Abstract
Objective
This study examines two dimensions of racial segregation across hospitals, using a disease for which substantial disparities have been documented.
Data Sources
Black (n=32,289) and white (n=244,042) patients 67 years and older admitted for acute myocardial infarction during 2004–2005 in 105 hospital markets were identified from Medicare data. Two measures of segregation were calculated: Dissimilarity (i.e., dissimilar distribution by race across hospitals), and Isolation (i.e., racial isolation within hospitals). For each measure, markets were categorized as having low, medium, or high segregation.
Study Design
The relationship of hospital segregation to residential segregation and other market characteristics was evaluated. Cox proportional hazards regression was used to evaluate disparities in the use of revascularization within 90 days by segregation level.
Results
Agreement of segregation category based on Dissimilarity and Isolation was poor (κ=0.12), and the relationship of disparities in revascularization to segregation differed by measure. The hazard of revascularization for black relative to white patients was lowest (i.e., greatest disparity) in markets with low Dissimilarity, but it was unrelated to Isolation.
Conclusions
Significant racial segregation across hospitals exists in many U.S. markets, although the magnitude and relationship to disparities depends on definition. Dissimilar distribution of race across hospitals may reflect divergent cultural preferences, social norms, and patient assessments of provider cultural competence, which ultimately impact utilization.
Keywords: Racial disparities, revascularization, acute myocardial infarction, risk adjustment
Racial disparities in outcomes and processes of health care have been documented for a wide spectrum of illnesses (Institute of Medicine 2002). Recent studies demonstrate that disparities may be attributed, in part, to differences in clinical practice across regions (Skinner et al. 2003; Baicker et al. 2004; Haas et al. 2004; Groeneveld, Heidenreich, and Garber 2005;) or differences in the use of high-quality providers within regions (Gregory et al. 1999; Bradley et al. 2004; Schelbert et al. 2005). Such disparities may, in turn, be a symptom of racial segregation that occurs in the health care delivery system or by residence.
Previously, residential segregation has been linked to higher mortality for black populations (Hart et al. 1998; Collins and Williams 1999;), and, to specific health conditions, including cardiovascular disease (Cooper 2001). Residential segregation is believed to affect health indirectly through environmental and individual factors such as poor housing conditions, lack of information, health behaviors, and stress (Schulz et al. 2005; Payne-Sturges et al. 2006;). Utilization and outcomes of specific health services may also be impaired if racially concentrated neighborhoods face limited access to high-quality providers, or if social pressures within such neighborhoods deter the use of services deemed unacceptable by neighborhood norms.
While residential segregation likely plays a key role in segregating the health delivery system, other factors contribute to segregation of hospital services independent of residence. Historically, hospitals were racially segregated in the South and most northern cities before the 1960s (Halperin 1988; Smith 1998, 2004). While sanctioned forms of hospital segregation were essentially eliminated during the 1960s, de facto segregation remains, partly due to social and economic pressures that are unique to health care. First, hospitals are predominantly voluntary in ownership and thus insulated from public scrutiny and political control. Until recently, external monitoring efforts, such as those by JCAHO, focused almost exclusively on structure (e.g., staff credentials). Second, medical staffs within hospitals have wide latitude in assigning clinical privileges. If such latitude results in fewer admitting privileges for physicians who treat blacks, access to hospitals for patients of black physicians may be compromised. Hospitals and individual physicians may also limit the patients they treat through managed care contracting, payment criteria, or physical location. Such activities may make sense from a “business” perspective, but they can have divisive effects on patient populations. Other factors that may divide hospitals racially include racial differences in physician referrals, transportation systems, hospital emergency department capacity, institutional discrimination, and patient preferences. Hospital segregation may, in turn, impact health outcomes and service utilization differently than residential segregation through racial differences in access to high-quality providers, specialized services, and medical practice patterns.
Sociological literature defines multiple dimensions of segregation (Massey and Denton 1988). The most popular dimension reflects the evenness of the population distribution across units. Using this dimension, hospital segregation exists if patients are distributed unevenly by race across hospitals. A second dimension of segregation reflects the isolation of a minority group to the majority. Markets in which black patients are unlikely to be exposed to white patients within hospitals are segregated on this dimension. A geographic market can be segregated on multiple dimensions, or it may be segregated on one dimension but not another. For example, black patients may be evenly distributed across hospitals in a market but experience little exposure to white patients if a large proportion of the market is black.
Isolation and uneven distribution may impact disparities uniquely, although there is likely significant overlap. Isolation may impact disparities if hospitals in which blacks have little exposure to whites are under-funded, limiting the availability of specialized services. Isolated black patients may also have lower levels of trust for white providers or feel unwelcome by white providers, making them disinclined to use the services of majority white hospitals, even if such hospitals are available to them (Marschall and Stolle 2004). Physicians treating those patients may be equally isolated, and therefore unlikely to refer patients to majority white hospitals with more services. Finally, an isolated black medical community may develop medical practice patterns that reflect the norms of the populations they serve—such practices may differ from those of less isolated communities. Dissimilar distribution of blacks across hospitals may impact disparities similarly, if the uneven distribution results in racial isolation. In contrast, uneven distribution may actually facilitate utilization if minority populations perceive a cultural affinity with a particular hospital.
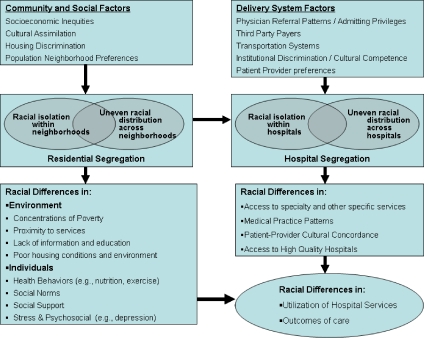
Figure 1 provides a framework for investigating the relationship between segregation and disparities in the utilization and outcomes of health services. Residential unevenness and isolation impact racial differences in environmental and individual factors such as poverty, proximity to services, housing conditions, individual stress, and social norms. These residential factors also impact hospital unevenness and isolation, which may be magnified further by other delivery system factors, including physician referral patterns, third-party payer contracts, and patient preferences. Ultimately, disparities in health care utilization and outcomes result.
Figure 1.
Framework for Evaluating Segregation and Disparities in Utilization and Outcomes of Health Care
This study investigates segregation, defined as racial unevenness and racial isolation, and disparities in treatment of acute myocardial infarction (AMI). Hospital discharge data for black and white patients admitted for AMI during 2004–2005 were used to calculate two measures of hospital segregation. Analyses investigated the relationship of hospital segregation measures to each other, as well as to residential segregation, and the relationship to disparities in the use of revascularization after AMI. AMI was chosen as the disease paradigm because of the extensive evidence documenting disparities in treatment of AMI (Barnato et al. 2005; Cromwell et al. 2005; Vaccarino et al. 2005; Popescu, Vaughan-Sarrazin, and Rosenthal 2007;).
METHODS
Patient Population
Patients were identified using Medicare Provider Analysis and Review (MedPAR) data files. MedPAR files contain all Medicare fee-for-service hospitalizations and include patient demographics and zip code, ICD-9-CM diagnoses and procedure codes, admission source (e.g., transfer from another hospital), admission and discharge dates, hospital discharge disposition, date of death, and hospital identifier. Records for black and white patients with primary ICD-9-CM diagnosis codes 410.xx admitted during 2004–2005 were identified in the MedPAR files (N=607,664). Patients were excluded if they were transferred from another acute care facility (n=89,243) or resided outside the market of the admitting hospital (n=60,051), leaving 458,370 patients.
Health Care Markets
Regional markets for hospital care were defined using hospital referral regions (HRR's), which represent 306 geographic markets for tertiary health care and were defined using zip codes to reflect patient flows for major surgery (http://www.dartmouthatlas.org). HRR characteristics were obtained from the American Hospital Association 2005 Survey (e.g, number of acute care hospitals, teaching hospitals, and acute care hospital beds per 1,000 population), the U.S. 2,000 Census Summary Tape File 3 (STF) available at http://factfinder.census.gov, and Medicare Denominator files for 2004–2005 (e.g., Medicare Part A and managed care enrollment).
We restricted the sample to 105 HRRs with a minimum of 5 acute care hospitals and 50 black AMI admissions. The 105 HRRs included 32,289 black and 244,042 white patients, and 2,065 total acute care hospitals. Sensitivity analyses evaluated four alternative market selection criteria: (1) minimum of five hospitals and 25 black patients (n=135 markets), (2) minimum of 5 hospitals and 100 black patients (n=64), (3) minimum of 10 hospitals and 50 black patients (n=79), and (4) minimum of 10 hospitals and 100 black patients (n=53).
Measurement of Segregation
In their classic article, Massey and Denton (1988) identified the Dissimilarity Index as the best measure of evenness, because it is popular, easy to interpret, and easy to compute. The index represents the fraction of black patients that must change hospitals to achieve equal proportions of blacks in each hospital, and it is calculated as
where ti and pi are the total number of patients and proportion of black patients in hospital i, and T and P are the total number of patients and proportion of black patients in the entire market. The index varies between 0 (no segregation) and 1 (complete segregation). Perfect desegregation exists if every hospital in the market has exactly the same share of black patients.
The most popular and straightforward measure of isolation is the Isolation Index. This index represents the proportion of patients who are black in the hospital in which the “average” black patient is admitted, and it is calculated as
where ti and xi are the total numbers of patients and black patients in hospital i, and X is the total number of black patients in the entire market. The index varies between 0 (no isolation) and 1 (complete isolation).
The Dissimilarity Index and Isolation Index were calculated separately for each HRR using MedPAR data for black and white patients admitted for AMI during 2004–2005 (n=276,811). Markets were categorized as having low, medium, or high hospital segregation, so that each category contained one third of the total 105 markets (n=35). Indices for residential segregation within HRRs were also calculated, using U.S. 2,000 Census Tract data. Census tracts were assigned to HRRs using U.S. Census Zip Code Tabulation Areas (ZCTA), which aggregate census tracts to approximate U.S. zip codes.
Sample Selection for Evaluating Disparities in Revascularization
Four additional patient exclusions were applied before evaluating disparities in revascularization. Patients were excluded if they (1) had a previous AMI admission within 2 years of the index admission (n=54,788) to limit the sample to patients with initial episodes of AMI; (2) were <67 years at the time of AMI admission, to ensure at least 2 years of Medicare data for identifying prior admissions (n=11,584); (3) did not have a valid zip code for which zip code-level median household income could be identified (n=5,150); (4) were admitted after September 30, 2005, to ensure sufficient time for identifying subsequent revascularizations (n=23,499). The remaining sample included 18,984 black and 153,712 white AMI patients who resided in and were admitted to a hospital in one of the 105 markets during January 2004–September 2005.
For each patient we created a longitudinal record including the index admission and transfers or readmissions for coronary revascularization. Revascularization included percutaneous coronary intervention (PCI; ICD-9-CM procedure codes 36.01, 36.02, 36.05–36.07) and coronary artery bypass graft (CABG; ICD-9-CM 36.10–36.19). The number of days from the index AMI admission date to the first revascularization procedure within 90 days was identified. Other patient characteristics included age (categorized as <70, 70–74, 75–79, 80–84, and 85 or older), gender, zip code–level median household income, and distance from patient residence to nearest hospital with revascularization services (based on miles between zip codes). Clinical variables included comorbid conditions defined elsewhere (Quan et al. 2005); AMI location (defined by the fourth digit of the primary ICD-9-CM code as anterior or lateral, inferior or posterior, subendocardial, or other); and an indicator of severity (mechanical ventilation on the day of admission).
Analysis
Analyses were conducted in three steps. First, we examined agreement of hospital segregation based on the Dissimilarity and Isolation Index. Second, we examined the relationship of hospital segregation to other market characteristics, including residential segregation and region of the country (i.e., Northeast, South, Midwest, and West). All analyses were conducted first using categorical segregation measures (i.e., high, medium, and low), and subsequently using continuous measures.
Finally, we applied each segregation measure to explain disparities in the use of revascularization. For these analyses, the relationship between race and revascularization was evaluated using Cox regression models to censor patients who die before revascularization and to adjust for patient sociodemographic characteristics, comorbidity, disease severity, and distance from patient residence to the nearest revascularization hospital (Appendix SA2). Patient risk factors for the Cox models were identified using previous literature, bivariate analyses, and stepwise selection in multivariable models (p<.01). After identifying patient risk factors, individual Cox models were generated for each of the 105 markets, and the hazard of revascularization for black relative to white patients (i.e., hazard ratio [HR]) was calculated for each market. Differences in the HRs for markets with high, medium, and low hospital segregation, defined in alternative analyses as Isolation or Dissimilarity, were evaluated with a statistical criterion of p<.05 for a two-sided test. The relationship of residential Isolation and Dissimilarity to HRs was also investigated.
Cox regression models were estimated with and without conditioning on the admitting hospital. Conditioning on the admitting hospital accounts for variation in the use of revascularization across hospitals. If the admitting hospital is an important determinant of disparities, the relationship between segregation and disparities should diminish after accounting for the hospital. Additional models were also estimated that only included patients admitted to hospitals with revascularization capabilities.
RESULTS
The mean Dissimilarity Index and mean Isolation Index for AMI admissions across the 105 markets were 0.37 (SD=0.15) and 0.22 (SD=0.14), respectively (Table 1). Of the 105 markets included in the sample, 62, 19, 16, and 8 were located in the South, Midwest, Northeast, and West, respectively. The proportions of markets categorized as having high hospital segregation differed by region of the country and by measure. The South had the lowest proportion with high Dissimilarity (15 percent), while 74 percent, 62 percent, and 44 percent of Midwest, West, and Northeast markets had high Dissimilarity. The proportions of markets having high Isolation were 42 percent, 25 percent, 34 percent, and 25 percent in the Midwest, Northeast, South, and West, respectively.
Table 1.
Mean Dissimilarity and Isolation Index, and Numbers of Markets Categorized with Low, Medium, and High Segregation by the Dissimilarity and Isolation Index, Overall and by Region of the Country
| Region of the Country |
|||||
|---|---|---|---|---|---|
| Overall | Midwest | Northeast | South | West | |
| Number of markets | 105 | 19 | 16 | 62 | 8 |
| Hospital Dissimilarity category | |||||
| Low | 35 (33%) | 2 (10%) | 2 (12%) | 31 (50%) | 0 (0%) |
| Medium | 35 (33%) | 3 (16%) | 7 (44%) | 22 (35%) | 3 (38%) |
| High | 35 (33%) | 14 (74%) | 7 (44%) | 9 (15%) | 5 (62%) |
| Mean Dissimilarity (SD) | 0.37 (0.15) | 0.53 (0.15) | 0.40 (0.12) | 0.30 (012) | 0.47 (0.11) |
| Hospital Isolation category | |||||
| Low | 35 (33%) | 5 (26%) | 9 (56%) | 18 (29%) | 3 (37%) |
| Medium | 35 (33%) | 6 (32%) | 3 (19%) | 23 (37%) | 3 (37%) |
| High | 35 (33%) | 8 (42%) | 4 (25%) | 21 (34%) | 2 (25%) |
| Mean Isolation (SD) | 0.22 (0.14) | 0.29 (0.20) | 0.19 (0.16) | 0.22 (0.11) | 0.21 (0.12) |
The Dissimilarity and Isolation indices were modestly correlated (r=0.48 [p<.001]), and agreement of markets were categorized as low, medium, or highly segregated using the two measures was poor (weighted κ=0.12). Of the 35 markets classified as highly segregated using the Dissimilarity Index, 12 (34 percent), 13 (37 percent), and 10 (29 percent) were classified as having high, medium, and low segregation by the Isolation Index, respectively.
Relationship of Segregation to Other Market Characteristics
The correlation between hospital segregation and residential segregation was 0.71 (p<.001) using the Dissimilarity Index and 0.75 (p<.001) using the Isolation Index. Greater Dissimilarity across hospitals was also associated (p<.01) with greater numbers of CMS enrollees, more hospitals, greater proportions of the population with income exceeding US$75,000, and greater CMS managed care enrollment (Table 2). The Isolation Index was only associated with greater proportions of black patients.
Table 2.
Characteristics of Markets with Low, Medium, and High Segregation of AMI Admissions across Hospitals as Determined by the Dissimilarity Index and by the Isolation Index
| Dissimilarity Based on AMI Admissions |
|||||
|---|---|---|---|---|---|
| Mean (SD) |
|||||
| Market Characteristic | Low (0.11–0.28) | Medium (>0.28–0.42) | High (>0.42–0.77) | p Value* | Correlation (Pearson's r) for Market Characteristic and Isolation (p Value)† |
| Residential dissimilarity index | 0.50 (0.11) | 0.58 (0.13) | 0.71 (0.09) | <.001 | 0.71 (p<.001) |
| Total CMS part A enrollees (1,000) | 102 (77) | 190 (117) | 291 (168) | <.001 | 0.53 (p<.001) |
| Percent part A enrollees who are black | 17.6% (8.6) | 11.6% (8.3) | 11.9% (9.0) | .006 | −0.15 (p=.12) |
| Number of hospitals admitting AMI | 11.8 (5.2) | 18.8 (11.3) | 28.0 (17.6) | <.001 | 0.48 (p<.001) |
| Number of hospitals performing revascularization | 3.5 (2.0) | 7.3 (4.5) | 12.4 (7.3) | <.001 | 0.61 (p<.001) |
| Proportion of hospitals with membership in the Council of Teaching Hospitals (COTH) | 7.6% (7.2) | 7.8% (6.8) | 10.1% (9.3) | .20 | 0.23 (p=.02) |
| Hospital beds per 1,000 population | 3.5 (0.8) | 2.9 (0.6) | 3.0 (0.6) | .002 | −0.18 (p=.07) |
| Persons per square mile | 913 (4,315) | 482 (719) | 1,641 (3,842) | .37 | 0.16 (p=.11) |
| Percent of population with income US$75,000 | 21.1% (6.1) | 29.4% (9.9) | 30.9% (7.8) | <.001 | 0.43 (p<.001) |
| Percent enrollment in CMS Managed Care | 4.7% (7.3) | 13.3% (13.8) | 16.0% (14.5) | <.001 | 0.29 (p=.003) |
| Isolation Based on AMI Admissions |
|||||
| Mean (SD) |
|||||
| Market Characteristic | Low (0.05–0.14) | Medium (>0.14–0.27) | High (>0.27–0.77) | p Value* | Correlation (Pearson's r) for Market Characteristic and Isolation (p Value)† |
| Residential Isolation category | 0.40 (0.12) | 0.51 (0.10) | 0.66 (0.12) | <.001 | 0.75 (p<.001) |
| Total CMS part A enrollees (1,000) | 195 (138) | 167 (115) | 222 (180) | .43 | 0.14 (p=.14) |
| Percent CMS enrollees who are black | 6.4 (2.8) | 12.5 (6.5) | 22.2 (8.1) | <.001 | 0.75 (p<.001) |
| Number of hospitals admitting AMI | 16.6 (10.3) | 18.1 (11.3) | 23.9 (18.4) | .20 | 0.22 (p=.02) |
| Number of hospitals performing revascularization | 6.3 (4.5) | 7.0 (5.6) | 9.9 (7.8) | .14 | 0.28 (p=.004) |
| Proportion of hospitals with membership in the Council of Teaching Hospitals (COTH) | 7.6 (7.2) | 5.8 (5.5) | 10.9 (13.6) | .12 | 0.30 (p=.002) |
| Hospital beds per 1,000 population | 2.9 (0.6) | 3.1 (0.6) | 3.3 (0.9) | .18 | 0.17 (p=.09) |
| Persons per square mile | 309 (272) | 504 (902) | 2,222 (5,607) | .04 | 0.36 (p<.001) |
| Percent of population with income US$75,000 | 27.8% (9.5) | 27.0% (9.8) | 26.9% (8.2) | .99 | 0.04 (p=.69) |
| Percent enrollment in CMS managed care | 12.6% (13.0) | 11.4% (14.1) | 10.0% (12.4) | .54 | −0.05 (p=.69) |
p value for comparing market characteristics across segregation categories was determined using F-test with ANOVA procedure.
p value for Pearson's r.
AMI, acute myocardial infarction.
Relationship to Revascularization
Black patients had lower overall rates of revascularization within 90 days compared with white patients (28.3 percent versus 37.8 percent; p<.001), as well as for PCI and CABG separately (7.0 percent versus 9.6 percent for CABG; p<.001, and 21.8 percent versus 29.0 percent for PCI; p<.001). In Cox regression models adjusting for patient sociodemographic and clinical factors, the likelihood of revascularization was nearly 30 percent lower for black compared to white patients (HR=0.72; 95 percent CI, 0.71–0.75; p<.001). The relative hazards of PCI and CABG were 0.77 (95 percent CI, 0.74–0.79, p<.001) and 0.69 (95 percent CI, 0.66–0.73; p<.001), respectively.
The 105 HRs generated separately for each market differed significantly across markets with low, medium, and high hospital Dissimilarity (Table 3; p<.01). The mean HR was lower (i.e., more disparity) in markets with low Dissimilarity (HR=0.66), compared with markets with medium or high Dissimilarity (HR=0.79 and 0.73, respectively). Results were similar using residential Dissimilarity (HR=0.67, 0.73, and 0.78 in markets with low, medium, and high residential Dissimilarity [p=.03]). Overall, the correlation between the Dissimilarity Index and HRs for the 105 markets (measured as Pearson's r) was not statistically significant based on hospital Dissimilarity, and was 0.26 (p=.008) based on residential Dissimilarity. Within region, HRs differed across segregation categories only in the South (p=.001).
Table 3.
Mean Risk-Adjusted Relative Hazard of Revascularization within 90 Days for Black and White Patients in 105 Markets Categorized as Low, Medium, and High Segregation by the Dissimilarity or Isolation Index, and Correlation of Market-Level Relative Hazard Ratios to the Dissimilarity and Isolation Index*,
| Low | Medium | High | p Value† | Pearson's r‡ | |
|---|---|---|---|---|---|
| Dissimilarity segregation category | |||||
| All markets (n=105) | |||||
| Dissimilarity across hospitals | 0.65 | 0.79 | 0.73 | .002 | r=0.16; p=.10 |
| (0.60–0.71; p<.001) | (0.74–0.84; p<.001) | (0.68–0.78; p<.001) | |||
| Dissimilarity by residence | 0.67 | 0.73 | 0.78 | .03 | r=0.26, p<.008 |
| (0.62–0.73; p<.001) | (0.67–0.78; p<.001) | (0.72–0.83; p<.001) | |||
| Hospital Dissimilarity by region | |||||
| Midwest (n=19 markets) | 0.90 | 0.93 | 0.75 | .25 | r=0.36; p=.13 |
| (0.63–1.18; p=.25) | (0.70–1.15; p=.27) | (0.65–0.85; p<.001) | |||
| Northeast (n=16 markets) | 0.72 | 0.71 | 0.76 | .83 | r=0.11; p=.70 |
| (0.49–0.95; p=.009) | (0.58–0.84; p<.001) | (0.64–0.89; p<.001) | |||
| South (n=63 markets) | 0.63 | 0.78 | 0.71 | .001 | r=0.26; p=.04 |
| (0.59–0.68; p<.001) | (0.72–0.84; p<.001) | (0.61–0.80; p<.001) | |||
| West (n=8 markets) | — | 0.89 | 0.67 | .17 | r=0.20; p=.64 |
| (0.62–1.17; p=.23) | (0.45–0.88; p=.001) | ||||
| Isolation segregation category | |||||
| All markets (n=105) | |||||
| Isolation across hospitals | 0.78 | 0.71 | 0.69 | .06 | r=0.14; p=.15 |
| (0.72–0.83; p<.001) | (0.65–0.76; p<.001) | (0.64–0.74; p<.001) | |||
| Isolation by residence | 0.71 | 0.76 | 0.71 | .33 | r=0.01, p=.98 |
| (0.65–0.76; p<.001) | (0.70–0.81; p<.001) | (0.65–0.76; p<.001) | |||
| Hospital Isolation by region | |||||
| Midwest (n=18 markets) | 0.94 | 0.77 | 0.73 | .13 | r=0.38; p=.11 |
| (0.77–1.11; p=.24) | (0.61–0.92; p=.002) | (0.59–0.86; p<.001) | |||
| Northeast (n=16 markets) | 0.77 | 0.57 | 0.77 | .09 | r=0.10; p=.72 |
| (0.68–0.87; p=.05) | (0.41–0.74; p=.05) | (0.63–0.91; p=.04) | |||
| South (n=63 markets) | 0.76 | 0.68 | 0.66 | .10 | r=0.21; p=.10 |
| (0.69–0.83; p<.001) | (0.62–0.74; p<.001) | (0.60–0.72; p<.001) | |||
| West (n=8 markets) | 0.62 | 0.91 | 0.71 | .27 | r=0.10; p=.82 |
| (0.33–0.91; p=.005) | (0.62–1.20; p=.28) | (0.36–1.07; p=.06) | |||
Hazard of revascularization in black patients relative to white patients was estimated separately for 105 HRR markets, using Cox regression models to adjust for sociodemographic factors (age, gender, zip code–level median income), comorbidities (diabetes, congestive heart failure, chronic obstructive lung disease, peripheral vascular disease, cerebrovascular disease, valvular disease, metastatic cancer, arrhythmia, neurological disease, renal failure, fluid and electrolyte imbalance, and weight loss), AMI location (anterior/lateral, inferior/posterior, subendocardial, and other site), the use of mechanical ventilation on day of admission, and distance to nearest hospital performing revascularization.
p value for comparing market characteristics across segregation categories was determined using F-test with ANOVA procedure.
p value for Pearson's r.
AMI, acute myocardial infarction; HRR, hospital referral regions.
In contrast, the mean HR decreased modestly, although not significantly (p=.06) with increasing hospital Isolation (HR=0.78, 0.71, and 0.69 in markets with low, medium, and high hospital Isolation), and it showed no change by residential Isolation category (p=.33) or within region of the country.
HRs based on CABG procedures only did not differ significantly across segregation levels using either the Dissimilarity or Isolation Index. For PCI, results were similar to those obtained for all revascularizations.
We note that results reported thus far do not control for the admitting hospital in the Cox regression models. Using conditional Cox regression models to control for the effects of the admitting hospital on the relative hazard of revascularization, HRs still increased with increasing hospital Dissimilarity (HR=0.67, 0.77, and 0.73 for markets with low, medium, and high hospital Dissimilarity; p=.02), as well as in analyses that only included revascularization hospitals (HR=0.68, 0.80, and 0.74; p=.01). Results based on residential Dissimilarity were similar. HRs were not related to either hospital or residential Isolation after controlling for the admitting hospital or in analyses that only included hospitals performing revascularization.
Sensitivity analyses using alternative selection criteria for markets provided similar conclusions. For example, mean HRs in markets with low, medium, and high Dissimilarity were 0.59, 0.78, and 0.71 (p<.001) using 64 markets with a minimum of five hospitals and 100 black patients, and they were 0.60, 0.78, and 0.72 (p=.001) using 53 markets with a minimum of 10 hospitals and 100 blacks. The HRs were generally unrelated to Isolation regardless of market selection criteria.
DISCUSSION
This study represents the first comprehensive analysis of hospital segregation. Using AMI as the disease paradigm, we find significant variation in segregation levels in 105 markets, with substantial disagreement using the Dissimilarity and Isolation Index. For example, seven markets with high hospital Dissimilarity had low Isolation. These markets tended to have one or two large hospitals serving the majority of black persons in the market (accounting for the high Dissimilarity), but a low overall proportion of black patients, suggesting that blacks are not isolated from the white majority. There were also 10 markets with low Dissimilarity but high Isolation for AMI—all but one of these markets was located in the South. In these markets, black patients may be spread approximately evenly across hospitals (i.e., low Dissimilarity), but the overall proportion of blacks patients is high.
Not surprisingly, hospital segregation was correlated with residential segregation, using either measure. Nevertheless, only slightly more than 50 percent of either hospital segregation measure was explained by residential segregation, suggesting the importance of factors other than geographic proximity in hospital selection. Notably, only 57 percent of white and 52 percent of black patients in our study were admitted to the hospital closest to their residence zip code—a surprising finding given the expectation that patients experiencing AMI be admitted to the closest hospital. The frequent use of diversion status by inner-city hospitals may play a role in the use of distant hospitals (Hoot and Aronsky 2008), but this does not explain the uneven distribution by race. Hospital Dissimilarity also increased with higher managed care penetration and income, suggesting that the racial distribution across hospitals is explained in part by payer status. Finally, hospital Dissimilarity increases significantly as the number of hospitals and population increase—an expected finding given that the potential for segregation increases in larger markets. The strongest correlate to hospital Isolation was the percent of the population black—so strong, in fact, that the Isolation Index may provide little information beyond a more simple measure of the proportion of the population black.
Overall, we found significantly lower rates of revascularization for blacks compared with whites—consistent with previous studies. This lower use may be related to differences in access to specialized providers (Gregory et al. 1999), racial bias in physician referral patterns (Ibrahim et al. 2003), preferences for treatment (Oddone et al. 2002), and medical system mistrust (LaVeist, Nickerson, and Bowie 2000).
The relationship of the magnitude of revascularization disparity to segregation was somewhat mixed. Using the Dissimilarity Index, we found that disparity in markets with medium and high hospital segregation was modestly lower (i.e., higher HRs), compared with markets with low hospital segregation. Results for residential Dissimilarity were slightly stronger. Moreover, this relationship persisted even after controlling for the admitting hospital, and in analyses limited to patients admitted to revascularization hospitals, suggesting that other environmental or social factors drive the relationship. If the uneven distribution of black and white patients across hospitals reflects populations choosing hospitals based on cultural identification and trust, utilization may be improved. Indeed, racial identity is an important determinant of individual actions for blacks (Philogene 2004) and may facilitate or hinder health-seeking behavior depending on perceived concordance with providers in the black community. Moreover, cultural concordance may also impact patient compliance and outcomes (Saha et al. 1999, 2000; Johnson et al. 2004). Racially concentrated neighborhoods within segregated markets may also have greater social cohesion, leading to greater social support that facilitates access to health services (Wen, Browning, and Cagney 2003), and the presence of autonomous institutions and social networks within segregated areas may mitigate the effects of racial mistrust and bias (Geronimus 2000). Finally, we acknowledge the possibility that HRs in markets with high Dissimilarity may be overestimated, if our models do not adequately capture the need for revascularization among blacks in those communities.
In contrast to Dissimilarity, we found a modest increase in disparity (i.e., decrease in the relative HRs), as hospital Isolation increased, although this relationship was not statistically significant and disappeared entirely after controlling for the admitting hospital. Moreover, revascularization disparity was not related at all to residential isolation and was not evident using alternative market selection criteria. We hypothesized that Isolation would impact disparities through under-resourced hospitals, patient mistrust, and practice patterns of an isolated community. While these factors may limit the availability of revascularization in isolated communities, the relative disparity between blacks and whites remains consistent, regardless of isolation (i.e., if black patients are less likely to receive revascularization in isolated medical communities, so are white patients).
Our analysis required decisions about market selection criteria, patient sample selection, and segregation measurement. The limitations of these decisions, and possible variations, should be noted. First, the opportunity for segregation is lower in small markets (i.e., Dissimilarity across hospitals cannot exist in a market with only one hospital or with no black patients). Therefore, a minimum number of black patients and/or hospitals had to be identified. We selected markets with a minimum of 50 black patients and five hospitals, and conducted sensitivity analyses using four alternative market selection criteria (e.g., 25 black patients and five hospitals). Our conclusions were similar, regardless of market selection criteria.
Second, we excluded patients who did not reside in the same market as the hospital, which may affect the calculation of segregation for markets with large flows of patients into the market. However, segregation indices based on all patients were highly correlated to those we used (r>0.98 for both Isolation and Dissimilarity Indices).
Third, hospital services markets for acute medical conditions, such as AMI, may follow different patterns compared with hospitalizations for chronic conditions (e.g., CHF). In addition, this study focuses on elderly Medicare patients only; results based on all-payer data may differ.
Fourth, these analyses investigated only two dimensions of segregation—unevenness and isolation. Other dimensions include concentration (i.e., concentration of minorities within a spatial area) and centralization (i.e., centralization of minorities in large cities) (Massey and Denton 1988). Finally, most segregation indices compare a single minority population to the majority (usually white) population, but multirace indices may be useful in markets with large proportions of other ethnic groups.
In summary, this study proposes a framework for studying pathways that create segregation of the health delivery system, as well as the link between segregation and disparities in utilization and outcomes of care. Our framework emphasizes pathways through which segregation may be both positively and negatively associated with health services and outcomes. We also evaluated the relationship between two dimensions of segregation and disparities in the use of revascularization—procedures for which substantial disparities have been documented. We found marked differences between the two dimensions of segregation and their relationship to revascularization disparities.
It is unlikely that the full racial and ethnic heterogeneity of health care markets can be summarized in a single number. Nevertheless, segregation indices, as overall measures of market structure, may have policy implications. First, if disparities are attributable to differential access to providers, then strategies to create parity in access may be beneficial. However, such efforts must consider the degree to which access of diverse populations depends on the community culture. Enhancing access for minorities goes beyond traditional competency training, and encompasses forces that create social cohesion, as well as distrust and fractionalization in the medical delivery system. Second, programs to encourage the use of high-quality hospitals, such as public dissemination of hospital performance measures, may be futile if black patients are limited in their choice of hospital due to social factors, choose certain hospitals because of cultural identification, or place a higher priority on perceived cultural competence than processes of care. Third, one must also consider the degree to which patient choice creates racial separation of health services. While the importance of preserving patient choice is generally recognized, U.S. history shows us that racially separate health delivery systems are not likely to be equal. Thus, segregation due to lack of options is undesirable; but segregation that reflects patient preferences may not be undesirable, as long as quality of care is maintained. Finally, the fact that significant segregation still exists in the health delivery system may warrant a civil rights approach to eliminating disparities. However, the underlying problem is not likely overt racial discrimination by health service organizations, but rather the failure to account for the needs of minority populations in health systems planning. Ultimately, the solution lies in a delivery system that respects the cultures of diverse populations, who often have diverse patterns of care, while addressing the health needs of those populations in a manner that is efficacious and constructive.
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This study was supported by a grant from the National Institute on Aging (NIA R03 AG027286). Mary Vaughan Sarrazin and Kelly Richardson also receive support from the Health Services Research and Development Service, Veterans Health Administration, Department of Veterans Affairs (HFP-04-149). The views expressed in this paper are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Disclosures: None.
Disclaimers: None.
Supporting Information
Additional supporting information may be found in the online version of this article:
Appendix SA1: Author Matrix.
Appendix SA2: Characteristics of 18,984 Black and 153,712 White Patients Admitted to Hospitals for Acute Myocardial Infarction from 2004 to September 2005.
Appendix SA3: Hospital Dissimilarity and Hospital Isolation for 105 Hospital Referral Regions by Census Region of the Country.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- Baicker K, Chandra A, Skinner JS, Wennberg JE. Who You Are and Where You Live: How Race and Geography Affect the Treatment of Medicare Beneficiaries. Health Affairs (Millwood) 2004 doi: 10.1377/hlthaff.var.33. Suppl Web Exclusives: Var33–44. [DOI] [PubMed] [Google Scholar]
- Barnato AE, Lucas FL, Staiger D, Wennberg DE, Chandra A. Hospital-Level Racial Disparities in Acute Myocardial Infarction Treatment and Outcomes. Medical Care. 2005;43:308–19. doi: 10.1097/01.mlr.0000156848.62086.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EH, Herrin J, Wang Y, McNamara RL, Webster TR, Magid DJ, Blaney M, Peterson ED, Canto JG, Pollack CV, Jr, Krumholz HM. Racial and Ethnic Differences in Time to Acute Reperfusion Therapy for Patients Hospitalized with Myocardial Infarction. Journal of the American Medical Association. 2004;292(13):1563–72. doi: 10.1001/jama.292.13.1563. [DOI] [PubMed] [Google Scholar]
- Collins C, Williams DR. Segregation and Mortality: The Deadly Effects of Racism. Social Forum. 1999;14:495–523. [Google Scholar]
- Cooper R. Social Inequality, Ethnicity, and Cardiovascular Disease. International Journal of Epidemiology. 2001;30:S48–52. doi: 10.1093/ije/30.suppl_1.s48. [DOI] [PubMed] [Google Scholar]
- Cromwell J, McCall NT, Burton J, Urato C. Race/Ethnic Disparities in Utilization of Lifesaving Technologies by Medicare Ischemic Heart Disease Beneficiaries. Medical Care. 2005;43:330–7. doi: 10.1097/01.mlr.0000156864.80880.aa. [DOI] [PubMed] [Google Scholar]
- Geronimus AT. To Mitigate, Resist, or Undo: Addressing Structural Influences on the Health of Urban Populations. American Journal of Public Health. 2000;90:867–72. doi: 10.2105/ajph.90.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PM, Rhoads GG, Wilson AC, O'Dowd KJ, Kostis JB. Impact of Availability of Hospital-Based Invasive Cardiac Services on Racial Differences in the Use of These Services. American Heart Journal. 1999;138:507–17. doi: 10.1016/s0002-8703(99)70154-7. [DOI] [PubMed] [Google Scholar]
- Groeneveld PW, Heidenreich P, Garber A. Trends in Implantable Cardioverter-Defibrillator Racial Disparity: The Importance of Geography. Journal of the American College of Cardiology. 2005;45:72–8. doi: 10.1016/j.jacc.2004.07.061. [DOI] [PubMed] [Google Scholar]
- Haas JS, Phillips KA, Sonneborn D, McCulloch CE, Baker LC, Kaplan CP, Perez-Stable EJ, Liang SY. Variation in Access to Health Care for Different Racial/Ethnic Groups by the Racial/Ethnic Composition of an Individual's County of Residence. Medical Care. 2004;42:707–14. doi: 10.1097/01.mlr.0000129906.95881.83. [DOI] [PubMed] [Google Scholar]
- Halperin EC. Desegregation of Hospitals and Medical Societies in North Carolina. New England Journal of Medicine. 1988;318:58–63. doi: 10.1056/NEJM198801073180127. [DOI] [PubMed] [Google Scholar]
- Hart KD, Kunitz SJ, Sell RR, Mukamel DB. Metropolitan Governance, Residential Segregation, and Mortality among African Americans. American Journal of Public Health. 1998;88:434–8. doi: 10.2105/ajph.88.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoot NR, Aronsky D. Systematic Review of Emergency Department Crowding: Causes, Effects, and Solutions. Annals of Emergency Medicine. 2008;52:126–36. doi: 10.1016/j.annemergmed.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim SA, Whittle J, Bean-Mayberry B, Kelley ME, Good C, Conigliaro J. Racial/Ethnic Variations in Physician Recommendations for Cardiac Revascularization. American Journal of Public Health. 2003;93:1689–93. doi: 10.2105/ajph.93.10.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: The National Academies Press; 2002. Available at http://www.nap.edu[accessed on May 15, 2007] [PubMed] [Google Scholar]
- Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and Ethnic Differences in Patient Perceptions of Bias and Cultural Competence in Health Care. Journal of General Internal Medicine. 2004;19:101–10. doi: 10.1111/j.1525-1497.2004.30262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist TA, Nickerson KJ, Bowie JV. Attitudes about Racism, Medical Mistrust, and Satisfaction with Care among African American and White Cardiac Patients. Medical Care Research Review. 2000;57:146–61. doi: 10.1177/1077558700057001S07. [DOI] [PubMed] [Google Scholar]
- Marschall M, Stolle D. Race and the City: Neighborhood Context and the Development of Generalized Trust. Political Behavior. 2004;26:125–54. [Google Scholar]
- Massey D, Denton N. The Dimensions of Residential Segregation. Social Forces. 1988;67:281–315. [Google Scholar]
- Oddone EZ, Horner RD, Johnston DC, Stechuchak K, McIntyre L, Ward A, Alley LG, Whittle J, Kroupa L, Taylor J. Carotid Endarterectomy and Race: Do Clinical Indications and Patient Preferences Account for Differences? Stroke. 2002;33:2936–43. doi: 10.1161/01.str.0000043672.42831.eb. [DOI] [PubMed] [Google Scholar]
- Payne-Sturges D, Gee GC, Crowder K, Hurley BJ, Lee C, Forello-Frosch R, Rosenbaum A, Schulz A, Wells C, Woodruff T, Zenick H. Workshop Summary: Connecting Social and Environmental Factors to Measure and Track Environmental Health Disparities. Environmental Research. 2006;102:146–53. doi: 10.1016/j.envres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Philogene G. Racial Identity in Context: The Legacy of Kenneth B. Clark. Washington, DC: American Psychological Association; 2004. [Google Scholar]
- Popescu I, Vaughan-Sarrazin MS, Rosenthal GE. Differences in Mortality and Use of Revascularization in Black and White Patients with Acute MI Admitted to Hospitals with and without Revascularization Services. Journal of the American Medical Association. 2007;297:2489–95. doi: 10.1001/jama.297.22.2489. [DOI] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Medical Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient–Physician Racial Concordance and the Perceived Quality and Use of Health Care. Archives of Internal Medicine. 1999;159:997–1004. doi: 10.1001/archinte.159.9.997. [DOI] [PubMed] [Google Scholar]
- Saha S, Taggart SH, Komaromy M, Bindman AB. Do Patients Choose Physicians of Their Own Race? Health Affairs (Millwood) 2000;19:76–83. doi: 10.1377/hlthaff.19.4.76. [DOI] [PubMed] [Google Scholar]
- Schelbert E, Rosenthal GE, Welke K, Vaughan Sarrazin M. Treatment Variation in Older Black and White Patients Receiving Aortic Valve Replacement. Circulation. 2005;112:2347–53. doi: 10.1161/CIRCULATIONAHA.104.530550. [DOI] [PubMed] [Google Scholar]
- Schulz AH, Kannan S, Dvonch JT, Israel BA, Allen A, James SA, House JS, Lepkowski J. Social and Physician Environments and Disparities in Risk for Cardiovascular Disease: The Healthy Environments Partnership Conceptual Model. Environmental Health Perspectives. 2005;113:1817–25. doi: 10.1289/ehp.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, Ethnic, and Geographic Disparities in Rates of Knee Arthroplasty among Medicare Patients. New England Journal of Medicine. 2003;349:1350–9. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- Smith DB. Addressing Racial Inequities in Health Care: Civil Rights Monitoring and Report Cards. Journal of Health, Politics, Policy and Law. 1998;23:75–105. doi: 10.1215/03616878-23-1-75. [DOI] [PubMed] [Google Scholar]
- Smith DB. Population Ecology and the Racial Integration of Hospitals and Nursing Homes in the United States. Milbank Quarterly. 2004;68:591–6. [PubMed] [Google Scholar]
- Vaccarino V, Rathore SS, Wenger NK, Frederick PD, Abramson JL, Barron HV, Manhapra A, Mallik S, Krumholz HM. Sex and Racial Differences in the Management of Acute Myocardial Infarction, 1994 through 2002. New England Journal of Medicine. 2005;353:671–82. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen M, Browning CR, Cagney KA. Poverty, Affluence, and Income Inequality: Neighborhood Economic Structure and Its Implications for Health. Social Science and Medicine. 2003;57:843–60. doi: 10.1016/s0277-9536(02)00457-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.