Abstract
The neuropathology of Alzheimer's disease (AD) and other tauopathies is characterized by filamentous deposits of the microtubule-associated protein tau, but the relationship between tau polymerization and neurotoxicity is unknown. Here, we examined effects of filamentous tau on fast axonal transport (FAT) using isolated squid axoplasm. Monomeric and filamentous forms of recombinant human tau were perfused in axoplasm, and their effects on kinesin- and dynein-dependent FAT rates were evaluated by video microscopy. Although perfusion of monomeric tau at physiological concentrations showed no effect, tau filaments at the same concentrations selectively inhibited antero-grade (kinesin-dependent) FAT, triggering the release of conventional kinesin from axoplasmic vesicles. Pharmacological experiments indicated that the effect of tau filaments on FAT is mediated by protein phosphatase 1 (PP1) and glycogen synthase kinase-3 (GSK-3) activities. Moreover, deletion analysis suggested that these effects depend on a conserved 18-amino-acid sequence at the amino terminus of tau. Interestingly, monomeric tau isoforms lacking the C-terminal half of the molecule (including the microtubule binding region) recapitulated the effects of full-length filamentous tau. Our results suggest that pathological tau aggregation contributes to neurodegeneration by altering a regulatory pathway for FAT.
Keywords: Alzheimer's disease, axonal transport, tau filament, GSK3, PP1, kinesin, tau
Tau is a microtubule-associated protein (MAP) involved in microtubule dynamics and maintenance (Amos and Schlieper, 2005), and insoluble filamentous tau aggregates form in Alzheimer's disease (AD) and several other neurodegenerative tauopathies (for review see Gamblin et al., 2003a). Despite the strong positive correlation between the appearance of filamentous tau and neuronal dysfunction (for review see Binder et al., 2005), no toxic mechanism has been directly tied to these structures, and as a result the toxicity of tau filaments remains a subject of debate (King, 2005).
In AD, degenerating neurons exhibit alterations in synaptic function (Bell and Claudio Cuello, 2006; Yoshiyama et al., 2007), the appearance of neuritic varicosities, and the mislocalization of various membrane-bound organelles (MBOs), all of which indicate that intracellular transport is disrupted in this disease (for review see Morfini et al., 2002a). Given these observations, and the central role of tau in AD pathology, several investigators have explored the effects of monomeric tau on microtubule-dependent fast axonal transport (FAT). Although reports have been published arguing both for (Ebneth et al., 1998; Seitz et al., 2002; Vershinin et al., 2007) and against (Morfini et al., 2007a) the idea that high levels of soluble tau can reduce anterograde FAT by interfering with the attachment of the molecular motor kinesin, there is no evidence that such levels of tau are seen in normal or pathological neurons. Remarkably, even though the hallmark of AD and other tauopathies is the presence of intracellular tau filaments, the biological effects of filamentous tau on FAT have not been assessed previously.
In this study, we have examined the effects of tau filaments on FAT using isolated squid axoplasm. Although at low, physiologically relevant ratios of tau to tubulin monomeric tau has no effect, tau filaments selectively inhibit anterograde, conventional kinesin-dependent FAT. Furthermore, deletion experiments indicate that this effect requires the first 18 a.a. at the amino terminus of tau, which becomes abnormally exposed upon polymerization. Consistently with this notion, monomeric tau isoforms lacking the microtubule-binding region (MTBR) and the C-terminus mimicked the effect of tau filaments on FAT. Finally, we show that tau filaments do not act at the microtubule surface but rather inhibit anterograde FAT by activating protein phosphatase 1 (PP1) and glycogen synthase kinase 3 (GSK-3). Taken together, our findings reveal a novel gain-of-function mechanism by which the formation of tau filaments may play a critical role in AD pathogenesis.
MATERIALS AND METHODS
Reagents
CREBpp was synthesized and purified (95%) by New England Peptide (Gardner, MA). Inhibitor-2 (I-2), SB203580, and okadaic acid were purchased from Calbiochem (San Diego, CA). Arachidonic acid (AA; Cayman Chemical, Ann Arbor, MI) was stored at −20°C, and working solutions were prepared in 100% ethanol immediately prior to use. Mammalian protease inhibitor cocktail was from Sigma (St. Louis, MO). ING-135 was synthesized as described previously (Kozikowski et al., 2007).
Recombinant Proteins
The full-length tau used in this study (hTau40) corresponds to the longest isoform in adult human brain, containing 441 a.a. and four microtubule-binding repeats (MTBRs). K23 is a tau construct lacking both alternatively spliced N-terminal exons and all four MTBRs. Tau6D and Tau6P are tau isoforms lacking the MTBR region and the C-terminus of canonical tau. The alternative splicing that generates these isoforms occurs in exon 6 and introduces a unique 11-a.a. sequence followed by a stop codon. The specific 11 a.a. vary depending on whether the splice site is proximal or distal to the beginning of exon 6 (Luo et al., 2004). Tau6D and Tau6P isoforms were generated by restriction digestion and ligation of constructs previously described (Luo et al., 2004) and hTau40 (Gustke et al., 1994; Carmel et al., 1996). All other constructs used in this study have been described elsewhere:, Δ2−18 (Gamblin et al., 2003b), 1−421 (Gamblin et al., 2003c), K23 (Preuss et al., 1997). All proteins were expressed in Escherichia coli and purified by means of an N-terminal poly-histidine tag (Carmel et al., 1996; Abraha et al., 2000).
Immunoblots
Tau constructs were spotted onto nitrocellulose membranes (1 ng/μl, 1 μl per spot), blocked with 5% nonfat dry milk in Tris-buffered saline, pH 7.4, and probed with the monoclonal antibodies Tau12 (2 ng/ml), Tau5 (20 ng/ml), and Tau46.1 (20 ng/ml), which recognize a.a. 9−18, 210−230, and 428−441, respectively (Kosik et al., 1988; Carmel et al., 1996; Ghoshal et al., 2002). Primary antibody binding was detected with HRP-conjugated anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA) and ECL developing solution (GE Healthcare, Amersham, United Kingdom).
Microtubule-Binding Assays
Squid optic lobes were dissected and flash frozen in liquid nitrogen (Morfini et al., 2007a). One and one-half grams of freshly thawed squid optic lobes was homogenized in 2.5 ml of BRB80 buffer (80 mM Pipes, 1 mM MgCl2, and 1 mM EGTA) and 1/100 mammalian protease inhibitor cocktail (Sigma), plus phosphatase and kinase inhibitors (Calbiochem) as follows: 1/200 phosphatase inhibitor cocktail II, 200 mM sodium orthovanadate, 200 nM mycrocystin RR, 50 nM okadaic acid, 100 nM K252a, 100 nM staurosporine. Squid optic lobe homogenate was prepared at 4°C using a glass Dounce homogeneizer. This homogenate was centrifuged at 12,500g for 20 min at 4°C. The supernatant fraction was transferred to a new tube and centrifuged at 125,000g for 5 min at 4°C in a TL100.3 rotor (Beckman, Fullerton, CA). The supernantant (cytosol) was transferred to a new tube, adjusted to 20 μM taxol, and incubated at 37°C for 15 min to allow for microtubule polymerization. After this step, 200-μl aliquots of microtubule-containing cytosol were incubated alone or with htau40 or K23 tau constructs (5 μM final concentration) for 20 min at 37°C. Samples were loaded on top of a 60-μl BRB80 buffer plus 20% sucrose cushion and 20 μM taxol using 1.5-ml microcentrifuge tubes, and centrifuged for 5 min at 125,000g at 4°C using a TLA100.3 rotor (Beckman). Microtubule pellets were resuspended in 200 μl of BRB80. Pellets and supernatant fractions were adjusted to 1× gel loading buffer (GLB) using a 5× GLB stock (0.35 M Tris-HCl, pH 6.8, 10% w/v SDS (Sequanal grade; Pierce, Rockford, IL), 36% glycerol, 5% β-mercaptoethanol, 0.01% bromophenol blue). Membranes were also probed with an antibody against tubulin (DM1a; Sigma) to demonstrate the presence of microtubules in the pellet samples.
Tau Polymerization
Tau polymerization was induced using AA as previously described (King et al., 1999), except that KCl was substituted for NaCl in the polymerization buffer. This substitution did not prevent filament formation (see Fig. 5b–d). Briefly, tau protein (4 μM) was incubated at room temperature in reaction buffer (50 mM HEPES, pH 7.6, 50 mM KCl, 5 mM DTT) in the presence of 75 μM AA (in ethanol vehicle). Samples of soluble tau were prepared for perfusion in the same manner, except that arachidonic acid was excluded from the polymerization buffer. Control mixtures containing AA but lacking tau were prepared in parallel. Final ethanol concentration in all samples was 3.8%.
Fig. 5.
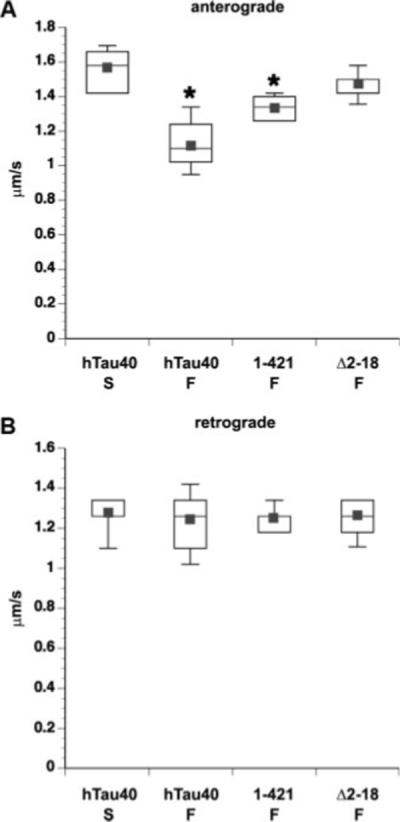
Removal of the extreme N-terminus of tau abolishes the effects of tau filaments on FAT. Box plots of FAT rates in axoplasm perfused with 2 μM soluble or filamentous tau. Data represent pooled measurements taken between 30 and 50 min of observation. Perfusion with filaments composed of hTau40 (hTau40 F) and 1−421 (1−421 F) significantly reduced anterograde FAT compared with hTau40 monomer (hTau40 M) rate at (*P ≤ 0.0001 by a two-sample t-test). In contrast, Δ2−18 filaments (Δ2−18 F) failed to inhibit anterograde FAT (A). Retrograde FAT was unaffected under all conditions (B). Rates for monomeric hTau40 were indistinguishable from axoplasm perfused with buffer alone (data not shown).
Electron Microscopy
Polymerization reactions were allowed to proceed for 6 hr, fixed with 2% glutaraldehyde, spotted onto 300 mesh formvar/carbon-coated copper grids (Electron Microscopy Sciences, Hatfield, PA), and negatively stained with 2% uranyl acetate (King et al., 1999). Samples were examined with a JEOL JEM-1220 electron microscope at 60 kV and ×12,000 magnification, and photographs were taken with a MegaScan 794/20 digital camera and DigitalMicrograph software version 3.9.3 (Gatan, Pleasanton, CA).
Squid Axoplasm Motility Assays
Axoplasm from squid giant axons (Loligo pealii; Marine Biological Laboratory, Woods Hole, MA) was extruded as previously described (Brady et al., 1985). All proteins and inhibitors were diluted in ATP-supplemented X/2 buffer (175 mM potassium aspartate, 65 mM taurine, 35 mM betaine, 25 mM glycine, 10 mM HEPES, 6.5 mM MgCl2, 5 mM EGTA, 1.5 mM CaCl2, 0.5 mM glucose, pH 7.2) for perfusion. For experiments involving tau and their controls, reaction mixtures (containing tau alone, tau and AA, and AA alone) were diluted 1:1 in ATP-supplemented X/2 buffer (final tau concentration 2 μM, when present). Motility was analyzed using a Zeiss Axiomat microscope equipped with a ×100, 1.3 N.A. objective and DIC optics. Organelle velocities were measured by matching calibrated cursor movements to the speed of vesicles moving in the axoplasm (Morfini et al., 2006).
Purification of Membrane Vesicle Fractions From Squid Axoplasms
Two “sister” axoplasms were prepared from the same animal and incubated with the appropriate effectors (control buffer, active GSK-3β, monomeric tau, or filamentous tau) as for motility assays in X/2 buffer plus 1 mM ATP in 25 μl final volume. Active GSK-3β was from Sigma (No. G1663). After 40 min of incubation, axoplasms were transferred, along with perfusion buffer, to low protein binding 1.5 ml centrifuge tubes containing 200 μl of homogenization buffer [0.25 mM sucrose, 1 mM EDTA, 10 mM Hepes, pH 7.4, 1/100 protease inhibitor cocktail (Sigma; No. P8340), 1/200 phosphatase inhibitor cocktail set II (Calbiochem; No. 524627), 2 μM K252a (Calbiochem; No. 420298), 1 μM PKI (Upstate, Lake Placid, NY; No. 12−151)], and carefully homogenized by two passages through a 23-G syringe needle and five passages through a 27-G syringe needle using a 1-ml Hamilton pipette. Axoplasm homogenates were adjusted to 30% iodixanol by mixing 200 μl of axoplasm homogenates with 300 μl of solution D [50% (w/v) iodixanol, 10 mM MgCl2 in 250 mM sucrose]. A 500-μl layer of solution E [25% (w/v) iodixanol, 10 mM MgCl2 in 250 mM sucrose] and a 100-μl layer of solution F [5% (w/v) iodixanol, 10 mM MgCl2 in 250 mM sucrose] were loaded on top of the axoplasm homogenates. Samples were centrifuged at 250,000g max for 1 hr at 4°C in an RP55-S Sorvall rotor. Three hundred microliters containing floating vesicles were collected from immediately below the 5% iodixanol interface, and 60 μl of 6× loading sample buffer was added. In separate experiments, 0.1% Triton X-100 was added to the axoplasm homogenates prior to centrifugation to confirm the membranous nature of this fraction. Immunoblots were developed using antibodies against kinesin-1 heavy chain (H2; Morfini et al., 2007a), dynein intermediate chain (rabbit polyclonal V3, a generous gift from Kevin Vaughan), and SNAP-25 (Synaptic Systems; No. 111−002). Quantitative immunoblotting was performed as described elsewhere (Morfini et al., 2006).
Statistical Analysis
All experiments were repeated at least three times. Unless otherwise stated, the data were analyzed by ANOVA followed by post hoc Student-Newman-Keul's test in order to make all possible comparisons. Comparison of transport data from axoplasm under different conditions was done using a two-sample t-test of μ1-μ2 with Datadesk statistical software (Data Description, Inc., Ithaca, NY). Data were expressed as mean ± SEM, and significance was assessed at P values as noted.
RESULTS
To evaluate the effect of tau on microtubule-dependent FAT, we used vesicle motility assays in isolated squid axoplasm. In this experimental system, the bidirectional transport of membrane-bound organelles (MBOs) can be directly observed by video-enhanced differential interference contrast (DIC) microscopy. This preparation preserves the ionic strength and complex environment of the cell, and, because the axoplasm is isolated from the cell body, nuclear effects can be ignored (Brady et al., 1985). Also, the absence of plasma membrane in this preparation allows for the introduction of experimental agents at tightly controlled concentrations (Morfini et al., 2007a). This system was instrumental in the original discovery of kinesin-1 (Brady, 1985), novel regulatory pathways for FAT (Morfini et al., 2002b, 2004, 2006), and axonal-specific phosphorylation events (Grant et al., 1999).
Monomeric hTau40 Binds to Squid Microtubules but Does Not Affect FAT
Alternative splicing produces six major tau isoforms in the adult human central nervous system. In a previous study, we assayed monomeric tau constructs derived from the shortest tau isoform (352 a.a., hTau23) and established their effects on FAT (Morfini et al., 2007a). In the present study, we chose to use the longest isoform (441 a.a., hTau40), because of its greater propensity to form filaments (Gamblin et al., 2003a). Unlike hTau23, hTau40 contains two alternatively spliced N-terminal exons (E2 and E3) and four microtubule binding repeats (R1–R4). A schematic of each tau construct used in the present study is shown in Figure 1A.
Fig. 1.
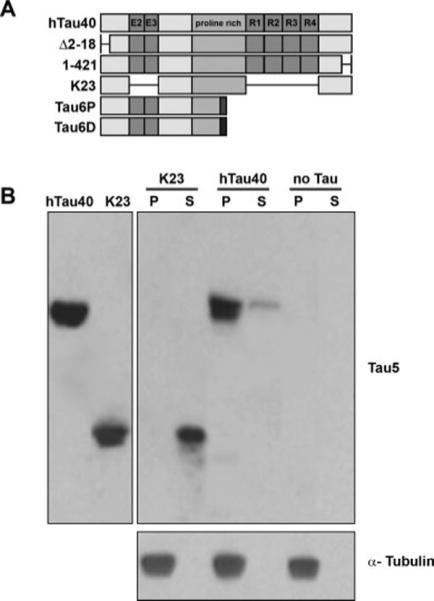
Schematic and microtubule binding of tau constructs used in this study. A: The longest isoform of tau in the human central nervous system is referred to as hTau40. Boxes represent alternatively spliced exons (E2 and E3), the proline-rich region, and the microtubule-binding repeats (R1–R4). Δ2−18 and 1−421 are constructs containing N-terminal and C-terminal deletions, respectively, and K23 is a construct lacking N-terminal exons and microtubule-binding repeats. Tau6P and Tau6D are isoforms that are identical to canonical tau from a.a. 1−144, at which point alternative splicing introduces 11 unique a.a. (represented by dark boxes) followed by a stop codon. The specific amino acids differ between Tau6P and Tau6D. B: Tau constructs (5 μM) were incubated with microtubule-containing squid optic lobe cytosol as described in Materials and Methods. Microtubule pellets (P) and supernatant (S) fractions were prepared by centrifugation and analyzed by immunoblot using antibodies against tau (Tau5) and tubulin. Note that the K23 tau construct lacking MTBRs is mostly recovered in supernatant fractions, consistent with its weak binding to microtubules. In contrast, hTau40 is recovered in association with microtubules. Neither construct was recovered in pellet fractions when centrifuged in the absence of squid microtubules (data not shown).
Previously, we demonstrated that monomeric hTau23 binds to axonal squid microtubules (Morfini et al., 2007a). To rule out species-related artifacts, we assayed the ability of monomeric hTau40 to interact with endogenous squid microtubules. Microtubule-enriched fractions from squid optic lobe were prepared in the presence or absence of hTau40. After taxol-induced microtubule assembly, microtubules and associated proteins were sedimented by centrifugation and the resulting fractions (supernatants and microtubule-enriched pellets) probed with anti-tau antibodies. A tau construct with low binding affinity (K23) was assayed in parallel to control for nonspecific sedimentation. As observed previously (Morfini et al., 2007a), most K23 remained in the supernatant fraction. In contrast, hTau40 was depleted from the supernatant fraction and found in association with the microtubule-enriched pellet fraction (Fig. 1B), indicating that monomeric hTau40 can bind to endogenous squid microtubules.
Microtubule rigidity is increased when tau binds to the microtubule surface, and this effect is observable even at low, nonsaturating tau concentrations (Felgner et al., 1997; Morfini et al., 2007a). As an additional indicator of hTau40's ability to interact with squid microtubules, we perfused axoplasm with tau and then examined the morphology of microtubules at the axoplasm periphery. In the absence of exogenous tau, many of these microtubules exhibited a curved appearance (Fig. 2a). However, perfusion of monomeric hTau40 (2 μM) caused peripheral microtubules to acquire a straight, rigid appearance (Fig. 2b), consistent with binding of hTau40 to the microtubule surface (Morfini et al., 2007a). Together with the sedimentation assay, these results demonstrate that hTau40 is capable of binding to squid microtubules.
Fig. 2.
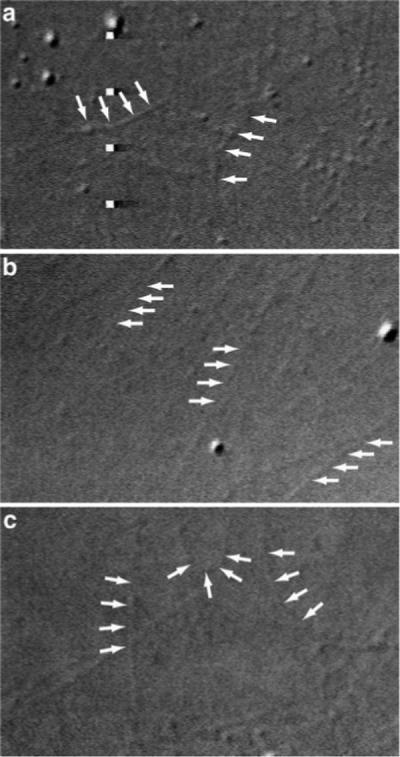
Effect of hTau40 on the morphology of axoplasmic squid microtubules. a: After perfusion of squid axoplasm with control buffer, individual microtubules can be observed at the axoplasm periphery. Many of these microtubules display a gently curving, freely bending morphology (arrows). b: Perfusion of hTau40 at 2 μM dramatically changes the morphology of peripheral microtubules to a stiff, linear appearance (arrows). This effect of tau and other MAPs on microtubule stiffness has been previously characterized in vitro (Felgner et al., 1997) and indicates direct binding of hTau40 to endogenous squid microtubules. c: In contrast to monomeric hTau40, perfusion of filamentous hTau40 at 2 μM does not change the morphology of peripheral microtubules.
We next examined the effects of monomeric hTau40 on FAT. We perfused axoplasm with hTau40 at 2 μM, which is within the physiological range for neurons (2−5 μM; Drubin et al., 1985). The concentration of tubulin in squid axoplasm is 50 μM (Morris and Lasek, 1984), resulting in a tau to tubulin ratio of approximately 1:25. FAT rates measured between 30 and 50 min postperfusion were pooled and compared with axoplasms perfused with control buffer alone. As observed for other monomeric tau constructs (Morfini et al., 2007a), perfusion of hTau40 at 2 μM showed no effect on either anterograde or retrograde FAT (Fig. 3A), demonstrating that physiological levels of monomeric hTau40 do not impair FAT in this system.
Fig. 3.
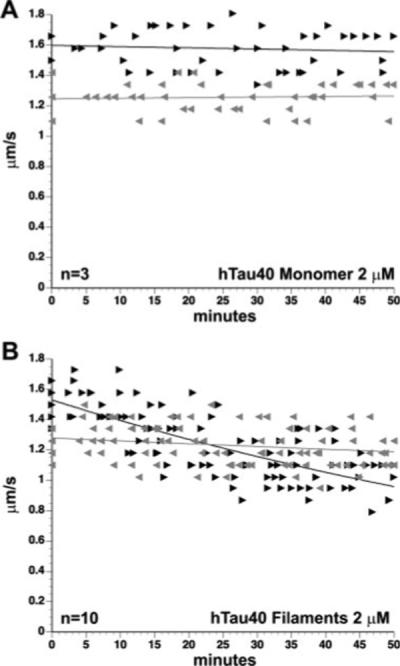
Effects of soluble and filamentous tau on FAT. Axoplasms were perfused with soluble or filamentous recombinant tau (hTau40) and FAT rates (μm/sec) monitored over a period of 50 min. Each arrowhead represents a measurement of the average velocity at which particles move in the anterograde (black arrowheads) or retrograde (gray arrowheads) direction. Graphs depict compiled data from at least three separate trials. Although soluble hTau40 (2 μM) had no observable effect on either FAT direction (A), hTau40 filaments (2 μM) specifically inhibited anterograde FAT in a time-dependent manner (B).
Perfusion of Filamentous Tau Selectively Inhibits Anterograde FAT
Recombinant hTau40 forms filaments in vitro when incubated with AA (for review see Gamblin et al., 2003a), and these filaments are morphologically similar to those isolated from AD neuronal tissue (King et al., 1999). To determine the effects of filamentous tau on FAT, we perfused hTau40 filaments (2 μM) into squid axoplasm and monitored anterograde and retrograde FAT rates. Unlike monomeric tau, hTau40 filaments inhibited anterograde, kinesin-dependent FAT rates (Fig. 3B; P = 0.001) when perfused at the same concentration as monomeric hTau40 (2 μM). Perfusion of axoplasms with polymerization buffer alone (see Materials and Methods) had no effect on FAT (data not shown), demonstrating that the effects on FAT were due to the presence of filamentous tau. Significantly, retrograde FAT rates remained unaffected, suggesting that the effects of hTau40 filaments on anterograde FAT were not due to alterations in microtubule integrity. Supporting this idea, htau40 filaments (2 μM) did not produce changes in microtubule morphology when perfused into axoplasm, which also suggests that filamentous tau does not bind to microtubules (Fig. 2c). Similarly, perfusion of equivalent amounts of polymerization buffer (containing AA but no tau) had no effect on FAT (not shown).
The Effect of Tau Filaments on FAT Depends on the Extreme N-Terminus of Tau
Several studies suggest important functional roles for both the amino and carboxy termini of tau (Brandt et al., 1995; Amadoro et al., 2004, 2006; Lee, 2005), and the study of selected modifications of these domains constitutes an emerging area of interest in AD (Gamblin et al., 2003c; Guillozet-Bongaarts et al., 2005). To determine whether these domains are involved in FAT inhibition, we assayed two tau constructs, Δ2−18 and 1−421, containing deletions at the extreme amino- and carboxy-termini, respectively (see Figs. 1A, 4A). These deletions do not normally prevent tau from forming filaments (Berry et al., 2003; Gamblin et al., 2003b). However, the buffer conditions required for axoplasm perfusion (see Materials and Methods) differ from the buffer conditions used in previous tau assembly assays. This led us to evaluate the formation of tau filaments under these experimental conditions by electron microscopic analysis. As shown in Figure 4B–D, hTau40, Δ2−18, and 1−421 all form morphologically undistinguishable filaments under these buffer conditions (Fig. 4B–D).
Fig. 4.
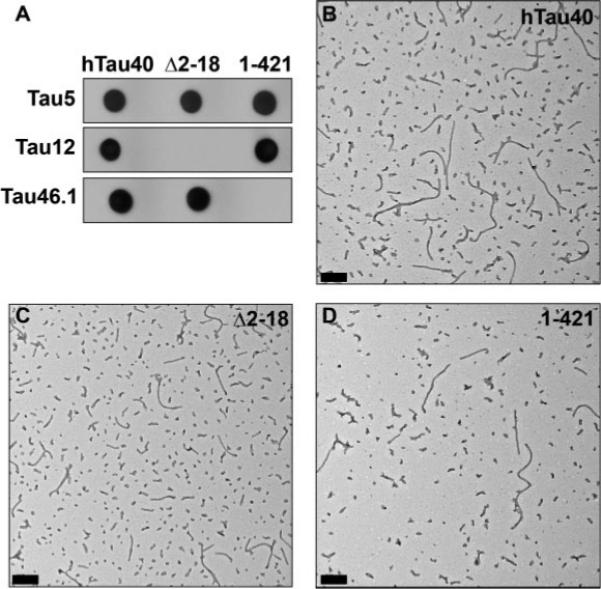
Characterization of tau filaments. Tau constructs hTau40, Δ2−18, and 1−421 were all recognized by Western blot analysis with the antibody Tau5, which recognizes an epitope near the middle of the protein (top panel). Δ2−18 is not recognized by the N-terminal antibody Tau12, and the C-terminal antibody Tau46.1 does not recognize 1−421 (lower panel; A). Filaments generated from 4 lM hTau40 (B), Δ2−18 (C), and 1−421 (D) following 5 hr of polymerization with achidonic acid. Fixed samples were negatively stained and viewed by electron microscopy. Representative digital micrographs are shown. Scale bars = 200 nm.
We then evaluated the effects of Δ2−18 and 1−421 filaments on FAT. Axoplasms perfused with monomeric hTau40 (2 μM) were used as an experimental control, because this treatment results in FAT rates that are indistinguishable from control buffer alone (see Fig. 3A). Filaments composed of hTau40 (hTau40 F) and 1−421 (1−421 F) significantly reduced anterograde FAT rates, compared with hTau40 monomer (hTau40 M; *P = 0.0001 by a two-sample t-test). In contrast, Δ2−18 filaments (Δ2−18 F) had no effect on FAT (Fig. 5A). Retrograde transport was unaffected in all conditions (Fig. 5B). These results suggest that the first 18 a.a. of tau are necessary for the inhibitory effect of tau filaments on anterograde FAT. Although the magnitude of the 1−421 filament effect was not as great that of hTau40 filaments in the time period under analysis, the pattern of inhibition was similar (see Supp. Info. Fig. 1A) and the effect of 1−421 filaments was not significantly different from that of full-length hTau40 filaments in a t-test. It remains to be determined whether the apparent difference is the result of variability in the effective concentration of the two types of filaments or whether some small difference in the structure of the 1−421 filaments affects the presentation of the N-terminus.
Monomeric Tau Constructs Lacking the C-Terminal Half of the Protein Recapitulate the Effects of Tau Filaments on FAT
Results from deletion experiments described above suggested that the first 18 a.a. at the amino terminus of tau are required to elicit the inhibitory effect of tau filaments on FAT. However, various full-length tau constructs including the amino terminal 18 a.a. domain do not affect FAT when perfused in monomeric, soluble form (Fig. 2; Morfini et al., 2007a), suggesting that this domain is abnormally exposed in filamentous hTau40. Supporting this idea, biochemical studies identified a intramolecular interaction between the amino and C termini of monomeric tau constructs (Horowitz et al., 2006; Jeganathan et al., 2006). These observations led us to evaluate the effect of endogenous tau isoforms lacking the C-terminal half of the protein on FAT. These isoforms, Tau6P and Tau6D, are the products of two cryptic splice sites in exon 6 (see Fig. 1A). They are identical to canonical tau from a.a. 1−144, at which point splicing introduces a unique 11-a.a. sequence followed by a stop codon. The specific 11 a.a. differ depending on whether the splice site is proximal or distal to the beginning of exon 6 (Luo et al., 2004). Tau6P and Tau6D terminate prior to the MTBR region and so are not expected to interact with microtubules (Lee et al., 1989) or to form filaments (Abraha et al., 2000; von Bergen et al., 2000). When axoplasm was perfused with monomeric Tau6P or Tau6D (2 μM), anterograde FAT was inhibited (P = 0.0001), but retrograde transport remained unchanged. Because the effects of these two isoforms were indistinguishable, data from Tau6P and Tau6D were pooled (Fig. 6). The inhibitory effect of these tau isoforms was indistinguishable from that of hTau40 filaments, indicating that the amino terminus of tau is sufficient to trigger FAT inhibition (see Discussion).
Fig. 6.
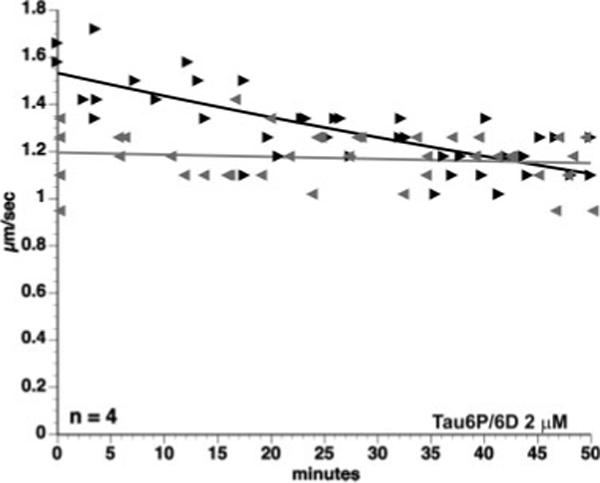
The N-terminus of tau is sufficient to inhibit anterograde FAT. Graph representing pooled data from axoplasms perfused with either Tau6P or Tau6D (2 μM). The N-termini of both isoforms are identical to the N-terminus of hTau40, but they lack the MTBR region and the C-terminus. When perfused as monomer into isolated axoplasm, these isoforms inhibit anterograde FAT. The effect was not statistically different from inhibition caused by hTau40 filaments.
The Effects of Tau Filaments on FAT Are Mediated by GSK-3 Activity
The effect of tau filaments and monomeric 6P/6D tau isoforms on anterograde FAT raised questions about the underlying molecular mechanisms. The effect of tau filaments on FAT is unlikely to be due to steric hindrance of kinesin by tau at the microtubule surface, because filamentous tau is not expected to bind to microtubules (Fig. 2c) and Tau6P/6D isoforms lack an MTBR domain. Instead, we suspected that FAT inhibition might occur through one of the signaling pathways that regulate the activity of the major anterograde motor in this system, conventional kinesin.
Several axonal kinases have been identified that play a role in regulating conventional kinesin-dependent FAT, including JNK (Morfini et al., 2006), and GSK-3 (Morfini et al., 2002b) kinases. JNK phosphorylates kinesin heavy chains (KHCs), which inhibits kinesin-1 binding to microtubules and results in reduced anterograde FAT (Morfini et al., 2006). The effects of abnormal JNK activation can be blocked by SB203580 (Morfini et al., 2006), a pharmacological inhibitor that acts on JNK2/3 and other members of the stress-activated protein kinase (SAPK)/JNK family (Coffey et al., 2002; Morfini et al., 2006). To determine whether tau filaments inhibit anterograde FAT through a mechanism involving JNK activation, we coperfused tau filaments with SB203580 (5 μM). Coperfusion of tau filaments with SB203580 did not block the effect of tau filaments on FAT (Fig. 7C; different from soluble tau at P = 0.001), indicating that this effect is independent of JNK and other SAPK/JNK kinases (i.e., p38s).
Fig. 7.
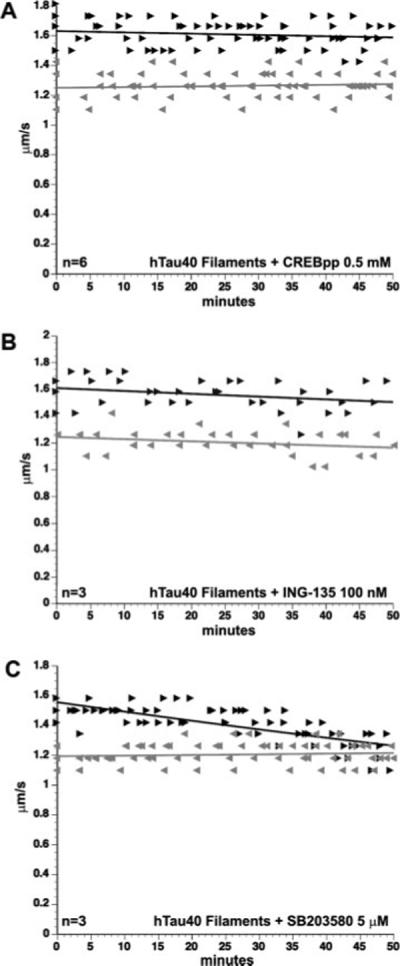
The effect of tau filaments is mediated by the activity of GSK-3. Coperfusion of hTau40 filaments with phospho-CREB peptide (0.5 mM), a competitive inhibitor of GSK-3, blocked the effects of hTau40 filaments on FAT (A). The inhibitory effect of tau filaments on FAT was also blocked by coperfusion with another specific GSK-3 inhibitor, ING-135 (100 nM; B). SB203580 (5 μM), a stress-activated protein kinase inhibitor, did not prevent the effects of hTau40 filaments on FAT (C). Perfusion of any of these inhibitors alone showed no effect on FAT (data not shown).
To evaluate whether tau filaments inhibit anterograde FAT through a mechanism involving GSK-3 activation, we coperfused hTau40 filaments (2 μM) with cAMP response element-binding protein phosphopeptide (CREBpp; 0.5 mM). Although many kinases phosphorylate intact CREB protein, the peptide fragment employed here (KRREILSRRPpSYR) is selectively phosphorylated by GSK-3 and therefore acts as a competitive inhibitor of other GSK-3 substrates (Wang et al., 1994b). Perfusion of CREBpp in squid axoplasm alone has no effect on FAT, although it effectively blocks the effects of active GSK-3 on kinesin-1-based motility (Morfini et al., 2002b, 2004). Remarkably, coperfusion of tau filaments and CREBpp blocked tau filament-induced effects on FAT (Fig. 7A), suggesting that the effects of the filaments are dependent on GSK-3 activation. CREBpp also blocked the effects of 1−421 tau filaments, indicating that these filaments act through the same mechanism as filaments of full-length hTau40 (see Supp. Info. Fig. 1).
To confirm that GSK-3 activity is required for tau filaments to inhibit kinesin-dependent FAT, we coperfused tau filaments with the lithium mimetic ING-135 (100 nM), a highly specific inhibitor of GSK-3 (Kozikowski et al., 2007; for the structure of this compound in complex with GSK-3β; see Supp. Info. Fig. 2). As with CREBpp, coperfusion with ING-135 blocked the effect of tau filaments (Fig. 7B), and perfusion of ING-135 alone had no effect on FAT (data not shown). Together, these results suggest that the effect of tau filaments on anterograde FAT involves activation of axonal GSK-3.
Phosphorylation of kinesin light chains (KLCs) by GSK-3 triggers the chaperone-dependent dissociation of kinesin-1 from its cargo (Morfini et al., 2002b; Pigino et al., 2003). To determine whether tau filaments induce the dissociation of kinesin-1 from cargo, we obtained “sister” axoplasms from individual animals and treated them with monomeric or filamentous hTau40 (2 μM). For comparison, we treated other axoplasm pairs with control buffer or with recombinant, active GSK-3β. After 40 min of incubation, we isolated axoplasmic vesicle fractions and evaluated kinesin-1 levels with an antibody against KHCs. Antibodies recognizing the synaptic integral membrane protein SNAP-25 served as a control for equal vesicle protein loading. The recovery of SNAP-25, kinesin-1, and dynein from vesicles was blocked by addition of 0.1% Triton X-100 to axoplasm homogenates prior to centrifugation, confirming the membranous nature of this fraction (not shown). Perfusion of either filamentous tau or active GSK-3β resulted in an ∼50% decrease in the amount of kinesin-1 associated with vesicles relative to monomeric tau or control buffer, respectively (P = 0.05 for filamentous tau). In contrast, levels of dynein intermediate chain were unaffected (Fig. 8). These results demonstrate that both tau filaments and GSK-3 selectively inhibit anterograde FAT by leading to dissociation of conventional kinesin-1, but not dynein, from its transported vesicular cargo.
Fig. 8.
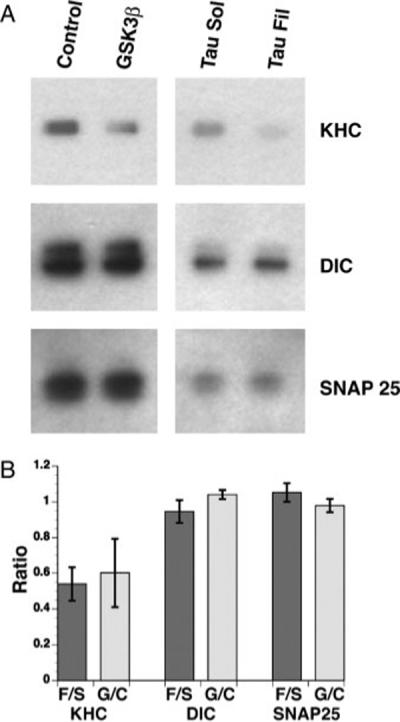
Perfusion of active GSK-3β or filamentous tau induces kinesin-1 release from squid vesicle fractions. A: Purified vesicle fractions from individual axoplasms perfused with buffer control or active GSK-3β and with soluble tau or filamentous tau were immunobloted for kinesin-1 heavy chain (KHC), dynein intermediate chain (DIC), and SNAP-25 antibodies. Note the dramatic reduction of kinesin-1 from membrane fractions of axoplasms perfused with GSK-3β and filamentous tau. B: Perfusion of active GSK-3β and filamentous tau reduces kinesin-1 membrane association by ∼50%. Membrane binding values of KHC, DIC, and SNAP-25 are expressed as the ratio between axoplasms perfused with active GSK-3β over buffer control and fibrillar over soluble tau. Values were obtained by using the Image J software from NIH and represent the mean ± SEM from three independent experiments. The difference between soluble and filamentous tau was significant at P = 0.05 by two sample t-test of μ1-μ2.
PP1 Activity Mediates the Inhibitory Effect of Tau Filaments on FAT
GSK-3 is inactive when phosphorylated (Wang et al., 1994a) and can be activated by axonal phosphatases (Wang et al., 1994a; Morfini et al., 2004). This prompted us to evaluate whether the inhibitory effects of tau filaments involve the activity of axoplasmic phosphatases. To this end, we coperfused axoplasm with tau filaments and okadaic acid (100 nM). Okadaic acid inhibits two major serine-threonine phosphatases, namely, protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A). Concentrations of okadaic acid alone up to 1 μM have no effect on FAT (Bloom et al., 1993). Remarkably, okadaic acid blocked the effect of tau filaments on FAT (Fig. 9A), suggesting that tau filament-mediated inhibition of anterograde FAT involves the activity of a major serine-threonine phosphatase. To distinguish between PP1 and PP2A activity, we coperfused filaments with 50 nM inhibitor-2 (I-2). I-2 selectively inhibits PP1, but has no effect on PP2A, even at micromolar concentrations (Cohen, 1991). When coperfused with tau filaments, I-2 prevented FAT inhibition (Fig. 9B), suggesting that axonal PP1 activity mediates the effect of tau filaments on FAT.
Fig. 9.
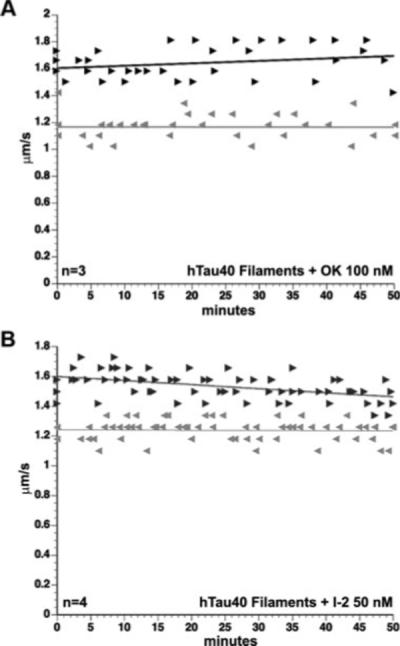
The effect of tau filaments depends on PP1 activity. The inhibitory effect of tau filaments on FAT was blocked by coperfusion with the serine-threonine phosphatase inhibitor okadaic acid (100 nM; A) and the PP1-specific inhibitor I-2 (50 nM; B). Perfusion of either of these inhibitors alone had no effect on FAT (data not shown).
DISCUSSION
We have demonstrated that hTau40 filaments selectively impair anterograde FAT in isolated axoplasm, whereas monomeric hTau40 has no effect at the same concentration. Our results provide a novel link between tau aggregation and neuronal dysfunction and identify a specific gain-of-function mechanism conferred by the aggregation process. Furthermore, our studies suggest that inhibition of kinesin-1-based motility represents an important pathogenic event in AD and other tauopathies.
We have previously described a signaling pathway that regulates conventional kinesin-based motility. In this pathway, increased PP1 activity results in the dephosphorylation and activation of axonal GSK-3 (Morfini et al., 2004). Activated GSK-3 phosphorylates KLCs, prompting a chaperone-dependent dissociation of kinesin-1 and cargo (Morfini et al., 2002b, 2004). Results presented here suggest that tau filaments inhibit anterograde FAT by triggering this pathway (Fig. 10). It is possible that tau filaments directly activate PP1 in this cascade, because tau reportedly binds to PP1 and stimulates its activity (Liao et al., 1998). However, more work is necessary to determine whether tau filaments act directly on PP1 or whether additional intermediate components remain undiscovered. Additionally, some feedback from GSK-3 is conceivable, insofar as GSK-3 is a major tau kinase that can also bind directly to tau (Sun et al., 2002) and is reported to be abnormally activated in AD (Ferrer et al., 2005).
Fig. 10.
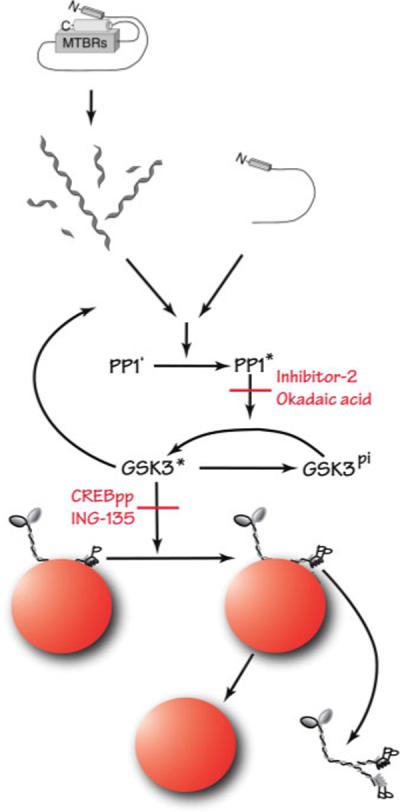
Proposed mechanism underlying tau filament-induced inhibition of anterograde FAT. Pharmacological experiments presented here indicate that FAT inhibition requires the activity of PP1 and GSK-3. We have previously described a pathway in which PP1 dephosphorylates and activates GSK-3, which subsequently phosphorylates kinesin light chains (KLCs). Phosphorylation of KLCs promotes a chaperone-mediated detachment of kinesin-1 from its transported cargo. Our results suggest that tau can trigger this cascade when the amino terminus of tau is abnormally exposed in the filamentous form. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We demonstrated that the inhibitory effect of tau filaments on FAT requires the extreme amino terminus of the protein. A pivotal role for this part of the protein is consistent with studies in other experimental systems. For example, overexpression of tau amino terminus induces cell death in cultured neurons through a mechanism involving abnormal kinase activity (Amadoro et al., 2004, 2006), and expression of the N-terminus of tau induced microtubule disassembly in cells exposed to beta-amyloid (King et al., 2006). Immunological studies indicate that loss of the N-terminus is an early event in the maturation of tau fibrillar lesions in AD (Horowitz et al., 2004). Our results suggest that such cleavage events may modulate the toxicity of filamentous tau on FAT. The amino terminus is also the site of AD-related phosphorylation (Lee et al., 2004) and nitration (Reynolds et al., 2006) events and of the FTDP-17-associated mutations R5L (Poorkaj et al., 2002) and R5H (Hayashi et al., 2002). Analysis of how these modifications influence the effects of tau filaments on FAT may provide further insights into disease progression.
Although hTau40 monomer had no effect on FAT at the concentrations tested, monomers of Tau6P and Tau6D inhibited anterograde FAT as effectively as hTau40 filaments. This result demonstrates that the N-terminus of tau is sufficient to produce the observed effects on FAT, insofar as these isoforms lack the MTBR region and the C-terminal tail of canonical tau. Additionally, this raises the question of why hTau40 monomer failed to show the same inhibitory effect, even though it contains an intact amino terminus. Although monomeric hTau40 was first thought to exist in an extended conformation (Schweers et al., 1994; Syme et al., 2002), recent evidence suggests that tau in solution adopts a globally folded conformation in which the N-terminus folds in close proximity to the C-terminus (Horowitz et al., 2006; Jeganathan et al., 2006). It is possible that this conformation shields the extreme amino terminus, thus preventing hTau40 monomer from inhibiting FAT. In this scenario, polymerization would freeze hTau40 in a different conformation in which the N-terminus is exposed.
With these results, tau joins a growing list of proteins whose pathogenic forms alter regulatory pathways for FAT. As with tau filaments, AD-associated mutations in presenilin-1 inhibit kinesin-1-based motility through GSK-3 activation (Pigino et al., 2003), whereas pathogenic forms of androgen receptor and huntingtin inhibit kinesin-1-dependent transport through JNK activation (Morfini et al., 2006). The current study suggests that AD represents an example of a dysferopathy, in which alterations in FAT lead to a dying back neuropathy (Morfini et al., 2007b), providing further evidence that alterations in regulatory pathways for FAT represent a common pathogenic event in multiple, otherwise apparently unrelated neurodegenerative diseases (Morfini et al., 2002a, 2005).
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Natalia Marangoni, Sarah Pollema, Kelly Monk, and Hannah Brown for assistance with axoplasm experiments; Dr. Athena Andreadis for the Tau6P and Tau6D constructs; and especially Dr. Angela Guillozet-Bongaarts for a critical reading of the manuscript.
Contract grant sponsor: NIH; Contract grant number: NS049834 (to N.E.L.); Contract grant number: AG14453 (to L.I.B.); Contract grant sponsor: NINDS; Contract grant number: NS23868; Contract grant number: NS23320; Contract grant number: NS41170; Contract grant number: NS43408 (to S.T.B.); Contract grant sponsor: MDA (to S.T.B.); Contract grant sponsor: ALSA (to G.M., S.T.B.); Contract grant sponsor: HDSA (to G.M.).
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- Abraha A, Ghoshal N, Gamblin TC, Cryns V, Berry RW, Kuret J, Binder LI. C-terminal inhibition of tau assembly in vitro and in Alzheimer's disease. J Cell Sci. 2000;113:3737–3745. doi: 10.1242/jcs.113.21.3737. [DOI] [PubMed] [Google Scholar]
- Amadoro G, Serafino AL, Barbato C, Ciotti MT, Sacco A, Calissano P, Canu N. Role of N-terminal tau domain integrity on the survival of cerebellar granule neurons. Cell Death Differ. 2004;11:217–230. doi: 10.1038/sj.cdd.4401314. [DOI] [PubMed] [Google Scholar]
- Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–298. doi: 10.1016/S0065-3233(04)71007-4. [DOI] [PubMed] [Google Scholar]
- Bell KF, Claudio Cuello A. Altered synaptic function in Alzheimer's disease. Eur J Pharmacol. 2006;545:11–21. doi: 10.1016/j.ejphar.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Berry RW, Abraha A, Lagalwar S, LaPointe N, Gamblin TC, Cryns VL, Binder LI. Inhibition of tau polymerization by its carboxy-terminal caspase cleavage fragment. Biochemistry. 2003;42:8325–8331. doi: 10.1021/bi027348m. [DOI] [PubMed] [Google Scholar]
- Binder LI, Guillozet-Bongaarts AL, Garcia-Sierra F, Berry RW. Tau, tangles, and Alzheimer's disease. Biochim Biophys Acta. 2005;1739:216–223. doi: 10.1016/j.bbadis.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Richards BW, Leopold PL, Ritchey DM, Brady ST. GTP gamma S inhibits organelle transport along axonal microtubules. J Cell Biol. 1993;120:467–476. doi: 10.1083/jcb.120.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST. A novel brain ATPase with properties expected for the fast axonal transport motor. Nature. 1985;317:73–75. doi: 10.1038/317073a0. [DOI] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, Allen RD. Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 1985;5:81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- Brandt R, Leger J, Lee G. Interaction of tau with the neural plasma membrane mediated by tau's amino-terminal projection domain. J Cell Biol. 1995;131:1327–1340. doi: 10.1083/jcb.131.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50's selectivity for Alzheimer's disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- Coffey ET, Smiciene G, Hongisto V, Cao J, Brecht S, Herdegen T, Courtney MJ. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J Neurosci. 2002;22:4335–4345. doi: 10.1523/JNEUROSCI.22-11-04335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–398. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M. Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol. 1997;138:1067–1075. doi: 10.1083/jcb.138.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Gomez-Isla T, Puig B, Freixes M, Ribe E, Dalfo E, Avila J. Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies. Curr Alzheimer Res. 2005;2:3–18. doi: 10.2174/1567205052772713. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Berry RW, Binder LI. Modeling tau polymerization in vitro: a review and synthesis. Biochemistry. 2003a;42:15009–15017. doi: 10.1021/bi035722s. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Berry RW, Binder LI. Tau polymerization: role of the amino terminus. Biochemistry. 2003b;42:2252–2257. doi: 10.1021/bi0272510. [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003c;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal N, Garcia-Sierra F, Wuu J, Leurgans S, Bennett DA, Berry RW, Binder LI. Tau conformational changes correspond to impairments of episodic memory in mild cognitive impairment and Alzheimer's disease. Exp Neurol. 2002;177:475–493. doi: 10.1006/exnr.2002.8014. [DOI] [PubMed] [Google Scholar]
- Grant P, Diggins M, Pant HC. Topographic regulation of cytoskeletal protein phosphorylation by multimeric complexes in the squid giant fiber system. J Neurobiol. 1999;40:89–102. doi: 10.1002/(sici)1097-4695(199907)40:1<89::aid-neu8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Guillozet-Bongaarts AL, Garcia-Sierra F, Reynolds MR, Horowitz PM, Fu Y, Wang T, Cahill ME, Bigio EH, Berry RW, Binder LI. Tau truncation during neurofibrillary tangle evolution in Alzheimer's disease. Neurobiol Aging. 2005;26:1015–1022. doi: 10.1016/j.neurobiolaging.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Gustke N, Trinczek B, Biernat J, Mandelkow EM, Mandelkow E. Domains of tau protein and interactions with microtubules. Biochemistry. 1994;33:9511–9522. doi: 10.1021/bi00198a017. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Toyoshima Y, Hasegawa M, Umeda Y, Wakabayashi K, Tokiguchi S, Iwatsubo T, Takahashi H. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol. 2002;51:525–530. doi: 10.1002/ana.10163. [DOI] [PubMed] [Google Scholar]
- Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer's disease. J Neurosci. 2004;24:7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz PM, LaPointe N, Guillozet-Bongaarts AL, Berry RW, Binder LI. N-terminal fragments of tau inhibit full-length tau polymerization in vitro. Biochemistry. 2006;45:12859–12866. doi: 10.1021/bi061325g. [DOI] [PubMed] [Google Scholar]
- Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E. Global hairpin folding of tau in solution. Biochemistry. 2006;45:2283–2293. doi: 10.1021/bi0521543. [DOI] [PubMed] [Google Scholar]
- King ME. Can tau filaments be both physiologically beneficial and toxic? Biochim Biophys Acta. 2005;1739:260–267. doi: 10.1016/j.bbadis.2004.06.019. [DOI] [PubMed] [Google Scholar]
- King ME, Ahuja V, Binder LI, Kuret J. Ligand-dependent tau filament formation: Implications for Alzheimer's disease progression. Biochemistry. 1999;38:14851–14859. doi: 10.1021/bi9911839. [DOI] [PubMed] [Google Scholar]
- King ME, Kan HM, Baas PW, Erisir A, Glabe CG, Bloom GS. Tau-dependent microtubule disassembly initiated by prefibrillar beta-amyloid. J Cell Biol. 2006;175:541–546. doi: 10.1083/jcb.200605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Orecchio LD, Binder L, Trojanowski JQ, Lee VM, Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988;1:817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, Blond SY, Fedolak A, Caldarone B, McGonigle P. Structure-based design led to the identification of lithium mimetics that block mania-like effects in rodents. possible new GSK-3beta therapies for bipolar disorders. J Am Chem Soc. 2007;129:8328–8332. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]
- Lee G. Tau and src family tyrosine kinases. Biochim Biophys Acta. 2005;1739:323–330. doi: 10.1016/j.bbadis.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Lee G, Neve RL, Kosik KS. The microtubule binding domain of tau protein. Neuron. 1989;2:1615–1624. doi: 10.1016/0896-6273(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM, Do LH, Andreadis A, Van Hoesen G, Ksiezak-Reding H. Phosphorylation of tau by fyn: implications for Alzheimer's disease. J Neurosci. 2004;24:2304–2312. doi: 10.1523/JNEUROSCI.4162-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Li Y, Brautigan DL, Gundersen GG. Protein phosphatase 1 is targeted to microtubules by the microtubule-associated protein Tau. J Biol Chem. 1998;273:21901–21908. doi: 10.1074/jbc.273.34.21901. [DOI] [PubMed] [Google Scholar]
- Luo MH, Tse SW, Memmott J, Andreadis A. Novel isoforms of tau that lack the microtubule-binding domain. J Neurochem. 2004;90:340–351. doi: 10.1111/j.1471-4159.2004.02508.x. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Beffert U, Busciglio J, Brady ST. Fast axonal transport misregulation and Alzheimer's disease. Neuromol Med. 2002a;2:89–99. doi: 10.1385/NMM:2:2:089. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002b;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Brady ST. Polyglutamine expansion diseases: failing to deliver. Trends Mol Med. 2005;11:64–70. doi: 10.1016/j.molmed.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Mizuno N, Kikkawa M, Brady ST. Tau binding to microtubules does not directly affect microtubule-based vesicle motility. J Neurosci Res. 2007a doi: 10.1002/jnr.21154. (in press) [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Opalach K, Serulle Y, Moreira JE, Sugimori M, Llinas RR, Brady ST. 1-Methyl-4-phenylpyridinium affects fast axonal transport by activation of caspase and protein kinase C. Proc Natl Acad Sci U S A. 2007b;104:2442–2447. doi: 10.1073/pnas.0611231104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JR, Lasek RJ. Monomer-polymer equilibria in the axon: direct measurement of tubulin and actin as polymer and monomer in axoplasm. J Cell Biol. 1984;98:2064–2076. doi: 10.1083/jcb.98.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J Neurosci. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, Schellenberg GD. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Preuss U, Biernat J, Mandelkow EM, Mandelkow E. The “jaws” model of tau-microtubule interaction examined in CHO cells. J Cell Sci. 1997;110:789–800. doi: 10.1242/jcs.110.6.789. [DOI] [PubMed] [Google Scholar]
- Reynolds MR, Lukas TJ, Berry RW, Binder LI. Peroxynitrite-mediated tau modifications stabilize preformed filaments and destabilize microtubules through distinct mechanisms. Biochemistry. 2006;45:4314–4326. doi: 10.1021/bi052142h. [DOI] [PubMed] [Google Scholar]
- Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994;269:24290–24297. [PubMed] [Google Scholar]
- Seitz A, Kojima H, Oiwa K, Mandelkow EM, Song YH, Mandelkow E. Single-molecule investigation of the interference between kinesin, tau and MAP2c. EMBO J. 2002;21:4896–4905. doi: 10.1093/emboj/cdf503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Qureshi HY, Cafferty PW, Sobue K, Agarwal-Mawal A, Neufield KD, Paudel HK. Glycogen synthase kinase-3beta is complexed with tau protein in brain microtubules. J Biol Chem. 2002;277:11933–11940. doi: 10.1074/jbc.M107182200. [DOI] [PubMed] [Google Scholar]
- Syme CD, Blanch EW, Holt C, Jakes R, Goedert M, Hecht L, Barron LD. A Raman optical activity study of rheomorphism in caseins, synucleins and tau. New insight into the structure and behaviour of natively unfolded proteins. Eur J Biochem. 2002;269:148–156. doi: 10.1046/j.0014-2956.2001.02633.x. [DOI] [PubMed] [Google Scholar]
- Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A. 2007;104:87–92. doi: 10.1073/pnas.0607919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bergen M, Friedhoff P, Biernat J, Heberle J, Mandelkow E. Assembly of tau protein into Alzheimer paired helical filaments depends on a local sequence motif (306VQIVYK311) forming beta structure. Proc Natl Acad Sci U S A. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994a;269:14566–14574. [PubMed] [Google Scholar]
- Wang QM, Roach PJ, Fiol CJ. Use of a synthetic peptide as a selective substrate for glycogen synthase kinase 3. Anal Biochem. 1994b;220:397–402. doi: 10.1006/abio.1994.1356. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


