Abstract
Malignant brain tumors are among the most lethal cancers, and conventional therapies are largely limited to palliation. Novel therapies targeted against specific molecular pathways may offer improved efficacy and reduced toxicity compared to conventional therapies, but initial clinical trials of molecular targeted agents in brain cancer therapy have been frequently disappointing. In brain tumors and other cancers, subpopulations of tumor cells have recently been characterized by their ability to self-renew and initiate tumors. Although these cancer stem cells, or tumor initiating cells, are often only present in small numbers in human tumors, mounting evidence suggests that cancer stem cells contribute to tumor maintenance and therapeutic resistance. Thus, the development of therapies that target cancer stem cell signal transduction and biologies may improve brain tumor patient survival. We now demonstrate that populations enriched for cancer stem cells are preferentially sensitive to an inhibitor of Akt, a prominent cell survival and invasion signaling node. Treatment with an Akt inhibitor more potently reduced the numbers of viable brain cancer stem cells relative to matched non-stem cancer cells associated with a preferential induction of apoptosis and a suppression of neurosphere formation. Akt inhibition also reduced the motility and invasiveness of all tumor cells but had a greater impact on cancer stem cell behaviors. Furthermore, inhibition of Akt activity in cancer stem cells increased survival of immunocompromised mice bearing human glioma xenografts in vivo. Together, these results suggest that Akt inhibitors may function as effective anti-cancer stem cell therapies.
Keywords: glioma, Akt, targeted therapy, small molecule inhibitor
INTRODUCTION
Glioblastomas are the most common and deadly primary tumors of the central nervous system in adults1. Current therapy consists of maximal surgical resection followed by cytotoxic therapies that non-specifically damage DNA or inhibit mitosis2. Despite the significant toxicities of these therapies, a large fraction of brain cancer patients suffer tumor recurrence due to the resistance of tumors to therapy2. The rapidity of tumor regrowth even after evidence of tumor response suggests that some tumor cells are resistant to therapy at treatment initiation. We and other groups have demonstrated that a subset of tumor cells (called cancer stem cells or tumor initiating cells) are functionally defined by their abilities to undergo sustained self-renewal and form tumors recapitulating the phenotypes of the parental tumors 3-8 and are relatively resistant to radiotherapy and chemotherapy3,9-13. While the degree to which cancer stem cells contribute to therapeutic resistance remains to be defined, the identification of a tumor cell subpopulation with an enriched capacity to promote tumor growth3-8, angiogenesis4, and metastasis12 may better inform cancer biology. Clarke has hypothesized that current therapies might target the bulk of the tumor (the majority of which is the non-stem cancer cell compartment), but unless the cancer stem cells themselves are targeted, they may remain viable and repopulate the tumor following treatment14,15. Thus, it is paramount to devise therapies that target cancer stem cells if the tumor is to be eliminated entirely.
Glioblastomas commonly display hyperactivation of the phosphatidylinosital-3-kinase (PI3K)-AKT (also known as protein kinase B) pathway, a pro-tumorigenic signaling cascade that contributes to the pathogenesis of several human cancers16-21. The PI3K-AKT pathway can be activated in tumors through a number of mechanisms, including activation of upstream growth factor receptors, mutations of the PI3K catalytic subunit (PIK3CA), overexpression or amplification of Akt family members, or inactivation of the inhibitory effects of the phosphatase and tensin homolog (PTEN) tumor suppressor16-20. Hyperactive Akt signaling promotes tumorigenic cell behaviors by increasing cell survival, proliferation, invasion, and angiogenesis, and it has been directly associated with in vitro conversion of grade III anaplastic astrocytoma to grade IV glioblastoma16-21.
Because of the association of Akt activity with a wide range of tumorigenic properties, we hypothesized that brain cancer stem cells may exhibit a dependence on the Akt pathway. Indeed, chemoresistance in hepatocarcinoma stem cells may be conferred by activation of Akt11 and Akt regulates the survival of tumor cells in the perivascular niche bearing stem cell markers in mouse medulloblastoma models21. To further investigate the dependence of brain cancer stem cells on Akt signaling, we pharmacologically treated matched populations of glioblastoma cancer stem cells and non-stem cells with a small molecule inhibitor of Akt. We sought to determine if preferential targeting of brain tumor stem cells could be achieved through inhibition of Akt by decreasing the capacity of these cells to survive, proliferate, and invade, thereby decreasing their malignant potential.
MATERIALS AND METHODS
Isolation of CD133+ and CD133− tumor cells
T3359 cultures were isolated from primary glioblastoma samples transiently amplified in immunocompromised mice. Tumor specimens were obtained from surgical biopsies of consenting patients under a protocol approved by the Duke University Medical Center Institutional Review Board. D456MG xenografts were originally derived from a pediatric glioblastoma biopsy specimen and have maintained in immunocompromised mice under a Duke Institutional Animal Care and Use approved protocol. Of note, T3359 and D456MG express wildtype PTEN. Tumors were dissociated into single cells using an enzyme dissociation kit (Worthington Biochemical, Lakewood, NJ). For fluorescence-activated cell sorting (FACS) into CD133+ and CD133− enriched populations, cells were labeled with an allophycocyanin-conjugated CD133 antibody (Miltenyi Biotec, Auburn, CA) before sorting by FACS. For magnetic bead sorting (MACS) into CD133+ and CD133− enriched cell populations, cells were incubated with CD133 antibodies conjugated with biotin and magnetic beads that bind biotin prior to separation by a magnetic column (Miltenyi Biotec, Auburn, CA). CD133+ cells were maintained in their undifferentiated state using Neurobasal Media supplemented with epidermal growth factor and fibroblastic growth factor (each at 10 μg/500 mL media), sodium pyruvate, glutamine, B27, non-essential amino acids and penicillin/streptomycin (Gibco, Grand Island, NY). CD133− cells were maintained in their differentiated state with Dulbecco's Modified Eagle Medium (DMEM, Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO) and penicillin/streptomycin.
Small Molecule Inhibitor
The small molecule inhibitors of Akt (AktIII/SH-6, AktII), PI3K (LY294002), and mTOR (rapamycin) were purchased from Calbiochem (San Diego, CA). For all assays, stock solutions created by dissolving the drug in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO) were stored at −80°C. Immediately prior to the experiment, stock solutions were diluted in DMSO to 1000x of the final concentrations indicated. For each experiment, 1 μL/mL of DMSO as a control or inhibitor 1000x stock solutions in DMSO were added to the media of cells to make the indicated final concentrations of inhibitor.
Antibodies and Western Blotting
CD133+ and CD133− cells were plated in appropriate media in six-well plates at 5×105 cells per well and allowed to recover overnight. CD133− media was changed to CD133+ growth media before each experiment for the indicated times. All cells were harvested together and then lysed in buffer (62.5 mM Tris-HCl, 2% w/v sodium dodecyl sulfate (SDS), 10% glycerol, 40 mM dithiothreitol (DTT) and protease inhibitors). Protein concentrations were quantified (Biorad Protein Assay Reagent, Hercules, CA), and equal amounts of protein were run on polyacrylamide gels (Invitrogen, Carlsbad, CA), followed by transfer to polyvinyldifluoride membranes (Millipore, Billerica, MA) which were then were probed with antibodies. Total Akt and phospho-Akt (Ser473) antibodies were purchased from Cell Signaling (Beverly, MA) while alpha-Tubulin antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Proteins were detected with an enhanced chemiluminescence system (Pierce Biotechnology, Rockford, IL).
Akt Kinase Assay
CD133+ and CD133− cells were plated in appropriate media in 10-cm dishes at 106 cells per plate and allowed to recover overnight. CD133− media was changed to CD133+ media before treatment with DMSO or increasing concentrations of the Akt inhibitor. After 24 hours of treatment, cells were changed to neurobasal media without growth factors for 12 hours. Cells were then stimulated for 20 minutes with EGF and FGF at a final concentration of 50 ng/mL before harvest and lysis. Equal amounts of total protein (50 μg in 200 μL) were utilized for the in vitro kinase assay, which was performed as per the manufacturer's instructions (R&D Systems).
Proliferation and Survival Assay
CD133+ and CD133− cells were plated in appropriate media in 6-well plates in triplicate at 1×105 cells per well and left to recover overnight. CD133− media was changed to CD133+ media before treatment with DMSO or increasing concentrations of the Akt inhibitor. After 48 hours of treatment, cells were harvested, and live and dead cells were quantified via a hemocytometer with Trypan Blue stain (Gibco, Grand Island, NY).
Flow Cytometric and Annexin V Analysis
CD133+ and CD133− cells were plated in appropriate media in 6-well plates in triplicate at 1×105 cells per well and were allowed to recover overnight. CD133− media was changed to CD133+ growth media before treatment with DMSO control or increasing concentrations of Akt inhibitor for 24 hours. Cells were then harvested with their conditioned media to ensure collection of floating cells along with adherent cells. Using an Annexin V kit, apoptotic cells were labeled with FITC while necrotic cells were labeled with propidium iodide as per the manufacturer's instructions (EMD Chemicals, San Diego, CA). Proportions of apoptotic cells were then quantified by FACScan gated to exclude cellular debris.
Neurosphere Formation Assay
CD133+ cells were plated in 24-well plates at 20 cells per well with DMSO or Akt inhibitor at increasing concentrations. Wells were inspected every three days, numbers of neurospheres were quantified at each timepoint, and individual neurospheres were imaged with an Olympus CK40 digital camera mounted to a light microscope.
Migration and Invasion Assay
Migration/Invasion plates were purchased and used according to the manufacturer's instructions (BD Biosciences, San Jose, CA). CD133+ and CD133− cells were pretreated for 1 hour with increasing concentrations of Akt inhibitor, then plated at 5 × 104 cells per well in upper transwell chambers of inserts uncoated (migration) or coated with Matrigel (invasion) in serum- and growth factor-free media. The bottoms of the chambers were filled with 500 μL media containing 2% fetal bovine serum. After 24 hours, migration inserts were fixed and stained with Diff-Quick Fixative Solutions (Dade Behring, Newark, DE). After 48 hours, Matrigel-coated inserts were fixed and stained. Attached cells were imaged with an Olympus CK40 digital camera mounted to a light microscope and quantified using ImageJ (http://rsb.info.nih.gov/ij/) software.
Statistical Analysis
Statistical significance was calculated with GraphPad Prism Software (GraphPad Software Inc.). Data is presented as the mean ± the standard error. Statistical significance in the human xenograft mouse models was calculated using Kaplan-Meier survival curves and statistical analysis with MedCalc Software (www.medcalc.be/).
RESULTS
Brain tumor stem cells exhibit greater sensitivity to Akt inhibition
Cancer stem cells are defined through functional assays to determine the capacity for sustained self-renewal and the ability to recapitulate the full diversity of the parental tumor upon xenotransplanation3-8. Several groups, including our own laboratory, have demonstrated that cancer stem cells can be enriched prospectively through the use of the CD133 (Prominin 1) cell surface marker3-8. The CD133 marker does not absolutely segregate for tumorigenesis as some tumors may contain CD133− cells that form tumors, although this frequently requires transplantation of very high numbers of cells22,23. However, we have found that CD133+ tumor cells from patient biopsy specimens display both potent neurosphere formation potential in cell culture and effective tumor generation in immunocompromised animal models while CD133− cells do not form neurospheres and rarely, if ever, form tumors upon xenotransplanation3. We, therefore, used models that we have previously characterized in functional assays (sustained neurosphere generation, multi-lineage differentiation, tumor initiation) to define “cancer stemness” in our studies. In addition, CD133+ cells utilized for these novel studies highly expressed stem cell markers such as Nestin, Oct4, Olig2, and Sox2, whereas CD13− cells did not (Suppl. Figs. 1, 2). Taken together these results suggest that CD133+ cells are enriched in cancer stem cells.
To determine whether CD133+ tumor cells exhibit differential activation of the PI3K-Akt pathway compared to their non-stem counterparts, the activation state of pathway components was examined through immunoblotting. In short term cultures (<5 passages) of T3359 (Fig. 1A, 1B; Suppl. Fig. 3A, 3B) or D456MG (Suppl. Fig. 3C, 3D) glioblastoma cells, total levels of Akt protein were similar between CD133+ and CD133− cells. In contrast, basal levels of phosphorylated (activated) Akt were higher in the CD133− populations (Fig. 1A, 1B; Suppl. Fig. 3). Consistent with these data, we found decreased Akt kinase activity in CD133+ cells in comparison to matched CD133− cells (Fig. 1C, 1D). Although we expected cancer stem cells to have a higher basal activation of Akt, the relatively lower activation of Akt in CD133+ cells may be due to differences in cell attachment. Cancer stem cells grow in three-dimensional neurospheres whereas non-stem cancer cells are adherent to tissue culture plates. As there may be differences in cell-cell and cell-matrix interactions between these growth conditions, we evaluated Akt phosphorylation in short term cultures of adherent and non-adherent CD133− cells to ensure survival of non-adherent CD133− cells (which is compromised by the lack of serum and cell adhesion). We found adherent CD133− cells had greater Akt phosphorylation than either non-adherent CD133− or CD133+ cells which had very similar levels of basal Akt phosphorylation (Suppl. Fig. 4).
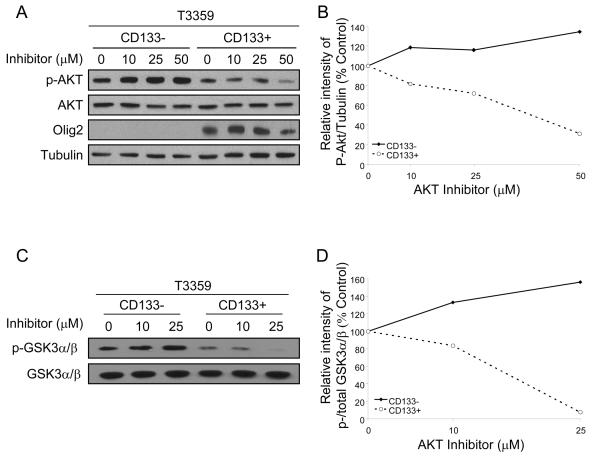
Figure 1. The Akt pathway is differentially targeted by an Akt inhibitor in CD133+ and CD133− brain tumor cells.
CD133+ and CD133− cells were isolated from a T3359 glioblastoma patient specimen passaged short-term in immunocompromised mice and treated with the indicated concentrations of Akt III inhibitor (SH-6, Calbiochem) for 2 hours. Lysates were analyzed by Western (A) and the intensities quantified using ImageJ software and normalized to the tubulin loading control (B). The levels of phospho-Akt, normalized to tubulin, decreased in a concentration-dependent manner in the CD133+ population but not the CD133− population. Akt was also immunoprecipitated from lysates and used for an in vitro Akt kinase assay with GSK3α/β fusion protein as the substrate (C, D). Western analysis(C) and intensities quantified using ImageJ software and normalized to total GSK3α/β indicated decreased basal and Akt-mediated phosphorylation of GSK3α/β in CD133+ cells in comparison to matched CD133− cells. AktIII inhibitor decreased the Akt-mediated phosphorylation of GSK3α/βin CD133+ cells, but not matched CD133− cells.
Several pharmacologic agents have been designed to inhibit Akt function. Phosphotidylinositol ether lipid analogues may target the pleckstrin homology domain of Akt to selectively inhibit cell survival in cancers with high Akt activity24. One of these drugs, the AktIII inhibitor (SH-6), reduced Akt activation in CD133+ glioma cells in concentration-dependent manner but had no beneficial effect on CD133− cells (Fig. 1A, B). The temporal course of AktIII inhibitor was also assessed in matched tumor cell populations and demonstrated transient effects in both populations with relatively greater effects on the CD133+ cells when normalized to the basal level of activation (Suppl. Fig. 3). This decrease in activated Akt is not due to a decrease in total Akt levels, nor to improper loading of protein samples, as assessed by tubulin controls (Fig. 1; Suppl. Fig. 3). Therefore, these data demonstrate that CD133+ and CD133− cells exhibit differential activation levels of the Akt pathway, and the CD133+ population has a greater sensitivity to the Akt inhibitor.
Akt activity is necessary for cancer stem cell proliferation and survival
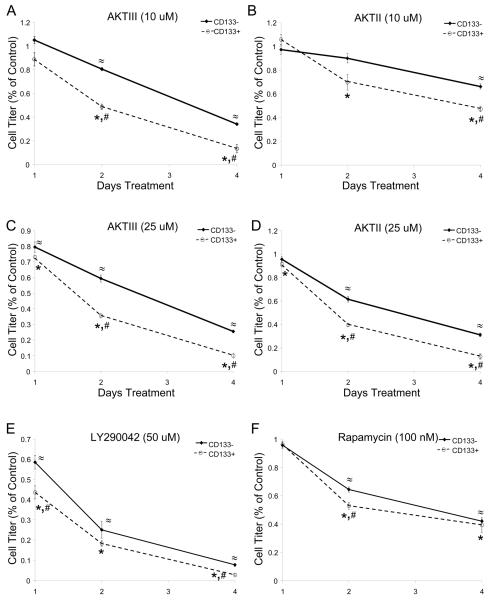
To determine if targeting AKT activity would preferentially decrease the pro-tumorigenic behaviors of CD133+ cells, the proliferation and survival of matched CD133+ and CD133− short term brain tumor cell cultures were interrogated. Despite having identical numbers of cells plated at the initiation of studies, at the end of the experiments CD133+ cultures yielded higher numbers of viable cells at baseline than the matched CD133− cells, likely due to the long term proliferative potential of these cells. However, the pharmacologic AktIII inhibitor demonstrated a significant concentration dependent effect in reducing the number of viable CD133+ cells with more modest effects on CD133− cells (Fig. 2A, 2B) as determined by trypan blue staining. The preferential decrease in CD133+ cell numbers is not likely to be due to potential differences in basal Akt phosphorylation in this assay with adherent CD133− cells. A similar preferential decrease in CD133+ cell growth was observed when both CD133− and CD133+ cells were cultured in stem cell media under non-adherent conditions with two different Akt inhibitors (Fig. 3, Suppl. Fig 5). Use of the PI3K inhibitor LY290042 to inhibit an upstream component of the Akt pathway also demonstrated greater effects on CD133+ cell growth with increasing concentrations of drug (Fig. 3, Suppl. Fig 5). When mammalian target of rapamycin (mTOR), a downstream target of Akt, was targeted with rapamycin there was a more modest preferential targeting of CD133+ cell growth (Fig. 3, Suppl. Fig 5). Together these results suggest that inhibition of PI3K or Akt more potently regulates the growth of CD133+ cells than CD133− cells.
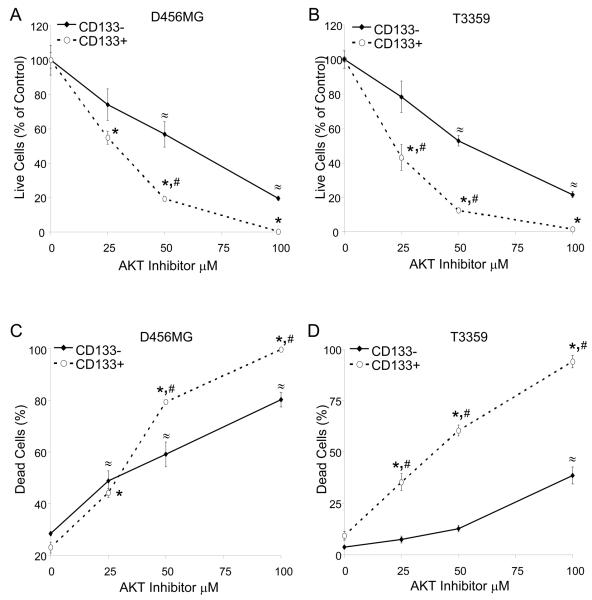
Figure 2. A small molecule inhibitor of Akt targets CD133+ cell growth and survival.
CD133+ and CD133− cells were isolated from an established D456MG pediatric glioblastoma xenograft (A, C) or a T3359 glioblastoma patient specimen passaged short-term in immunocompromised mice (B, D). Cells were treated with the indicated concentrations of AktIII inhibitor for 48 hours and the numbers of live cells (A, B) and the percentage of dead cells (C, D) as a fraction of the control determined through trypan blue staining. *, p<0.01 with ANOVA comparison of AktIII treated CD133+ cells to the DMSO control treated CD133+ cells; ≈, p<0.01 with ANOVA comparison of AktIII treated CD133− cells to the DMSO control treated CD133− cells;. #, p<0.01 with ANOVA comparison of CD133+ cells to identically treated CD133− cells.
Figure 3. Targeting Akt Preferentially Decreases CD133+ Brain Tumor Cell Growth Over Time.
CD133+ and CD133− cells isolated from a T3359 glioblastoma patient specimen passaged short-term in immunocompromised mice were plated in Neurobasal media with EGF and FGF, allowed to recover overnight, and then treated with 10 uM AktIII (A), 10 uM AktII (B), 25 uM AktIII (C), 25 uM AktII (D), 50 uM LY290042 (E), and 100 nM rapamycin (F) inhibitors. Cell growth was measured on the indicated days after inhibitor treatment began using the Cell Titer Glo assay (Promega) according to the manufacturer's instructions. The data for each time point were standardized to the DMSO treated controls for the same cell type on each day. *, p<0.05 with t-test comparison of inhibitor treated CD133+ cells to DMSO treated control CD133+ cells on the same day; ≈, p<0.01 with t-test comparison of inhibitor treated CD133− cells to DMSO treated control CD133− cells on the same day; #, p<0.05 with t-test comparison of CD133+ cells to similarly treated CD133− cells on the same day.
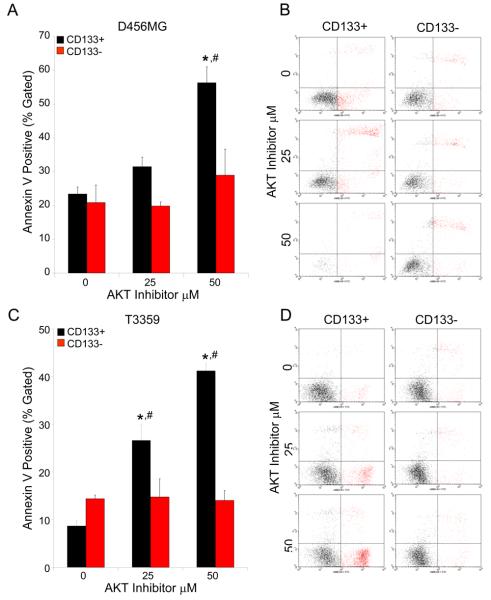
To evaluate the impact of pharmacologic Akt inhibition on CD133+ cell survival, the killing efficiency was measured over a range of inhibitor concentrations. Although the percentage of dead cells increased in a concentration-dependent manner with Akt inhibition in both the CD133+ and CD133− cells (Fig. 2C, 2D), the CD133+ populations were more significantly affected than the CD133− cells. After determining that AktIII preferentially targets CD133+ tumor cell survival, we investigated the mechanism of cell death using Annexin V staining to assess apoptosis in CD133+ and CD133− cells. In parallel with our earlier results, each CD133+ tumor cell culture demonstrated a concentration-dependent increase of apoptosis upon treatment with the Akt inhibitor (Fig. 4, Suppl. Fig. 6). In contrast, CD133− cells displayed little to no increase in the apoptotic cell fraction in response to AktIII inhibitor treatment (Fig. 4, Suppl. Fig. 6). These results indicate that the preferential cell death in CD133+ cancer stem cells is at least partially due to increased apoptosis, consistent with the known effects the Akt pathway has on cellular apoptosis and survival.
Figure 4. Targeting the Akt Pathway Results in Preferential Induction of CD133+ Cell Apoptosis.
CD133+ and CD133− cells isolated from an established D456MG pediatric glioblastoma xenograft (A, B) or a T3359 glioblastoma patient specimen passaged short-term in immunocompromised mice (C, D) were treated with the indicated concentration of AktIII inhibitor for 24 hours, trypsinized, labeled with an Annexin V kit according to manufacturer's instructions and analyzed by FACS. Apoptosis was induced in CD133+ cells at significantly higher levels than CD133− cells with increasing concentrations of Akt inhibitor. *p<0.001 with ANOVA comparison of AktIII treated CD133+ cells to DMSO control treated CD133+ cells; #, p<0.01 with ANOVA comparison of CD133+ cells to identically treated CD133− cells. Representative FACS gates of CD133+ and CD133− cells from D456MG (B) and T3359 (D) are shown.
Cancer stem cell neurosphere formation requires Akt activity
Both brain tumor stem cells and neural stem cells display the ability to form complex three-dimensional spherical structures (neurospheres) when cultured in serum-free media. In our studies, neurosphere formation potential is restricted to the prospectively enriched CD133+ tumor population, although other labs have generated neurospheres that did not express CD13320. To further investigate the effects of Akt inhibition on the cancer stem cell behaviors of CD133+ brain tumor cells, we examined the effects of the inhibitor on neurosphere formation in the presence of increasing concentrations of Akt inhibitor. CD133+ tumor cells displayed a striking concentration-dependent decrease in the ability to generate neurospheres across each time point examined (Fig. 5). When neurospheres that did form with Akt inhibitor treatment were further analyzed, there was a clear qualitative decrease in size and the cells were unable to form secondary neurospheres (Fig. 5 and data not shown). These results indicate that CD133+ brain tumor cells require Akt activity for neurosphere formation.
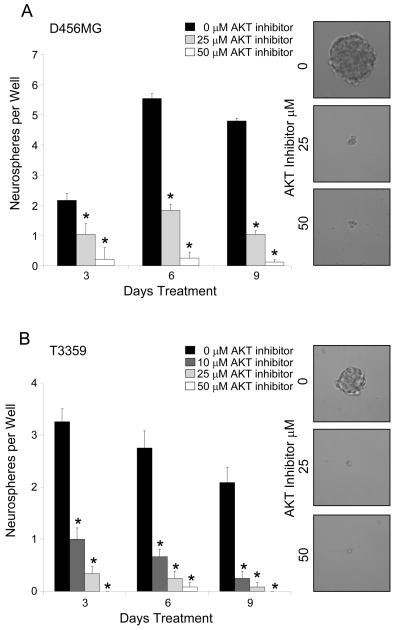
Figure 5. Targeting the Akt Pathway Decreases the Neurosphere Formation Efficiency of CD133+ Cells.
CD133+ cells isolated from an established D456MG pediatric glioblastoma xenograft (A) or a T3359 glioblastoma patient specimen passaged short-term in immunocompromised mice (B) were plated at an approximate density of 20 cells per well and treated with the indicated concentration of the AktIII inhibitor. The number of neurospheres per well was quantified over three time points. The number of neurospheres per well significantly decreased in the presence of Akt inhibitor. Representative images of neurospheres photographed at day 9 are shown and demonstrate a clear qualitative difference in size between those grown in the control conditions and those treated with the Akt inhibitor. *p<0.001 with ANOVA comparison of AktIII inhibitor treatment to the DMSO treated control on the same day.
Targeting Akt decreases cancer stem cell migration and invasion
The ability for malignant gliomas to invade into normal neural structures leads to the inability for these tumors to be completely surgically resected2. Although the mechanisms underlying brain tumor invasion remain incompletely understood, the PI3K-PTEN-Akt axis has been recognized as a contributor to invasion. Therefore, we expected that Akt inhibition may negatively regulate tumor cell invasion16,20. Using Boyden chamber assays, we evaluated the capacity of matched CD133+ and CD133− cells to migrate or invade the migration and invasion capacity of matched CD133+ and CD133− brain tumor cells was evaluated. Interestingly, there was a striking basal difference in the capacity of CD133+ and CD133− cells to migrate through either an uncoated member or a membrane coated with an artificial extracellular matrix (Matrigel). In one model (D456MG), the CD133− cells displayed much greater migratory and invasive potential (Fig. 6), perhaps due to the long term passage of these cells in a xenograft. In contrast, the CD133+ cells in the T3359 model were more invasive than the CD133− cells (Suppl. Fig. 7), consistent with the notion that CD133+ cells contribute to invasion and migration. Increasing concentrations of Akt inhibitor significantly attenuated the capacity of both CD133+ and CD133− cells to both migrate and invade (Figure 6) but in both models the CD133+ cells displayed greater sensitivity to the inhibitory effects of Akt inhibition than the cancer stem cell-depleted CD133−population (Fig. 6 and Suppl. Fig. 7). Thus, migratory and invasive potential of cancer stem cells relative to the non-stem cell population may depend on the tumor model, but cancer stem cells depend on Akt activity for these pro-invasive behaviors.
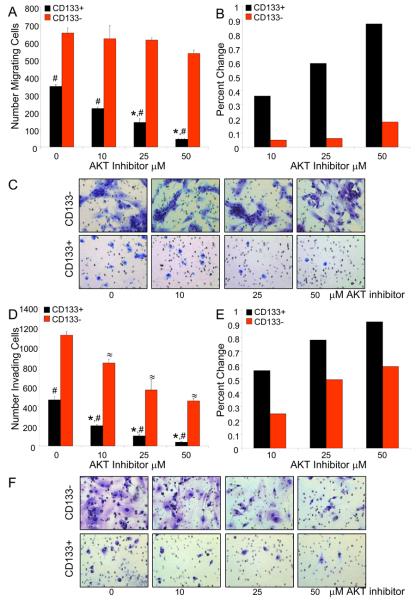
Figure 6. Targeting the Akt Pathway Decreases CD133+ Cell Migration and Invasion.
CD133+ cells isolated from an established D456MG pediatric glioblastoma xenograft were plated in serum-free media in the upper chambers of uncoated inserts (A-C) or Matrigel coated inserts (D-F) and were allowed to migrate toward 2% FBS for 48 hours. The migrating or invading cells were then stained and quantified with ImageJ, demonstrating both migration (A) and invasion (D) decrease in CD133+ cells with increasing concentrations of Akt inhibitor. When the percent change from baseline migration (B) or invasion (E) was calculated, CD133+ cells exhibited a greater sensitivity to the effects of Akt inhibitor. Representative images of migrating (C) or invading cells (F) are shown. *, p<0.05 with ANOVA comparison of AktIII inhibitor treated CD133+ cells to DMSO treated control CD133+ cells; ≈, p<0.05 with ANOVA comparison of AktIII inhibitor treated CD133− cells to DMSO treated control CD133−cells; #,, p<0.001 with ANOVA comparison of CD133+ cells to similarly treated CD133− cells.
Inhibition of Akt Activity in Cancer Stem Cells Increases Survival of Immunocompromised Mice Bearing Human Glioma Xenografts
To further determine the potential therapeutic benefit of targeting Akt activity in cancer stem cells, we determine the tumorigenic potential of cells treated with Akt III inhibitor. When 1×104 or 1×103 CD133+ cells were injected into the forebrains of immunocompromised mice, we observed neurologic signs due to the development of brain tumors regardless of Akt inhibitor treatment. These data demonstrate that inhibition of Akt activity alone for the treatment period was not sufficient to significantly reduce the tumor formation potential of cancer stem cells (Table 1). However, the time to the development of neurologic signs was increased with Akt inhibition. The median survival until the development of neurologic signs of animals bearing 1×104 CD133+ tumor cells treated with DMSO control was 24 days while animals bearing identical CD133+ cells treated with the Akt inhibitor survived for a median of 42 days (p<0.03). Similarly, the median survival until the development of neurologic signs of animals bearing 1×103 CD133+ cells was 35 for the DMSO control and 66 days for Akt inhibitor treatment (p<0.03). These data demonstrate that reducing Akt activity in CD133+ cells can increase survival of mice bearing intracranial xenografts. Prior work in our laboratory has implicated cancer stem cells in promoting tumor angiogenesis via elevated vascular endothelial growth factor (VEGF) secretion4. However, the increased survival of mice injected with Akt inhibitor treated CD133+ cells is unlikely to be due to changes in VEGF levels as the Akt inhibitor did not reduce VEGF expression (Suppl. Fig. 8) and is more likely to be attributed to induction of apoptosis (Figs. 2, 4, Suppl. Fig. 6)
Table 1.
| T3359 CD133+ | ||
|---|---|---|
| Treatment | 10,000 cells | 1,000 cells |
| Control (DMSO) | Incidence: 3/3 Median Survival: 24 days |
Incidence: 3/3 Median Survival: 35 days |
| 25 uM AktIII inhibitor | Incidence: 3/3 Median Survival: 40 days |
Incidence: 2/3 Median Survival: 66 days |
DISCUSSION
The essential pathways regulating cancer stem cell biology remain poorly defined, but molecular targets with defined roles in normal stem cell biology and aberrant activity or expression in cancers [including Notch, Hedgehog, Wnt/β-catenin, Bone Morphogenic Protein (BMP), Myc, Epidermal Growth Factor Receptor (EGFR), Fibroblast Growth Factor Receptor (FGFR), and PTEN] are likely to be important. Activation of these pathways by autocrine signals from the cancer stem cells themselves or paracrine signals from the cancer stem cell niche could be essential for stem cell maintenance25,26. Recognizing that these stem cell maintenance cues regulate cell survival and differentiation, these signals are logical targets for anti-cancer stem cell directed therapies.
The potential for inhibition of the PI3K-Akt pathway to target cancer stem cells is supported by the known involvement of Akt signaling in tumorigenesis and normal stem cell biology as well as the beneficial effects of Akt inhibition on glioma cell growth27-34. Multiple pro-tumorigenic behaviors (such as the promotion of cell proliferation, survival, and invasion) now suggested to be driven by cancer stem cells3-15 are known to be regulated by Akt signaling20. Activation of the PI3K-Akt pathway is common in malignant gliomas and associated with increased tumor grade and decreased glioma patient survival16-20. Genetically engineered glioma models also demonstrate that that constitutively activated Akt contributes to tumor initiation27,28.
In addition to roles in tumor formation, the PI3K-Akt pathway is implicated in stem cell biology. Loss of the PI3K inhibitor PTEN increases neural and hematopoeitic stem cell proliferation and survival29,30. Recent studies in the hematopoietic system have shown that activation of the PI3K/Akt pathway in normal hematopoietic stem cells can produce leukemia within weeks31. However, leukemic stem cells are also particularly sensitive to the effects of mTOR antagonism31, suggesting a potential therapeutic window for targeting the PI3K-Akt pathway in cancer stem cells with minimal effects on normal somatic stem cells. Until recently, this strategy has not been fruitfully applied to the cancer stem cells of solid tumors. Ma et al. demonstrated that the CD133+ cells from two established hepatocellular carcinoma cell lines are less sensitive to chemotherapy and express higher levels of survival proteins involved in the Akt and Bcl-2 pathway than the CD133− cells, and respond to Akt inhibition by reducing key survival proteins11. Hambardzumyan and co-workers used a murine model of medulloblastoma (a primary brain tumor distinct from gliomas that commonly occur in children and display greater radiosensitivity than gliomas) to show that a nestin-expressing perivascular tumor cell population survives radiation, activates downstream effectors of Akt, undergoes a p53-dependent cell cycle arrest, and re-enters the cell cycle at 72 hours13. In addition, inhibition of Akt signaling sensitizes cells in the perivascular region to radiation-induced apoptosis13. We have extended these findings into a cancer type in which the Akt/Pten axis plays an essential role using models derived from human specimens or maintained in vivo then only briefly cultured to maintain a cancer stem cell phenotype. We sought to further determine if inhibition of Akt signaling in glioma stem cells may be a beneficial mechanism for reducing cancer stem cell growth in vitro and increasing survival in vivo.
To evaluate the effects of Akt inhibition in cancer stem cells and non-stem cancer cells, we have built on prior investigations, including our own, that demonstrated that cancer stem cells may be enriched through the use of the CD133 (Prominin-1) cell surface marker3-8. The use of CD133 must be viewed with caution as CD133 has not been linked to a contributory role in “cancer stemness” and some tumors may have CD133− cells with tumor initiation abilities22,23. However, experiments in our laboratory find a striking enrichment of cancer stem cells in the CD133+ tumor cell population even though CD133 should not be considered a surrogate for a cancer stem cell phenotype.
When we compared the effects of Akt inhibition on populations of brain tumor cells, we found a preferential targeting of Akt activity in CD133+ cells in comparison to matched CD133− cells. The AktIII inhibitor that we employed for the majority of experiments is a phosphatidylinositol ether lipid analog that may also activate the stress kinase, p38α. This small molecule inhibitor of Akt effectively reduced the growth, survival, migration, and invasion of glioma cells and did so with greater potency in the CD133+ subpopulation than in matched CD133− cells. Inhibiting the Akt pathway in brain cancer stem cells also increased survival in animal models, suggesting a potential therapeutic benefit. Together these data suggest that many of the malignant characteristics of brain tumor stem cells are dependent on Akt signals.
We were somewhat surprised to find that CD133− tumor cells expressed higher basal levels of activated Akt than the CD133+ cells, considering the recognized role of Akt in survival and prior reports indicating higher levels of phosphorylated Akt in human hepatocellular carcinoma and mouse medulloblastoma stem cells11,13. As our studies were conducted in different tumor types and culture conditions, it is difficult to make direct comparisons. However, we found that differences in attachment between CD133− and CD133+ cells can alter basal Akt phosphorylation, suggesting that differences culture conditions may contribute to the discrepancy in basal Akt activation. All of our primary experiments were performed on short-term cultures in which CD133+ cells formed neurospheres (were non-adherent) and CD133− cells were adherent in the presence of identical media in an effort to make the best possible comparisons. We also used AktII and AktIII inhibitors rather than the previously utilized AktI inhibitor11 or perifosine13. Using these conditions and inhibitors, we found that CD133+ cells exhibited greater sensitivity to Akt inhibition. The precise molecular differences between CD133−and CD133+ cells which could contribute to the observed higher potency of Akt inhibition in CD133+ cells remain to be fully elucidated.
The concept of cancer stem cells is still evolving, but data implicating these cells in tumor maintenance and therapeutic resistance indicates the potential benefit of targeting cancer stem cells in combination with conventional therapies. While targeting Akt activation in CD133+ cells increased the survival of mice bearing intracranial human glioblastoma xenografts, Akt inhibition alone is unlikely to directly translate to therapeutic benefit for human patients. Monotherapies against any signaling pathway have been largely ineffective in the clinic. However, data from our laboratory and others suggests the utility of targeting Akt signaling components alone or in combination with other pathways in gliomas32-34. As these prior studies focus on the effects of Akt inhibitors in glioma cell lines that are passaged long term in the presence of serum (a condition which promotes the differentiation of cancer stem cells) future studies determining the effect of combining Akt inhibition with chemo− and radiotherapies on cancer stem cell biologies may prove enticing. Ensuring that both glioblastoma stem cells and the more prevalent CD133− cells are targeted may offer the opportunity to eliminate the last vestiges of the primary tumor after surgical resection, an absolute requirement for preventing recurrence.
In conclusion, CD133+ glioblastoma stem cells were shown to have increased sensitivity to the effects of a small molecule Akt inhibitor, despite exhibiting decreased baseline activation of the Akt pathway compared to the CD133− cells. Akt inhibition produced preferential reduction of CD133+ cell growth, survival, migration, and invasion in comparison to CD133− cells. The AktIII small molecule inhibitor also targeted characteristics unique to cancer stem cells, such as the ability to form neurospheres. Targeting Akt activity in CD133+ cells also increased the survival of immunocompromised mice bearing glioma xenografts, indicating a potential therapeutic benefit. While many pro-tumorigenic behaviors of CD133+ cells were reduced to a greater extent than in the CD133− cells, the secretion of VEGF was not one of them, indicating that not all the unique features of CD133+ cells are dependent on Akt signaling.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kathryn Lattimore, Pei Miao, Sarah Wickman, and Qiulian Wu for technical assistance and Dr. Mike Cook and Dr. Beth Harvat for assistance with flow cytometry.
Financial Support: Financial support was provided by the Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of the United States, Accelerate Brain Cancer Cure, Alexander and Margaret Stewart Trust, Brain Tumor Society, Goldhirsh Foundation, Duke Comprehensive Cancer Center Stem Cell Initiative Grant (J.R.), NIH grants NS047409, NS054276, CA129958, and CA116659 (J.R.). W.F. is a Howard Hughes Research Training Fellow. J.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon Runyon Cancer Research Foundation and a Sidney Kimmel Foundation for Cancer Research Scholar. C.E. receives Medical Scientist Training Program support from the National Institute of General Medical Sciences grant 2T32GM007171.
Footnotes
Disclaimers: We have no conflicts of interest to disclose.
REFERENCES
- 1.Central Brain Tumor Registry of the United States . 1997 annual report. CBTRUS; Chicago (IL): 1998. [Google Scholar]
- 2.Sathornsumetee S, Rich JN, Reardon DA. Diagnosis and treatment of high-grade astrocytoma. Neurol Clin. 2007;25:1111–1139. doi: 10.1016/j.ncl.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 4.Bao S, Wu Q, Sathornsumetee S, et al. Stem Cell-like Glioma Cells Promote Tumour Angiogenesis through Vascular Endothelial Growth Factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 5.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 7.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumor initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 9.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todaro M, Alea MP, Di Stefano AB, et al. Colon Cancer Stem Cells Dictate Tumor Growth and Resist Cell Death by Production of Interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Ma S, Lee TK, Zheng BJ, et al. CD133(+) HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2007 doi: 10.1038/sj.onc.1210811. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Hermann PC, Huber SL, Herrler T, et al. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Hambardzumyan D, Becher OJ, Rosenblum MK, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hajj M, Becker MW, Wicha M, et al. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–47. doi: 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 16.Castellino RC, Durden DL. Mechanisms of Disease: the PI3K-Akt-PTEN signaling node--an intercept point for the control of angiogenesis in brain tumors. Nat Clin Pract Neurol. 2007;3:682–693. doi: 10.1038/ncpneuro0661. [DOI] [PubMed] [Google Scholar]
- 17.Wlodarski P, Grajkowska W, Lojek M, et al. Activation of Akt and Erk pathways in medulloblastoma. Folia Neuropathol. 2006;44:214–220. [PubMed] [Google Scholar]
- 18.Knobbe CB, Trampe-Kieslich A, Reifenberger G. Genetic alteration and expression of the phosphoinositol-3-kinase/Akt pathway genes PIK3CA and PIKE in human glioblastomas. Neuropathol Appl Neurobiol. 2005;31:486–490. doi: 10.1111/j.1365-2990.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda Y, Ozawa T, Aldape KD, et al. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 20.Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim Biophys Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma Formation, Cancer Stem Cells, and Akt Signaling. Stem Cell Rev. 2008 doi: 10.1007/s12015-008-9021-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2007 doi: 10.1002/ijc.23130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Beier D, Hau P, Proescholdt M, et al. CD133+ and CD133− glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–40105. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 24.Gills JJ, Castillo SS, Zhang C, et al. Phosphatidylinositol ether lipid analogues that inhibit AKT also independently activate the stress kinase, p38alpha, through MKK3/6-independent and – dependent mechanisms. J Biol Chem. 2007;282:27020–27029. doi: 10.1074/jbc.M701108200. [DOI] [PubMed] [Google Scholar]
- 25.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 26.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 27.Holland EC, Celestino J, Dai C, et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 28.Uhrbom L, Dai C, Celestino JC, et al. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62:5551–5558. [PubMed] [Google Scholar]
- 29.Groszer M, Erickson R, Scripture-Adams DD, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci USA. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groszer M, Erickson R, Scripture-Adams DD, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 31.Follo MY, Mongiorgi S, Bosi C, et al. The Akt/mammalian target of rapamycin signal transduction pathway is activated in high-risk myelodysplastic syndromes and influences cell survival and proliferation. Cancer Res. 2007;67:4287–4294. doi: 10.1158/0008-5472.CAN-06-4409. [DOI] [PubMed] [Google Scholar]
- 32.Hjelmeland AB, Lattimore KP, Fee BE, et al. The combination of novel low molecular weight inhibitors of RAF (LBT613) and target of rapamycin (RAD001) decreases glioma proliferation and invasion. Mol Cancer Ther. 2007;6:2449–2457. doi: 10.1158/1535-7163.MCT-07-0155. [DOI] [PubMed] [Google Scholar]
- 33.Van Meter TE, Broaddus WC, Cash D, Fillmore H. Cotreatment with a novel phosphoinositide analogue inhibitor and carmustine enhances chemotherapeutic efficacy by attenuating AKT activity in gliomas. Cancer. 2006;107:2446–2454. doi: 10.1002/cncr.22248. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara K, Iwado E, Mills GB, et al. Akt inhibitor shows anticancer and radiosensitizing effects in malignant glioma cells by inducing autophagy. Int J Oncol. 2007;31:753–760. [PubMed] [Google Scholar]
- 35.Bayascas JR, Alessi DR. Regulation of Akt/PKB Ser473 phosphorylation. Mol Cell. 2005;18:143–145. doi: 10.1016/j.molcel.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 37.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun SY, Rosenberg LM, Wang X, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.