Abstract
Objective
Fine particulate matter < 2.5 µm (PM2.5) has been implicated in vasoconstriction and potentiation of hypertension in humans. We investigated the effects of short-term exposure to PM2.5 in the angiotensin II (AII) infusion model.
Methods and Results
Sprague-Dawley rats were exposed to PM2.5 or filtered air (FA) for 10 weeks. At week 9, minipumps containing AII were implanted and the responses studied over a week. Mean concentration of PM2.5 inside the chamber was 79.1±7.4 µg/m3. Following AII infusion, mean arterial pressure was significantly higher in PM2.5-AII vs. FA-AII group. Aortic vasoconstriction to phenylephrine was potentiated with exaggerated relaxation to the Rho-kinase (ROCK) inhibitor Y-27632 and increase in ROCK-1 mRNA levels in the PM2.5–AII group. Superoxide (O2 ˙−) production in aorta was increased in the PM2.5-AII compared to the FA group, inhibitable by apocynin and L-NAME with coordinate upregulation of NAD(P)H oxidase subunits p22phox and p47phox and depletion of tetrahydrobiopterin. In-vitro exposure to ultrafine particles (UFP) and PM2.5 was associated with an increase in ROCK activity, phosphorylation of myosin light chain and myosin phosphatase target subunit (MYPT1). Pre-treatment with the non-specific anti-oxidant N-Acetylcysteine and the Rho kinase inhibitors (Fasudil and Y-27632) prevented MLC and MYPT-1 phosphorylation by UFP suggesting a O2 ˙− mediated mechanism for PM2.5 and UFP effects.
Conclusions
Short-term air pollution exaggerates hypertension through O2 ˙− mediated up regulation of the Rho/ROCK pathway.
Keywords: Air pollution, NADPH oxidase, hypertension, free radicals, Rho/ROCK
Introduction
Fine particulate matter (aerodynamic diameter <2.5 µm, PM2.5) in ambient air has been implicated in the pathogenesis of cardiovascular disease.1–3 Recent studies have suggested that this risk is rapid and occurs within hours to days of exposure to high levels of PM2.5. 4–6 Increases in blood pressure may represent an important mechanism through which PM2.5 may modulate its effects. Data from recent epidemiological studies from North America and Europe are indeed consistent with this hypothesis and have associated short-term exposure to PM2.5 with elevations in blood pressure (BP). 7, 8 This effect seems to be exaggerated in pre-disposed individuals 9, an observation that has also been noted in relation to the association of PM2.5 with other chronic conditions such as atherosclerosis. 3, 6, 10 Although the precise mechanisms through which PM2.5 gains access to the systemic vasculature is still hotly debated, there is increasing evidence that particles in the fine and ultrafine range transgress into the systemic circulation and modulate vascular tone acutely, presumably through reactive oxygen species (ROS) dependent pathways.11, 12 We hypothesized that short term (weeks) increases in PM2.5 levels is associated with an increases in BP and that these responses are exaggerated in a model of angiotensin II (AII) dependent hypertension through up-regulation of ROS pathways.
Methods
Animals and BP Monitoring
All experimental procedures were approved by the Committees on Use and Care of Animals from New York University and Mount Sinai School of Medicine. Male Sprague-Dawley (SD) rats (500–650 g) were purchased from Charles River Laboratories Inc. (Wilmington, MA). The conscious systolic, diastolic and mean arterial pressure (MAP) was monitored by radio-telemetry method with the Dataquest IV system (Data Sciences International, St. Paul, MN).
PM2.5 Exposure and AII Infusion
The animal exposure and the monitoring of the exposure environment and ambient aerosol were performed as previously described.13, 14 Rats were randomly exposed to PM2.5 or filtered air (FA) for a total of 10 weeks. At the end of 9-week exposure, the rats were infused with 0.75 mg/kg/day of AII for 7 days. PM2.5 or FA exposure continued during AII infusion. Our exposure system allows for exposure to all particles <2.5µm in diameter and thus allows for both PM2.5 and ultrafine particles (UFP, particulate matter <0.1µm) exposure.
Myograph Experiments
The myograph experiments were performed with 2-mm thoracic aortic rings mounted in organ bath chambers as previously described.14
In situ Detection and Quantification of O2 ˙− Generation
In situ detection and quantification of O2 ˙− generation in aortic tissues were determined with dihydroethidium (DHE, Molecular Probes, Inc., Eugene, OR) staining and a modified high-throughput lucigenin chemiluminescence assay15, respectively.
High-Performance Liquid Chromatography Analysis of Tetrahydrobiopterin (BH4)
BH4 content was determined in the heart, mesenteric vasculature and liver samples by a modification of the method described previously.16
Cell Culture
Primary rat aortic smooth muscle cells (RASMCs) were maintained in Dulbeco modified Eagle medium with 10% fetal bovine serum in a humidified atmosphere in 5% CO2 at 37°C. Cells at passages 4–8 were used for the experiments. Cells were treated with UFP or AII for the indicated time.
RhoA Activation Assay
RhoA-GTP levels were determined with G-LISA™ RhoA activation assay kit (Cytoskeleton, Inc., Denver, CO) according to the manufacture’s instructions.
Quantitative RT-PCR and Immunoblotting
Total RNA was prepared from aortic tissues and subjected to real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR). Whole lysates of aortic samples were prepared and subjected to immunoblotting.
Data Analyses
All data are expressed as mean ± SE unless otherwise specified. Comparisons between groups of animals or treatments were made with one-way analysis of variance (ANOVA). When significance was indicated, a Student-Newman-Keuls post hoc analysis was used. Significance was considered at a value of P < 0.05. The Fishing License method was used to analyze blood pressure differences including mean arterial pressure between the PM2.5-AII and FA-AII groups as detailed previously.17 For details, please see the supplemental materials, available online at http://atvb.ahajournals.org.
RESULTS
PM2.5 Concentrations during the Study Period
The mean daily ambient PM2.5 concentration at the study site was 6.1 ± 0.4 µg/m3, while the mean concentration inside the PM2.5 chamber was 79.1 ± 7.4 µg/m3. During the exposure time period, the outdoor mean temperature was 5.9 ± 8.9 °F (median 5.8 °F), and the outdoor mean humidity was 63.2 ± 20.6% (median 58%). Because the rats were exposed for 6 hours a day, 5 days a week, the equivalent PM2.5 concentration to which the rats were exposed to in the chamber “normalized” over the 10-week period was 14.1 µg/m3 after taking into account non-exposed time and weekends, which is well within the annual average PM2.5 National Ambient Air Quality Standard of 15.0 µg/m3 (US Environmental Protection Agency).18
BP Change
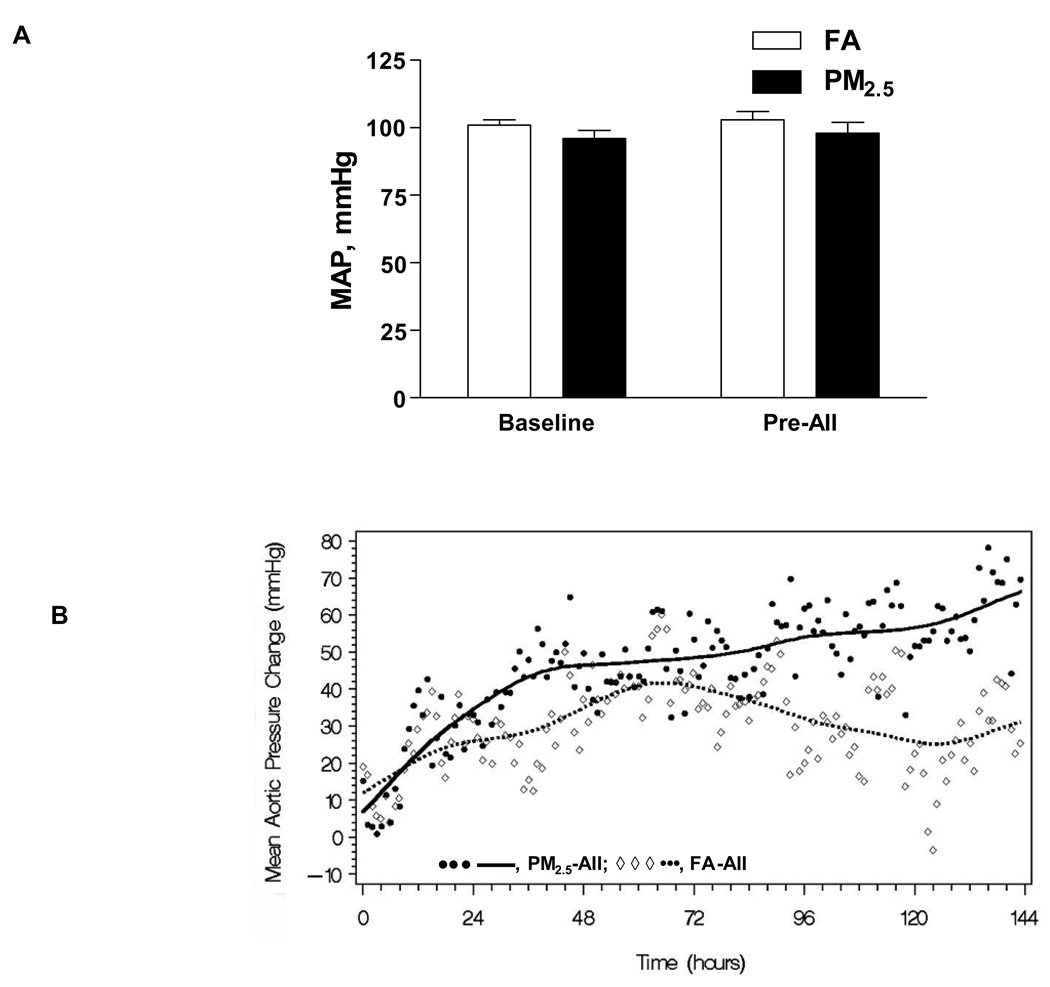
Figure 1A displays the mean arterial pressure at baseline following implantation of the radiotelemetry device and following 9 weeks of PM2.5 exposure (Pre-AII) compared to the FA exposed group. There was no change in mean arterial pressure following PM2.5 or FA exposure alone (96 ± 3 and 98 ± 4 mm Hg vs. 101 ± 2 and 103 ± 3 mm Hg following PM2.5 and FA exposure respectively). Figure 1B depicts changes in mean arterial pressure (MAP) in response to a 7-day infusion of AII. MAP was significantly higher following AII compared to baseline beginning at 24 hours. The MAP response was significantly different between FA-AII and PM2.5-AII groups, beginning at 93.0 ± 16.7 hours and lasting until the end of the monitoring period (hour 135.8 ± 5.2; P < 0.0001, Figure 1B). The slopes of the BP curves were significantly different with a persistently positive slope for the PM2.5-AII animals compared to the FA-AII group (Figure 1B).
Figure 1.
A, Twenty-four hour mean arterial blood pressure in the aorta (MAP) at baseline and after 9 weeks of PM2.5 exposure (Pre-AII) in SD rats (n = 6). There were no significant changes in mean arterial pressure following 9 weeks of PM2.5 exposure alone. B, MAP change in SD rats exposed to PM2.5-AII or FA-AII after the implant of AII osmotic minipumps (n = 6).
Vasomotor Responses
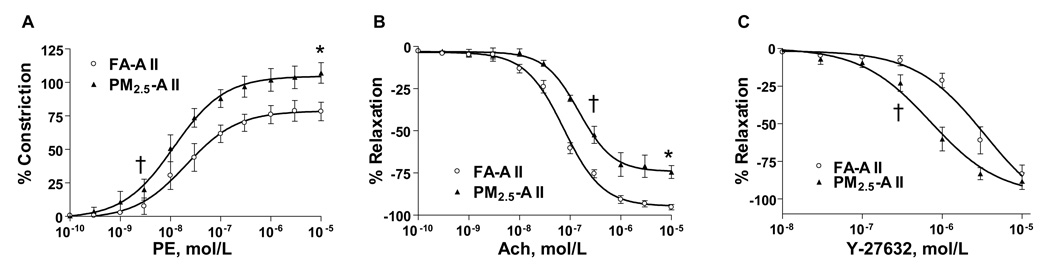
Figure 2A depicts responsiveness of thoracic aortic segments to the α-adrenergic agonist phenylephrine (PE). Responses to PE in the PM2.5-AII group were characterized by a shift in the half-maximal dose for constriction (EC50, 1.4 ± 0.1 × 10−8 vs. 2.5 ± 0.1 × 10−8 mol/L, P < 0.05) and an increase in peak constriction compared with FA-AII. Figures 2B and 2C demonstrate responses of preconstricted aortic segments to the endothelium dependent agonist acetylcholine (Ach) and the Rho-kinase (ROCK) inhibitor Y-27632. Peak responses to Ach were attenuated in the PM2.5-AII group with a right shift in the half-maximal dose for dilation (ED50, 2.3 ± 0.1 × 10−7 vs. 6.3 ± 0.1 × 10−8 mol/L in FA-AII, P < 0.05, Figure 2B). Aortic relaxation responses to Y-27632 were exaggerated in the PM2.5-AII group characterized by a significant decrease in ED50 (7.0 ± 0.2 × 10−7 vs. 2.1 ± 0.1 × 10−6 mol/L in FA-AII, P < 0.05, Figure 2C), indicating significant upregulation of ROCK activity in the aortic tissues of PM2.5-AII group compared with FA-AII. In additional experiments (see online supplement) performed in C57BL/6 mice with Fasudil (1mg/kg/day), we demonstrated that Fasudil administration concomitantly with AII and PM2.5 restored bioavailable NO levels as evidenced by improved constriction to L-NMMA (Supplemental Figure I, available online at http://atvb.ahajournals.org) and corrected abnormal sensitivity to PE (unpublished data).
Figure 2.
Aortic vasomotor responses in SD rats exposed to PM2.5-AII or FA-AII. Contraction of aortic rings in response to vasoconstrictor phenylephrine (A), and relaxation of aortic rings in response to endothelium dependent vasodilator acetylcholine (B) or ROCK inhibitor Y-27632 (C) (n = 6). *P < 0.05 vs. FA-AII for peak constriction or dilation, †P < 0.05 vs. FA-AII for EC50 or ED50.
Superoxide Generation
We used DHE staining (in-situ method) and lucigenin chemiluminescence assays to determine superoxide (O2 ˙−) generation in the aorta. O2 ˙− production in aortic rings was markedly enhanced in PM2.5-AII group compared with the FA-AII group. Pretreatment of aortic sections with O2 ˙− scavenger polyethylene glycol-superoxide dismutase (PEG-SOD) reduced the DHE fluorescence, confirming the authenticity of the signal (data not shown). The NAD(P)H oxidase inhibitor apocynin or the flavin protein inhibitor diphenyliodonium (DPI) significantly reduced the DHE fluorescence in tissue sections from both groups (supplemental Figure IIA). O2 ˙− generation in aortic ring by lucigenin chemilumiscence demonstrated a significant 2.2-fold increase in segments from the PM2.5-AII group compared with FA-AII (P < 0.001, supplemental Figure IIB). Increase of O2 ˙− production in aortic rings was prevented by apocynin. A nitric oxide synthase (NOS) inhibitor, N-omega-nitro-L-arginine methyl ester (L-NAME) also prevented O2 ˙− generation in aortic rings, suggesting the involvement of NOS dependent O2 ˙− generation in response to PM2.5.
Tetrahydrobiopterin (BH4) Levels in Response to PM2.5
Since we demonstrated L-NAME mediated inhibition of O2 ˙−, we investigated whether BH4 depletion was involved as a mechanism for eNOS uncoupling in response to PM2.5 mediated oxidant stress. BH4 levels in the mesenteric vasculature (resistance vessels) and heart were quantified. These were 6.5 ± 1.2 and 9.7 ± 1.3 pmol/mg protein in the FA-AII group compared with 3.5 ± 0.9 and 5.9 ± 0.8 pmol/mg protein, respectively in the PM2.5-AII group which represent a 46% and 41% reduction respectively (n = 6/group, p < 0.05 for both heart and mesenteric tissue). Additionally, BH4 levels in the liver, an important site of BH4 synthesis and a highly vascular organ were decreased in the PM2.5-AII vs. FA-AII (27.2 ± 2.1 vs. 15.8 ± 3.0 pmol/mg protein, P < 0.05), consistent with a systemic effect of PM2.5 on extra-pulmonary tissues.
NADPH Oxidase Subunit Expression
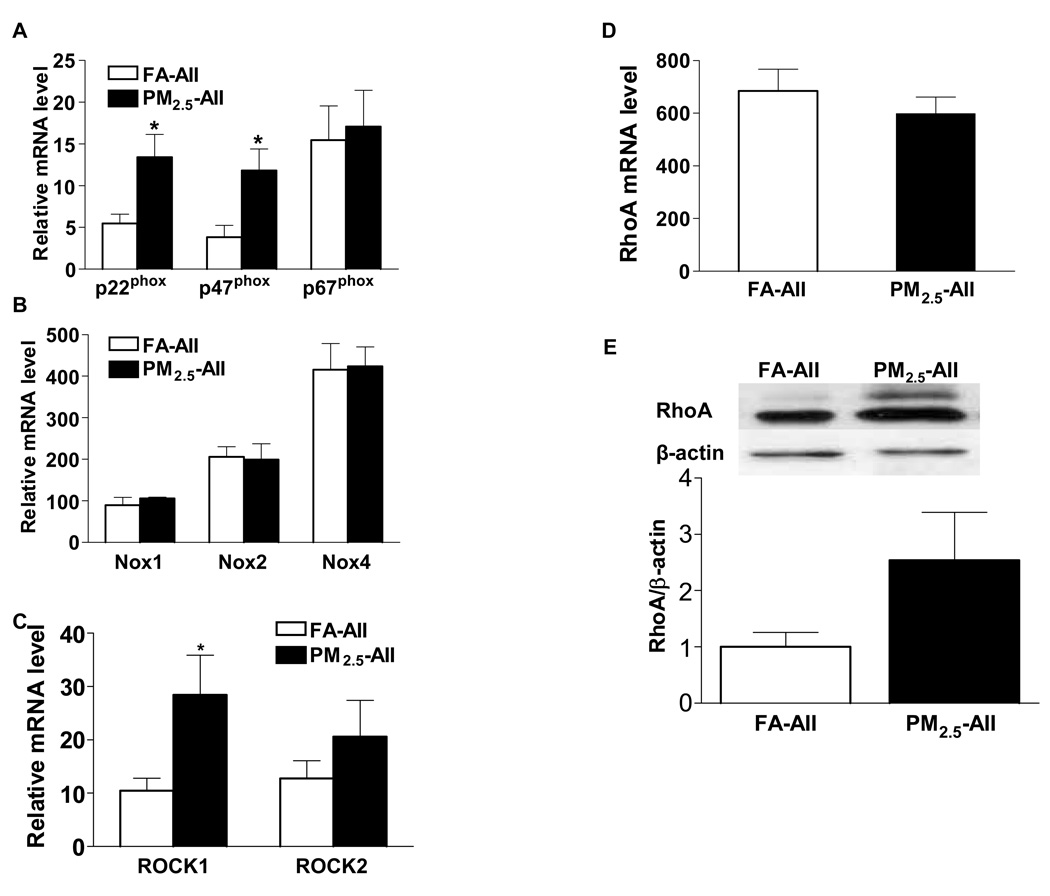
NAD(P)H oxidases in the vasculature, are composed of two membrane-associated subunits p22phox and gp91phox [also named Nox2 (neutrophil oxidase 2) in vascular smooth muscle cells, or Nox4 in endothelial cells], and the cytosolic components p47phox, p67phox, and the small GTP-binding protein Rac (Ras-related C3 botulinum toxin substrate). The mRNA expression levels of both the membrane-associated subunit p22phox and the cytoplasmic subunit p47phox were significantly increased in the aortic tissues of PM2.5-AII group compared with those of the FA-AII group (Figure 3A). No changes were observed in the expression levels of other components in these two groups, including p67phox, Nox2 (gp91phox), and other two members of the family of gp91phox-like proteins Nox1 and Nox4 (Figure 3B). There were no differences in the expression levels of the small GTP binding cytosolic units Rac-1 or Rac-2 (data not shown).
Figure 3.
Expression of NAD(P)H oxidase subunits, RhoA, and ROCKs in aortic tissue of rats exposed to FA-AII or PM2.5-AII. A and B, The mRNA expression of NAD(P)H oxidase subunits p22phox, p47phox, p67phox (A), and Nox1, Nox2, Nox4 (B) in aortic tissue. C and D, The mRNA expression of ROCK-1 and ROCK-2 (C), and RhoA (D) in aortic tissues. The mRNA levels were determined by real-time quantitative PCR, and normalized to that of β-actin mRNA. E, The expression of RhoA protein in aortic tissue. Values are expressed as mean ± SE (n= 6). *P < 0.05 vs. FA-AII.
Expression of RhoA/ROCK
To determine the effect of PM2.5 on the RhoA/ROCK expression, mRNA and protein levels of RhoA and ROCKs in aortic tissues were detected. Relative mRNA level of ROCK1 was 2.6-fold higher in the PM2.5-AII group vs. the FA-AII group (Figure 3C, P < 0.05), while ROCK-2 level (expressed predominantly in brain and skeletal muscle) was not different between the groups (P > 0.05). No difference was found in the expression level of RhoA mRNA (Figure 3D, P > 0.05). Although the protein level of RhoA protein in aortic tissues was 2.5 times higher in the PM2.5-AII group compared with the FA-AII group, this difference was not statistically significant (Figure 3E, P > 0.05).
Ultrafine Particles Mediates Myosin Light Chain (MLC) Activation through RhoA/ROCK Pathways
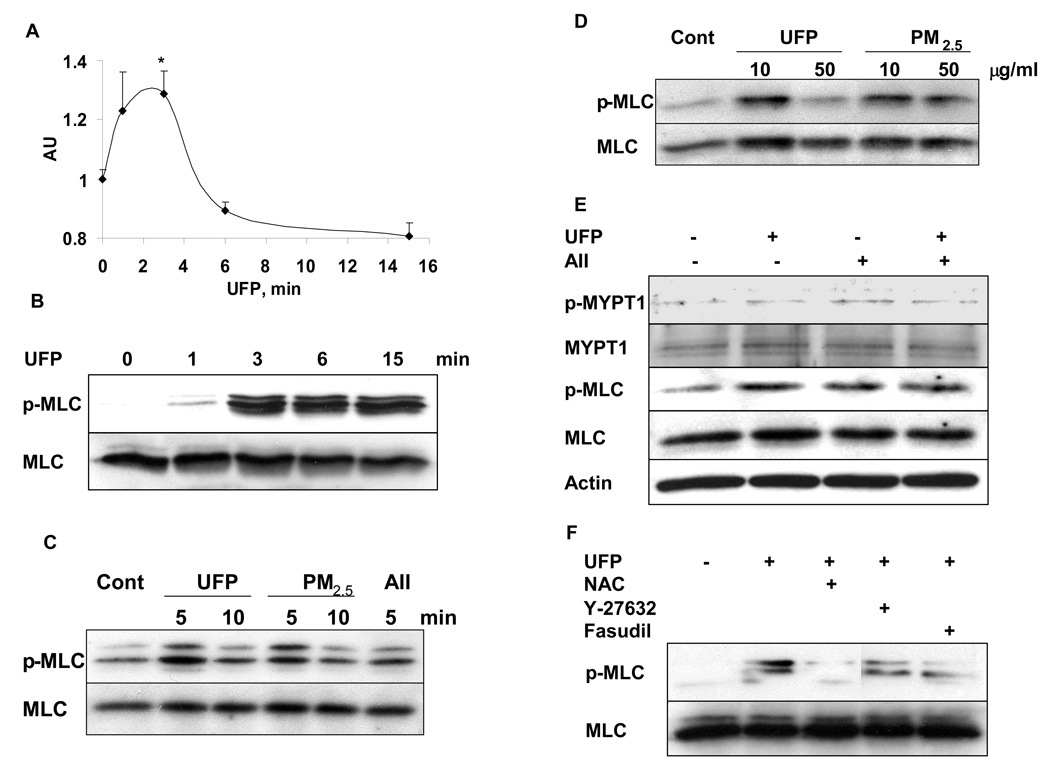
To further investigate the mechanism through which PM2.5 mediates smooth muscle vasoconstriction and hypertension and the involvement of ROS in RhoA/ROCK activation, we performed an in vitro study where we exposed rat aortic smooth muscle cells (RASMCs) with UFP. We chose these as particles in this size range have been shown to transgress into the systemic circulation. Further our exposure system allows for simultaneous exposure to PM2.5 and particles in the UFP range (PM0.1). Exposure of RASMCs to 10 µg/ml of UFP rapidly and significantly induced the activation of RhoA (Figure 4A), which was similar to the activation of RhoA induced by 100 nM of AII (supplemental Figure III). The effect of PM2.5 on Rho activity was considerably weaker than the effects of UFP (online supplement Figure III). Since the well-known myosin phosphatase target subunit (MYPT1) is a major effector of RhoA/ROCK-mediated Ca2+ sensitization and a regulator of MLC activation for the contraction in smooth muscle cells, we tested the ability of UFP and PM2.5 exposure to phosphorylate MLC and MYPT1. Figure 4B to 4E depict the results in response to acute exposure of UFP and PM2.5 in RASMCs. Exposure to 10 µg/ml of UFP rapidly induced the phosphorylation of MLC in RASMCs (Figure 4B). Both UFP and PM2.5 exposure exhibited similar magnitude of effect on MLC phosphorylation. These effects were not dose dependent with the lower concentration (10 µg/ml) showing more potent effect than high concentration (50 µg/ml) (Figure 4C and 4D). Moreover, both UFP and PM2.5 induced the phosphorylation of MLC and MYPT1 to the same extent of that induced by AII, a well known activator of ROCK (Figure 4C and 4E). The activation of MLC induced by UFP exposure in RASMCs was inhibited by ROCK inhibitors Y-27632 and fasudil and by the non-specific thiol antioxidant N-acetyl-L-cysteine (NAC, Figure 4F), implicating ROS mediated ROCK activation in response to PM.
Figure 4.
PM exposure induces MLC activation through RhoA/ROCKmediated pathways in cultured rat aortic smooth muscle cells. For all experiments, cells were serum starved and exposed to UFP or PM2.5 for the indicated time or dose. A. The level of “active” GTP-RhoA in cell lysates detected with a specific anti-RhoA antibody. Values are expressed as mean ± SE (n= 3) *P < 0.05 vs. 0 min. B. Time course of MLC phosphorylation with UFP. Cells were exposed to 10 µg/ml of UFP for the indicated time and MLC phosphorylation was determined by immunoblotting. C. Comparison of UFP with PM2.5 on MLC phosphorylation. Cells were exposed to 10 µg/ml of UFP or PM2.5, or to 100 nM of AII (positive control) for the indicated time. MLC phosphorylation was determined by immunoblotting. D. Effect of UFP and PM2.5 on MLC phosphorylation contrasted by dose. 10 or 50 µg/ml of UFP or PM2.5 were used for 5 min with MLC phosphorylation determined by immunoblotting. E. Effect of UFP compared with AII on phosphorylation of large sub-unit of myosin phosphatase-MYPT1. Cells were treated with 10 µg/ml of UFP and/or 100 nmol/L of AII for 3 min, and the phosphorylation of MYPT1 and MLC were detected. F. PM effects on MLC phosphorylation are mediated by ROS and Rho/ROCK pathways. Cells were exposed to 10 µg/ml of UFP for 3 min with or without pretreatment for 20 minutes with the thiol anti-oxidant N-acetylcysteine (NAC, 5 mM), Y-27632 (10 µM) and fasudil (10 µM). All data shown are representative of 3–4 independent experiments.
Discussion
We demonstrate in this paper that 10-weeks of exposure to concentrated ambient PM2.5 potentiates hypertension in response to AII and alters vasoconstrictor/vasodilator sensitivity. These alterations were accompanied by increased NAD(P)H oxidase and NOS-dependent generation of O2 ˙− and up-regulation of the RhoA/ROCK pathway.
Since exposure to PM2.5 alone did not alter BP, we did not pursue additional investigations in the PM2.5 group alone and investigated the impact of PM2.5 in conjunction with AII. An additional reason to examine the effect of PM2.5 in conjunction with AII is prior observations by us and others that suggest that PM2.5 has minimal effects by itself, but actively synergizes with other risk factors to influence outcomes. 3,6,9,10 Our data are consistent with this notion and suggests that although PM2.5 by itself had no discernible impact on BP, has an important effect in potentiating it, presumably by “sensitizing” the vasculature. The AII infusion model is a well characterized model of hypertension, where at least a portion of the BP elevation is related to the generation of reactive oxygen species (ROS) through an NAD(P)H oxidase dependent mechanism.19, 20 , 21 It also has a human analogue (renovascular hypertension) with the dose of AII used in this experiment, being comparable to that seen in these patients.22 Thus, the usage of this model to test the effects of PM2.5 exposure (a well known generator of ROS) was deliberate and planned. There is now increasing evidence that a number of components of PM2.5 may be facile mediators of redox cycling events such as polycyclic aromatic hydrocarbons, quinones, and transition metals.23 These events may be exaggerated in vulnerable patient populations such as diabetics, hypertensives and individuals with established cardiovascular diseases.
Both animal models and human studies have demonstrated a central role for ROS in the pathogenesis of hypertension.24–26 In the vasculature, the NA(D)PH oxidase system, a prototypical electron transport chain with both membrane (p22phox, Nox-1, 2, 3 or 4 depending on the tissue and species) and cytosolic units (p47phox, p67phox, Rac-1), have been shown to be functionally important in AII-mediated O2 ˙− production and in the genesis of hypertension.20, 27–29 We have shown up-regulation of key components of this oxidase (p22phox and p47phox) by PM2.5. The finding that PM2.5 activates the NAD(P)H oxidase system above and beyond what one may encounter with AII alone, likely represents a specific PM2.5 effect. Our observations extend recent experiments that confirm in-vitro activation of NAD(P)H oxidase by PM2.5. 30 An important additional finding in this study is that BH4 depletion and eNOS uncoupling is an additional major pathophysiologic consequence of PM2.5 exposure, providing a new mechanism for unbridled ROS generation through NOS dependent sources. It is well known that AII infusion as well as deoxycorticosterone acetate (DOCA) can result in O2 ˙− production through NOS uncoupling due to depletion of the NOS cofactor BH4, and this has been suggested to occur through upstream activation of NAD(P)H oxidases through the production of “kindling radicals”.31, 32 A NOS dependent mechanism for O2 ˙− by pollutant particles has been suggested in a prior study, where short term exposure to particles resulted in depletion of BH4 and enhanced endothelial cytotoxicity that could be rescued by exogenous BH4 supplementation.33 Thus our observations provide in-vivo confirmation of PM2.5 mediated vascular effects through dysregulation of two major homeostatic pathways. Based on our findings, it can be hypothesized that PM2.5 exposure in the presence of AII may activate NAD(P)H oxidases which then could lead to further BH4 depletion and NOS uncoupling.
The increased activity of Rho/ROCK in this model is a new finding and one that may provide additional mechanistic basis for increase in BP seen with PM2.5 in prior studies. 7–9, 34 The Rho/ROCK pathway is a key regulator of vascular smooth muscle tone through its effects on calcium sensitization of the contractile apparatus.35 Blockade of Rho/ROCK signaling through the usage of the ROCK inhibitors Y-27632 or hydroxyfasudil ameliorates BP and blood flow in hypertensive animals and humans, implicating this pathway in the pathogenesis of hypertension.36, 37 Rho/ROCK may potentially interact with the NAD(P)H oxidase system at multiple loci. Both ROS and AII, through NAD(P)H oxidase have been previously shown to activate Rho/ROCK.38, 39 Thus PM2.5 may potentially synergize with AII derived ROS generation to upregulate calcium sensitization pathways. Our in-vitro experiments, where UFP and PM2.5 derived O2 ˙− generation activates Rho/ROCK strongly implicates ROS generation as being a proximal signaling pathway. This is consistent with prior publications suggesting that ROS (primarily NADPH oxidase derived) is proximal and important for Rho/ROCK activation.40, 41 Our findings suggest that additional sources of ROS such as uncoupled eNOS may additionally be important.
The exposure pattern in our current study is environmentally relevant and allows for exposure to PM2.5 and UFP. The latter particles have been shown to transgress the pulmonary barrier and justify the use of the UFP in the in-vitro study.11,12 The peak daily levels of exposure, although higher than the recently revised daily PM2.5 NAAQS standards (<35 µg/m3, http://www.epa.gov/air/criteria.html) is regularly encountered in niches in urban areas or in close vicinity to automobiles and power plants. This situation at a global level is far worse as suggested by daily PM2.5 levels in urban areas in developing countries such as India and China where daily PM2.5 levels may exceed 200 µg/m3.42 The mean levels of exposure in our study of 14.1 µg/m3 is within the annual NAAQS standards, suggesting a discernible effect of PM2.5 at levels previously thought to be safe. Our findings thus have major implications for further regulations in PM levels.
In conclusion, exposure to PM2.5 may potentiate hypertension through NAD(P)H oxidase and eNOS dependent ROS generation that in turn activates the Rho/ROCK signaling pathway. These findings have important implications for PM2.5-mediated cardiovascular effects, and suggest that vascular effects of PM2.5 may modulate sensitivity to pressor stimuli.
Acknowledgments
The authors wish to acknowledge the contributions of Aixia Wang, Ximei Jin, Qiang Li, Damon Duquaine, Mianhua Zhong, and Dr. Morton Lippmann for superb technical and intellectual assistance and manuscript review. We also wish to acknowledge Dr. Terry Gordon for assistance with the radiotelemetry system.
Source of Funding
This study was supported partly by NIH R01ES013406 and R01ES015146 (Dr. Rajagopalan) and the Patel Family. The exposures were performed in facilities at New York University that were supported by Center Grants from EPA (R827351) and NIEHS (ES00260).
Footnotes
Disclosure
None.
References
- 1.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 3.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 4.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased Particulate Air Pollution and the Triggering of Myocardial Infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 5.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 6.Pope CA, 3rd, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 7.Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91:571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan-Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopalan S, Sun Q, Chen LC. Particulate pollution and endothelial function: deja vu all over again in the air. Circulation. 2005;111:2869–2871. doi: 10.1161/CIRCULATIONAHA.105.540872. [DOI] [PubMed] [Google Scholar]
- 11.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 12.Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17:189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. Jama. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 15.Chen WT, Tung CH, Weissleder R. Imaging reactive oxygen species in arthritis. Mol Imaging. 2004;3:159–162. doi: 10.1162/15353500200404124. [DOI] [PubMed] [Google Scholar]
- 16.Cardounel AJ, Xia Y, Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280:7540–7549. doi: 10.1074/jbc.M410241200. [DOI] [PubMed] [Google Scholar]
- 17.Nadziejko C, Chi Chen L, Nadas A, Hwang JS. The 'Fishing License' method for analysing the time course of effects in repeated measurements. Stat Med. 2004;23:1399–1411. doi: 10.1002/sim.1727. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous. Washington, DC: U.S. Environmental Protection Agency; Air quality criteria for particulate matter. 2004
- 19.Griendling K, Minieri C, Ollerenshaw J, Alexander R. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1817. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 20.Mehta PK, Griendling KK. Angiotensin II Cell Signaling: Physiological and Pathological Effects in the Cardiovascular System. Am J Physiol Cell Physiol. 2006 doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 21.Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. Journal of Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Flecha B. Oxidant mechanisms in response to ambient air particles. Mol Aspects Med. 2004;25:169–182. doi: 10.1016/j.mam.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 25.Lassegue B, Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 27.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H Oxidase : Role in Cardiovascular Biology and Disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 28.Fukui T, Ishizaka N, S, Laursen JB, Capers Qt, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circulation Research. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–866. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Hyseni X, Carter JD, Soukup JM, Dailey LA, Huang YC. Pollutant particles enhanced H2O2 production from NAD(P)H oxidase and mitochondria in human pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2006;291:C357–C365. doi: 10.1152/ajpcell.00365.2005. [DOI] [PubMed] [Google Scholar]
- 31.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 33.Bai Y, Suzuki AK, Sagai M. The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species. Free Radic Biol Med. 2001;30:555–562. doi: 10.1016/s0891-5849(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 34.Linn WS, Gong H, Jr, Clark KW, Anderson KR. Day-to-day particulate exposures and health changes in Los Angeles area residents with severe lung disease. J Air Waste Manag Assoc. 1999;49:108–115. doi: 10.1080/10473289.1999.10463890. [DOI] [PubMed] [Google Scholar]
- 35.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 36.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 37.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 38.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 2002;39:245–250. doi: 10.1161/hy0202.103271. [DOI] [PubMed] [Google Scholar]
- 39.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–H1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 40.Kim JS, Diebold BA, Kim JI, Kim J, Lee JY, Park JB. Rho is involved in superoxide formation during phagocytosis of opsonized zymosans. J Biol Chem. 2004;279:21589–21597. doi: 10.1074/jbc.M308386200. [DOI] [PubMed] [Google Scholar]
- 41.Kim JS, Kim JG, Jeon CY, Won HY, Moon MY, Seo JY, Kim JI, Kim J, Lee JY, Choi SY, Park J, Yoon Park JH, Ha KS, Kim PH, Park JB. Downstream components of RhoA required for signal pathway of superoxide formation during phagocytosis of serum opsonized zymosans in macrophages. Exp Mol Med. 2005;37:575–587. doi: 10.1038/emm.2005.71. [DOI] [PubMed] [Google Scholar]
- 42.Health Effects Institute. Health Effects of Outdoor Air Pollution in Developing Countries of Asia: A Literature Review. Special Report 15. 2004:31.