Abstract
SUMO conjugation to protein substrates requires the concerted action of a dedicated E2 ubiquitin conjugation enzyme (Ubc9) and associated E3 ligases. Although Ubc9 can directly recognize and modify substrate lysine residues that occur within a consensus site for SUMO modification, E3 ligases can redirect specificity and enhance conjugation rates during SUMO conjugation in vitro and in vivo. In this chapter, we will describe methods utilized to purify SUMO conjugating enzymes and model substrates which can be used for analysis of SUMO conjugation in vitro. We will also describe methods to extract kinetic parameters during E3-dependent or E3-independent substrate conjugation.
1. Introduction
Post-translational covalent modification of substrates by ubiquitin (Ub) and ubiquitin-like (Ubl) proteins can alter the activities of targeted substrates by affecting protein stability, catalytic activity, or by redirecting protein localization within the cell (1–6). Substrate modification by Ub/Ubl modifiers is carried out by sequential action of at least three enzymes or factors termed E1, E2, and E3 (1). The Ub/Ubl is first processed by a protease to reveal a conserved di-glycine motif which is subsequently adenylated by E1 in an ATP-dependent reaction. The Ub/Ubl is then transferred to a conserved E1 cysteine residue to form E1~Ub/Ubl adduct (where ~ indicates a thioester bond). The E1~Ub/Ubl is then transferred to a conserved E2 active site cysteine residue to form an E2~Ub/Ubl adduct. The Ub/Ubl modifier is then transferred from the E2~Ub/Ubl adduct to substrate lysine residues to form a stable isopeptide bond between the Ub/Ubl C-terminal glycine and the ε-amine atom from the substrate lysine residue. E3 ligases can facilitate this reaction between substrate and E2~Ub/Ubl either by enhancing the rate of transfer or by redirecting substrate specificity.
SUMO is a member of the Ubl family of proteins. Yeast encodes one SUMO ortholog termed Smt3 while human encodes four SUMO orthologs termed SUMO-1, SUMO-2, SUMO-3, and SUMO-4. It remains unclear whether SUMO-4 is capable of conjugation. SUMO activation requires a dedicated heterodimeric E1 (Aos1/Uba2 or SAE1/SAE2), a single E2 enzyme (Ubc9) and at least two distinct families of SUMO E3 ligases. Ubc9 can directly interact with and modify SUMO substrates containing an accessible consensus motif Φ-K-x-E/D where Φ is a hydrophobic, K is the lysine attached to SUMO, x is any amino acid, and E or D is an acidic residue (7, 8). Many SUMO substrates can be conjugated in vitro in an E3-independent manner, although SUMO E3 ligases enhance SUMO conjugation in vitro and in vivo. In addition, SUMO E3s can confer additional substrate specificity during modification in vitro and in vivo. There are two known families of SUMO E3 ligases. SP-RING E3 ligases share limited sequence similarity to ubiquitin RING E3s and include members from yeast (Siz1, Siz2, Mms21 and Zip3) and the human PIAS protein family in higher eukaryotes (9–16). The RanBP2/Nup358 protein encompasses the second type of SUMO E3 ligase and is unrelated to either RING or HECT E3 ligase families (17–19).
Recent analysis of the enzymes involved in SUMO conjugation has elucidated the biochemical and structural basis for SUMO conjugation in both E2- and E3-dependent reactions. We will describe procedures used to isolate SUMO conjugating enzymes and methods to extract kinetic parameters for SUMO conjugation in both E3-independent and E3-dependent reactions.
2. Materials
2.1 Protein expression and purification
Luria-Bertani (LB) media: 10 g bacto-tryptone, 10 g NaCl, 5 g bacto-yeast extract in 1 L water.
Super Broth (SB) media: 32 g tryptone, 20 g yeast extract, 5 g NaCl in 1 L water.
Antibiotics: Ampicillin, 200 mg/ml in water, filter sterilized; Kanamycin, 50 mg/ml in water, filter sterilized; Chloramphenicol, 34 mg/ml in ethanol.
Isopropyl-beta-D-thiogalactopyranoside (IPTG): 1 M in water, filter sterilized.
Plasmids: pET-15b, pET-11c, pET-28b, and pET-21b (Novagen). pSMT3 and TOPO-SMT3 (See Note 1).
Bacterial strains: Escherichia coli BL21(DE3) RIL Codon Plus (Stratagene) or E. coli BL21(DE3) pLysS (Novagen).
Fermentation: BioFlo-3000 fermentor equipped with a 14 L vessel (New Brunswick).
Site directed mutagenesis: QuikChange Mutagenesis Kit (Stratagene).
Fluorescence: Alexa Fluor 488 C5 maleimide dye (Molecular Probes; Invitrogen).
PCR Primers (Invitrogen); Polymerase (Pfu turbo; Strategene).
Bovine thrombin (Sigma): 1 U/μl (0.33 μg/μl) in 20 mM Tris-HCl pH 8.0, 350 mM NaCl, 1 mM BME or water and stored at −20°C.
Ulp1 protease catalytic domain (amino acids 403–621): 3 mg/ml in 20 mM Tris-HCl pH 8.0, 350 mM NaCl, 1 mM β-mercaptoethanol (BME), 10% glycerol at −80°C.
Suspension buffer: 20% sucrose, 50 mM Tris-HCl pH 8.0.
Lysis buffer: 20% sucrose, 50 mM Tris pH 8.0, 1 mM BME, 350 mM NaCl, 20 mM imidazole, 20 μg/ml lysozyme, 100 μg/ml DNAse I, 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1% IGEPAL CA-630 (Sigma).
Ni-NTA Superflow agarose resin (Qiagen).
Buffer A: 20 mM Tris-HCl pH 8.0, 350 mM NaCl, 1 mM BME, and 20 mM imidazole.
Buffer B: 20 mM Tris-HCl pH 8.0, 350 mM NaCl, 1 mM BME, and 400 mM imidazole.
Buffer C: 20 mM Tris-HCl pH 8.0, 50 mM NaCl, and 1 mM BME.
Buffer D: 20 mM Tris-HCl pH 8.0, 100 mM NaCl, and 1 mM BME.
Buffer E: 20 mM Tris-HCl pH 8.0, 1 M NaCl, and 1 mM BME.
Buffer F: 20 mM Tris-HCl pH 8.0, 350 mM NaCl, and 1 mM BME.
Buffer G: 20 mM Tris-HCl pH 8.0, 150 mM NaCl, and 1 mM BME.
AKTA-FPLC (GE Healthcare) equipped with gel filtration columns (Superdex-75 26/60 and Superdex-200 26/60) and ion exchange columns (Mono-Q 10/10 and Mono-S 10/10) (See Note 2).
Centricon or Centriprep micro-filtration devices (Amicon) with appropriate molecular weight cutoffs (10 kDa, 30 kDa or 50 kDa).
Bradford reagent (Bio-Rad) or BCA Protein Assay Reagent (Pierce).
2.2 SUMO-conjugation assays
Desalting column: Micro Bio-Spin Bio-gel P-6 (Bio-Rad).
Buffer I: 20 mM HEPES pH 7.5, 50 mM NaCl, 5 mM MgCl2, 0.1% (v/v) Tween-20.
Buffer II: 50 mM sodium citrate pH 5.5, 50 mM NaCl, and 5% glycerol.
Buffer III: 20 mM HEPES pH 7.5, 50 mM NaCl, 0.1% v/v Tween-20, 5 mM EDTA.
Buffer IV: 50 mM Tris-HCl pH 6.8, 2% SDS, 4 M Urea, 10% glycerol, and 0.25% bromophenol blue.
Buffer V: 50mM sodium citrate pH 6.8, 75mM NaCl, 5 mM MgCl2.
Buffer VI: 20 mM HEPES pH 7.5, 50 mM NaCl.
Buffer VII: 20 mM Bis-Tris propane (pH range from 7.07 to 10.6), 50 mM NaCl, 0.1% v/v Tween-20, 5 mM EDTA.
2.3 SDS-Polyacrylamide gel electrophoresis (SDS-PAGE)
NuPAGE system (Invitrogen) for SDS-PAGE analysis with either MES or MOPS running buffer (Invitrogen).
4–12% gradient polyacrylamide Bis-Tris gels (Invitrogen).
2.4 Protein detection and analysis by western-blot
Transfer buffer: 1x Tris glycine (Genemate; ISC Bioexpress) buffer with 20% methanol.
Wash buffer: Phosphate buffered saline (PBS) solution (10mM phosphate buffer pH 7.4, 2.7 mM potassium chloride, 137 mM Sodium chloride; Sigma) and 0.1% Tween-20 (Bio-rad).
Blocking buffer: 3% (w/v) non-fat dry milk in PBS.
Immun-Blot™ PVDF membranes (BioRad).
Primary Antibody: Antibody against human SUMO-1 (Boston Biochem) used at 1:1000 dilution in blocking buffer.
Secondary Antibody: Anti-rabbit IgG horseradish peroxidase linked whole antibody (donkey; GE Healthcare) used at 1:2500 dilution in blocking buffer.
Trans-Blot Semi-Dry Electrophoretic Transfer Cell (Bio-Rad).
Enhanced chemiluminescent (ECL) plus reagent (GE Healthcare).
Imaging western blots: Fujifilm LAS3000 chemiluminescence detector.
Image processing and data quantification: Multi Gauge v2.02 or Image Gauge v4.0 (Fujifilm).
2.5 Protein detection and analysis using fluorescence
Fujifilm FLA-5000 with a FITC filter.
Image processing and data quantification: Multi Gauge v2.02 or Image Gauge v4.0 (Fujifilm).
2.6 Data processing
Raw data were processed in EXCEL (Microsoft).
Data and regression analysis: SigmaPlot 9.0 (Systat Software Inc.).
3. Methods
3.1 Cloning and purification of human and yeast E1 (Aos1/Uba2)
The E1 enzyme for SUMO is heterodimeric and consists of two individually encoded polypeptides, Aos1 and Uba2 (a.k.a. SAE1 and SAE2 for human E1). Human or yeast genes were amplified by PCR from human cDNA or yeast genomic DNA, respectively.
The yeast Aos1 subunit was cloned into pET-15b using 5′ NcoI and 3′ BamHI and the human Aos1 subunit was cloned into pET-11c using 5′ NdeI and 3′ BamHI. In both instances, Aos1 was encoded as a native polypeptide.
Full-length human and yeast Uba2 were cloned into pET-28b using 5′ NheI and 3′ XhoI or 5′ NdeI and 3′ HindIII, respectively, to encode polypeptides fused to an N-terminal thrombin cleavable His6-tag.
Primers were designed to amplify the C-terminally truncated versions of the human and yeast Uba2 (ΔC-term; human 1–549; yeast 1–554) using 5′ BglII and 3′ SalI or 5′ NdeI and 3′ XhoI, respectively.
The two plasmids encoding respective E1 subunits (Aos1, Uba2, or Uba2ΔC-term) were co-transformed into E. coli BL21 (DE3) RIL Codon Plus.
A 10 L culture was grown by fermentation at 37°C to an A600 of 3.0, induced by addition of IPTG to a final concentration of 1 mM, and grown for 3 hours at 30°C.
Cells were harvested by centrifugation (7000 × g) and cell pellets were suspended in Suspension buffer to a final concentration of 2 ml/g cell wet weight. The cell suspension can be stored at −80°C for later use after snap freezing the suspended cells in liquid nitrogen.
Frozen cell pellets were thawed and equilibrated in Lysis buffer prior to sonication.
Cells were disrupted by sonication and the cell lysate was clarified by centrifugation (40000 × g) to obtain supernatant free from cell debris (See Note 3).
The lysate was applied to a chromatography column packed with Ni-NTA resin and washed using at least 5 column volumes of Buffer A prior to elution with Buffer B. The heterodimeric E1 enzyme was isolated by virtue of the His6-tag on Uba2. Fractions were collected and analyzed by SDS-PAGE. Protein content was quantified by Bradford analysis.
Protein fractions were analyzed by SDS-PAGE and those containing E1 were pooled and applied to a gel filtration column (Superdex-200) in Buffer F. The SUMO E1 heterodimer migrates as a monodisperse peak with an apparent molecular weight of ~120 kDa.
Fractions were analyzed by SDS-PAGE, and those containing E1 were pooled, desalted into Buffer D, and applied to an anion exchange column (Mono-Q). The protein was eluted using a gradient from Buffer D to 50% Buffer E over 20 column volumes. SUMO E1 eluted at approximately 200–250 mM NaCl.
Fractions were analyzed by SDS-PAGE and those containing E1 were pooled, desalted into Buffer H, concentrated to ~10 mg/ml, flash frozen in liquid nitrogen and stored at −80°C (See Note 7).
3.2 Cloning and purification of human and yeast Ubc9
The primers used to amplify the open reading frame of human and yeast UBC9 were designed to include NdeI and XhoI restriction sites at the 5′ or 3′ ends, respectively.
Human and yeast UBC9 were amplified by PCR from human cDNA or yeast genomic DNA, respectively. Yeast UBC9 contains an intron near the 5′ end, so the 5′ primer was designed to include the 5′ exon.
PCR products were digested with appropriate restriction endonucleases and ligated into pET-28b plasmid to encode Ubc9 N-terminally fused with a thrombin cleavable hexahistidine tag.
Yeast UBC9 containing the point mutation K153R was generated by site directed mutagenesis. This Ubc9 isoform will be utilized in assays described later.
Plasmids were transformed into E. coli BL21 (DE3) RIL Codon Plus.
2 L LB cultures were grown in baffled shaker flasks at 37°C until to an A600 of 1.0. Cultures were cooled to 30°C, and IPTG was added to a final concentration of 1 mM. Cultures were then incubated for 3 hours at 30°C.
Cells were harvested, cell pellets processed for sonication, and lysate applied to Ni-NTA resin as described in section 3.1.
The lysate from a 2 liter culture contains approximately 100 mg of His6-Ubc9, requiring ~10 ml of Ni-NTA resin.
Fractions containing Ubc9 were pooled. The His6-thrombin cleavable polypeptide was removed by incubation with a 1:1000 (w/w) ratio of bovine thrombin to protein. The extent of proteolysis was monitored by SDS-PAGE.
After proteolysis was complete (2–4 hours at room temperature or overnight at 4°C), the sample was applied to gel filtration (Superdex-75) in Buffer F. Ubc9 migrates as a monodisperse protein with an apparent molecular weight of ~20 kDa. Fractions were analyzed by SDS-PAGE and those containing Ubc9 were pooled and dialyzed or desalted into Buffer C. This mixture was loaded onto cation-exchange resin (Mono-S) and the protein was eluted using a gradient from Buffer C to 50% Buffer E over 20 column volumes. Ubc9 eluted from MonoS at approximately 150 mM NaCl. Fractions containing the protein peak were exchanged into Buffer C and concentrated to 5–10 mg/ml.
Protein was aliquoted and flash frozen in liquid nitrogen at stored at −80°C for later use.
3.3 Cloning, purification and fluorophore labeling of human SUMO-1 and yeast SUMO (Smt3)
In this section we discuss the purification protocol of native and mutant isoforms of SUMO-1 and Smt3. We also discuss the procedure for labeling mutant SUMO proteins with Alexa Fluor 488 C5 maleimide dye which covalently and irreversibly modifies cysteine residues. The mutant isoforms (SUMO-1 K9C and Smt3 K11C) were generated to introduce cysteine residues. Wild-type Smt3 contains no cysteine residues, although human SUMO-1 contains a cysteine residue at position 52. This residue was mutated to alanine by altering the respective codon within the SUMO-1 K9C construct. K9C and K11C are located in the structurally disordered N-terminal domain. We and others have determined that deletion of this region has no detectable deleterious effects during SUMO activation or during SUMO conjugation to substrates. These labeled SUMO proteins will be utilized in subsequent assays described in the text.
3.3.1 Purification of processed human SUMO-1
Full length SUMO-1 with a C-terminal hexahistidine tag was cloned and purified as described in the chapter detailing proteolysis with endogenous substrates (Reverter and Lima).
The human SUMO-1 isoform containing the point mutation K9C was obtained by site directed mutagenesis.
3.3.2 Purification of processed Smt3 K11C
Yeast SMT3 was inserted into pET-28b using NcoI and XhoI restriction sites to encode Smt3 with a C-terminal His6-tag.
The Smt3 isoform containing the point mutation K11C was obtained by site directed mutagenesis.
Plasmids used to express wild-type and mutant Smt3 proteins were transformed into E. coli BL21 (DE3) pLysS.
Strains were grown in LB media at 37°C to an O. D of 1.0 prior to addition of IPTG to a final concentration of 1 mM. Cultures were grown for an additional 3 hours at 30°C.
Cell pellets were processed for sonication as described above (section 3.1).
The lysate was applied to Ni-NTA resin in Buffer A and eluted from the chromatography column in Buffer B.
To remove the C-terminal His6-tag, fractions containing the protein were incubated with the Ulp1 protease at a 1:1000 (w/w) ratio for 2–4 hours at room temperature or overnight at 4°C.
This mixture was applied to gel filtration (Superdex-75) in Buffer F. Smt3 eluted as a monodisperse peak with an apparent molecular weight near 20 kDa. Fractions were analyzed by SDS-PAGE and those containing Smt3 were pooled and desalted into Buffer C, and applied to anion exchange resin (Mono-Q). Smt3 was eluted from the column using a gradient from Buffer C to 50% Buffer E over 20 column volumes. Smt3 eluted at ~150 mM NaCl.
Fractions were analyzed by SDS-PAGE and those containing Smt3 were pooled, concentrated to approximately 4 mg/ml, flash frozen using liquid nitrogen, and stored at −80°C for future use.
3.3.3 Labeling SUMO K9C or Smt3 K11C with Alexa Fluor 488 C5 maleimide
300 μM Smt3 K11C or SUMO-1 K9C (~4 mg/ml) were incubated with 10 molar excess of Alexa Fluor 488 C5 maleimide dye.
Dye was added to the protein solution in a drop wise manner and incubated overnight at 4°C.
The reaction was quenched and excess dye removed by applying the mixture to a desalting column equilibrated with Buffer D.
The resulting mixture was concentrated to at least 1 mg/ml, aliquoted, flash frozen in liquid nitrogen, and stored at −80°C for later use.
3.4 Cloning and purification of SUMO substrates
3.4.1 Cloning and purification of the human p53 C-terminal domain
Primers were designed to clone the C-terminal tetramerization domain of human p53 (residues 320-393) as a C-terminal fusion to Smt3. The PCR amplified ORF was cloned using pSMT3.
Plasmid DNA was transformed into E. coli BL21 (DE3) RIL Codon Plus cells.
A 10 L culture was grown by fermentation at 37°C to an A600 of 3.0, induced by addition of IPTG to a final concentration of 1 mM, and grown for an additional 3 hours at 30°C.
The cell pellets were processed as described previously (section 3.1).
The lysate is applied to Ni-NTA resin which was pre-equilibrated with Buffer A. The protein was eluted using Buffer B.
Fractions were analyzed by SDS-PAGE and those containing His6-Smt3-p53 were pooled and equilibrated by dialysis or desalting in Buffer G in the presence of Ulp1 protease (1:1000 w/w) to liberate p53 from the His6-Smt3 tag. Dialysis and cleavage can be carried out simultaneously by overnight incubation at 4°C or after desalting by incubation at room temperature for 3–4 hours. The extent of cleavage was assessed by SDS-PAGE before proceeding to step 7.
The sample was applied to cation exchange resin (Mono-S) and eluted using a gradient from Buffer G to 50% Buffer E over 20 column volumes. Human p53 eluted at ~325 mM NaCl. The His6-Smt3 tag does not interact with MonoS resin.
Fractions containing p53 were applied to gel filtration (Superdex-75) in Buffer F. The C-terminal p53 domain eluted as a tetramer with an apparent molecular weight of ~32 kDa. Fraction containing p53 were pooled, desalted into Buffer D, concentrated to ~6 mg/ml, aliquoted, frozen in liquid nitrogen, and stored at −80°C.
3.4.2 Cloning and purification of yeast PCNA (K127G)
Primers were designed to amplify wild-type PCNA from yeast genomic DNA using Pfu turbo polymerase. Primers included NdeI and XhoI restriction sites at the 5′ or 3′ ends of the gene, respectively.
PCR products were digested with appropriate restriction enzymes and ligated into pET-21b to encode yeast PCNA without any affinity tag.
A non-consensus lysine residue (K164) is the primary site for Siz1-dependent SUMO modification of PCNA (21, 22). However, in a Ubc9-depdendent reaction under some in vitro and in vivo conditions, a minor SUMO-modified product accumulates on a lysine residue within a SUMO consensus site (K127). To simplify kinetic analysis during E3-mediated conjugation to K164, we mutated this side chain to glycine, a residue observed in other PCNA family members. The K127G PCNA mutation was introduced by site directed mutagenesis.
The plasmid carrying PCNA was transformed into E. coli BL21 (DE3) RIL Codon Plus.
10 L cultures were grown by fermentation at 37°C to an A600 of 3.0, adjusted to an IPTG concentration of 1 mM, and grown at 30°C for an additional 3 hours.
Cell cultures were processed as described above (section 3.1).
Cell pellets for protein purification were processed in batches corresponding to approximately 4 L of bacterial culture.
PCNA was purified from the soluble fraction by slow addition of solid ammonium sulfate to the supernatant with stirring to 45% saturation. The precipitated material was removed by centrifugation at 7500 × g for 30 minutes. The supernatant was retrieved and a second ammonium sulfate precipitation was carried conducted to 70% saturation. The precipitated material contained yeast PCNA as a major species (confirmed by SDS-PAGE analysis). This fraction was suspended in Buffer F and dialyzed overnight at 4°C against the same buffer.
This mixture was applied to gel filtration (Superdex-200) in Buffer F. Fractions containing yeast PCNA were analyzed by SDS-PAGE, pooled, and dialyzed against Buffer C.
This mixture was applied to anion-exchange resin (Mono-Q) and PCNA was eluted using a gradient from Buffer C to 50% Buffer E over 20 column volumes. PCNA eluted at ~300 mM NaCl. Peak fractions containing PCNA were analyzed by SDS-PAGE, pooled, concentrated to ~3 mg/ml as estimated by the BCA protein assay, flash frozen in liquid nitrogen, and stored at −80°C.
3.5 Cloning and purification of SUMO E3 ligases
3.5.1 Cloning and purification of RanBP2/Nup358 IR1
Nup358/RanBP2 (residues 2632-2695) was cloned with the TOPO-SMT3 vector (19). This Nup358/RanBP2 fragment was named IR1*.
The plasmid was transformed into E. coli strain BL21 (DE3) RIL Codon Plus.
2 L LB cultures were grown at 37°C to an A600 of 1.0 prior to addition of IPTG to a final concentration of 1mM. Cultures were incubated at 30°C for 3–4 hours.
His6-Smt3-Nup358 was purified from cell lysate by applying the mixture to Ni-NTA resin in Buffer A. His6-Smt3-Nup358 eluted from the resin in Buffer B.
The sample was applied to gel-filtration (Superdex-75) in Buffer F and fractions containing His6-Smt3-Nup358 were analyzed by SDS-PAGE, pooled, and the His6-Smt3 tag was liberated from IR1* by incubating His6-Smt3-Nup358 with Ulp1 at a 1000:1 (w/w) ratio, respectively.
The sample was denatured in 6 M guanidine hydrochloride and applied to Ni-NTA resin to remove the His6-Smt3 tag. Nup358 IR1* was collected in the flow-through fractions.
Guanidine hydrochloride was removed from the sample by applying the mixture to a desalting column equilibrated with Buffer F in which BME was substituted with 2 mM DTT. The sample was then applied to gel filtration (Superdex-75) in Buffer F.
Fractions containing Nup358 IR1* were analyzed by SDS-PAGE, pooled, concentrated to 5–10 mg/ml, flash frozen in liquid nitrogen, and stored at −80°C.
3.5.2 Cloning and purification of the yeast E3 ligase Siz1
A minimal Siz1 fragment encoding E3 ligase activity (residues 172-443) was amplified by PCR from yeast genomic DNA and cloned using the directional TOPO-SMT3 vector.
Plasmids were transformed into E. coli BL21 (DE3) RIL Codon Plus.
10 L cultures were grown by fermentation at 37°C to an A600 of 3.0 before inducing protein expression at 30°C for 3–4 hrs with the addition IPTG to a final concentration of 1 mM.
Cell cultures were processed for storage and sonication as described before (section 3.1).
The lysate was applied to Ni-NTA resin in Buffer A and His6-Smt3-Siz1 was eluted from the column using Buffer B.
Fractions were analyzed by SDS-PAGE, and those containing His6-Smt3-Siz1 were pooled and dialyzed against Buffer F. Ulp1 protease was added to the mixture at a 1000:1 (w:w) ratio of protein to Ulp1 and incubated overnight at 4°C.
To remove His6-Smt3, the mixture was applied to fresh Ni-NTA resin in Buffer A. Siz1 was recovered in the flow-through fractions.
Fractions containing Siz1 were pooled and applied to gel filtration (Superdex-200) in Buffer F. Siz1 migrates as a mono-disperse peak with an apparent molecular weight of ~35 kDa. Fractions were analyzed by SDS-PAGE, and those containing Siz1 were pooled and dialyzed against Buffer C.
Fraction containing Siz1 were applied to cation exchange resin (Mono-S) in Buffer C and eluted by applying a gradient from Buffer C to 50% Buffer E. Siz1 eluted at ~125 mM NaCl.
Fractions were analyzed by SDS-PAGE, and those containing Siz1 were pooled, concentrated to 15 mg/ml, aliquoted, flash frozen in liquid nitrogen, and stored at −80°C.
3.6 Assays for substrate conjugation under single turnover conditions
SUMO can be conjugated to many substrates using only E1 and E2 (Ubc9) in the presence of SUMO, the substrate, magnesium, and ATP. These assays contain a complex mixture of reagents, and each reactant must interact with at least one or more of the other reactants. As such, extraction of relevant kinetic parameters during substrate conjugation remains difficult under conditions of multiple turnover. To address this issue, we have utilized single turnover assays for SUMO conjugation. This is achieved by isolating Ubc9~SUMO (where ‘~’ indicates a thioester adduct) in the absence of E3 and substrate using E1, E2, SUMO and ATP. In this section we describe the methods to conduct single turnover assays using Ubc9~SUMO in conjunction with substrate titrations in the presence or absence of an E3 ligase. We will also describe methods to determine pK values during conjugation. In addition, we will describe methodologies to extract kinetic parameters from these assays.
3.6.1 Preparation of Ubc9-SUMO thioester and single turnover conjugation assay for the human SUMO conjugation system
3.6.1.1 Assay utilizing native SUMO-1
Formation of the Ubc9~SUMO adduct was carried out in a reaction Buffer I with 1 μM E1, 10 μM mature SUMO-1 and 5 μM Ubc9.
The reaction was initiated by addition of 10 μM ATP and incubated for 20 minutes at 37°C.
The reaction was quenched by removing magnesium and excess ATP by applying the mixture to a desalting column equilibrated with Buffer II. The lower pH of Buffer II increases the stability of Ubc9~SUMO for long term storage at −80°C.
Single turnover reactions were conducted by adding 0.6 μl of Ubc9~SUMO to reaction Buffer III with substrate (reaction volume: 50 μl). This usually results in a final Ubc9~SUMO concentration that ranges between 5–20 nM. In this case, we utilized the human p53 C-terminal domain (see above) at concentrations ranging from 2 μM to 94 μM at 37°C or 4°C. These assays were also carried out in the presence of IR1*, a SUMO E3 ligase, at a concentration of 60 nM. Reactions containing E3 ligase were carried out 4°C because reactions were too fast to reproducibly measure rates at higher temperatures.
Samples were removed at various time points (at least 3 time points per substrate concentration) and quenched by addition of Buffer IV, snap frozen with liquid nitrogen, and stored at −80°C.
Reaction products were separated by non-reducing SDS-PAGE carried out in MES buffer at a constant voltage of 180v for 60 minutes and subsequently transferred to PVDF membranes (See Note 4). The semi-dry transfer was conducted at 20 V for 40 minutes at room-temperature.
PVDF membranes were blocked for 1 hour at room temperature or overnight at 4°C in Blocking buffer.
PVDF membranes were probed with a primary antibody against human SUMO-1 in Blocking buffer at room temperature for 1 hour or overnight at 4°C.
PVDF membranes were washed for 30 minutes with Wash buffer with three changes of buffer to remove excess unbound primary antibody.
PVDF membranes were then incubated with secondary antibody for 1 hour at room temperature. Excess secondary antibody was removed by rinsing 3 times in Wash buffer.
PVDF membranes were incubated with ECL-Plus reagent and imaged using a LAS-3000 chemiluminescence detector (Fig. 1A) (See Note 5).
Images were processed and data quantified using either MultiGauge v2.02 or ImageGauge v4.0.
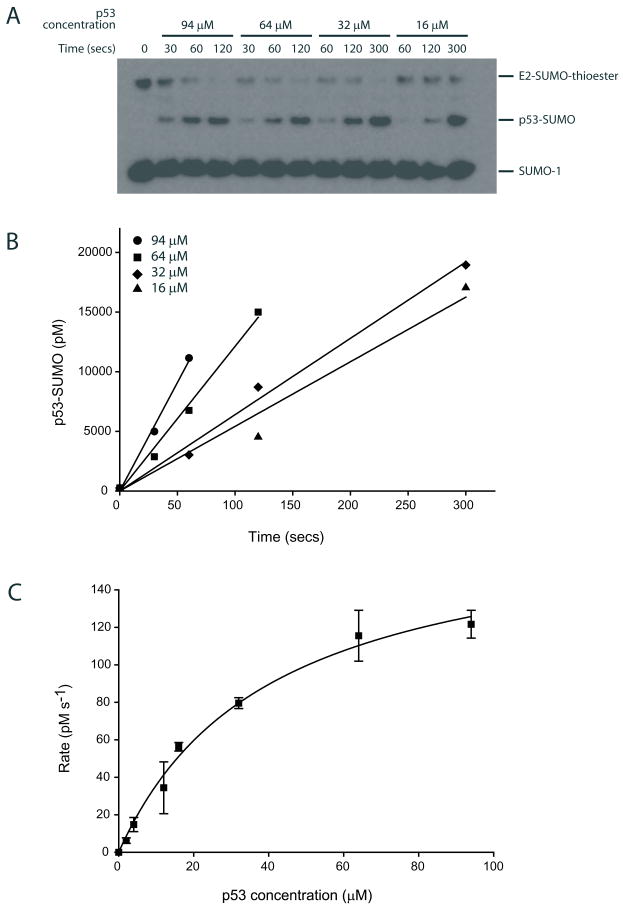
Raw data was processed with EXCEL to extract the rates of reaction at various substrate concentrations using linear regression analysis (Fig 1B).
Initial rate values obtained at different substrate concentrations were subsequently used to derive the maximal rate and apparent dissociation constant by fitting the data non-linearly to a two parameter rectangular hyperbolic function (Fig. 1C) (See Note 6). The hyperbolic function used to fit the data is of the form v = Vmax [S]/(Kd + [S]), where Vmax = k2[E]t, k2 is the rate constant, [E]t is the E2-SUMO thioester concentration, Kd is the apparent dissociation constant, and [S] is the substrate concentration. The apparent rate constant k2 could be calculated by dividing Vmax by [E]t.
The concentration of E2~SUMO ([E]t) was determined by quantifying the fraction of the E2~SUMO with respect to known input SUMO-1 concentrations. In conjugation reactions, the final concentration of E2~SUMO ranged between 5–20 nM depending on the assay conditions and date of preparation.
Figure 1. Ubc9 mediated SUMO conjugation to human p53.
(A) Time course for a single turnover assay for SUMO transfer from the E2-SUMO thioester to the C-terminal tetramerization domain of human p53 at various p53 concentrations after separation by SDS-PAGE and detection with an anti-SUMO-1 antibody. (B) Data in A depicted graphically to calculate the reaction rates at various p53 concentrations by linear regression analysis. (C) Reaction rates (Y-axis) plotted against various p53 concentrations (X-axis) and data fit to a rectangular hyperbola of the form y=ax/(b+x). The data points represent the mean of three independent experiments and error bars denote one standard deviation.
3.6.1.2 pH titration analysis of Ubc9~SUMO-1 with and without E3
Reactions were carried out at 4°C as reactions at higher pH conditions were too fast to be reproducibly measured at 22°C or 37°C.
Ubc9~SUMO thioester was generated as described in 3.6.1.1.
Single turnover assays at different pH values were conducted as described in section 3.6.1.1, but in Buffer VII to facilitate analysis across a broad pH range.
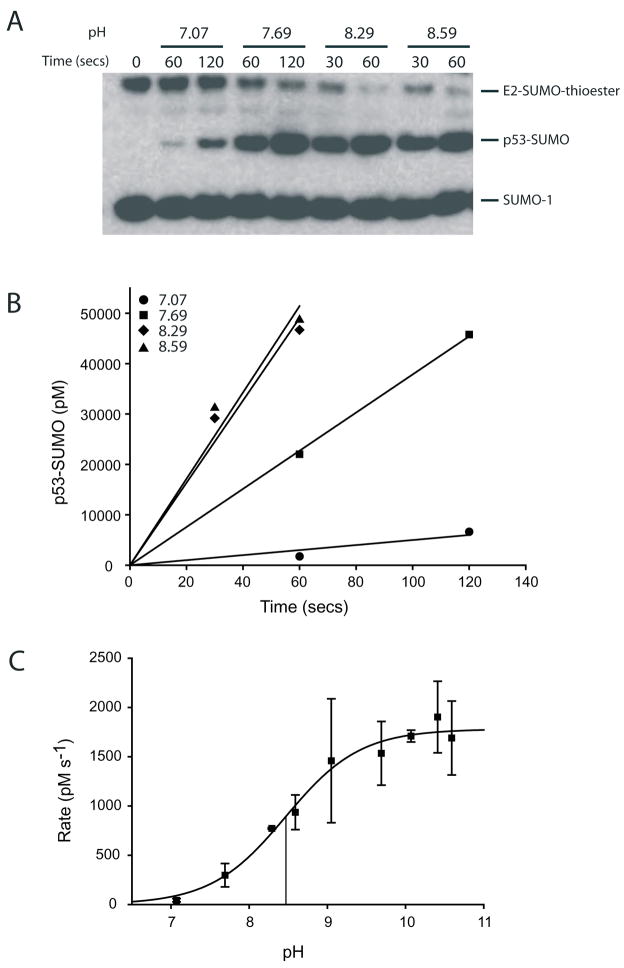
Human p53 was used as the substrate for pH analysis in assays using p53 at 94 μM in the absence of IR1* and at 1 μM in the presence of IR1*. Reactions were analyzed and processed as described in the previous section (Fig. 2A and 2B).
The pH titration data was fit to sigmoidal function of the form LH = (LHA [H+] + LA- K)/(K + [H+]) (where LH is the rate of the reaction at a particular pH; LHA and LA- are constants that denote the contribution of the acid or base form of the reactive group towards rate of the reaction; K is the equilibrium constant of dissociation of an acid; and LHA = 0 if only the basic form of the species contributes to the reaction rate). The pK of the titratable group was estimated by non-linear curve fitting (8, 23).
Figure 2. Titration of pH and analysis of Ubc9 mediated SUMO conjugation to human p53.
(A) Time course for SUMO conjugation to p53 at 94 μM under single turnover conditions at various pH values at 4°C after separation by SDS-PAGE and detection with an anti-SUMO-1 antibody. (B) Data in A depicted graphically to calculate the reaction rates at various pH values. The reaction rates are calculated by linear regression analysis. (C) Initial reaction rates (Y-axis) plotted as a function of pH and data fit to a sigmoidal function (see text for details). The vertical lines mark the pH at half-maximal activity (pK of the titratable group). The data points represent the mean of two independent experimental trials and error bars denote one standard deviation.
3.6.1.3 Assays for conjugation using Alexa Fluor 488 labeled SUMO-1
Ubc9~SUMO thioester was generated at 37°C in Buffer V with 0.27 μM E1, 12.5 μM Alexa Fluor 488 labeled SUMO-1 and 33 μM Ubc9. The reactions are initiated by addition of ATP to a final concentration of 5 mM.
The reaction was quenched by applying the reaction to a desalting column equilibrated with Buffer II to remove excess magnesium and ATP. The mix was diluted 3.3 fold to yield a final SUMO concentration of 3.8 μM and a final Ubc9 concentration of 10 μM, snap frozen in liquid nitrogen, and stored at −80°C until further use.
Single turnover SUMO conjugation to human p53 in the presence or absence of an SUMO E3 ligase IR1* was initiated by adding 0.6 μl of the E2~SUMO to 50 μl reaction Buffer VI, and human p53 at concentrations ranging from 512 μM to 0.25 μM. E3 ligase reactions contained IR1* at 100 nM.
Samples were removed at different time points (at least 3 time points per concentration) and quenched by addition of Buffer IV. Samples were snap frozen in liquid nitrogen and stored at −80°C until analysis.
Reactants were resolved by non-reducing SDS-PAGE and immediately imaged to mitigate diffusion in the gel using a FLA-5000. Alexa 488 fluorophore was excited with blue laser (473 nm) and fluorescent signal detected through a FITC filter.
Image and data processing was conducted in a manner similar to that described for analysis of chemiluminescence in section 3.6.1.1.
3.6.2 Preparation of Ubc9~SUMO and single turnover conjugation assays for the yeast SUMO conjugation system
3.6.2.1 Assays for conjugation using Alexa Fluor 488 C5-maleimide labeled Smt3
Yeast Ubc9~Smt3 thioester formation was conducted as described above (section 3.6.1.3).
Reaction mix included Buffer V with 0.27 μM yeast E1, 12.5 μM Alexa Fluor 488 labeled Smt3K11C and 33 μM yeast Ubc9 which contained the K153R point mutation to suppress auto-conjugation of SUMO to the E2 at K153.
Reactions were initiated by addition of ATP to a final concentration of 5 mM.
Reactions were quenched by applying the reaction to a desalting column equilibrated with Buffer II to remove excess magnesium and ATP. The mix was diluted 3.3-fold to yield a final Smt3 concentration of 3.8 μM and a final Ubc9 concentration of 10 μM, snap frozen in liquid nitrogen, and stored at −80°C for future use.
Single turnover conjugation of PCNA K127G in the presence of Siz1(172–443) was initiated by addition of 0.6 μl of the E2-Smt3 thioester reaction to a 50 μl reaction Buffer VI with PCNA at concentrations ranging from 40 μM to 2.5 μM.
Aliquots removed at time points ranging from seconds to minutes (at least 2 time points per concentration) and quenched by addition of Buffer IV. Samples were snap frozen with liquid nitrogen.
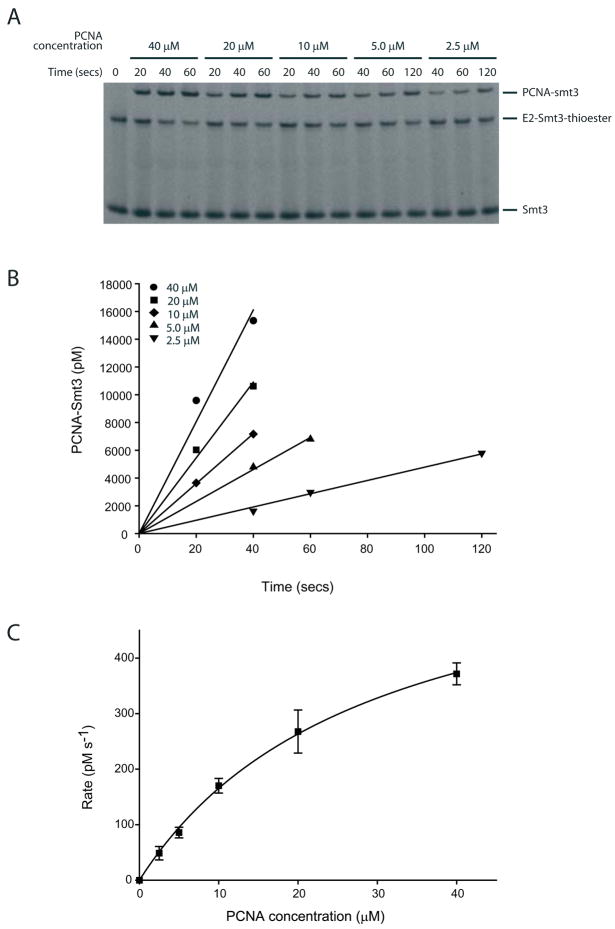
Reaction products were resolved, imaged, and processed as described in section 3.6.1.3 (Fig. 3)
Figure 3. Siz1-dependent Smt3-Alexa Fluor 488 conjugation to yeast PCNA.
(A) Time course of a single turnover assay for Smt3 transfer from the E2~Smt3 thioester to the yeast PCNA mutant K127G at various PCNA concentrations after separation by SDS-PAGE and detection of the fluorescent signal. (B) Data in A depicted graphically to calculate the reactions rates at various substrate concentrations using linear regression analysis. (C) Initial reaction rates (Y-axis) plotted against various yeast PCNA concentration and data fit to a rectangular hyperbola of the form y=ax/(b+x). The data points represent the mean of three independent experiments and error bars denote one standard deviation.
4. Notes
The pSMT3 vectors are derived from pET-28b and include an N-terminal hexahistidine fusion to S. cerevisiae Smt3 (20). pSMT3 enables cloning of the gene of interest into the MCS, usually using the BamHI site to fuse the protein of interest (POI) in-frame with the N-terminal His6-Smt3 polypeptide to generate the His6-Smt3-gly-gly-ser-POI fusion polypeptide. The Ulp1 protease can then be used to cleave His6-Smt3 from the protein of interest C-terminal to the Smt3 di-glycine sequence to liberate the POI with a non-native N-terminal serine residue. The TOPO-SMT3 vector is based on pSMT3, but custom TOPO-adapted to facilitate directional flap ligation (TOPO-adapted by Invitrogen). N-terminal Smt3 fusions to proteins can enhance expression and solubility for difficult to express proteins.
To ensure reproducibility and to protect the columns from undo wear and tear, all chromatographic steps were performed using filtered buffer solutions prepared from MilliQ (Millipore) water or the equivalent. All buffers should were degassed under vacuum for at least 1 hour prior to use.
All protein purification was conducted at 4°C to avoid degradation and/or aggregation. All protein preparations were passed through a 0.2 μm filter prior to application to chromatography media. All proteins were flash-frozen in liquid nitrogen prior to storage at −80°C.
While immunoblotting and detection has provided adequate signal to quantify protein bands, uneven transfer of protein to PVDF membranes can result in substantive artifacts. Duplicate gels and transfer steps are recommended for each experiment to ensure reproducibility.
Integration of signal requires proper background selection.
For the extraction of the kinetic parameters, it is important to obtain velocities at substrate concentrations that are at least ten-fold higher than the binding constant to ensure that the reaction is approaching saturation.
SUMO conjugating enzymes are prone to oxidation and damage. After preparation, enzymes should be aliquoted in small volumes to avoid repeated freeze-thaw cycles.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- 3.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 4.Laney JD, Hochstrasser M. Substrate targeting in the ubiquitin system. Cell. 1999;97:427–430. doi: 10.1016/s0092-8674(00)80752-7. [DOI] [PubMed] [Google Scholar]
- 5.Melchior F. SUMO--nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 6.Muller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–210. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 7.Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell. 2002;108:345–356. doi: 10.1016/s0092-8674(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 8.Yunus AA, Lima CD. Lysine activation and functional analysis of E2-mediated conjugation in the SUMO pathway. Nat Struct Mol Biol. 2006;13:491–499. doi: 10.1038/nsmb1104. [DOI] [PubMed] [Google Scholar]
- 9.Cheng CH, Lo YH, Liang SS, Ti SC, Lin FM, Yeh CH, Huang HY, Wang TF. SUMO modifications control assembly of synaptonemal complex and polycomplex in meiosis of Saccharomyces cerevisiae. Genes Dev. 2006;20:2067–2081. doi: 10.1101/gad.1430406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochstrasser M. SP-RING for SUMO: new functions bloom for a ubiquitin-like protein. Cell. 2001;107:5–8. doi: 10.1016/s0092-8674(01)00519-0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell. 2001;106:735–744. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 12.Kahyo T, Nishida T, Yasuda H. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol Cell. 2001;8:713–718. doi: 10.1016/s1097-2765(01)00349-5. [DOI] [PubMed] [Google Scholar]
- 13.Potts PR, Yu H. Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol Cell Biol. 2005;25:7021–7032. doi: 10.1128/MCB.25.16.7021-7032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt D, Muller S. PIAS/SUMO: new partners in transcriptional regulation. Cell Mol Life Sci. 2003;60:2561–2574. doi: 10.1007/s00018-003-3129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi Y, Toh-e A, Kikuchi Y. A novel factor required for the SUMO1/Smt3 conjugation of yeast septins. Gene. 2001;275:223–231. doi: 10.1016/s0378-1119(01)00662-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Blobel G. A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci U S A. 2005;102:4777–4782. doi: 10.1073/pnas.0500537102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 18.Pichler A, Knipscheer P, Saitoh H, Sixma TK, Melchior F. The RanBP2 SUMO E3 ligase is neither HECT- nor RING-type. Nat Struct Mol Biol. 2004;11:984–991. doi: 10.1038/nsmb834. [DOI] [PubMed] [Google Scholar]
- 19.Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature. 2005;435:687–692. doi: 10.1038/nature03588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell. 2000;5:865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- 21.Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 22.Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO–modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- 23.Fersht A. Structure and Mechanism in Protein Science. W. H Freeman and Company; New York: 2000. The pH dependence of enzyme catalysis. [Google Scholar]