This project was approved by the National Institute of Mental Health (NIMH) and the University of Florida Institutional Review Boards.
Demographic characteristics of the twins are presented in Table 1. The age range was 6.9–16.4 years for the MZ group and 6.1–15.0 years in the DZ group. MZ children were older than DZ children by 1.7 years, but this difference was not statistically significant [t(1,37) = 1.85, P < 0.10]. Socioeconomic status (SES) was determined using the Hollingshead inventory (Hollingshead, 1975) and did not differ between the MZ (range, 20–77) and DZ (range, 20–73) groups. Quantitative handedness measures were not available for this study. Handedness was defined by writing hand. Operationally defining handedness by writing hand overestimates right-handedness and underestimates the proportion of ambidextrous participants. There were no zygosity differences for SES [t(1,37) = 0.43, n.s.] or writing hand [χ2(1,74) = 0.47, n.s.].
Table 1.
Demographic characteristics of the MZ and DZ twins
| MZ twins | DZ twins | |
|---|---|---|
| Age (years) | 11.9 (2.7) | 9.89 (2.8) |
| SES (Hollingshead) | 42.9 (16.1) | 34.9 (13.1) |
| Writing hand (% right) | 83% (41/47) | 92% (22/24) |
| Writing hand concordance | 74% (17/23) | 83% (10/12) |
Writing hand information missing for one MZ twin.
Records indicating whether twins were monochorionic, a risk factor for TTTS, were not available for many twins. It has been suggested (Tan et al., 1979) that a birth weight difference >20% can be used as a proxy for TTTS. MZ twins in this study were classified as having birth weight differences >20% as an index of twin transfusion syndrome. Birth weights and gestation length were determined by parental report and corroborated with medical records when medical records were available.
MRI Protocol and Measurement Methods
MRI Acquisition
Volumetric 1.5 mm thick axial images were acquired using a GE 1.5 T Signa scanner. Scan parameters consisted of a repetition time of 24 ms, an echo time of 5 ms, flip angle of 45°, a 24 cm field of view and a 192 × 256 matrix. These images were acquired at the National Institutes of Health.
Image Processing
Brain structure data collection for this study was performed at the University of Florida Mcknight Brain Institute. The images were reformatted into 1 mm thick sagittal sections to correct for tip in the coronal, axial and sagittal planes of section. Parameter files were created that stored the distance between the anterior commissure and borders of the brain. Talairach coordinates were used to identify the same medial to lateral locations in each brain. These coordinates are reliable for sagittal positions. The Talairach system standardizes positions by relating them to a brain atlas where the horizontal plane intersects the anterior and posterior commissure. The images were not warped or altered during the reformatting process. Each image was assigned a new random number to ensure that raters were blind to the zygosity and pairing of the twins.
Data Collection Procedures
Surface area measurements were obtained for the PT (Leonard, 2001). The Sylvian fissure is surrounded by horizontal and vertical planes of cortical tissue. The PT is defined by the horizontal bank, which extends from Heschl’s sulcus to the origin of the vertical bank or posterior ascending ramus (PP). The PP, also called the planum parietale, rises from the termination of the PT into the parietal cortex. The surface area of the PT and PP was measured between 46 and 56 cm lateral to the midline in sagittal sections. Asymmetry of the planum is most dramatic in this lateral region of the PT (Best and Demb, 1999). These coordinates were also chosen in order to replicate the methods of previous magnetic resonance imaging (MRI) studies showing cognitive associations with PT measures (Foundas et al., 1994; Leonard et al., 1996; Gauger et al., 1997; Eckert et al., 2001). Raters were blind to zygosity and twin pairs. Intra-rater reliability was 0.94. Inter-rater reliability was 0.87.
In addition, the morphology of the left and right Sylvian fissure was categorized according to Witelson and Kigar classification criteria (Witelson and Kigar, 1992). Witelson and Kigar classify Sylvian fissure morphology into three categories based on the orientation of the Sylvian fissure, posterior to Heschl’s gyrus. The horizontal and vertical type (HV) is defined by the presence of both a horizontal and a vertical branch of the Sylvian fissure. The horizontal type (H) is determined by the absence of a vertical branch. The vertical type (V) does not exhibit a horizontal branch. The V type is more frequently found in the right hemisphere when there is a very small PT. Intra-rater classification reliability was 0.94 and 90% of cases were consistently classified between raters.
A non-language-related brain asymmetry was also examined. Surface area measurements of the central sulcus (CS) were made in sagittal sections that included the hand bump (Penfield and Boldrey, 1937; Kim et al., 1993; Yousry et al., 1997). The boundaries for this region were Talairach coordinates 32–55 mm. The anterior bank of the CS was measured from the base of the CS to the peak of the posterior segment of the precentral gyrus. The posterior bank of the CS was measured from the base of the CS to the peak of the anterior segment to the postcentral gyrus. The anterior and posterior bank measurements are highly correlated and because there is no clear anatomical boundary between motor and sensory cortex within the CS, the anterior and posterior measures were added together. Intra-rater reliability was 0.97 and inter-rater reliability was 0.90.
Total cerebral volume (TCV) was also measured to determine if co-twin relations in brain asymmetries were driven by brain size. Quantitative analysis of TCV was performed using the Montreal Neurological Institute ANIMAL and INSECT methods. These methods produce brain volumes using both a voxel-intensity-based artificial neural network technique and information from a non-linear registration-based regional segmentation approach (Collins et al., 1999).
Statistical Analyses
Chi-square, Pearson correlations and t-tests were performed to determine if variables such as writing hand, birth order and SES might confound the interpretation of the co-twin results between zygosity groups. Pearson correlations were used to examine the co-twin anatomical relations. Results from all the exploratory demographic analyses are presented and should be viewed with caution due to the possibility for type 1 error.
Results
Writing Hand and Sylvian Fissure Morphology
All left-handed DZ (n = 2) and MZ (n = 6) participants were second born [χ2(1,37) = 31.8, P < 0.0001] and had co-twins who were right-handed. MZ twins discordant for handedness had a shorter gestation than MZ twins concordant for writing hand [t(1,25) = 2.24, P < 0.05].
Partial correlations, controlling for twin pair, were performed to examine the relation of the planum measures to writing hand. Table 2 shows that writing hand was related to PT asymmetry in MZ and DZ twins. This finding was due to the high prevalence of a right hemisphere type V Sylvian fissure morphology in participants with a left writing hand. Six of eight left-hand writers had a type V compared to 4 of 68 right-hand writers [controlling for twin pair: partial r(1,73) = 0.619, P < 0.001].
Table 2.
Writing hand predicts to planum measures
| LPT | RPT | PTA | LPP | RPP | PPA | |
|---|---|---|---|---|---|---|
| DZ | 0.259 | −0.273 | 0.509a | −0.242 | 0.166 | −0.334 |
| MZ | 0.175 | −0.357a | 0.478b | −0.084 | 0.281a | −0.203 |
P < 0.05;
P < 0.005; L, left hemisphere; R, right hemisphere; A, asymmetry.
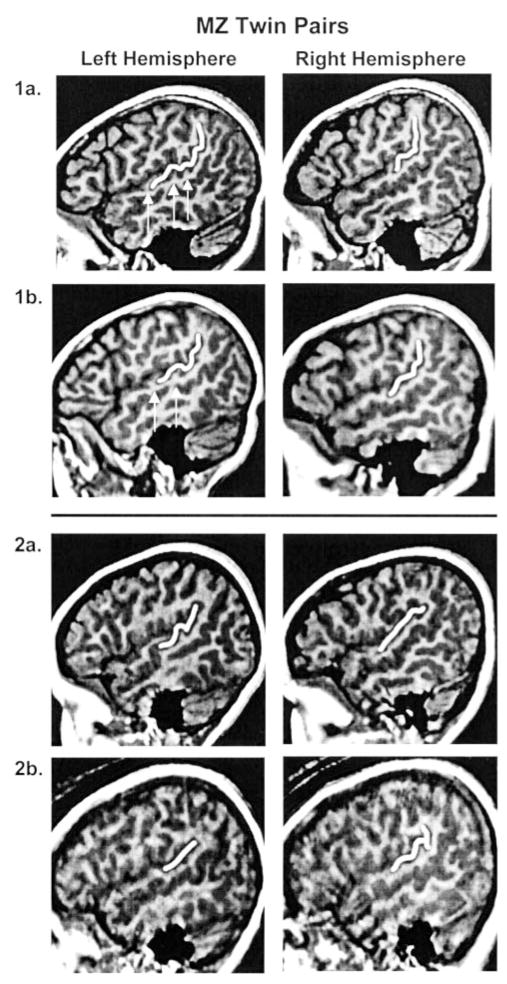
The Witelson and Kigar classification method was also used to determine if the left and right Sylvian fissures of one twin exhibited the opposite hemispheric morphology of the co-twin. There were no hemispheric reversals of Sylvian fissure morphology in twins discordant for writing hand. One pair of right-handed MZ twins exhibited reversals of Sylvian fissure morphology. The morphology of surrounding perisylvian cortex of one twin was not, however, a reversed mirror image of the co-twin’s morphology (Fig. 1).
Figure 1.
Sagittal images of MZ twin pairs. Twin pair 1a,b exhibit similar Sylvian fissure morphology. Also note the similarity in brain shape, but variation in minor gyri and sulci (arrows). Twin pair 2a,b exhibit hemispheric reversals in Witelson and Kigar Sylvian fissure classification, but they are not mirror image reversals. Images created at Talairach position 50 for the right hemisphere and 47 for the left hemisphere. The PT and PP are outlined in each image.
MZ twins were more likely than DZ twins to be concordant for right hemisphere [χ2(1,38) = 3.79, P < 0.10] and bilateral Sylvian fissure morphology [χ2(1,38) = 4.79, P < 0.05]. There was not a significant difference between the percentage of MZ twins (19%) and DZ twins (33%) that were discordant for left hemisphere Sylvian fissure morphology [χ2(1,38) = 1.03, n.s.].
Neuroanatomical Concordance
Pearson correlations between co-twin anatomical measures are presented in Table 3. As expected, total cerebral volume was significantly correlated between MZ and DZ co-twins. The planar asymmetry correlation coefficient approached significance in the MZ twins. Power analysis for the planar asymmetry relation in MZ twins indicated there was low power (PT asymmetry β= 0.34).
Table 3.
PTA ICC for DZ and MZ twins
| LPT | RPT | PTA | LPP | RPP | PPA | LCS | RCS | CSA | TCV | |
|---|---|---|---|---|---|---|---|---|---|---|
| DZ twins | −0.171 | 0.105 | −0.235 | −0.052 | 0.366 | −0.193 | 0.225 | 0.184 | −0.343 | 0.571b |
| MZ twins | 0.218 | 0.161 | 0.303 | 0.133 | −0.149 | 0.162 | 0.331a | 0.563c | 0.098 | 0.944d |
P < 0.10;
P < 0.05;
P < 0.005;
P < 0.001; L, left hemisphere; R, right hemisphere; A, asymmetry.
Demographic and Perinatal Factors Affecting Planum Concordance
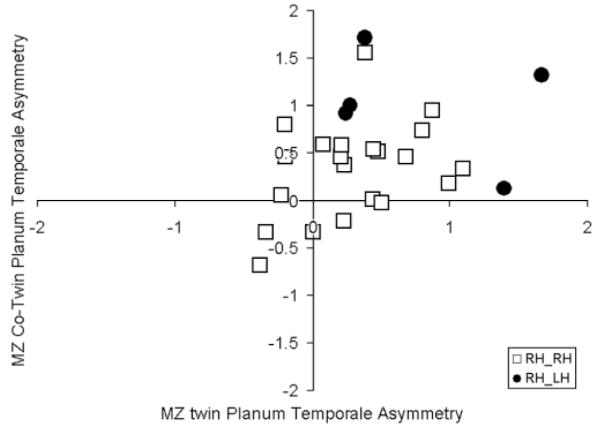
MZ twin pairs discordant for writing hand exhibited significantly greater absolute differences in PT asymmetry [t(24) = 2.08, P < 0.05]. Figure 2 shows the planar asymmetry relation between MZ co-twins, coded by writing hand discordance. Excluding twins discordant for writing hand improved the planar asymmetry correlation between MZ co-twins [r(20) = 0.377, n.s.], but not the significance value.
Figure 2.
PT asymmetry relation between MZ twins by writing hand concordance (RH_RH = right-handed twin 1 and right-handed twin 2; RH_LH = right-handed twin 1 and left-handed twin 2).
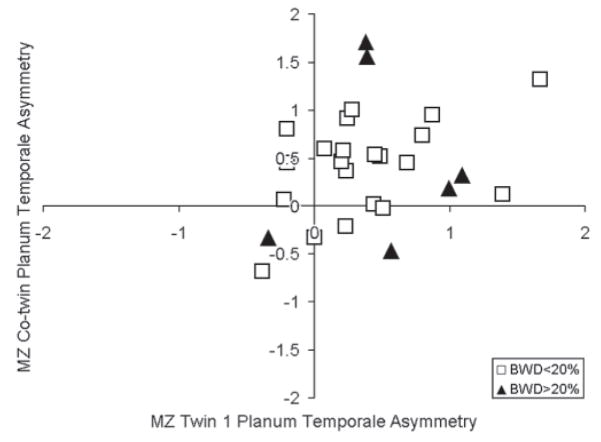
MZ twin pairs with birth weight differences >20% exhibited greater absolute differences in PT asymmetry [t(25) = 2.67, P < 0.05]. Figure 3 shows the planar asymmetry relation between MZ co-twins, coded by birth weight differences. Excluding twins with birth weight differences (>20%) improved the planar asymmetry correlation between MZ co-twins [r(21) = 0.437, P < 0.05]. The increase in planar asymmetry relation was due to the exclusion of twins with birth weight differences (>20%) that exhibited discrepant left Sylvian fissure morphology.
Figure 3.
PTA relation between MZ twins by writing birth weight differences greater than 20% (BWD > 20%).
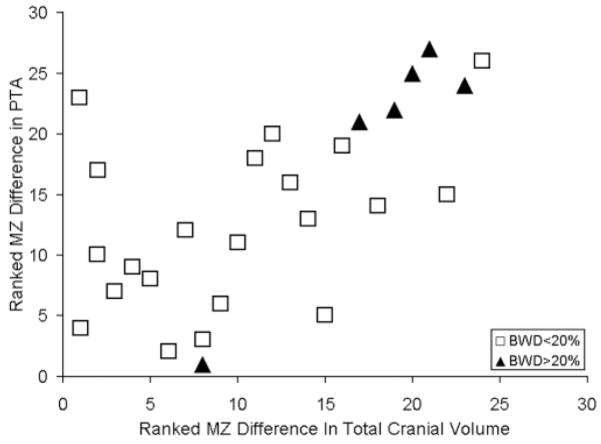
MZ twin pairs with birth weight differences >20% also exhibited the greatest differences in TCV [t(25) = 2.70, P < 05]. Figure 4 shows that MZ twins with the greatest differences in TCV had the greatest differences in planar asymmetry [r(26) = 0.612, P < 0.001]. Figure 4 also shows that this relation was due, in part, to twins with birth weight differences (>20%). Discord- ance for central sulcus asymmetry was not explained, however, by birth weight differences [t(25) = 0.48, n.s.].
Figure 4.
MZ twins exhibiting the largest PT asymmetry differences also exhibit the largest total cerebral volume differences in the group.
Discussion
The gyral and sulcal features of monozygotic twins are surprisingly dissimilar. Monozygotic twin discordance in brain structure appears to be due, in part, to intrauterine events that could lead to divergent morphological development. Weak relations for co-twin PT measures were found for MZ and DZ twins. There was an increase in MZ twin planum similarity when twins with birth weight differences (>20%) were excluded from the analyses. These findings suggest that perinatal pathological events can disrupt concordant expression of brain morphology in MZ twins.
The Twin Design and Perinatal Events
The twin paradigm for estimating genetic effects for various phenotypes has received criticism for over 50 years (Price, 1950). Genetic estimates may be inflated for some traits mediated by parental care (Saudino et al., 2000), but it is also likely that genetic effects for other traits, such as brain structure, are underestimated. Although MZ twins are frequently described as identical, antenatal environmental events and post-zygotic genetic effects produce discordance in MZ twins (Martin et al., 1997). Teratogens, infection, length of delivery, post-zygotic non-disjunction, differential imprinting, skewed X-inactivation and major gene malformations have been related to phenotypic differences in twins. These are some reasons power analyses indicate that at least 200 twin pairs are necessary to estimate a highly heritable quantitative phenotype (Martin et al., 1978).
The uniqueness of twinning is another criticism. Events related to placentation are unique for twins and limit strict comparisons to singleton development. TTTS is an example of a developmental problem largely specific to monochorionic twins (Gaziano et al., 2000). In TTTS, the arterial vasculature of one twin is shared with the venous vasculature of the other twin. This causes one twin to transfuse the co-twin, leading to hypoxia, reduced concentrations of essential amino acids, growth retardation (Gall, 1996) and depressed IQ (Munsinger, 1977) in the donor twin and hyperperfusion of the recipient twin. TTTS could alter normal brain development for the donor or recipient twin.
The increased frequency of perinatal risk factors in monozygotic monochorionic twins has led some to suggest that heritability estimates should be based on comparisons of MZ dichorionic twins to DZ twins (Corey et al., 1979). The relation between twin birth weight difference (>20%) and differences in planar asymmetry supports this point. Valid estimates of brain structure heritability must take into account intrauterine events unique to twins and chorionic status in particular.
Alternatively, MZ twins provide a model for identifying environmental variables that influence human cortical development. For example, maternal drug use has been related to discordance for congenital structural anomalies in a pair of MZ twins (Reitnauer et al., 1997). Examining the relation between brain structure concordance and the timing of pathological events could provide evidence as to when particular neuroanatomical features are most susceptible to perinatal insults.
Comparison to Other Imaging Twin Studies
The heritability findings for planar asymmetry are similar to findings from other twin studies for gyral and sulcal topography. It has been estimated (Bartley et al., 1997) that 7–17% of gyral patterning was due to genetic influences in 10 MZ and nine DZ adult twin pairs. In another small study, low heritability estimates were also reported for temporal lobe regions (Tramo et al., 1998). Figure 1 illustrates these findings. Even MZ twins that exhibit the same Sylvian fissure morphology demonstrate differences in tertiary gyri and sulci.
Planar asymmetry discordance and the high frequency of left hemisphere Sylvian fissure discordance in twins exhibiting birth weight differences (>20%) suggest that Sylvian fissure morphology can be affected by perinatal events. In addition, Ajayi-Obe and coworkers found that a group of infants born prematurely exhibited less gyral and sulcal complexity than full term infants, despite their similar cerebral volumes and age corrected for prematurity (Ajayi-Obe et al., 2000). The association of perinatal events with cortical development helps to explain the low heritability estimates reported by previous small sample twin studies.
Only one other study has examined PT concordance in twins (Steinmetz et al., 1995). They did not find significant concordance for planar asymmetry in 20 monozygotic twin pairs. Steinmetz et al. did report that twins discordant for handedness exhibited large discrepancies in planar asymmetry. All but one of the left-handers in the Steinmetz et al. study exhibited symmetry or reversed planar asymmetry. In contrast, the left-handed twins in this study exhibited leftward asymmetry. These left-hand writers were all second-born and more likely to be born prematurely. Hopkins et al. have shown that chimpanzee handedness is heritable (Hopkins et al., 2001), but the degree of heritability is modified by offspring parity (developmental instability). An uncoupling of handedness and planar asymmetry in this study could have occurred because of pathological events. There are too few cases, however, to discount a more stochastic explanation for the handedness findings.
Anatomical Specificity
Birth weight differences accounted for differences in MZ twin Sylvian fissure morphology, planar asymmetry and total cerebral volume. There was not a similar explanation for discordance in central sulcus asymmetry, however. Variation in central sulcus asymmetry was also unrelated between co-twins. This replicated finding (Bonan et al., 1998) suggests there are not strong genetic effects on central sulcus asymmetry. The modulating effects of perinatal events on development may be most easily seen for genetically mediated phenotypes.
If TTTS explains the birth weight differences, then the timing of TTTS inf luences could provide insight into planum development. The development of TTTS is not well understood, however. The influences of TTTS may be greatest after 20 weeks gestation, when placental vascular patterns are stabilized (Sebire et al., 2001). This timing appears to coincide with the period of gestation when the Sylvian fissure is developing and planar asymmetry is first seen (Wada et al., 1975; Chi et al., 1977; Bernard et al., 1988).
Developing Asymmetry
Individuals with situs inversus (reversed asymmetry of visceral organs) have been studied to determine if the same mechanisms for visceral organ asymmetry affect cerebral asymmetry. Three cases of situs inversus exhibited reversed frontal and occipital petalia (Kennedy et al., 1999). Other evidence of anomalous laterality was not found. All three subjects were right-handed, had a left hemisphere language dominance and 2/3 had leftward planar asymmetry. Although one case of right hemisphere aphasia has been reported in a stroke patient with situs inversus (Cohen et al., 1993), most situs inversus studies do not report an increased incidence of left-handedness or anomalous language organization (Woods, 1986; Tanaka et al., 1999). These studies do not support the idea that mechanisms affecting visceral organ asymmetries are related to cortical asymmetries. Perhaps similar, but different genetic mechanisms direct the development of cerebral asymmetry than for visceral organ asymmetry (Alexander and Annett, 1996).
Although little is known about the events that produce cerebral asymmetry, considerable progress has been made in understanding the cascade of events leading to asymmetric development of visceral organs. Visceral organ asymmetry is dependent on asymmetric expression of key regulatory proteins. Activin inhibits the expression of sonic hedgehog (Shh) on the right, but not left side of the primitive streak (Levin et al., 1995). Left-sided expression of Shh leads to the expression of transforming growth factor beta (TGF-β) by Nodal, which has downstream effects on the expression of Pitx2 (Levin et al., 1995). Asymmetric expression of Pitx2 leads to asymmetric development of the heart, lungs, pituitary and pineal body (Lin et al., 1999; Liang et al., 2000). The conservation of these molecular events across frogs, chickens and mice is strong evidence that similar mechanisms could regulate human asymmetries.
Discordant asymmetry may occur in twins because of placentation and orientation of embryos. Obliquely conjoined twins provide support for this hypothesis. In conjoined twins, the twin on the right frequently exhibits laterality deficits (Levin et al., 1996). Levin suggests the parallel orientation of the two primitive streaks allows activin on the right side of the left embryo to inhibit Shh on the left side of the right embryo. This mechanism could explain mirrored lateralization for brain function in MZ twins (Sommer et al., 1999), but only for monochorionic monoamniotic MZ twins who make up <1% of twins (Hill et al., 1996).
The Epigenesis of Planum Temporale Asymmetry
The cascade of regulatory events guiding left and right plana development could begin around 21 weeks when the Sylvian fissure develops (Bernard et al., 1988) and taper off around 29–31 weeks when planar asymmetry is first discernible (Wada et al., 1975; Chi et al., 1977). The weak heritability estimates in this study suggest that large numbers of families would be necessary to find genetic linkage for planum development. Identification of genetic expression patterns regulating planum development may require microarray studies of post-mortem human or chimpanzee fetal brains.
A large number of factors, including nutrition, probably affect genomic to synaptic levels of planum development. For example, vitamin A deficient quail embryos exhibit cardiac situs inversus. Administration of retinoic acid rescues the expression of nodal and Pitx2, and produces normal cardiac development (Zile et al., 2000). Teratogens might also affect planar asymmetry. Prenatal alcohol exposure produces neuronal migration errors in rat cortex (Hirai et al., 1999) and has been related to changes in white and grey matter in the left hemisphere temporal-parietal region of children (Sowell et al., 2001).
Summary
This study supports suggestions that PT development is mediated by genetic and experiential factors (Habib and Galaburda, 1986). Monozygotic twins exhibit weak concordance for planar asymmetry, due in part to modulating factors related to birth weight differences and writing hand discordance. These findings suggest that intrauterine events can alter the direction of brain development between twins and highlight the importance of having large twin pair samples when estimating neuroanatomical heritability. On the other hand, MZ twins may be a good model for identifying specific perinatal events and the timing of those events that negatively affect brain development and function.
Acknowledgments
We would like to thank the NIDCD (F32 00393-1) for their generous support of this research.
References
- Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356:1162–1163. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Annett M. Crossed aphasia and related anomalies of cerebral organization: case reports and a genetic hypothesis. Brain Lang. 1996;55:213–239. doi: 10.1006/brln.1996.0102. [DOI] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120:257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Bernard C, Droulle P, Didier F, Gerard H, Larroche JC, Plenat F, Bomsel F, Roland J, Hoeffel JC. Echographic aspects of cerebral sulci in the ante- and perinatal period. J Radiol. 1988;69:521–532. [PubMed] [Google Scholar]
- Best M, Demb J. Normal planum temporale asymmetry in dyslexics with a magnocellular deficit. Neuroreport. 1999;10:607–612. doi: 10.1097/00001756-199902250-00030. [DOI] [PubMed] [Google Scholar]
- Biondi A, Nogueira H, Dormont D, Duyme M, Hasboun D, Zouaoui A, Chantome M, Marsault C. Are the brains of monozygotic twins similar? A three-dimensional MR study. Am J Neuroradiol. 1998;19:1361–1367. [PMC free article] [PubMed] [Google Scholar]
- Bonan I, Argenti AM, Duyme M, Hasboun D, Dorion A, Marsault C, Zouaoui A. Magnetic resonance imaging of cerebral central sulci: a study of monozygotic twins. Acta Genet Med Gemellol (Roma) 1998;47:89–100. doi: 10.1017/s000156600000026x. [DOI] [PubMed] [Google Scholar]
- Charlemaine C, Duyme M, Ville Y, Aurengo A, Tremblay R, Frydman R, Pons JC. Fetal biometric parameters, twin type and birth weight difference. A longitudinal study. Eur J Obstet Gynecol Reprod Biol. 2000;93:27–32. doi: 10.1016/s0301-2115(00)00239-6. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left, right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Cohen L, Geny C, Hermine O, Gray F, Degos JD. Crossed aphasia with visceral situs inversus. Ann Neurol. 1993;33:215–218. doi: 10.1002/ana.410330213. [DOI] [PubMed] [Google Scholar]
- Collins DI, Zijdenbos AD, Baare WFL, Evans AC. ANIMAL and INSECT: improved cortical structure segmentation. In: Kuba A, Samal M, Todd-Prokropek A, editors. Proceedings of the sixteenth international conference on information processing in medical imaging (IPMI); 1999. pp. 210–223. [Google Scholar]
- Corey LA, Nance WE, Kang KW, Christian JC. Effects of type of placentation on birthweight and its variability in monozygotic and dizygotic twins. Acta Genet Med Gemellol (Roma) 1979;28:41–50. doi: 10.1017/s0001566000009326. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM. Structural imaging in dyslexia; the planum temporale. Ment Retard Dev Disabil Res Rev. 2000;6:198–206. doi: 10.1002/1098-2779(2000)6:3<198::AID-MRDD7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Lombardino LJ, Leonard CM. Tipping the environmental playground: who is at risk for reading failure. Child Dev. 2001;72:988–1002. doi: 10.1111/1467-8624.00330. [DOI] [PubMed] [Google Scholar]
- Foundas A, Leonard CM, Gilmore R, Fennell E, Heilman KM. Planum temporale asymmetry and language dominance. Neuropsychologia. 1994;32:1225–1231. doi: 10.1016/0028-3932(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Gall S. Multiple pregnancy and delivery. St Louis, MO: Mosby; 1996. [Google Scholar]
- Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. J Speech Lang Hear Res. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- Gaziano EP, De Lia JE, Kuhlmann RS. Diamnionic monochorionic twin gestations: an overview. J Matern Fetal Med. 2000;9:89–96. doi: 10.1002/(SICI)1520-6661(200003/04)9:2<89::AID-MFM1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Galaburda A. Cerebral lateralization: biological mechanisms, association and pathology. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- Habib M, Galaburda AM. Biological determinants of cerebral dominance. Rev Neurol. 1986;142:869–894. [PubMed] [Google Scholar]
- Hill LM, Chenevey P, Hecker J, Martin JG. Sonographic determination of first trimester twin chorionicity and amnionicity. J Clin Ultrasound. 1996;24:305–308. doi: 10.1002/(SICI)1097-0096(199607/08)24:6<305::AID-JCU4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hirai K, Yoshioka H, Kihara M, Hasegawa K, Sawada T, Fushiki S. Effects of ethanol on neuronal migration and neural cell adhesion molecules in the embryonic rat cerebral cortex: a tissue culture study. Brain Res Dev Brain Res. 1999;118:205–210. doi: 10.1016/s0165-3806(99)00159-5. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social skills. New Haven, CT: Yale University; 1975. [Google Scholar]
- Hopkins W, Dahl J, Pilcher D. Genetic influence on the expression of hand preferences in chimpanzees (Pan troglodytes): evidence in support of the right-shift theory and developmental instability. Psychol Sci. 2001;12:299–303. doi: 10.1111/1467-9280.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WH. The IQ advantage of the heavier twin. Br J Psychol. 1982;73:513–517. doi: 10.1111/j.2044-8295.1982.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen AL, Philip J, Raskind WH, Matsushita M, Christensen B, Dreyer V, Motulsky AG. Different patterns of X inactivation in MZ twins discordant for red–green color-vision deficiency. Am J Hum Gen. 1992;51:291–298. [PMC free article] [PubMed] [Google Scholar]
- Kennedy DN, O’Craven KM, Ticho BS, Goldstein AM, Makris N, Henson JW. Structural and functional brain asymmetries in human situs inversus totalis. Neurology. 1999;53:1260–1265. doi: 10.1212/wnl.53.6.1260. [DOI] [PubMed] [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellerman JM, Merkle H, Ugurbil K, Georgopoulos AD. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Leonard CM. Structural imaging in children. Differentiating language disability and reading disability. Learn Disab Quart. 2001;24:158–176. [Google Scholar]
- Leonard CM, Lombardino LJ, Mercado LR, Browd SR, Breier JI, Agee OF. Cerebral asymmetry and cognitive development in children: a magnetic resonance imaging study. Psychol Sci. 1996;7:79–85. [Google Scholar]
- Leonard CM, Eckert MA, Lombardino LJ, Oakland T, Kranzler J, Mohr CM, King WM, Freeman AJ. Anatomical risk factors for phonological dyslexia. Cereb Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. A molecular pathway determining left–right asymmetry in chick embryogenesis. Cell. 1995;82:803–814. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- Levin M, Roberts DJ, Holmes LB, Tabin C. Laterality defects in conjoined twins. Nature. 1996;384:321. doi: 10.1038/384321a0. [DOI] [PubMed] [Google Scholar]
- Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- Lin CR, Kioussi C, O’Connell S, Briata P, Szeto D, Liu F, Izpisua-Belmonte JC, Rosenfeld MG. Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature. 1999;401:279–282. doi: 10.1038/45803. [DOI] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cereb Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Machin G, Bamforth F, Innes M, McNichol K. Some perinatal characteristics of monozygotic twins who are dichorionic. Am J Med Genet. 1995;55:71–76. doi: 10.1002/ajmg.1320550119. [DOI] [PubMed] [Google Scholar]
- Martin NG, Boomsma D, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997;17:387–392. doi: 10.1038/ng1297-387. [DOI] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- Munsinger H. The identical-twin transfusion syndrome: a source of error in estimating IQ resemblance and heritability. Ann Hum Genet. 1977;40:307–321. doi: 10.1111/j.1469-1809.1977.tb00195.x. [DOI] [PubMed] [Google Scholar]
- O’Brien PJ, Hay DA. Birthweight differences, the transfusion syndrome and the cognitive development of monozygotic twins. Acta Genet Med Gemellol (Roma) 1987;36:181–196. doi: 10.1017/s0001566000004414. [DOI] [PubMed] [Google Scholar]
- Ojemann G. Brain organization for language from the perspective of electrical stimulation mapping. Brain Behav Sci. 1983;6:189–230. [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, Filley CM, Galaburda A, DeFries JC. A twin MRI study of size variations in human brain. J Cogn Neurosci. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Plante E, Swisher L, Vance R, Rapcsak S. MRI findings in boys with specific language impairment. Brain Lang. 1991;41:52–66. doi: 10.1016/0093-934x(91)90110-m. [DOI] [PubMed] [Google Scholar]
- Plante E, Boliek C, Binkiewicz A, Erly WK. Elevated androgen, brain development and language/learning disabilities in children with congenital adrenal hyperplasia. Dev Med Child Neurol. 1996;38:423–437. doi: 10.1111/j.1469-8749.1996.tb15100.x. [DOI] [PubMed] [Google Scholar]
- Price B. Primary biases in twin studies. Am J Hum Genet. 1950;2:293–352. [PMC free article] [PubMed] [Google Scholar]
- Reitnauer PJ, Callanan NP, Farber RA, Aylsworth AS. Prenatal exposure to disulfiram implicated in the cause of malformations in discordant monozygotic twins. Teratology. 1997;56:358–362. doi: 10.1002/(SICI)1096-9926(199712)56:6<358::AID-TERA3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Donahue BC, Brady DR, Nace K, Giedd JN, Andreason P. A magnetic resonance imaging study of planum temporale asymmetry in men with developmental dyslexia. Arch Neurol. 1997;54:1481–1489. doi: 10.1001/archneur.1997.00550240035010. [DOI] [PubMed] [Google Scholar]
- Saudino KJ, Cherny SS, Plomin R. Parent ratings of temperament in twins: explaining the ‘too low’ DZ correlations. Twin Res. 2000;3:224–233. doi: 10.1375/136905200320565193. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Talbert D, Fisk NM. Twin-to-twin transfusion syndrome results from dynamic asymmetrical reduction in placental anastomoses: a hypothesis. Placenta. 2001;22:383–391. doi: 10.1053/plac.2001.0631. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Bouma A, Kahn RS. Cerebral mirror-imaging in a monozygotic twin. Lancet. 1999;354:1445–1446. doi: 10.1016/s0140-6736(99)04130-6. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Herzog A, Schlaug G, Huang Y, Jancke L. Brain (A) symmetry in monozygotic twins. Cereb Cortex. 1995;5:296–300. doi: 10.1093/cercor/5.4.296. [DOI] [PubMed] [Google Scholar]
- Tan KL, Tan R, Tan SH, Tan AM. The twin transfusion syndrome. Clinical observations on 35 affected pairs. Clin Pediatr. 1979;18:111–114. doi: 10.1177/000992287901800206. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kanzaki R, Yoshibayashi M, Kamiya T, Sugishita M. Dichotic listening in patients with situs inversus: brain asymmetry and situs asymmetry. Neuropsychologia. 1999;37:869–874. doi: 10.1016/s0028-3932(98)00144-4. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RL, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50:1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- Victoria A, Mora G, Arias F. Perinatal outcome, placental pathology, and severity of discordance in monochorionic and dichorionic twins. Obstet Gynecol. 2001;97:310–315. doi: 10.1016/s0029-7844(00)01111-x. [DOI] [PubMed] [Google Scholar]
- Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Kigar D. Sylvian fissure morphology and asymmetry in men and women: bilateral differences in relation to handedness in men. J Comp Neurol. 1992;323:326–340. doi: 10.1002/cne.903230303. [DOI] [PubMed] [Google Scholar]
- Woods RP. Brain asymmetries in situs inversus. A case report and review of the literature. Arch Neurol. 1986;43:1083–1084. doi: 10.1001/archneur.1986.00520100087021. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Akadhi H, Schmidt D, Peraud A, Buettner A, Winkler D. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Zile MH, Kostetskii I, Yuan S, Kostetskaia E, St Amand TR, Chen Y, Jiang W. Retinoid signaling is required to complete the vertebrate cardiac left/right asymmetry pathway. Dev Biol. 2000;223:323–338. doi: 10.1006/dbio.2000.9754. [DOI] [PubMed] [Google Scholar]