Abstract
A potentially less toxic approach for cancer therapy comprises induction of tumor cells to lose growth potential irreversibly and terminally differentiate. Combining this scheme termed ‘differentiation therapy of cancer’ with subtraction hybridization to human melanoma cells resulted in the cloning of melanoma differentiation associated (mda) genes displaying elevated expression as a consequence of induction of terminal differentiation. One originally novel gene, mda-7, was found to display elevated expression in normal melanocytes and nevi with progressive loss of expression as a consequence of melanoma development and progression to metastasis. Based on structure, biochemical properties and chromosomal location, mda-7 has now been reclassified as interleukin (IL)-24 a member of the expanding IL-10 family of cytokines. In vitro cell culture and in vivo animal studies indicate that mda-7/IL-24 selectively induces programmed cell death (apoptosis) in multiple human cancers (including melanomas), without harming normal cells, and promotes profound anti-tumor activity in nude mice containing human tumor xenografts. Based on these remarkable properties, a Phase I Clinical trial was conducted to test the safety of administration of mda-7/IL-24 by a replication incompetent adenovirus (Ad.mda-7; INGN 241) in patients with advanced solid cancers including melanoma. mda-7/IL-24 was found to be safe and to promote significant clinical activity, particularly in the context of patients with metastatic melanoma. These results provide an impetus for further clinical studies, and document a central paradigm of cancer therapy, namely translation of basic science from the “bench to the bedside.”
Keywords: mda-7/IL-24, apoptosis, metastatic melanoma, Phase I Clinical Trial
Introduction
Mortality from skin cancer is most frequently associated with malignant melanoma of the skin. Despite intensive study and a rapid increase in the incidence of malignant melanoma, current interventional therapeutic approaches have not translated into superior treatment options (Atallah and Flaherty, 2006; Atkins, 2006; Kirkwood et al., 2006) without evidence of improvement of survival after more than 27 phase III studies of diverse chemo- and biotherapies (Eggermont, 2006). Even with aggressive prevention campaigns and attempts to promote early detection, ~20 to 25% of all melanoma patients ultimately die as a consequence of disseminated metastases (Hauschild et al., 2006). Spread of melanoma to regional lymph nodes is a poor indicator of survival. When this occurs survival decreases to ~30 to 55%. Moreover, once melanoma metastasizes to various organs there is no effective curative treatment. These unfavorable statistics highlight the need for innovative and improved therapeutic options to treat patients with advanced metastatic melanoma (Leszczyniecka et al., 2001; Herlyn, 2006).
Cloning of mda-7/IL-24: Application of Differentiation Induction Subtraction Hybridization (DISH)
Melanoma is a melanocyte-derived skin cancer, which progresses through well defined stages, including melanocyte, benign nevus (common mole), dysplastic nevus, radial growth phase (RGP) melanoma, vertical growth phase (VGP) melanoma and ultimately metastatic melanoma (Clark, 1991). Although a readily treatable disease during early stages and even in the early VGP melanoma stage, universally effective therapies for advanced disease are non-existent. Our studies have focused on this disease as one involving aberrant differentiation, in which melanoma cells have lost the ability to respond to environmental cues normally resulting in controlled proliferation and differentiation (Jiang et al., 1994; Leszczyniecka et al., 2001). We have tested the hypothesis that by appropriate pharmacological manipulation it is possible to induce or upregulate expression of threshold levels of defined genes that restore growth control and induce terminal differentiation. This was in fact found to be the case, since treatment with a combination of recombinant fibroblast interferon (IFN-β) and the protein kinase C activator, mezerein (MEZ), resulted in terminal differentiation in essentially 100% of treated HO-1 metastatic human melanoma cells (Fisher et al., 1985; Jiang et al., 1993). By combining this unique differentiation model system with subtraction hybridization, an approach pioneered in our laboratory (Jiang and Fisher, 1993; Kang et al., 1998; Jiang et al., 2000) called differentiation induction subtraction hybridization (DISH) (Huang et al., 1999), we successfully identified and cloned a number of mda genes displaying elevated expression during terminal differentiation in human melanoma cells (Fig. 1). These genes included the originally novel gene mda-6, which is the ubiquitous cyclin dependent kinase inhibitor p21 (Jiang and Fisher, 1993; Jiang et al., 1995b), mda-7/IL-24, a novel apoptosis-inducing cytokine and the focus of this review (Jiang et al., 1995a; Fisher, 2005), mda-5, a gene important in the process of innate immunity (Kang et al., 2002; Lin et al., 2006) and mda-9/syntenin, a PDZ-containing molecule that is a positive regulator of metastasis (Sarkar et al., 2004; Boukerche et al., 2005). These findings support the robustness of this approach and its application in defining critical genes altered during important physiological processes.
Figure 1.
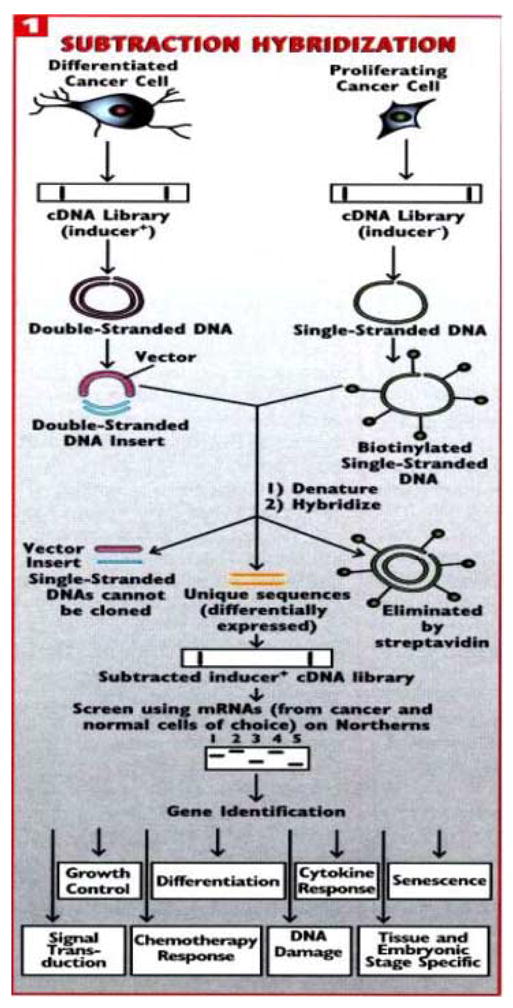
Strategy for identifying genes differentially expressed during induction of irreversible growth arrest and terminal differentiation in human melanoma cells. Subtraction hybridization was combined with a treatment protocol, IFN-β plus MEZ, that promotes terminal differentiation in metastatic HO-1 human melanoma cells. This approach, termed differentiation induction subtraction hybridization (DISH), has resulted in the cloning of melanoma differentiation associated (mda) genes involved in important physiological processes, including growth control, differentiation and apoptosis (mda-6/p21 and mda-7/IL-24), metastasis (mda-9/syntenin), cellular senescence (human polynucleotide phosphorylase) and innate immunity (mda-5). Experimental details of the subtraction hybridization and DISH approach can be found in Jiang and Fisher (1993) and Huang et al. (1999). (From Fisher et al., 2003).
mda-7/IL-24: A Novel Member of the of the IL-10 Gene Family Involved in Human Melanoma Growth, Differentiation and Progression
Induction of terminal differentiation in HO-1 human melanoma cells by treatment with a combination of IFN-β and MEZ results in profound changes in cell morphology with a concomitant irreversible loss in proliferative capacity and induction of terminal differentiation which correlates with induction of mda-7 an originally novel gene encoding a protein of 206-aa with a predicted size of ~23.8-kDa (Jiang et al., 1995a). Sequence analysis indicates that mda-7 is a highly conserved gene with homologous sequences in yeast, monkey, bovine, dog and cat. Initial studies documented de novo expression of mda-7 in normal immortal human melanocytes with a lack of de novo expression in most human melanoma cell lines, including RGP, VGP and metastatic melanoma (Jiang et al., 1995a). Accordingly, analysis of mda-7 versus GAPDH RNA levels by comparative-RT-PCR in a series of normal melanocytes, primary melanomas (RGP and VGP samples) and metastatic melanomas suggested an inverse relationship between mda-7 expression and melanoma development and progression (Jiang et al., 1995a; Gupta et al., 2006). In the case of a Matrigel-assisted melanoma progression model, mda-7 expression decreased in early VGP primary human melanoma cells selected for autonomous or enhanced tumor formation in nude mice. Moreover, when transfected into HO-1 and C8161 metastatic human melanoma cells, growth and colony formation were inhibited. These results suggest that mda-7 has antiproliferative properties in melanoma, but not in normal melanocytes, and in this context might contribute to growth suppression associated with terminal cell differentiation induced by IFN-β and MEZ. Additionally, we postulated that the mda-7 gene might also function as a negative regulator of melanoma progression with tumor suppressor functions.
Initial sequence analysis of mda-7 indicated a stretch of 21 amino acids, from position 101 to 121, corresponding to a signature motif for interleukin (IL)-10 family gene members (Jiang et al., 1995a). However, based on only ~23% amino acid sequence homology, further studies were obligatory to confirm a potential relationship between mda-7 and the IL-10 gene family. The association between mda-7 and the IL-10 gene family has now been confirmed based on structure (containing the IL-10 signature motif), chromosomal location (1q32-33, ~195-kb genomic region containing other IL-10 family gene members, including IL-10, IL-19, IL-20, mda-7) and biochemical properties (containing a secretory sequence and confirmed secretion from cells, including subsets of T-cells) resulting in its renaming as IL-24 (Huang et al., 2001; Caudell et al., 2002). In these contexts, mda-7/IL-24 is now considered a bona fide member of the IL-10 gene family.
Studies employing histochemistry document that MDA-7/IL-24 protein is found in primary melanocytes and early stage melanomas with decreased expression in more advanced melanomas (Ekmekcioglu et al., 2001; Ellerhorst et al., 2002). Using 41 primary melanomas and 41 metastases, including 24 paired samples, significant decreases in MDA-7/IL-24 staining were evident, as indicated by both the percentage of positive cells and overall intensity of MDA-7/IL-24 immunostaining, when comparing the intraepidermal and superficially invasive portions with the deeply invasive portions of primary tumors (Ellerhorst et al., 2002). Additionally, significant differences were also evident when comparing primary tumors to paired metastases (Ellerhorst et al., 2002). Taken together, these data and that of comparative-RT-PCR suggest that the level of expression of mda-7/IL-24 at both an RNA and protein level decreases during the process of melanocyte to melanoma progression, supporting the hypothesis that this gene may function as a negative regulator of melanoma growth and progression.
A novel mda-7 splice variant (mda-7s) has been reported that is detected by RT-PCR in nevi, but is absent in metastatic melanoma (Allen et al., 2004). The predicted open reading frame of mda-7s has 63-aa from mda-7/IL-24 derived from sequences located in exons 2, 4, 6 and 7 with an homology to wild type MDA-7/IL-24 protein of only 14 amino acids derived from exon 2. These 14 amino acids correspond to the initial 49 residues of the MDA-7/IL-24 N-terminal amino acid signal peptide sequence, without the signal peptide cleavage sites between residues 49 and 50 of the wildtype MDA-7/IL-24 (Sauane et al., 2003). This splice variant also lacks the IL-10 signature motif, N-glycosylation sites and protein kinase c and casein kinase II phosphorylation motifs present in the wildtype sequence (Sauane et al., 2003). Although some preliminary data is provided indicating that this variant can bind to wildtype MDA-7/IL-24 and reduce secretion of this cytokine, the involvement of this variant in melanoma development and progression requires further study and clarification.
Jiang et al. (1995a, 1996) provided the first experimental proof that forced expression of mda-7/IL-24 in human melanoma and other cancer cells, following transfection, resulted in growth suppression and a decrease in colony formation in monolayer culture. Subsequent studies employing a replication incompetent adenovirus (Ad.mda-7) to transduce mda-7/IL-24 into melanoma cells also indicated a capacity to suppress growth and decrease survival (Ekmekcioglu et al., 2001; Lebedeva et al., 2002). Studies employing Ad.mda-7 were particularly informative, indicating that mda-7/IL-24 did not significantly alter growth or decrease survival in primary or immortal normal human melanocytes (Ekmekcioglu et al., 2001; Lebedeva et al., 2002). Infection of a series of metastatic human melanoma cells, but not primary or immortal normal human melanocytes, with Ad.mda-7 resulted in accumulation of cells in the G2/M phase of the cell cycle and induction of apoptosis (Ekmekcioglu et al., 2001; Lebedeva et al., 2002). Additionally, infection with Ad.mda-7 induced changes in the ratio of pro-apoptotic (BAX and BAK) to anti-apoptotic (BCL-2 and/or BCL-XL) members of the BCL-family, promoting a shift from survival to programmed cell death (Lebedeva et al., 2002). These studies in melanoma, namely the ability of mda-7/IL-24 to induce changes in expression of BCL-family proteins and induce apoptosis selectively in cancer have also been confirmed in additional cancers, including brain (malignant glioma), breast, cervical, colon, ovarian and prostate (reviewed in Fisher, 2005, Gupta et al., 2006). Additional studies employing human tumor xenografts demonstrated significant activity of intratumoral injection of Ad.mda-7 in inhibiting both primary tumor growth and progression (reviewed in Fisher, 2005, Gupta et al., 2006). Recent approaches using conditionally replication competent adenoviruses to deliver mda-7/IL-24 have demonstrated profound activity in treating (and even curing) both primary tumors and tumors at a distant site in athymic nude mice exceeding that observed using a replication incompetent adenovirus to deliver this cytokine to cancer cells (Sarkar et al., 2005; Zhao et al., 2005; Sarkar et al., 2006).
Signal transduction pathways contributing to mda-7/IL-24-induction of apoptosis selectively in melanoma cells
A profoundly important question is the mechanism by which mda-7/IL-24 selectively induces apoptosis in cancer cells without altering the physiology or survival of normal cells. This question has been addressed in human melanoma cells by defining potential signal transduction pathways that may modulate this differential response (Sarkar et al., 2002). Infection of human melanoma cells, but not normal immortal melanocytes, with Ad.mda-7 resulted in a time- and dose-dependent increase in the expression, both mRNA and protein, of the growth arrest and DNA damage inducible (GADD) family of genes, including GADD153, GADD45α and GADD34. Additionally, treatment of melanoma cells with SB203580, a selective pharmacological inhibitor of the p38 mitogen-activated protein kinase (MAPK) pathway, efficiently inhibited Ad.mda-7-induced apoptosis. Further confirmation of an involvement of the p38 MAPK pathway in mda-7/IL-24-mediated apoptosis was provided by studies employing an adenovirus expressing a dominant negative mutant of the p38 MAPK pathway, which effectively blocked killing of melanoma cells by Ad.mda-7 (Sarkar et al., 2002). Infection of melanoma cells with Ad.mda-7 resulted in increased phosphorylation of p38 MAPK and heat shock protein 27 in melanoma cells, but not in normal immortal astrocytes. Moreover, SB203580 effectively inhibited Ad.mda-7-mediated induction of the GADD family of genes in both a time- and –dose dependent manner, and it significantly blocked downregulation of the antiapoptotic protein BCL-2. Additionally, inhibition of GADD genes using an antisense strategy either alone or in combination also effectively inhibited Ad.mda-7-induced apoptosis in melanoma cells. These results support an hypothesis that Ad.mda-7 mediates induction of the GADD family of genes through the p38 MAPK pathway, thereby promoting the selective induction of apoptosis in human melanoma cells (Sarkar et al., 2002) (Fig. 2).
Figure 2.
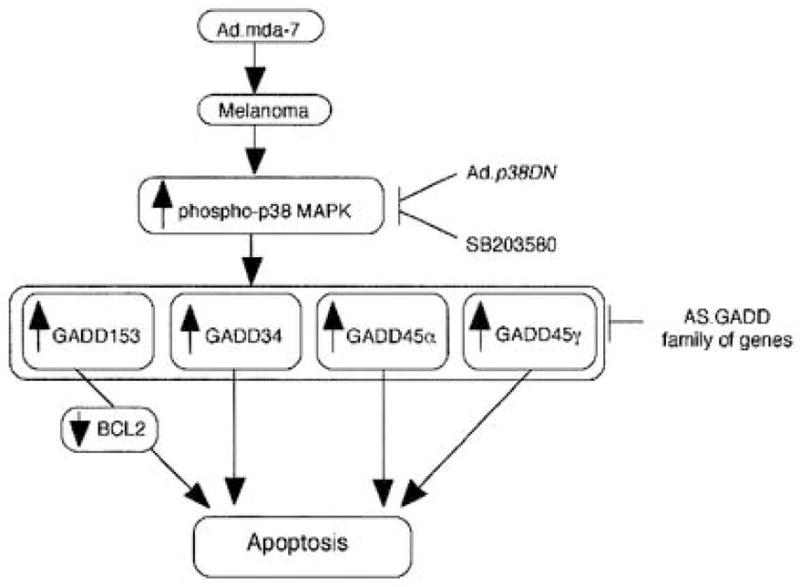
A hypothetical model of the involvement of p38 MAPK pathway and the GADD family members of genes in mediating apoptosis in human melanoma cells by Ad.mda-7. Ad.p38DN, Ad delivered dominant-negative mutant of p38 MAPK; SB203580, pharmacological inhibitor of p38 MAPK; GADD, growth arrest and DNA damage inducible gene; AS.GADD, antisense of GADD gene. (From Sarkar et al., 2002).
An inconsistent effect was reported in chronic lymphocytic leukemia (CLL)-B cells in which MDA-7/IL-24 promoted p38 MAPK phosphorylation and actually promoted the survival of these malignant cells (Sainz-Perez et al., 2006). Both mda-7/IL-24 mRNA and protein were overexpressed in the CLL B-cells examined, and p38 MAPK, a confirmed downstream mda-7/IL-24 signaling target, was highly phosphorylated in all CLL cells, but not in normal B-cells. In the CLL cells studied, phospho-p38 MAPK had no pro-apoptotic functions and instead may be required for survival, as suggested from these studies. Correspondingly, phosphorylation of p38 MAPK following transfection of CLL cells with mda-7/IL-24 promotes CLL cell survival (Sainz-Perez et al., 2006). These studies do not exclude the possibility of mda-7/IL-24 signaling through a p38 MAPK-independent mechanism; however, their data suggests a competition between mda-7/IL-24 and SB203580 (a specific inhibitor of p38 MAPK) for p38 MAPK activation. In recent studies, using a GST-MDA-7 protein produced in bacteria and tropism modified adenoviruses expressing mda-7/IL-24 (and capable of infecting leukemic cells), a loss in viability and induction of apoptosis was evident in a subset of leukemic cells (Lebedeva, Gupta, Dmitriev, Curiel and Fisher, unpublished data). Further studies are required to determine if this effect is a result of supraphysiological levels of MDA-7/IL-24 and if it results from a mechanism(s) independent of p38 MAPK activation.
Although GADD family gene induction, through activation of p38, is clearly a pathway of relevance in apoptosis induction by mda-7/IL-24 in melanoma cells, and other tumor cells including malignant glioma and breast, ovarian, prostate and pancreatic cancers, other signaling pathways are also activated by this cytokine and implicated in promoting apoptosis in specific cancer cell types, such as non-small-cell lung carcinoma (reviewed in Fisher, 2005, Gupta et al., 2006). These studies, performed in several laboratories, provide support for the hypothesis that multiple signaling pathways and processes are being impinged upon by mda-7/IL-24 in cancer cells, which are not being activated in normal cells, that ultimately lead to the definitive phenotype, death of the cancer cells. Intensive investigations are now in progress to further elucidate and understand these multiple parameters in a cancer cell biology and physiology that are altered selectively by mda-7/IL-24 (reviewed in Fisher, 2005, Gupta et al., 2006).
mda-7/IL-24 induces potent “bystander” antitumor activity promoting enhanced therapeutic potential
A phenomenon augmenting the therapeutic efficacy of mda-7/IL-24, first recognized in the context of pancreatic carcinoma cells (Su et al., 2001), involves potent “bystander” antitumor activity of this cytokine (Su et al., 2001; Su et al., 2005; Lebedeva et al., 2006). Unlike most cancer cell types, human pancreatic carcinoma cells did not show growth suppression or apoptosis induction when infected with doses of Ad.mda-7 that elicit this response in other human cancers (Su et al., 2001). Exploration of this phenomenon indicated that infection with Ad.mda-7 resulted in robust generation of mda-7/IL-24 mRNA, without production of significant protein. Based on the hypothesis that inability to translate mda-7/IL-24 mRNA into protein might be a consequence of pre-existing genetic changes in pancreatic tumor cells, we tested the potential role of K-ras, which is mutated in >90% of pancreatic tumors, as a possible inhibitor of translational efficiency of this cytokine in pancreatic cancer cells (Su et al., 2001). Blocking of K-ras expression using antisense phosphorothioate oligonucleotides targeting K-ras or an antisense K-ras expression construct resulted in elimination of the mda-7/IL-24 ‘translational block’ and production of MDA-7/IL-24 protein with concomitant induction of growth suppression and apoptosis (Su et al., 2001; Lebedeva et al., 2006). When used in combination, Ad.mda-7 infection and K-ras expression plasmid transfection, it was shown that even though a small number of cells received both agents, the majority of pancreatic carcinoma cells were killed, supporting the concept of a ‘bystander” antitumor effect (Su et al., 2001). The mechanism underlying this effect involves an alteration in the physical status of mda-7/IL-24 mRNA from free to polysome-associated, thereby resulting in its translation into protein (Lebedeva et al., 2006). Additional support for potent antitumor ‘bystander’ activity has come from studies employing an MDA-7/IL-24 fusion protein consisting of GST and MDA-7 (GST-MDA-7) (Sauane et al., 2004), Ad.mda-7 infection in animals containing tumors on both flanks in which only one flank was injected (Sarkar et al., 2005) and in a Phase I Clinical trial with Ad.mda-7 (INGN 241) (Cunningham et al., 2005).
The therapeutic activity of mda-7/IL-24 is significantly enhanced by its potent antitumor ‘bystander” activity (reviewed in Fisher al., 2003; Fisher, 2005; Gupta et al., 2006). A significant question is how mda-7/IL-24 “bystander” antitumor activity is initiated? Several studies have directly investigated the mechanism underlying this phenomenon (Chada et al., 2004; Su et al., 2005; Sauane et al., 2006). In a study by Chada and colleagues (2004) the activity of glycosylated secreted MDA-7/IL-24 against human melanoma cells was investigated. It was shown that addition of glycosylated MDA-7/IL-24 to melanoma cells resulted in phosphorylation and nuclear translocation of STAT3 through both type I (IL-20R1/IL-20R2) and type II (IL-22R1/IL-20R2) receptors. This interaction resulted in upregulation of BAX and induction of cell death in melanoma cells that was shown to involve STAT3-independent and PKR-independent signaling pathways. In contrast, other members of the IL-10 gene family, including IL-10, -19, -20 and –22, also activated STAT3, but they did not induce death in human melanoma cells. Similarly, in normal cells STAT3 is activated by glycosylated MDA-7/IL-24 without inducing cell death. These studies provide direct evidence that exogenously applied glycosylated MDA-7/IL-24 can promote melanoma-specific ‘bystander’ antitumor activity. In the context of in vivo animal models and clinical trials, immune modulation may also play a prominent role in MDA-7/IL-24’s potent ‘bystander’ antitumor activity (reviewed in Fisher et al., 2003; Fisher, 2005; Tong et al., 2005; Gupta et al., 2006; Miyahara et al., 2006).
The requirement for N-glycosylation of MDA-7/IL-24 in inducing biological activity, including ‘bystander’ antitumor activity, has recently been experimentally addressed by Sauane et al. (2006). An adenovirus vector expressing a non-secreted and non-glycosylated version of MDA-7/IL-24 protein was generated via deletion of its signal peptide and point mutations of its three N-glycosylated sites. This intracellular non-glycosylated protein was as effective as wild-type MDA-7/IL-24 protein in inducing apoptosis in multiple tumor cell lines. Both constructs: (1) displayed transformed cell specificity and localization to the endoplasmic reticulum (ER) compartment; (2) mediated apoptosis through JAK/STAT-independent and p38MAPK-dependent pathways; (3) induced sustained ER stress as evidenced by expression of ER stress markers (BiP/GRP78, GRP94, XBP-1, eIF2α); and (4) generated proteins that physically interacted with BiP/GRP78. Additionally, an expression construct containing the mda-7/IL-24 signal peptide linked to the mutated non-glycosylated mda-7/IL-24 gene retained the ability to induce ‘bystander’ antitumor activity. These studies reveal that MDA-7/IL-24 glycosylation is not mandatory for inducing cell death or “bystander” activities in different cancer cells, providing new insights into the mechanism by which MDA-7/IL-24 induces apoptosis and ER-stress.
Initial Phase I clinical studies employed a non-replicating adenovirus to deliver mda-7/IL-24 to tumor cells under transcriptional regulation by a cytomegalovirus (CMV) early promoter (Ad.mda-7; INGN 241) (Fisher et al., 2003; Cunningham et al., 2005; Lebedeva et al., 2005; Tong et al., 2005). This promoter expresses effectively in both cancer and normal cells and would be predicted to produce mda-7/IL-24 in both cell types. Based on this consideration, the role of normal cells in the MDA-7/IL-24 ‘bystander’ antitumor effect was evaluated by Su and colleagues (2005). Immortal normal human cells, including primary human fetal astrocytes (PHFA-IM), melanocytes (FM516-SV) and prostate epithelial cells (P69), infected with Ad.mda-7 produce and secrete MDA-7/IL-24 which modifies the anchorage-independent growth, invasiveness, survival and sensitivity to radiation of cancer cells that contain functional IL-20/IL-22 receptors. In contrast, secreted MDA-7/IL-24 from normal cells does not effect cancer cells that lack a full repertoire of functional IL-20/IL-22 receptors. Additionally, a combination of secreted MDA-7/IL-24 and radiation provokes a ‘bystander’ antitumor effect not only in cancer cells that are sensitive to either MDA-7/IL-24 or radiation used as a single therapy, but also in prostate tumor cells overexpressing the antiapoptotic proteins bcl-2 or bcl-xL and displaying resistance to either treatment alone. These observations support the value of normal cells as a means of producing MDA-7/IL-24 and also indicate that the antitumor activity of secreted MDA-7/IL-24 can be potentiated by radiation.
Phase I Clinical Experience with Ad.mda-7 (INGN 241) in the Context of Advanced Carcinoma and Metastatic Melanoma
A translational phase I study was designed to assess the clinical and local biological effectiveness of intratumoral injection of Ad.mda-7 in patients with injection-accessible advanced carcinoma (including melanoma) using as vector a replication-defective Ad5 backbone with E1 and partial E3 deletions (INGN 241) housing an expression cassette comprising the CMV immediate early promoter and wild-type mda-7 in the E1 region (Cunningham et al., 2005; Tong et al., 2005). The first three of eight patient cohorts (n=5) received sequential single-needle pass dose escalations of 2 × 1010 to 2 × 1012 viral particles (vp) with resection of the injected lesions 24 hours later. All of the remaining cohorts continued to receive 2 × 1012 vp followed by resections at 48 hours (cohort 4, n=3), 96 hours (cohort 5, n=4), multiple deposit injections with resection at 48 hours (cohort 6, n=1), single-needle pass injection with resection at 30 days (cohort 7, n=7), and single-needle pass injections twice weekly for 3 weeks every 28 days to a maximum of 2 cycles (cohort 8, n=8). Twenty-two of the 28 enrolled patients completed at least one cycle of therapy. All tumors demonstrated transgene transduction, MDA-7 protein expression and apoptosis induction. Post-treatment increases in CD3+CD8+ T-cells suggested a systemic Th1 cytokine response. Stable disease was documented in the three cohort 7 patients available for tumor measurements at the end of study, including one with melanoma. Five of the cohort 8 patients completed at least one cycle of therapy with clinically significant responses documented in two of them, both with melanoma. In one of these two patients with more than 10 discrete lesions, INGN 241 injection of the initial target 2 × 2 cm. right supraclavicular lesion resulted in significant perilesional erythema by day 4 and complete regression by the end of cycle 1 (see Fig. 3). A second 1.8 × 2.3 cm target lesion on the dorsum of the right hand was then treated with regression by injection 5 followed by excision after the sixth injection which, on pathology review, showed a marked inflammatory lymphoplasmacytic infiltrate with extensive coagulative necrosis. Notably, several distant non-injected lesions demonstrated erythema, although without demonstrable regression, concurrent with primary target therapy. This patient was alive, experiencing excellent performance status, at last follow-up at 491 days (Lebedeva et al. 2005). The second melanoma patient achieved a 33% target reduction (partial response). Overall, of the nine lesions injected in patient cohort 8, four (44%) demonstrated objective responses (CR or PR).
Fig. 3.
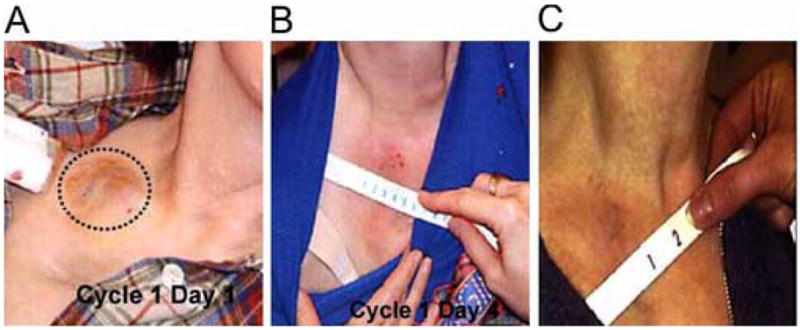
INGN 241 (Ad.mda-7) treatment of a melanoma metastasis in a right supraclavicular lymph node. (A) Pretreatment. (B) Inflammation by post-injection day 4. (C) Complete regression post-injection day 30. (From Cunningham et al., 2005).
Summary and Future Perspectives: MDA-7/IL-24 a Novel Cancer-Specific Apoptosis-Inducing Cytokine with Significant Clinical Potential
The present brief review, describes studies which began in 1990 that have now progressed from “bench to bedside” with the successful completion of a Phase I Clinical trial and initiation of Phase II clinical studies in patients with metastatic melanoma. What began as an endeavor to define a less toxic mode of treating melanoma by inducing tumor cells to undergo terminal differentiation, i.e., ‘differentiation therapy of cancer’, has culminated through subtraction hybridization in the identification of the novel cytokine mda-7/IL-24 (reviewed in Fisher, 2005; Gupta et al., 2006). Studies in multiple laboratories demonstrate that mda-7/IL-24 can selectively induce apoptosis in a majority of cancer cells, is non-toxic to a wide panel of normal cells and tissues, promotes potent ‘bystander’ antitumor activity in vitro and in vivo in animal models, inhibits tumor angiogenesis, is a potent immune modulating agent and enhances cancer-selective toxicity when combined with radiation, chemotherapy or monoclonal antibodies (reviewed in Fisher, 2005; Gupta et al., 2006). These unique properties combined with a Phase I clinical trial that documents safety when administered by means of a replication incompetent adenovirus (Ad.mda-7; INGN 241) and significant clinical activity in patients with advanced carcinomas and metastatic melanoma instill confidence that mda-7/IL-24 might become an effective frontline gene therapy for multiple cancers (Fisher et al., 2003; Cunningham et al., 2005; Lebedeva et al., 2005; Tong et al., 2005). It is improbable that mda-7/IL-24 will illicit a cancer cure when used alone, especially in the context of a replication incompetent adenovirus and intratumoral administration. However, combinatorial approaches (with other therapeutic agents) and employing improved vector delivery systems, especially those involving conditionally replication competent adenoviruses to promote viral replication and mda-7/IL-24 production in tumors (Sarkar et al., 2005; Zhao et al., 2005), offers potential for significantly improving the therapeutic index of this gene. Further laboratory development, animal modeling and expanded clinical trials will be necessary to determine if mda-7/IL-24 will become a standard of care for treating patients with primary and metastatic cancer.
Acknowledgments
The present study was supported in part by National Institutes of Health grants R01 CA035675, R01 CA097318, R01 CA098712, R01 CA108520 and P01 CA104177, the Samuel Waxman Cancer Research Foundation and the Chernow Endowment. PBF is the Michael and Stella Chernow Urological Cancer Research Scientist and a SWCRF Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen M, Pratscher B, Roka F, Krepler C, Wacheck V, Schofer C, Pehamberger H, Muller M, Lucas T. Loss of novel mda-7 splice variant (mda-7s) expression is associated with metastatic melanoma. J Invest Dermatology. 2004;123:583–588. doi: 10.1111/j.0022-202X.2004.23321.x. [DOI] [PubMed] [Google Scholar]
- Atallah E, Flaherty L. Treatment of metastatic malignant melanoma. Curr Treat Options Oncol. 2005;6:185–193. doi: 10.1007/s11864-005-0002-5. [DOI] [PubMed] [Google Scholar]
- Atkins MB. Cytokine-based therapy and biochemotherapy for advanced melanoma. Clin Cancer Res. 2006;12:2343s–2358s. doi: 10.1158/1078-0432.CCR-05-2503. [DOI] [PubMed] [Google Scholar]
- Boukerche H, Su Z-z, Emdad L, Baril P, Balme B, Thomas L, Randolph A, Valerie K, Sarkar D, Fisher PB. Mda-9/Syntenin: a positive regulator of melanoma metastasis. Cancer Res. 2005;65:10901–10911. doi: 10.1158/0008-5472.CAN-05-1614. [DOI] [PubMed] [Google Scholar]
- Clark WH. Tumor progression and the nature of cancer. Br J Cancer. 1991;64:631–644. doi: 10.1038/bjc.1991.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, Retter MW, Hill P, Chada S, Grimm EA. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, Zheng M, Grimm EA, Ekmekcioglu S. Bystander activity of Ad-mda7: human MDA-7 protein receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10:1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Eggermont AMM. Randomized trials in melanoma: an update. Surg Oncol Clin N Am. 2006;15:439–451. doi: 10.1016/j.soc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, Chada S, Grimm EA. Down-regulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int J Cancer. 2001;94:54–59. doi: 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, Grimm EA. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069–1074. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a ‘magic bullet’ for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, A novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–37. [PubMed] [Google Scholar]
- Fisher PB, Prignoli DR, Hermo H, Jr, Weinstein IB, Pestka S. Effects of combined treatment with interferon and mezerein on melanogenesis and growth in human melanoma cells. J Interferon Res. 1985;5:11–22. doi: 10.1089/jir.1985.5.11. [DOI] [PubMed] [Google Scholar]
- Gupta P, Su Z-z, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, Fisher PB. mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol & Therapeut. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild A, Egberts F, Russo P, Kahler KC. Individualized therapy of disseminated cancer using malignant melanoma as a model. Cancer Metastasis Rev. 2006;25:253–256. doi: 10.1007/s10555-006-8505-5. [DOI] [PubMed] [Google Scholar]
- Huang F, Adelman J, Jiang H, Goldstein NI, Fisher PB. Differentiation induction subtraction hybridization (DISH): an approach for cloning genes differentially expressed during growth arrest and terminal differentiation in human melanoma cells. Gene. 1999;236:125–131. doi: 10.1016/s0378-1119(99)00244-9. [DOI] [PubMed] [Google Scholar]
- Herlyn M. Molecular targets in melanoma: strategies and challenges for diagnosis and therapy. Int J Cancer. 2006;118:523–526. doi: 10.1002/ijc.21605. [DOI] [PubMed] [Google Scholar]
- Jiang H, Fisher PB. Use of a sensitive and efficient subtraction hybridization protocol for the identification of genes differentially regulated during the induction of differentiation in human melanoma cells. Mol Cell Different. 1993;1:285–299. [Google Scholar]
- Jiang H, Su ZZ, Boyd J, Fisher PB. Gene expression changes associated with reversible growth suppression and the induction of terminal differentiation in human melanoma cells. Mol Cell Different. 1993;1:41–66. [Google Scholar]
- Jiang H, Lin J, Fisher PB. A molecular definition of terminal cell differentiation in human melanoma cells. Mol Cell Different. 1994;2:221–239. [Google Scholar]
- Jiang H, Lin JJ, Su Z-z, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995a;11:2477–2486. [PubMed] [Google Scholar]
- Jiang H, Lin J, Su ZZ, Kerbel RS, Herlyn M, Weissman RB, Welch D, Fisher PB. The melanoma differentiation associated gene mda-6, which encodes the cyclin-dependent kinase inhibitor p21, is differentially expressed during growth, differentiation and progression in human melanoma cells. Oncogene. 1995b;10:1855–1864. [PubMed] [Google Scholar]
- Jiang H, Su Z-z, Lin JJ, Goldstein NI, Young CSH, Fisher PB. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA. 1996;93:9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Kang D-c, Alexandre D, Fisher PB. RaSH, A rapid subtraction hybridization approach for identifying and cloning differentially expressed genes. Proc Natl Acad Sci USA. 2000;97:12684–12689. doi: 10.1073/pnas.220431297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-c, La France R, Su Z-z, Fisher PB. Reciprocal subtraction differential RNA display: an efficient and rapid procedure for isolating differentially expressed gene sequences. Proc Natl Acad Sci USA. 1998;95:13788–13793. doi: 10.1073/pnas.95.23.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-c, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5, an interferon-inducible putative RNA helicase with dsRNA-dependent ATPase activity and melanoma growth suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood JM, Moschos S, Wang W. Strategies for the development of more effective adjuvant therapy of melanoma: current and future explorations of antibodies, cytokines, vaccines, and combinations. Clin Cancer Res. 2006;12:2331s–2336s. doi: 10.1158/1078-0432.CCR-05-2538. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su Z-z, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sauane M, Gopalkrishnan RV, Sarkar D, Su Z-z, Gupta P, Nemunaitis J, Cunningham C, Yacoub A, Dent P, Fisher PB. mda-7/IL-24: exploiting cancer’s Achilles’ heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Sarkar D, Su Z-z, Gopalkrishnan RV, Athar M, Randolph A, Valerie K, Dent P, Fisher PB. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006;66:2403–2413. doi: 10.1158/0008-5472.CAN-05-3510. [DOI] [PubMed] [Google Scholar]
- Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher PB. Differentiation therapy of cancer: basic science and clinical applications. Pharmacol & Therapeut. 2001;90:105–156. doi: 10.1016/s0163-7258(01)00132-2. [DOI] [PubMed] [Google Scholar]
- Lin L, Su Zz, Lebedeva IV, Gupta P, Boukerche H, Rai T, Barber GN, Dent P, Sarkar D, Fisher PB. Activation of Ras/Raf protects cells from melanoma differentiation associated gene-5-induced apoptosis. Cell Death & Differentiation. 2006 doi: 10.1038/sj.cdd.4401899. in press. [DOI] [PubMed] [Google Scholar]
- Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, Ioannides CG, Chada S, Ramesh R. Melanoma differentiation-associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model. Cancer Gene Therapy. 2006;13:753–761. doi: 10.1038/sj.cgt.7700954. [DOI] [PubMed] [Google Scholar]
- Sainz-Perez A, Gary-Gouy H, Portier A, Davi F, Merle-Beral H, Galanaud P, Dalloul A. High mda-7 expression promotes malignant cell survival and p38 MAP kinase activation in chronic lymphocytic leukemia. Leukemia. 2006;20:498–504. doi: 10.1038/sj.leu.2404073. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su Z-z, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A 2002. 2002;99:10054–10059. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Boukerche H, Su Z-z, Fisher PB. mda-9/syntenin: recent insights into a novel cell signaling and metastasis-associated gene. Pharmacol & Ther. 2004;104:101–115. doi: 10.1016/j.pharmthera.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102:14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D, Su Z-z, Fisher PB. Unique conditionally replication competent bipartite adenoviruses-cancer terminator viruses (CTV): efficacious reagents for cancer gene therapy. Cell Cycle. 2006;5:1531–1536. doi: 10.4161/cc.5.14.3095. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Sarkar D, Su Z-z, Lebedeva IV, Dent P, Pestka S, Fisher PB. MDA-7/IL-24: novel cancer growth suppressing and apoptosis inducing cytokine. Cytokine Growth Factor Rev. 2003;14:35–51. doi: 10.1016/s1359-6101(02)00074-6. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gopalkrishnan RV, Choo HT, Gupta P, Lebedeva IV, Yacoub A, Dent P, Fisher PB. Mechanistic aspects of mda-7/IL-24 cancer cell selectivity analysed via a bacterial fusion protein. Oncogene. 2004;23:7679–7690. doi: 10.1038/sj.onc.1207958. [DOI] [PubMed] [Google Scholar]
- Sauane M, Gupta P, Lebedeva IV, Su Zz, Sarkar D, Randolph A, Valerie K, Gopalkrishnan RV, Fisher PB. N-glycosylation of MDA-7/IL-24 is dispensable for tumor cell-specific apoptosis and “bystander” anti-tumor activity. Cancer Res. 2006 doi: 10.1158/0008-5472.CAN-06-1887. in press. [DOI] [PubMed] [Google Scholar]
- Su Z-z, Lebedeva IV, Gopalkrishnan RV, Goldstein NI, Stein CA, Reed JC, Dent P, Fisher PB. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98:10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Zhao L, Gu J, Dong A, Zhang Y, Zhong L, He L, Wang Y, Zhang J, Zhang Z, Huiwang J, Qian Q, Qian C, Liu X. Potent antitumor activity of oncolytic adenovirus expressing mda-7/IL-24 for colorectal cancer. Hum Gene Ther. 2005;16:845–858. doi: 10.1089/hum.2005.16.845. [DOI] [PubMed] [Google Scholar]


