Abstract
The longitudinal growth of long bones occurs in growth plates where chondrocytes synthesize cartilage that is subsequently ossified. Altered growth and subsequent deformity resulting from abnormal mechanical loading is often referred to as mechanical modulation of bone growth. This phenomenon has key implications in the progression of infant and juvenile musculoskeletal deformities, such as adolescent idiopathic scoliosis, hyperkyphosis, genus varus/valgus, tibia vara/valga, as well as neuromuscular diseases and clinical management of these deformities is often directed at modifying the mechanical environment of affected bones. However, there is limited quantitative and physiological understanding of how bone growth is regulated in response to mechanical loading. This review of published work addresses the state of knowledge concerning key questions about the mechanisms underlying biomechanical modulation of bone growth. The longitudinal growth of bones is apparently controlled by modifying the numbers of growth plate chondrocytes in the proliferative zone, their rate of proliferation, the amount of chondrocytic hypertrophy and the controlled synthesis and degradation of matrix throughout the growth plate. These variables may be modulated to produce a change in growth rate in the presence of sustained or cyclic mechanical load. Tissue and cellular deformations involved in the transduction of mechanical stimuli depend on the growth plate tissue material properties that are highly anisotropic, time-dependent, and that differ in different zones of the growth plate and with developmental stages. There is little information about the effects of time-varying changes in volume, water content, osmolarity of matrix, etc. on differentiation, maturation and metabolic activity of chondrocytes. Also, the effects of shear forces and torsion on the growth plate are incompletely characterized. Future work on growth plate mechanobiology should distinguish between changes in the regulation of bone growth resulting from different processes such as direct stimulation of the cell nuclei, physico-chemical stimuli, mechanical degradation of matrix or cellular components and possible alterations of local blood supply.
Keywords: growth plate, bone, endochondral growth, mechanical loading, mechanobiology
1. How do bones grow in length?
Bones, like all other tissues and organs of the body, grow to their adult size and also undergo continuous remodeling and turnover. Both the modeling and remodeling processes are sensitive to the surrounding mechanical environment. At the simplest level, bone remodeling is said to be governed by Wolff’s Law, while mechanical influence on longitudinal bone growth is said to be controlled by the Hueter-Volkmann law. Wolff’s law relates to the adaptation of bone to its mechanical environment, involving bone apposition stimulated by intermittent increased stress, and bone resorption following reduced intermittent stress. In contrast, the Hueter-Volkmann ‘law’ relates to immature bone growth suppression by sustained additional compressive loading and acceleration by reduced loading. Both ‘laws’ represent complex physiological relationships whose underlying mechanisms are incompletely understood. This is particularly true for the Hueter-Volkmann law of mechanical modulation of bone growth, which refers to changes in the longitudinal size of the bone due to loading. This is the topic of this review, which was conducted by searching the Medline (1968-August 2008) index using search terms Growth plate, Biomechanics, Bone staples, and Growth. Published articles and articles in their bibliographies were then reviewed and further Medline searches were conducted using selected authors’ names.
The biomechanical influences on bone must be viewed within the overall context of the control and regulation of skeletal growth. In humans, growth rate overall declines until skeletal maturity at the end of the second decade of life, despite sporadic growth spurts, such as in adolescence. Bones grow longitudinally primarily by synthesizing cartilaginous tissue that is subsequently transformed into bone by endochondral ossification. Longitudinal growth in long bones occurs in growth plates that lie between the epiphysis and the metaphysis at both ends of most bones, while transverse growth takes place in the periosteum via the process of intramembranous ossification. At skeletal maturity, bones are normally of the correct length, relative proportions, and there is symmetry between the two sides of the body. This is achieved by regulation of growth that involves genetic, hormonal, nutritional and environmental factors (Farnum and Wilsman, 1998a). However, the level of physical activity apparently has little influence on the eventual size and stature of an individual, which suggests that the mechanical factors, if maintained within physiological magnitude and frequency ranges, normally have limited effect relative to genetic, nutritional and hormonal controls as well as the overall health of the individual. There is a remarkable level of intrinsic control and adaptation regulating the differential growth processes that ensure normal bone development.
Mechanical influences on growth have been implicated in the development of the shapes of bones, including the femur (Shefelbine et al., 2002; Shefelbine and Carter, 2004b; Shefelbine and Carter, 2004a). The shapes of joint profiles may be determined in part by mechanical loading (Carter and Wong, 1988; Lerner et al., 1998). However, there are a number of clinical conditions of the skeleton that are thought to result from abnormal mechanical loading conditions influencing longitudinal growth prior to skeletal maturity. These include clubfoot (associated with limb position in utero), slipped capital femoral epiphysis, tibia vara, spondylolisthesis, and scoliosis. The Hueter-Volkmann law of mechanical modulation of bone growth has key implications in the pathogenesis as well as the treatment of these infant and juvenile progressive musculoskeletal deformities.
2. Bone growth process
2.1 How does the growth plate produce longitudinal growth?
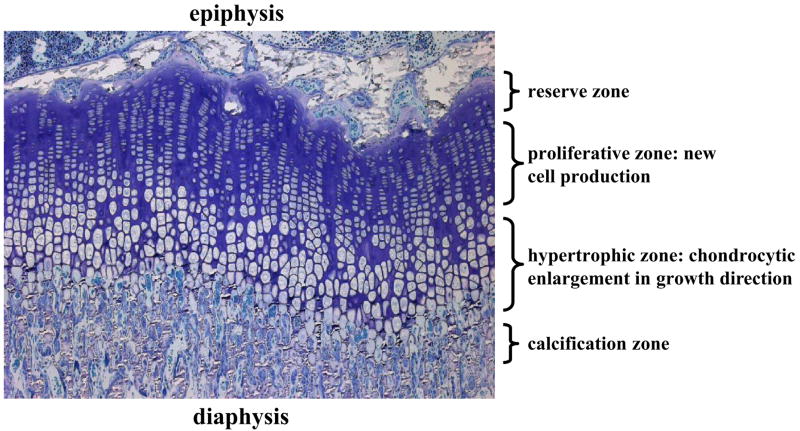
The growth plate is avascular, aneural and consists of cells (chondrocytes) embedded in an abundant extra-cellular matrix. In most species, the non-ossified growth plate disappears at skeletal maturity, although some species such as the rat retain an essentially inactive growth cartilage (Horton et al., 2008). A section of a growth plate reveals ordered columns of chondrocytes that are smaller and flatter at the top (epiphyseal end) and become larger and rounder towards the metaphysis (Figure 1). There is a highly ordered differentiation of these chondrocytes into three distinct zones, the reserve, proliferative and hypertrophic zones. The relative proportions of the three zones vary from species to species. In larger animals, the reserve zone occupies the greatest relative volume, with a size equal or greater than the two other zones combined (Farnum and Wilsman, 1998a). Sergerie et al. (2009) (Sergerie et al., 2009b) found proportions of 70%, 17% and 13% respectively for the reserve, proliferative and hypertrophic zones in newborn ulnar porcine growth plates, while Hunziker and Schenk (1989) (Hunziker and Schenk, 1989) found average proportions of the corresponding zones to be 6%, 35% and 59% in the proximal tibiae of rats aged 21 days, and 9%, 36% and 55% at age 80 days.
Figure 1.
Micrograph of a 2 μm thick section of a rat proximal tibial growth plate showing the reserve zone, as well as the proliferative and hypertrophic zones where chondrocytes firstly proliferate and subsequently enlarge (undergo hypertrophy).
Longitudinal bone growth results from a complex interplay between cell division (proliferation), cell enlargement (hypertrophy) as well as extra-cellular matrix synthesis and controlled degradation (Hunziker and Schenk, 1989; Wilsman et al., 1996; Breur et al., 1997; Farnum et al., 2000). Starting from the pool of chondrocytes in the reserve zone, there is cellular proliferation (division) in the proliferative zone that lies at the top (epiphyseal end) and several-fold volumetric enlargement of chondrocytes in the hypertrophic zone towards the diaphysis (metaphysis?) (Hunziker et al., 1987; Wilsman et al., 1996). Eventually the chondrocytes undergo apoptosis, the tissue is invaded by blood vessels and undergoes calcification in the zone of provisional calcification. Growth plates are considered to be monopolar since all the growth is directed away from the proliferative zone, whereas other growth cartilages such as apophyses are bipolar with reserve chondrocytes in the middle, and proliferative and hypertrophic chondrocytes on both sides (thus generating growth in both directions). Matrix synthesis and degradation occur in both the proliferative and hypertrophic zones, complementary to the increases in number of chondrocytes and their size. These processes control in a large measure the events of chondrocyte proliferation and hypertrophy within the growth plate (Greco et al., 1989; Hunziker and Schenk, 1989; Breur et al., 1991; Wilsman et al., 1996; Breur et al., 1997; Alvarez et al., 2000; Farnum et al., 2000). Type II collagen and aggrecan are the two main components of the growth plate extracellular matrix. Type X collagen is also found, but exclusively in the matrix of the hypertrophic zone. Despite the need to synthesise matrix required for growth, some degradation of the matrix also occurs. Collagenase 3 (or MMP-13) degrades type II and X collagens as well as aggrecan and other proteoglycans (Welgus et al., 1990; Knauper et al., 1996; Mitchell et al., 1996; Keeling and Herrera, 2008). In addition, type II and X collagens are degraded by MMP-3 (Keeling and Herrera, 2008), which is also a collagenase activator (Murphy et al., 1988; Suzuki et al., 1990) and a degrader of proteoglycans (Gunja-Smith et al., 1989). Aggrecanases, mainly ADAMTS-4 and -5, are also present (Abbaszade et al., 1999; Tortorella et al., 2000).
2.2 What factors influence bone growth rate?
The rate of longitudinal bone growth depends on the rate at which cells divide in the proliferative zone, the rate at which they enlarge in the hypertrophic zone and the rate at which they synthesize and degrade matrix (Hunziker and Schenk, 1989; Farnum and Wilsman, 2001; Ballock and O’Keefe, 2003). The height of the growth plate, as well as that of the proliferative and hypertrophic zones, correlates with the corresponding rate of growth (Wilsman et al., 1996). Kember (1985) (Kember, 1985) presented an analysis of bone growth in which the product of cell production and cell enlargement combine to control the resulting growth rate. Further, he identified the number (pool) of proliferative cells and the ultimate terminal size of hypertrophic chondrocytes as the key parameters of growth plate performance, since the rate of cell division (or cycle time) varies less than these two factors, at least within a given individual. This was confirmed by Wilsman et al. (1996) (Wilsman et al., 1996) in an analysis of relative contributions of key parameters of growth plate performance to regulating differential growth. They compared histological measurements at various anatomical locations of the same animal (rat) in a volumetric analysis of a unit cylinder of growth plate, using three parameters of growth plate performance: cell proliferation, chondrocytic cell sizes and matrix volume fraction. Most of the variability in growth rate between different growth plates was explained by differences in chondrocytic enlargement (44 to 59%) and associated matrix synthesis in the hypertrophic zone (14 to 28%), while differences in cellular duplication (7–10%) and matrix synthesis in the proliferative zone (16–21%) made a lesser contribution (Figure 2).
Figure 2.
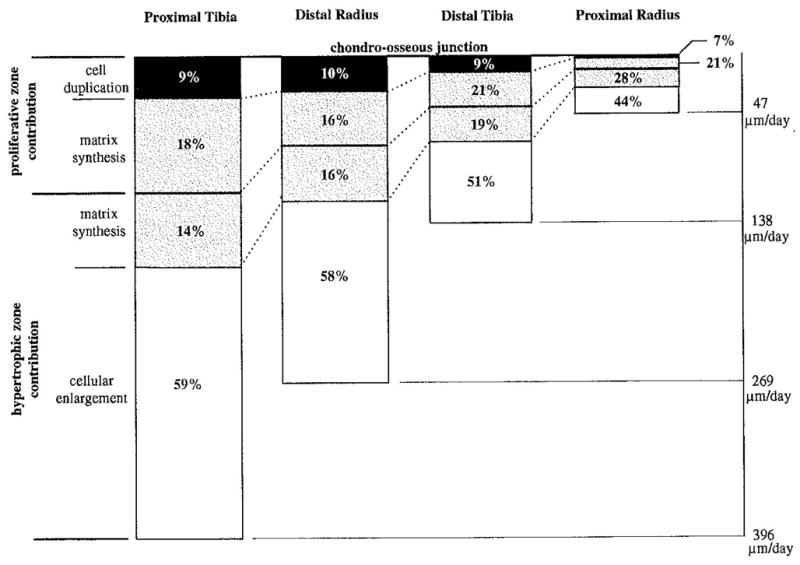
Relative contributions of four parameters of growth plate performance to the daily total elongation at the chondro-osseous junction for four rat growth plates. (Reproduced from (Wilsman et al., 1996) – with permission)
Based on the fact that growth plates produce essentially linear (uni-dimensional) growth, a simplification of this approach was used by Stokes et al. (2005) (Stokes et al., 2005) to analyze mechanically-modulated growth, based on the analysis of Kember (1985) (Kember, 1985). This analysis considers the contribution of columns of cells to the increase in bone length in the growth direction, rather than a volumetric analysis as made by Wilsman et al. (1996) (Wilsman et al., 1996). At a steady state, linear growth can then be expressed as:
where Growth (μm/day) refers to the 24-hour (daily) growth, N (cells/day) is the number of new chondrocytes created per day in the proliferative zone, which in turn is the product of the number of proliferating cells and their rate of division (Kember, 1985), and hmax (μm/cell) represents the average height achieved by fully mature chondrocytes in the hypertrophic zone.
Thus the regulation of growth rate whether by normal maturation, as altered by pathology, or as modulated by mechanical loading would be achieved by a combination of (1) a change in the rate of proliferation (cell cycle time), (2) a change in the number (pool) of chondrocytes undergoing proliferation, and (3) a different final height of hypertrophic cells in the growth direction. This simplified analysis assumes that all cells that are produced in the proliferative zone complete their differentiation into mature hypertrophic cells, and also that matrix synthesis between cells in the growth direction is negligible. Chondrocytic enlargement and matrix synthesis are strongly correlated since chondrocytes enlarge mostly in the growth direction, with little increase in their width. Hence matrix synthesis is required to fill the increased volume laterally surrounding each cell as it grows in the longitudinal direction (Figure 3).
Figure 3.
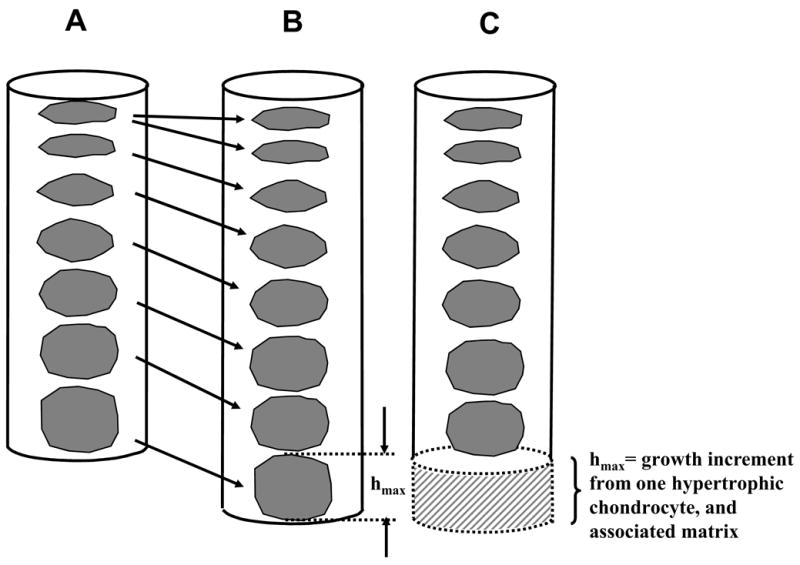
Bone growth increment associated with the creation of a new chondrocyte in the proliferative zone followed by chondrocytic hypertrophy (A to B), and eventual ossification of the fully enlarged cell having height hmax (B to C). Here a single column of cells and its matrix domain is considered before and after this cycle of events. Daily growth typically includes several of these cycles.
Regulation of bone growth rate is poorly understood, and probably complex. It likely involves numerous factors, including (1) genetic controls, possibly limiting the number of cell divisions permitted, (2) systemic hormonal levels, local action of growth factors and cytokines, nutritional status and blood supply (Trueta and Trias, 1961), (3) mechanical loading (LeVeau and Bernhardt, 1984; Farnum and Wilsman, 1998a; Stokes et al., 2006; Stokes et al., 2007; Cancel et al., 2009) and soft tissue (e.g. periosteum) constraints (Taylor et al., 1987; Wilson-MacDonald et al., 1990), (4), transport of solutes such as nutrients and signaling molecules, and (5) autocrinal and pericrinal influences, possibly influenced by mechanical factors by direct action on the cell nuclei and consequent regulation of gene expression. Among these regulatory factors, the feedback loop IHH-PTHrP (Indian HedgeHog/ParaTHyroid-related protein) plays an important role in endochondral bone growth within the growth plates (Farnum and Wilsman, 1998a; Vortkamp et al., 1998; van der Eerden et al., 2000; van der Eerden et al., 2003).
3. Growth plate behavior under mechanical load
The transduction of mechanical signals to growth plate chondrocytes probably results from cellular stresses and/or strains as well as depending on the mechanical state of the surrounding extra-cellular matrix. The thin growth plate, which is embedded within stiff bone tissue undergoing large gravitational and muscular loads, experiences primarily compression under normal physiological conditions, with slow rates of fluid exchange because of the low permeability of the adjacent bone. Regional variations in tissue properties have been determined by confocal micrscopy (Villemure et al., 2007), (Bachrach et al., 1995) and by atomic force microscopy (Radhakrishnan et al., 2004).
3.1 What are the biomechanical characteristics of the growth plate under compression?
Growth plate tested in confined or unconfined compression along the growth direction shows typical viscoelastic behavior (Figure 4), with better curve fitting obtained using the nonlinear biphasic model (Cohen et al., 1994) or the transversely isotropic biphasic model (Cohen et al., 1998; Sergerie et al., 2009b) as compared to the isotropic biphasic model. The growth plate is about ten times more compliant in the axial (longitudinal growth) direction than in its transverse (radial) direction, and the permeability is of similar range in the axial and transverse directions but shows greater variability in the axial direction (Table 1). The transverse Poisson’s ratio is at least two or three times greater than the out-of-plane (axial/transverse) Poisson’s ratio (Table 1). Growth plate mechanical behavior is comparable to articular cartilage, as both cartilaginous tissues show important similarities in their structure and composition.
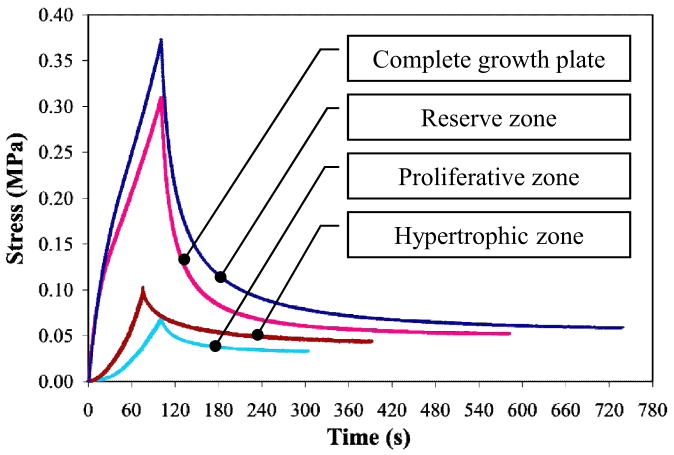
Figure 4.
Experimental stress relaxation time histories from unconfined compression tests on the complete growth plate and the reserve, proliferative and hypertrophic zones of newborn ulnar porcine growth plate samples. (Reproduced from (Sergerie et al., 2009b) - with permission).
Table 1.
Summary of reported growth plate intrinsic mechanical properties in compression and tension. Compression: E3=axial modulus; E1=transverse modulus; k3=axial permeability; k1=transverse permeability; v21=transverse Poisson’s ratio; v31= out-of-plane (i.e. axial/transverse) Poisson’s ratio. Tension: Et: tangent modulus; σu=ultimate stress; εu=ultimate strain. (Data from (Cohen et al., 1992; Cohen et al., 1994; Cohen et al., 1998; Fujii et al., 2000; Williams et al., 2001; Sergerie et al., 2009a; Sergerie et al., 2009b))
|
Compression | |||||
| E3 | E1 | K3 | K1 | v21 | v31 | |
| (Mpa) 0.3 – 1.1 |
(MPa) 4.6 – 10.6 |
(× 10−15 m4 /Ns) 0.9 – 25.0 |
(× 10−15 m4 /Ns) 1.8 – 5.0 |
( − ) 0.24 – 0.30 |
( − ) ≤ 0.10 |
|
| Tension | ||||||
| Et | σu | εu | ||||
| (MPa) 4 – 49 |
(MPa) 1.0 – 4.1 |
0.1 – 1.3 | ||||
Compressive mechanical properties are non uniform at different depths of the growth plate. Although this non uniformity differs among tested species and developmental stages, the hypertrophic zone is the less rigid region. Higher compressive strains are found mainly in regions overlapping the reserve and hypertrophic zones with lower compressive strains in the proliferative zone (Villemure et al., 2007). In the bovine ulnar growth plate, the combined chondroepiphysis/reserve zone was about twice as stiff as the combined proliferative/hypertrophic zone along the longitudinal axis as well as in the transverse plane (Cohen et al., 1998). The proliferative and hypertrophic zones of newborn porcine ulnar growth plates are half as stiff as the reserve zone along the compression axis and about three times less stiff in the transverse plane (Sergerie et al., 2009b) (Figure 4). These two zones are also three times as permeable as the reserve zone in the radial direction. It is hypothesized that the more rigid reserve zone, which also constitutes a greater proportion of growth plate height in large animals, might provide mechanical support in growth plates of large species that are undergoing slower growth rates for long periods of time together with greater mechanical loading (Kember and Sissons, 1976).
There is a significant increase in the extracellular matrix elastic moduli from the reserve zone to the chondro-osseous junction (Radhakrishnan et al., 2004) Tissue strains are non-uniform within different zones and there are differences between cell and intercellular strains in both the proliferative and hypertrophic zones (Bachrach et al., 1995). Regional variations in the compressive mechanical properties are also found in the transverse plane of the growth plate. In samples of bovine femoral growth plates, interior samples were about 40% more rigid and about 75% less permeable than samples located at the periphery, and this was attributed to the higher cellularity and water content found at the peripheral regions of the growth plate. (Cohen et al., 1994). Growth plate axial compressive rigidity also varies with developmental stage, although growth plate thickness progressively diminishes during bone growth process. In rat tibial growth plates studied at four developmental stages, the overall effective stiffnesses, combining structural and material effects, were decreased by 12% in 35 day-old rats and increased by 20 and 94% in 56 and 80 day-old rats as compared to 21 day-old rats (Villemure et al., 2004). Similarly, the equilibrium axial compressive moduli of 4, 8 and 18-week-old porcine ulnar growth plates in unconfined compression were respectively increased by 40% and then decreased by 12 and 39% when compared to the newborn growth plates (Sergerie et al., 2009b).
3.2 What are the biomechanical characteristics of the growth plate under tension?
Tangent moduli as well as ultimate stress and strain measured from uniaxial tension tests increase with faster displacement rate, indicating that the growth plate is also viscoelastic in tension (Table 1). Growth plate tensile rigidity also varies with anatomical regions of the growth plate. The lateral region of the bovine tibial growth plate was found to be stronger and more rigid than the medial and central regions in tension (Williams et al., 2001). Similarly, the posterior/medial and central regions were reported as the weakest and most compliant compared to anterior and posterior/lateral sites (Cohen et al., 1992). Also, the three zones of rabbit radial and ulnar growth plates have tangent tensile modulus 75% stiffer in the reserve zone as compared to the proliferative and hypertrophic zones, with the latter zones showing comparable tensile moduli (Fujii et al., 2000). The ultimate strain was lower (about 2 times) in the reserve zone as compared to the proliferative and hypertrophic zones. Fujii et al. (2000) (Fujii et al., 2000) speculated that the randomly oriented collagen network in the reserve zone, as compared to its longitudinal orientation in the proliferative and hypertrophic zones, might partly explain these zonal differences. Growth plate tensile mechanical properties probably change with skeletal development. Tensile moduli decrease and ultimate stress and strain increase with development (5 to 12–18 months in bovine growth plate) (Williams et al., 2001). Tensile properties (stiffness and strength) increase during development of rabbits from 20% to 80% of their weight at skeletal maturity (Guse et al., 1989).
4. Changes in bone growth from mechanical loading
The Hueter-Volkmann ‘law’ (increased compression of a growth plate reduces the growth rate, and vice versa) is qualitative and does not take into account the load history (loading rate, static versus cyclic) nor factors such as the growth plate dimensions, stage of maturity and underlying growth rate that might influence the sensitivity to mechanical loading. However, it should be noted that the Hueter-Volkmann ‘Law’ expresses a relationship that is different from that described by Wolff’s Law, which relates primarily to alterations in the internal architecture of bones, especially the trabeculae, as well as their transverse profiles. Changes in the orientation and size of trabeculae are related to the direction and magnitude of prevailing cyclic mechanical stress, with greater stress producing greater bone density and apposition. In contrast, growth plates are affected by static, sustained loads, with increased compression reducing the longitudinal growth (Stokes et al., 2005; Stokes et al., 2006; Stokes et al., 2007; Cancel et al., 2009). Although numerous experiments show empirically that bone growth is influenced by sustained load according to the Hueter-Volkmann Law, growth differences in individuals with differing activity levels are very small (Niehoff et al., 2004), indicating that growth is not much influenced by physiological dynamic loading.
4.1 How are bone growth rates modified with static loading?
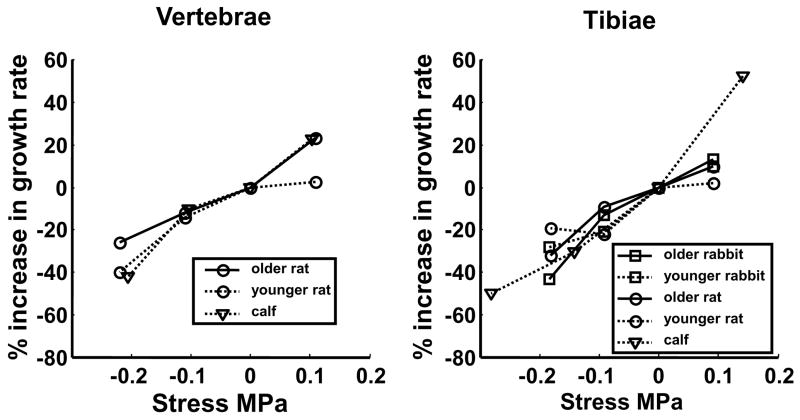
In vivo experiments using loading apparatus applied on immature animal growth plates have been used to quantify mechanical modulation of growth. The growth can be measured by comparisons of bone lengths measured directly post mortem, radiographically, or by administering one or more fluorochromes that label ossifying bone in vivo (e.g. (Mente et al., 1999). The form of the relationship between sustained stress (both tensile and compressive) and percentage change in growth rate is apparently linear (Stokes et al., 2006). The stress-growth relationship was determined in both vertebral and proximal tibial growth plates of three species (rats, rabbits and cattle). These growth plates had very different intrinsic growth rates, varying from 30 μm/day for rat vertebrae to 366 μm/day for rabbit proximal tibiae. There was no evidence of any threshold stress below which growth rate would be unchanged by the mechanical loading. The regression relationship between proportional modulation of growth and actual stress (growth-rate sensitivity to stress) did not differ by species and age of animal, but was somewhat greater in tibiae than vertebrae; 18.6% and 15% per 0.1 MPa, respectively, with an overall mean (for all animals and anatomical locations) of 17.1% per 0.1 MPa (Figure 5). This relationship is expressed in a linear formulation as:
where G (μ/day) is the actual growth, Gm (μ/day) is the mean baseline growth (unaltered stress), σ is the actual stress on growth plate (compression negative), and σm (MPa) is the mean prevailing (baseline) stress on growth plate.
Figure 5.
Relationship between applied stress and the percentage alteration in growth (relative to control) for the growth plates at two anatomical sites. The mean values from typically five animals are plotted. In each case, the mean values obtained from sham animals were subtracted and hence all mean values at 0 MPa are zero. (Reproduced from (Stokes et al., 2006) - with permission).
In the relationship shown in Figure 5, the applied stress was either tension (0.1 MPa) or compression (0.1 or 0.2 MPa) relative to a sham (unloaded) condition, which was within the normal physiological range for sustained loading. Based on published equilibrium elastic growth plate moduli (Cohen et al., 1998; Radhakrishnan et al., 2004), these sustained loading conditions would produce growth plate strains on the order of 10%. Rat growth plates subjected to compressive stress of 0.1 MPa for only 12 of the 24-hour cycle had growth suppressed by approximately half that measured in rats experiencing 24 hours of the same magnitude of compression (Stokes et al., 2005).
While the above analysis represents linear bone growth, subject to compressive or tensile stress (a one-dimensional analysis), a model of mechanically modulated bone ossification process that included both hydrostatic and octahedral (deformational) stresses and strains was proposed by Carter and Wong (1988) (Carter and Wong, 1988). Their 2-D planar stress analyses indicated that octahedral stresses would promote endochondral ossification, and hydrostatic stresses would promote preservation of cartilaginous tissue and further development of articular cartilage. However, while this model takes two-dimensional stress stimuli into account, it does not intrinsically incorporate the resulting growth orientation and the highly anisotropic nature of the growth plate (Lin et al., 2009).
Very large compressive stresses are known to arrest growth. Extrapolation from the average rate of growth suppression of 17.1% per 0.1 MPa stress suggests that a sustained compressive stress of 0.6 MPa would result in a 100% reduction in growth rate, which is growth arrest. This assumes that the stress–growth relationship follows the same linear relationship for higher compressive stresses. This estimate is supported by studies directly addressing the stress required to arrest growth. These studies give values of 0.51 and 1 MPa (Bylski-Austrow et al., 2001), more than 0.3 MPa (Bonnel et al., 1983), and more than 0.5 MPa (Safran et al., 1992). Growth arrest can be produced surgically by applying staples across a growth plate, yielding major disruption of the growth plate (Farnum et al., 2000) and its eventual calcification (Ehrlich et al., 1972). Measurements of the deformation of staples that caused growth arrest in human proximal tibial growth plates indicate that the stress was about 1 MPa (Bylski-Austrow et al., 2001).
There is very little information about the growth response to shear forces or torsion (stress perpendicular to growth direction). A study in rabbits (Moreland, 1980) indicated that there was no change in the longitudinal growth, although some evidence that the columns of chondrocytes had been deformed and produced a consequential change in growth direction. The torque and resulting shear stress were stated as ‘just less than that which would produce sufficient shear to fracture the bone’ suggesting that growth plate might have been injured during these experiments.
4.2 How are bone growth rates modified with dynamic loading?
The effects of physical (dynamic) activity on skeletal growth, i.e. whether it enhances or suppresses longitudinal bone growth, are presently not clearly determined, with growth rate following growth plate dynamic loading reported as either decreased or unchanged. Ohashi et al. (2002) (Ohashi et al., 2002) applied controlled dynamic forces (4, 8.5 or 17 N) at 2 Hz for 10 minutes per day for eight days in vivo in a rat ulnar model. Longitudinal bone growth, as measured by calcein and alizarin complexone labeling, was suppressed in a dose dependent manner with the increased applied loading when compared to their contralateral controls. Using rat caudal vertebrae, Arkyuz et al. (2006) (Akyuz et al., 2006) compared the effects of asymmetrical sustained static and dynamic (1.0 Hz) compression loading (55% body weight) over a three week period. Longitudinal growth suppression, which was measured from radiographs, was greater for dynamic loading (76%) as compared to sustained loading (67%) with respect to control animals, although these differences were not statistically significant. Using microtransducers implanted in lamb tibiae, Noonan et al. (2004) (Noonan et al., 2004) monitored bone lengths in vivo for 21 to 25 days and showed that 90% of bone lengthening occurs during recumbency phases, when growth plate loading is minimal, compared to phases of physical activities. In a study by Niehoff et al. (2004) (Niehoff et al., 2004), rats assigned to three different physical activity levels showed no significant difference in their femoral bone lengths, which were measured using an electronic caliper.
In adolescent humans, there is concern whether high skeletal loading of immature growth plates in competitive sports alters growth. There is evidence that gymnasts have altered relative growth of the radius and ulna, which might be associated with repetitive growth plate injuries and/or altered loadings selectively retarding radial or ulnar growth (De Smet et al., 1994; Caine et al., 1997). Professional tennis players studied by Krahl et al. (1994) (Krahl et al., 1994) had wider bones on their playing-side arms. Also, the length of the playing-side ulnar and 2nd metacarpal were respectively 3% and 3.7% longer than on the contralateral side, while control subjects had no measurable bone length differences between dominant and non-dominant arms.
4.3 How do mechanical loads alter chondrocyte metabolism?
The causes of the altered growth rate in mechanically loaded growth plates appear to result from a complex interaction of changes in the proliferation, differentiation and hypertrophy of the chondrocytes. These changes have been documented by histological examination and quantification of loaded growth plate morphology as well as by changes in marker genes or proteins related to chondrogenesis or osteogenesis following mechanical loading. These findings give some clues as to the mechanisms by which the loading state is transduced, and the signaling mechanisms that produce the alterations in growth plate function under load.
How do mechanical loads alter cell and matrix dimensions?
The overall mechanisms by which static (or sustained) loading alters growth plate function appears to be similar to the mechanisms underlying normal physiological regulation of differential growth at different developmental stages, for example. Sustained compressive loading affects both proliferative and hypertrophic tissue and cellular shape. Sustained compression was found to reduce the numbers and proportions of proliferating chondrocytes (Alberty et al., 1993). Stokes et al. (2007) (Stokes et al., 2007) reported that much of the proportional change in growth rate of loaded bones was explained by the sum of proportional changes in the number of proliferating chondrocytes and of the amount of chondrocytic enlargement in the hypertrophic zone. Increased pressure causes growth plate thinning in both the proliferative (Alberty et al., 1993) and hypertrophic zones (Alberty et al., 1993; Bachrach et al., 1995; Farnum and Wilsman, 1998a; Farnum et al., 2000; Stokes et al., 2002) and altered alignment of the columns of chondrocytes (Ehrlich et al., 1972; Alberty et al., 1993; Farnum and Wilsman, 1998a). Under increased compressive loading, growth plate chondrocytes reduce, but do not cease, their proliferative activity (Ehrlich et al., 1972; Alberty et al., 1993; Farnum and Wilsman, 1998a; Farnum et al., 2000), and the extent of chondrocyte hypertrophy (in terms of number, height, and volume) is also reduced (Farnum and Wilsman, 1998a; Farnum et al., 2000; Stokes et al., 2002).
Conversely, a sustained pressure reduction on the growth-plate (or growth plate distraction) results in overall thickening of the growth plate (Alberty et al., 1993; Apte and Kenwright, 1994; Stokes et al., 2002) as well as disruption of the chondrocyte columns (Alberty et al., 1993; Apte and Kenwright, 1994). Chondrocyte proliferation is usually unchanged by distraction (Alberty et al., 1993; Apte and Kenwright, 1994; Farnum and Wilsman, 1998a) together with thickening of the proliferative zone (Alberty et al., 1993; Apte and Kenwright, 1994), which would be independent of increased cell division. However, a study by (Wang and Mao, 2002) found enhanced chondrocytic proliferation following static and cyclic tensile loading. Hypertrophic zone thickening is the major contributor to the growth plate thickening (Taylor et al., 1987; Alberty et al., 1993; Apte and Kenwright, 1994; Farnum and Wilsman, 1998a; Stokes et al., 2002) with an increase in chondrocyte number, height and volume (Taylor et al., 1987; Stokes et al., 2002).
Reports concerning changes in histomorphometric parameters in dynamic loading on growth plates are inconsistent. Niehoff et al. (2004) (Niehoff et al., 2004) found significant decrease in thicknesses of the growth plate with contributions from both the proliferative and hypertrophic zones following voluntary exercise in rat femoral growth plates. These histomorphometric changes were similar to those observed in growth plates under sustained compression (Alberty et al., 1993; Farnum and Wilsman, 1998b; Farnum et al., 2000; Stokes et al., 2007; Cancel et al., 2009). Ohashi et al. (2002) (Ohashi et al., 2002) found somewhat opposite results, with thicker growth plate and hypertrophic zone, combined with an increased number of hypertrophic chondrocytes. The latter observations under compression loading were associated with an early response to pressure loading compatible with ischemia of the metaphyseal vasculature initially affecting blood supply (Farnum and Wilsman, 1998b; Farnum and Wilsman, 1998a). These results are similar to those obtained for intermittent cyclic tensile loading on cranial base growth plates (Wang and Mao, 2002), where significant increase in thicknesses of the growth plate in both proliferative and hypertrophic zones was observed in dynamically loaded rats when compared to sham rats. In addition, loading was also associated with a significantly greater number of proliferating chondrocytes and an increase, although not significant, in the number of hypertrophic chondrocytes (Wang and Mao, 2002).
How do mechanical loads alter gene and protein expressions of growth plate chondrocytes?
The effects of static and dynamic mechanical loading on marker genes or proteins related to chondrogenesis have been reported within the growth plate and surrounding tissues. There is in vitro evidence that sustained compressive loading narrowed the gene expression distribution of type II collagen, mostly in the hypertrophic zone, and of type X collagen in the lower hypertrophic zone of rat tibial growth plates (Villemure et al., 2005). These changes would potentially target the extracellular matrix of the hypertrophic zone as key players in the response of growth plate to mechanical loading. In an in vivo study of chickens loaded by carrying sand bags and thus increasing compression of their lower limb growth plates, Reich et al. (2005; 2008) (Reich et al., 2005; Reich et al., 2008) also reported narrowed protein expression zones for both types of collagen although no significant alteration of their relative gene expressions was observed. Similarly, Cancel et al. (2009) (Cancel et al., 2009) found no significant change in the mRNA expression of both Type II and Type X collagens following sustained compression of rat caudal vertebrae, but they reported lower immunostaining for both collgenous proteins in 83% of the loaded rats as compared to the control rats. In this study, extracellular matrix enzyme MMP-3 increased significantly in compressed rat caudal growth plates, which could potentially explain the reduction in type X and II collagens proteins due to mechanical loading, while no significant changes were observed in the mRNA expression of the proteolytic enzyme MMP-13 (Cancel et al., 2009). However, the loaded chicken growth plates studied by Reich et al. (2005) (Reich et al., 2005) produced a 50% increase in MMP-13 mRNA expression compared to control. Cancel et al. (2009) (Cancel et al., 2009) suggested three possible explanations for the cause of the change observed in the protein level for the collagens: (1) collagen mRNAs were not translated in proteins, (2) the lifetime of the mRNAs could be reduced, and (3) the applied load could mechanically damage the collagen fibrils. Other MMPs related to vascular invasion at the chondro-osseous junction showed moderate (MMP-2) or large increase (MMP-9) following sustained compression loading of sand bag loaded chickens (Reich et al., 2005). The number of vascular vessels across the chondro-osseous junction was indeed significantly higher in these loaded growth plates (Reich et al., 2005). Cancel et al. (2009) (Cancel et al., 2009) also reported no change in aggrecan and aggrecanases, ADAMTS-4 and -5, gene expression following sustained growth plate compression. Expression of mRNA for the parathyroid receptor (PTH-PTHrP), involved in the Indian Hedgehog-PTHrP feedback loop, remained unaffected by short-term in vitro static loading in rat tibial growth plates, suggesting that the process of differentiation from proliferative cells to hypertrophic chondrocytes is relatively insensitive to short-term mechanical loading (Villemure et al., 2005).
The effects of dynamic loading on marker genes or proteins related to chondrogenesis show some similarities and some differences with sustained compression. For growth plates undergoing in vivo dynamic loading, no changes were observed for type II and X collagens both at the gene (Tang and Mao, 2006) or protein (Ohashi et al., 2002; Niehoff et al., 2004; Tang and Mao, 2006) levels following intermittent cyclic (Ohashi et al., 2002; Tang and Mao, 2006) or exercise oriented (Niehoff et al., 2004) compressive loading of rat ulnar or cranial base growth plates. Aggrecan protein expression did not vary either following intermittent cyclic compression loading of cranial base growth plates. The gene expressions for the cartilaginous extracellular matrix proteoglycan molecules biglycan and versican were also insensitive to dynamic loading (Tang and Mao, 2006). However, versican protein expression was found to increase in the subchondral bone region with dynamic loading (Tang and Mao, 2006). Gene expression for the proteoglycan decorin was mechanically induced after short periods of cyclic loading in cranial base growth plates (Tang and Mao, 2006). However, this protein expression did not change within the growth plate zones but was markedly expressed in the perichondrium and adjacent subchondral bone (Tang and Mao, 2006). Niehoff et al. (2004) (Niehoff et al., 2004) also reported no difference in the localization of the matrilin-3 protein, which is involved in the collagen network interconnecting chondrocytes, following voluntary exercise in young rats. The vascular endothelial growth factor (VEGF), also a regulator of chondrogenesis, increased in rat ulnar growth plates cyclically compressed over physiological loading ranges, but remained unchanged under physiological cyclic compression level (Ohashi et al., 2002).
Key components related to osteogenesis within the growth plate and surrounding tissues have also been investigated in response to static and dynamic mechanical loading. Activity of proteins involved in cartilage ossification (alkaline phosphatase) as well as mRNA expression and protein level of osteopontin were upregulated in the growth plates exposed to sustained compression, suggesting an accelerated ossification as compared to controls (Reich et al., 2005; Reich et al., 2008). Also, osteopontin was most upregulated in the hypertrophic zone and at the bone-cartilage interface (Reich et al., 2005), which could be associated with premature mineralization in these regions. Gene expression of matrix Gla protein, a marker of cartilage and bone calcification, was downregulated after sustained compressive loading (Reich et al., 2008). Bone Gla protein gene expression, also a marker of mineralization, was slightly downregulated in the compact bone, while it was substantially upregulated in the trabecular bone (Reich et al., 2008). In response to intermittent cyclic loading in rat cranial base growth plates, no change was observed for osteopontin and osteocalcin gene and protein expressions (Tang and Mao, 2006). The protein expression of osteonectin, involved in the mineralization process, was more intense following voluntary exercise in rat femoral growth plates, suggesting a higher mineralization rate. Ossification is associated with capillary invasion. Rat ulnar growth plates exposed to load magnitudes over physiological ranges had fewer and irregular capillary invasion patterns than those exposed to physiological intermittent cyclic compression (Ohashi et al., 2002).
5. Discussion and conclusions
There is substantial evidence that mechanical loading affects the process of endochondral bone growth, where increased pressure on the physis retards growth and reduced pressure accelerates growth. Experimental observations indicate that chondrocytic hypertrophy is a key player in growth plate mechanobiology, as it is also in normal regulation of growth. The zone of hypertrophy is the least rigid growth plate region and would experience the greatest deformations under growth loading; it is therefore most likely to be a critical mechanotransductive zone. Growth plate compression reduces hypertrophic zone thickness, reduces hypertrophic chondrocyte volume as well as producing a loss of hypertrophic columnar arrangement and parallel reduced expression of the principal collagenous the extracellular matrix proteins (type II and X collagens). The cause of collagenous degradation (mechanical versus enzymatic) is not known. The sequence of changes (matrix composition, tissue and cellular morphometry) is not determined either, but clearly these factors are closely interdependent.
Longitudinal bone growth is also modulated by dynamic compression or tension applied on the growth plates, where increasing dynamic loading correlates with decreasing bone growth rate, and cyclic traction produces greater proliferation rate. Probably different mechanisms reduce the growth rate in dynamic loading situations, since the resulting histomorphometric changes differ, and no changes are observed in type II and X collagens protein expressions. However, there is limited experimental information about effects of dynamic loading, and these experiments have been less well controlled. Identifying the loading frequency and/or average stress (or strain) that has the greatest effect on growth would provide improved understanding of growth plate mechanobiology and the orthopaedic treatment of the progressive musculoskeletal deformities.
Several other aspects of the mechanical properties of growth plates and their physiological response to stresses remain to be explored and characterized. Little is known about the level of cellular strains associated with sustained and transient compressive loading. We need to know the effects of time-varying changes in volume, water content, osmolarity of matrix, etc. on growth plates and their function. Also, the effects of shear forces and torsion on the growth plate are incompletely known.
Understanding the mechanisms by which the differentiation, maturation and metabolic activity of chondrocytes is altered by mechanical stresses will require information about the relative importance and mode of action of different types of loading. There may be sensory mechanisms whereby bone length is sensed e.g. by tension in the periosteum. We will need to distinguish between cell regulation by (1) direct stimulation of the cell nuclei (regulation of expression of the genes involved in proliferation, hypertrophy and matrix synthesis/degradation and apoptosis, as well and autocrinal and pericrinal signaling molecules), (2) physico-chemical stimuli (e.g. mechanical effects on tissue volume, water content and resulting changes in concentrations and transport of solutes), (3) mechanical degradation of extracellular matrix components or chondrocytes, as well as (4) possible effects of mechanical loading on local blood supply.
In clinical medicine and surgery, there are several situations in which it is important to know how bone growth can be modified by mechanical loading. Many skeletal deformities are thought to progress as a consequence of altered or asymmetrical loading. These deformities include leg-length inequalities, angular deformities (scoliosis, tibia vara) and deformities resulting from malunion of fractures. Both external (such as braces) and internal (surgically implanted) devices are used in attempts to correct the abnormal loads or to compensate for the resulting deformity. For example, in the treatment of scoliotic spinal deformities, minimally invasive techniques, such as vertebral body stapling, are currently emerging as an alternative to currently used surgical procedures, which are more invasive and involving vertebral fusion. These techniques aim at stabilizing and correcting deformities by directly exploiting the process of bone growth modulation. Quantitative characterization and basic knowledge of the underlying mechanisms involved in the mechanical modulation of bone growth will be helpful in designing these new optimal devices, and in planning the timing of interventions relative to residual growth. Also, at some time in the future, modulating the signaling pathways by which mechanical forces alter growth may develop as a new emerging strategy for these progressive deformities. However, the information about these pathways remains to be determined. In the future, any attempts to engineer growth cartilages must take into account the sensitivity of these tissues to their surrounding mechanical environment.
Acknowledgments
The preparation of this manuscript was supported by the Canada Research Chair in Mechanobiology of the Pediatric Musculoskeletal System (I.V.) and by the National Institutes of Health grant R01 AR 053132 (I.A.F.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbaszade I, Liu RQ, Yang F, Rosenfeld SA, Ross OH, Link JR, Ellis DM, Tortorella MD, Pratta MA, Hollis JM, Wynn R, Duke JL, George HJ, Hillman MC, Jr, Murphy K, Wiswall BH, Copeland RA, Decicco CP, Bruckner R, Nagase H, Itoh Y, Newton RC, Magolda RL, Trzaskos JM, Burn TC, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem. 1999;274(33):23443–50. doi: 10.1074/jbc.274.33.23443. [DOI] [PubMed] [Google Scholar]
- Akyuz E, Braun JT, Brown NA, Bachus KN. Static versus dynamic loading in the mechanical modulation of vertebral growth. Spine. 2006;31(25):E952–8. doi: 10.1097/01.brs.0000248810.77151.22. [DOI] [PubMed] [Google Scholar]
- Alberty A, Peltonen J, Ritsila V. Effects of distraction and compression on proliferation of growth plate chondrocytes. A study in rabbits. Acta Orthop Scand. 1993;64(4):449–55. doi: 10.3109/17453679308993665. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Balbin M, Santos F, Fernandez M, Ferrando S, Lopez JM. Different bone growth rates are associated with changes in the expression pattern of types II and X collagens and collagenase 3 in proximal growth plates of the rat tibia. J Bone Miner Res. 2000;15(1):82–94. doi: 10.1359/jbmr.2000.15.1.82. [DOI] [PubMed] [Google Scholar]
- Apte SS, Kenwright J. Physeal distraction and cell proliferation in the growth plate. J Bone Joint Surg Br. 1994;76(5):837–43. [PubMed] [Google Scholar]
- Bachrach NM, Valhmu WB, Stazzone E, Ratcliffe A, Lai WM, Mow VC. Changes in proteoglycan synthesis of chondrocytes in articular cartilage are associated with the time-dependent changes in their mechanical environment. J Biomech. 1995;28(12):1561–1569. doi: 10.1016/0021-9290(95)00103-4. [DOI] [PubMed] [Google Scholar]
- Ballock RT, O’Keefe RJ. The biology of the growth plate. J Bone Joint Surg Am. 2003;85-A(4):715–26. [PubMed] [Google Scholar]
- Bonnel F, Peruchon E, Baldet P, Dimeglio A, Rabischong P. Effects of compression on growth plates in the rabbit. Acta Orthop Scand. 1983;54(5):730–3. doi: 10.3109/17453678308996619. [DOI] [PubMed] [Google Scholar]
- Breur GJ, Lapierre MD, Kazmierczak K, Stechuchak KM, McCabe GP. The domain of hypertrophic chondrocytes in growth plates growing at different rates. Calcif Tissue Int. 1997;61(5):418–25. doi: 10.1007/s002239900358. [DOI] [PubMed] [Google Scholar]
- Breur GJ, VanEnkevort BA, Farnum CE, Wilsman NJ. Linear relationship between the volume of hypertrophic chondrocytes and the rate of longitudinal bone growth in growth plates. J Orthop Res. 1991;9(3):348–59. doi: 10.1002/jor.1100090306. [DOI] [PubMed] [Google Scholar]
- Bylski-Austrow DI, Wall EJ, Rupert MP, Roy DR, Crawford AH. Growth plate forces in the adolescent human knee: a radiographic and mechanical study of epiphyseal staples. J Pediatr Orthop. 2001;21(6):817–23. [PubMed] [Google Scholar]
- Caine D, Howe W, Ross W, Bergman G. Does repetitive physical loading inhibit radial growth in female gymnasts? Clin J Sport Med. 1997;7(4):302–8. doi: 10.1097/00042752-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone. 2009;44(2):306–15. doi: 10.1016/j.bone.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Carter DR, Wong M. Mechanical stresses and endochondral ossification in the chondroepiphysis. J Orthop Res. 1988;6(1):148–154. doi: 10.1002/jor.1100060120. [DOI] [PubMed] [Google Scholar]
- Cohen B, Chorney GS, Phillips DP, Dick HM, Buckwalter JA, Ratcliffe A, Mow VC. The microstructural tensile properties and biochemical composition of the bovine distal femoral growth plate. J Orthop Res. 1992;10(2):263–75. doi: 10.1002/jor.1100100214. [DOI] [PubMed] [Google Scholar]
- Cohen B, Chorney GS, Phillips DP, Dick HM, Mow VC. Compressive stress-relaxation behavior of bovine growth plate may be described by the nonlinear biphasic theory. J Orthop Res. 1994;12(6):804–13. doi: 10.1002/jor.1100120608. [DOI] [PubMed] [Google Scholar]
- Cohen B, Lai WM, Mow VC. A transversely isotropic biphasic model for unconfined compression of growth plate and chondroepiphysis. J Biomech Eng. 1998;120(4):491–6. doi: 10.1115/1.2798019. [DOI] [PubMed] [Google Scholar]
- De Smet L, Claessens A, Lefevre J, Beunen G. Gymnast wrist: an epidemiologic survey of ulnar variance and stress changes of the radial physis in elite female gymnasts. Am J Sports Med. 1994;22(6):846–50. doi: 10.1177/036354659402200618. [DOI] [PubMed] [Google Scholar]
- Ehrlich MG, Mankin HJ, Treadwell BV. Biochemical and physiological events during closure of the stapled distal femoral epiphyseal plate in rats. J Bone Joint Surg Am. 1972;54(2):309–22. [PubMed] [Google Scholar]
- Farnum CE, Nixon A, Lee AO, Kwan DT, Belanger L, Wilsman NJ. Quantitative three-dimensional analysis of chondrocytic kinetic responses to short-term stapling of the rat proximal tibial growth plate. Cells Tissues Organs. 2000;167(4):247–58. doi: 10.1159/000016787. [DOI] [PubMed] [Google Scholar]
- Farnum CE, Wilsman NJ. Effects of Distraction and Compression on Growth Plate Function. In: Buckwalter JA, Ehrlich MG, Sandell LJ, Trippel SB, editors. Skeletal Growth and Development. AAOS; Rosemont: 1998a. pp. 517–530. [Google Scholar]
- Farnum CE, Wilsman NJ. Growth Plate Cellular Function. In: Buckwalter JA, Ehrlich MG, Sandell LJ, Trippel SB, editors. Skeletal Growth and Development. AAOS; Rosemont: 1998b. pp. 203–243. [Google Scholar]
- Farnum CE, Wilsman NJ. Converting a differentiation cascade into longitudinal growth: stereology and analysis of transgenic animals as tools for understanding growth plate function. Curr Opin Orthop. 2001;12(5):428–433. [Google Scholar]
- Fujii T, Takai S, Arai Y, Kim W, Amiel D, Hirasawa Y. Microstructural properties of the distal growth plate of the rabbit radius and ulna: biomechanical, biochemical, and morphological studies. J Orthop Res. 2000;18(1):87–93. doi: 10.1002/jor.1100180113. [DOI] [PubMed] [Google Scholar]
- Greco F, de Palma L, Specchia N, Mannarini M. Growth-plate cartilage metabolic response to mechanical stress. J Pediatr Orthop. 1989;9(5):520–4. doi: 10.1097/01241398-198909010-00004. [DOI] [PubMed] [Google Scholar]
- Gunja-Smith Z, Nagase H, Woessner JF., Jr Purification of the neutral proteoglycan-degrading metalloproteinase from human articular cartilage tissue and its identification as stromelysin matrix metalloproteinase-3. Biochem J. 1989;258(1):115–9. doi: 10.1042/bj2580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse RJ, Connolly JF, Alberts R, Lippiello L. Effect of aging on tensile mechanical properties of the rabbit distal femoral growth plate. J Orthop Res. 1989;7(5):667–73. doi: 10.1002/jor.1100070506. [DOI] [PubMed] [Google Scholar]
- Horton JA, Bariteau JT, Loomis RM, Strauss JA, Damron TA. Ontogeny of skeletal maturation in the juvenile rat. Anat Rec (Hoboken) 2008;291(3):283–92. doi: 10.1002/ar.20650. [DOI] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK. Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J Physiol. 1989;414:55–71. doi: 10.1113/jphysiol.1989.sp017676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69(2):162–73. [PubMed] [Google Scholar]
- Keeling J, Herrera GA. Human matrix metalloproteinases: characteristics and pathologic role in altering mesangial homeostasis. Microsc Res Tech. 2008;71(5):371–9. doi: 10.1002/jemt.20565. [DOI] [PubMed] [Google Scholar]
- Kember NF. Comparative patterns of cell division in epiphyseal cartilage plates in the rabbit. J Anat. 1985;142:185–190. [PMC free article] [PubMed] [Google Scholar]
- Kember NF, Sissons HA. Quantitative histology of the human growth plate. J Bone Joint Surg Br. 1976;58-B(4):426–35. doi: 10.1302/0301-620X.58B4.1018028. [DOI] [PubMed] [Google Scholar]
- Knauper V, Will H, Lopez-Otin C, Smith B, Atkinson SJ, Stanton H, Hembry RM, Murphy G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124–31. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- Krahl H, Michaelis U, Pieper HG, Quack G, Montag M. Stimulation of bone growth through sports. A radiologic investigation of the upper extremities in professional tennis players. Am J Sports Med. 1994;22(6):751–7. doi: 10.1177/036354659402200605. [DOI] [PubMed] [Google Scholar]
- Lerner AL, Kuhn JL, Hollister SJ. Are regional variations in bone growth related to mechanical stress and strain parameters? J Biomech. 1998;31(4):327–335. doi: 10.1016/s0021-9290(98)00015-3. [DOI] [PubMed] [Google Scholar]
- LeVeau BF, Bernhardt DB. Developmental biomechanics. Effect of forces on the growth, development, and maintenance of the human body. Phys Ther. 1984;64(12):1874–82. doi: 10.1093/ptj/64.12.1874. [DOI] [PubMed] [Google Scholar]
- Lin H, Aubin CE, Parent S, Villemure I. Mechanobiological bone growth: comparative analysis of two biomechanical modeling approaches. Med Biol Eng Comput. 2009;47(4):357–66. doi: 10.1007/s11517-008-0425-9. [DOI] [PubMed] [Google Scholar]
- Mente PL, Aronsson DD, Stokes IA, Iatridis JC. Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res. 1999;17(4):518–24. doi: 10.1002/jor.1100170409. [DOI] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97(3):761–8. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland MS. Morphological effects of torsion applied to growing bone. An in vivo study in rabbits. J Bone Joint Surg Br. 1980;62B(2):230–237. doi: 10.1302/0301-620X.62B2.6988435. [DOI] [PubMed] [Google Scholar]
- Murphy G, Nagase H, Brinckerhoff CE. Relationship of procollagenase activator, stromelysin and matrix metalloproteinase 3. Coll Relat Res. 1988;8(4):389–91. doi: 10.1016/s0174-173x(88)80009-8. [DOI] [PubMed] [Google Scholar]
- Niehoff A, Kersting UG, Zaucke F, Morlock MM, Bruggemann GP. Adaptation of mechanical, morphological, and biochemical properties of the rat growth plate to dose-dependent voluntary exercise. Bone. 2004;35(4):899–908. doi: 10.1016/j.bone.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Noonan KJ, Farnum CE, Leiferman EM, Lampl M, Markel MD, Wilsman NJ. Growing pains: are they due to increased growth during recumbency as documented in a lamb model? J Pediatr Orthop. 2004;24(6):726–31. doi: 10.1097/00004694-200411000-00024. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Robling AG, Burr DB, Turner CH. The effects of dynamic axial loading on the rat growth plate. J Bone Miner Res. 2002;17(2):284–92. doi: 10.1359/jbmr.2002.17.2.284. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan P, Lewis NT, Mao JJ. Zone-specific micromechanical properties of the extracellular matrices of growth plate cartilage. Ann Biomed Eng. 2004;32(2):284–91. doi: 10.1023/b:abme.0000012748.41851.b4. [DOI] [PubMed] [Google Scholar]
- Reich A, Jaffe N, Tong A, Lavelin I, Genina O, Pines M, Sklan D, Nussinovitch A, Monsonego-Ornan E. Weight loading young chicks inhibits bone elongation and promotes growth plate ossification and vascularization. J Appl Physiol. 2005;98(6):2381–9. doi: 10.1152/japplphysiol.01073.2004. [DOI] [PubMed] [Google Scholar]
- Reich A, Sharir A, Zelzer E, Hacker L, Monsonego-Ornan E, Shahar R. The effect of weight loading and subsequent release from loading on the postnatal skeleton. Bone. 2008;43(4):766–74. doi: 10.1016/j.bone.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Safran MR, Eckardt JJ, Kabo JM, Oppenheim WL. Continued growth of the proximal part of the tibia after prosthetic reconstruction of the skeletally immature knee. Estimation of the minimum growth force in vivo in humans. J Bone Joint Surg Am. 1992;74(8):1172–9. [PubMed] [Google Scholar]
- Sergerie K, Gennaro B, Eveno A-S, Dubois-Rioux M-C, Lacoursière M-O, Villemure I. Intrinsic mechanical properties of porcine growth plates vary with developmental stages. 55th Annual Meeting of the ORS; Las Vegas, NV, USA. 2009a. [Google Scholar]
- Sergerie K, Lacoursiere MO, Levesque M, Villemure I. Mechanical properties of the porcine growth plate and its three zones from unconfined compression tests. J Biomech. 2009b;42(4):510–6. doi: 10.1016/j.jbiomech.2008.11.026. [DOI] [PubMed] [Google Scholar]
- Shefelbine SJ, Carter DR. Mechanobiological predictions of femoral anteversion in cerebral palsy. Ann Biomed Eng. 2004a;32(2):297–305. doi: 10.1023/b:abme.0000012750.73170.ba. [DOI] [PubMed] [Google Scholar]
- Shefelbine SJ, Carter DR. Mechanobiological predictions of growth front morphology in developmental hip dysplasia. J Orthop Res. 2004b;22(2):346–352. doi: 10.1016/j.orthres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Shefelbine SJ, Tardieu C, Carter DR. Development of the femoral bicondylar angle in hominid bipedalism. Bone. 2002;30(5):765–770. doi: 10.1016/s8756-3282(02)00700-7. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Aronsson DD, Dimock AN, Cortright V, Beck S. Endochondral growth in growth plates of three species at two anatomical locations modulated by mechanical compression and tension. J Orthop Res. 2006;24(6):1327–34. doi: 10.1002/jor.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes IA, Clark KC, Farnum CE, Aronsson DD. Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone. 2007;41(2):197–205. doi: 10.1016/j.bone.2007.04.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes IA, Mente PL, Iatridis JC, Farnum CE, Aronsson DD. Enlargement of growth plate chondrocytes modulated by sustained mechanical loading. J Bone Joint Surg Am. 2002;84-A(10):1842–8. doi: 10.2106/00004623-200210000-00016. [DOI] [PubMed] [Google Scholar]
- Stokes IAF, Gwadera J, Dimock A, Farnum CE, Aronsson DD. Modulation of vertebral and tibial growth by compression loading: diurnal versus full-time loading. J Orthop Res. 2005;2(3):188–95. doi: 10.1016/j.orthres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29(44):10261–70. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Tang M, Mao JJ. Matrix and gene expression in the rat cranial base growth plate. Cell Tissue Res. 2006;324(3):467–74. doi: 10.1007/s00441-005-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JF, Warrell E, Evans RA. The response of the rat tibial growth plates to distal periosteal division. J Anat. 1987;151:221–31. [PMC free article] [PubMed] [Google Scholar]
- Tortorella M, Pratta M, Liu RQ, Abbaszade I, Ross H, Burn T, Arner E. The thrombospondin motif of aggrecanase-1 (ADAMTS-4) is critical for aggrecan substrate recognition and cleavage. J Biol Chem. 2000;275(33):25791–7. doi: 10.1074/jbc.M001065200. [DOI] [PubMed] [Google Scholar]
- Trueta J, Trias A. The vascular contribution to osteogenesis. IV. The effect of pressure upon the epiphysial cartilage of the rabbit. J Bone Joint Surg Br. 1961;43-B:800–13. doi: 10.1302/0301-620X.43B4.800. [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Gevers EF, Lowik CW, Wit JM. Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: evidence for a locally acting growth restraining feedback loop after birth. J Bone Miner Res. 2000;15(6):1045–55. doi: 10.1359/jbmr.2000.15.6.1045. [DOI] [PubMed] [Google Scholar]
- van der Eerden BC, Karperien M, Wit JM. Systemic and local regulation of the growth plate. Endocr Rev. 2003;24(6):782–801. doi: 10.1210/er.2002-0033. [DOI] [PubMed] [Google Scholar]
- Villemure I, Chung MA, Kimm MH, Matyas FR, Duncan NA. Mechanical properties of rat cartilaginous growth plates vary with developmental stages. In: S, editor. International Research Society of Spinal Deformities. 2004. pp. 227–230. [Google Scholar]
- Villemure I, Chung MA, Seck CS, Kimm MH, Matyas JR, Duncan NA. Static compressive loading reduces the mRNA expression of type II and X collagen in rat growth-plate chondrocytes during postnatal growth. Connect Tissue Res. 2005;46(4–5):211–219. doi: 10.1080/03008200500344058. [DOI] [PubMed] [Google Scholar]
- Villemure I, Cloutier L, Matyas JR, Duncan NA. Non-uniform strain distribution within rat cartilaginous growth plate under uniaxial compression. J Biomech. 2007;40(1):149–56. doi: 10.1016/j.jbiomech.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71(1–2):65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- Wang X, Mao JJ. Chondrocyte proliferation of the cranial base cartilage upon in vivo mechanical stresses. J Dent Res. 2002;81(10):701–5. doi: 10.1177/154405910208101009. [DOI] [PubMed] [Google Scholar]
- Welgus HG, Fliszar CJ, Seltzer JL, Schmid TM, Jeffrey JJ. Differential susceptibility of type X collagen to cleavage by two mammalian interstitial collagenases and 72-kDa type IV collagenase. J Biol Chem. 1990;265(23):13521–7. [PubMed] [Google Scholar]
- Williams JL, Do PD, Eick JD, Schmidt TL. Tensile properties of the physis vary with anatomic location, thickness, strain rate and age. J Orthop Res. 2001;19(6):1043–8. doi: 10.1016/S0736-0266(01)00040-7. [DOI] [PubMed] [Google Scholar]
- Wilsman NJ, Farnum CE, Green EM, Lieferman EM, Clayton MK. Cell cycle analysis of proliferative zone chondrocytes in growth plates elongating at different rates. J Orthop Res. 1996;14(4):562–72. doi: 10.1002/jor.1100140410. [DOI] [PubMed] [Google Scholar]
- Wilson-MacDonald J, Houghton GR, Bradley J, Morscher E. The relationship between periosteal division and compression or distraction of the growth plate. An experimental study in the rabbit. J Bone Joint Surg Br. 1990;72(2):303–8. doi: 10.1302/0301-620X.72B2.2312573. [DOI] [PubMed] [Google Scholar]